Abstract
Vaccination against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) represents our greatest hope to combat the devastating coronavirus disease 2019 (COVID-19) pandemic. Amid ongoing global vaccination efforts, rare cases of severe allergic reactions to COVID-19 mRNA vaccines have received significant attention. Although the exact nature of these reactions may be heterogeneous, various approaches exist to engage with patients, communities, public health departments, primary care providers, and other clinicians in a multidisciplinary approach to advance population health. Whereas it is optimal for patients to receive COVID-19 vaccination as outlined in emergency use authorizations, second-dose deferral of mRNA vaccines may be a consideration within a shared decision-making paradigm of care in select circumstances characterized by high durable first-vaccine–dose protection and significant elevations of vaccine anaphylaxis risk. Still, the durability of protection afforded by a single dose of a COVID-19 mRNA vaccine is uncertain, and alternative approaches to complete vaccination, including precautionary use of a COVID-19 viral vector vaccine, also remain patient-preference–sensitive options. There is an urgent need to define correlates of COVID-19 immunity and the level of longer-term protection afforded by COVID-19 vaccination.
Key words: COVID-19, Vaccine, Adverse effects, Anaphylaxis, Guideline, Shared decision making
Abbreviations used: CDC, U.S. Centers for Disease Control and Prevention; COVID-19, Coronavirus disease 2019; EUA, Emergency use authorization; SARS-CoV-2, Severe acute respiratory syndrome coronavirus 2
To Second Dose or Not to Second Dose—That is the Question
Within the context of a rapidly mobilizing global vaccination effort to combat coronavirus disease 2019 (COVID-19), questions have emerged regarding whether or not to proceed with a timely second COVID-19 mRNA vaccine dose for individuals who have had a potential allergic reaction to a first dose.1, 2, 3, 4 While guidance has evolved, as of this writing, the U.S. Centers for Disease Control and Prevention (CDC) considers a severe or immediate allergic reaction after a previous dose of a COVID-19 mRNA vaccine a contraindication to future administration of a COVID-19 mRNA vaccine and suggests evaluation with an allergist-immunologist for patients with immediate allergic reactions of any severity to an mRNA vaccine.5 Furthermore, the CDC advises that, if delays in administering the second dose are unavoidable, the Pfizer-BioNtech and Moderna COVID-19 mRNA vaccines can be administered up to 6 weeks following the initial dose.5
Ultimately, whether to provide or defer a second mRNA vaccine dose in patients at risk for anaphylaxis to a COVID-19 mRNA vaccine warrants an approach grounded in shared decision-making because this is a highly preference-sensitive care option given current data.6, 7, 8 While the allergist-immunologist engages the patient in shared decision-making, it is important that the clinician guard against a tendency toward risk aversion to “first, do no harm,” particularly given that COVID-19 mRNA first-dose effectiveness estimates range from 46% to 93% among various studies with uncertain duration of immunity.3 , 4 , 9, 10, 11, 12, 13, 14 Whereas the risk of anaphylaxis is real, it is easy to lose sight of the forest for the trees while striving for nonmaleficence through second-dose deferral and actually place a patient at greater risk for disease morbidity in the long term.15 Furthermore, it is quite possible for the allergist-immunologist to inadvertently exaggerate the patient's fears and hesitancy in the decision-making process or even replace a patient's own values with that of the clinician.8, 9, 10 Our specialty has a checkered past in trying to attenuate risk of anaphylaxis in the short term while sacrificing long-term health benefits, and we need not look far to find obvious examples, which range from overdiagnosis of anaphylaxis risk to vaccines, foods, and medications.16, 17, 18, 19, 20
Caveats of Single-Dose COVID-19 mRNA Vaccine Immunity
The risk of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and its associated morbidity is currently unclear when a single dose of a COVID-19 mRNA vaccine is not followed by the recommended second dose.1, 2, 3, 4 , 11, 12, 13 , 21 Early evidence is encouraging and suggests that a single dose (with certain vaccines) may reduce the risk of symptomatic COVID-19 infection by up to 90%.3 , 4 , 21 However, follow-up has been limited for single-dose recipients, and this may represent an upper limit of protection that, in reality, could be much lower.1, 2, 3, 4 , 11 The question of whether to delay the second dose of these vaccines to facilitate more rapid dissemination of first vaccine doses has been an area of debate, even in patients without a prior reaction to a first dose.2 At present, concern remains that deviating from recommended intervals could lead to a suboptimal vaccine immune response.2 Indeed, we do not yet even know for certain the duration of protection afforded by the full series.2 , 14 In addition, there should be consideration that the efficacy of a single dose could be comparatively reduced in the elderly and those at risk for high morbidity and mortality associated with COVID-19. Conversely, it is also important to consider that individuals with prior natural SARS-CoV-2 infection might have broader protection following a single dose.22 Thus, a decision regarding second-dose deferral is challenging because reliable correlates of immune protection and long-term follow-up on outcomes are lacking.
Anaphylaxis-Risk Thresholds for Second-Dose COVID-19 mRNA Vaccine Deferral
In weighing the risk versus benefit, could a management strategy consisting of deferral of the second dose of a COVID-19 mRNA vaccine in those with significantly increased anaphylaxis risk be a reasonable option for some? Answering such a question relies on several theoretical assumptions. Decisional modeling is possible if we presume durable immunity lasting at least 12 months from vaccination following a single mRNA vaccine dose and maintain the efficacy rates attributed to the COVID-19 mRNA vaccines from publications supporting emergency use. Adapting a previously published health-economic model of risk-stratification strategies relative to COVID-19 mRNA vaccine anaphylaxis to evaluate anaphylaxis-risk thresholds for deferral of a second dose of these vaccines reveals interesting findings (Table I ).23 Assuming a very high rate of first-dose durable protection of 90% against symptomatic COVID-19 infection over the ensuing year with a 5% additional second-dose benefit, deferring a second dose would be cost effective if anaphylaxis risk exceeds 0.13% (1,300 anaphylaxis cases/million vaccinations; willingness to pay threshold of $10,000,000 per death prevented). Although a 0.13% risk is quite low when considering second-dose risk in a patient with a convincing history of anaphylaxis to a first dose, it is well above the currently reported population risk (2.5–11.1 anaphylaxis cases per million) for COVID-19 mRNA vaccines.24 , 25 Furthermore, the durability of first (or even second) dose protection is uncertain, and it should be noted that, if either the durable immunological protection of a first vaccine dose falls (an assumption that may be more realistic)11 or the overall vaccine efficacy against COVID-19 (and present and future variants in the next year) decreases,26 the anaphylaxis-risk threshold for second-vaccine–dose deferral of an mRNA vaccine rises from 0.13% to an upper limit of 6.05% to 21.72% across estimates of overall vaccine protection (60,500–217,200 anaphylaxis cases/million; Table I).
Table I.
Simulation model-based guidance for COVID-19 vaccine second-dose deferral∗
| First-dose protection | Second-dose protection | Anaphylaxis risk threshold† |
|---|---|---|
| Very high overall vaccine protection | ||
| 90% | 5% | 0.13% |
| 75% | 20% | 1.27% |
| 50% | 45% | 5.72% |
| 30% | 65% | 11.57% |
| 5% | 90% | 21.72% |
| High overall vaccine protection | ||
| 70% | 5% | 0.38% |
| 50% | 25% | 3.18% |
| 30% | 45% | 8.01% |
| 5% | 70% | 16.02% |
| Moderate overall vaccine protection | ||
| 45% | 5% | 0.70% |
| 30% | 20% | 3.56% |
| 5% | 45% | 10.88% |
| Low overall vaccine protection | ||
| 25% | 5% | 0.96% |
| 5% | 25% | 6.05% |
This table demonstrates the anaphylaxis risk threshold projected using different modeled scenarios considering the efficacy of the first dose and the increase in second-dose protection against symptomatic COVID-19 infection. The degree to which the first and second doses offer protection to other contacts of the vaccined person was not specifically modeled and could increase the anaphylaxis threshold at which second-dose deferral would be cost effective.
Evaluation of cost-effectiveness threshold (willingness to pay: $10,000,000 per death prevented) of anaphylaxis risk for a second dose of an mRNA COVID-19 vaccination from a societal perspective. Anaphylaxis-risk threshold represents the value at which second-vaccine deferral is cost effective in an at-risk population who has received some degree of durable protection from a first vaccination for the ensuing 12 months.
Whereas a second-dose–deferral trade-off can be calculated, using theoretical assumptions about a durable immune response to the vaccine, it is important to realize that this only reaches thresholds for value-based care at anaphylaxis rates that are far greater relative to reported COVID-19 mRNA vaccination population risk.24 , 25 , 27 The science of COVID-19 is currently moving very rapidly, and we are seeing new SARS-CoV-2 variants in the community that are more transmissible and may impact the effectiveness of all COVID-19 vaccines in clinical use or under study that are currently based on a spike protein construct.26 Whereas estimates can account for variable overall effectiveness against new SARS-CoV-2 variants (Table I), it remains crucial to realize that cases of true COVID-19 mRNA vaccine anaphylaxis are very rare,24 , 25 , 28 and the allergist-immunologist is much more likely to encounter patients with nonanaphylactic reactions to mRNA vaccines for which second-dose–vaccine deferral beyond the accepted 6 weeks would not be in the patient's best interest.
Approaches to Administration of Second Doses of COVID-19 mRNA Vaccines
Any analysis of second-dose deferral is further limited by the fact that as of this writing few persons have received a second dose after an initial dose resulted in anaphylaxis, and we do not have accurate estimates for how likely anaphylaxis will recur following a second dose. The U.S. Centers for Disease Control and Prevention (CDC) currently advises against second dose or future administration of a COVID-mRNA vaccine for such individuals.5 However, our experience with other allergies importantly illustrates that reaction severity may not be reproducible, and reactions may not even consistently recur, especially if the mechanism is not immunoglobulin E–mediated.29 Graded-dose challenge/desensitization has historically been a cornerstone of management of immediate reactions to vaccines.29 However, given the novel vaccine platform of COVID-19 mRNA vaccines and their recent clinical use, it must be acknowledged that there is currently no evidence that splitting the dose of mRNA vaccines at any ratio will improve safety and it could also theoretically negatively affect vaccine efficacy.6 , 7 , 29 In addition, there would be no rationale to split the dose or use a graded-dose challenge/desensitization approach in individuals who have had only mild to moderate allergic reactions to the first dose if administration of the second dose of COVID-19 mRNA vaccines as a full single dose (as intended and outlined in the Pfizer-BioNTech and Moderna U.S. Food and Drug Administration emergency use authorization [EUA] of these yet to be licensed products) is shown to be safe and well tolerated for such patients.6 , 7 , 29 Furthermore, administering COVID-19 mRNA vaccines in accordance with EUA guidance is most likely to result in optimal vaccine efficacy, and allergist-immunologist supervised dosing may pose logistical challenges in accessing, storing, and utilizing vaccine supply in a timely fashion. Still, equipoise in vaccinating groups at high risk both for anaphylaxis and for severe COVID-19 is a delicate balance. As such, for patients at high risk for anaphylaxis to an mRNA vaccine, a graded-dose vaccine challenge/desensitization may be preferable to outright vaccine deferral in the midst of the ongoing pandemic, if the patient and/or clinician would not otherwise proceed with vaccination.6 , 30, 31, 32
Although utilization of another COVID-19 vaccine construct in the setting of a first-dose mRNA COVID-19 vaccine is being studied in the United Kingdom, there are currently no safety, efficacy, or effectiveness data for this approach. With the Janssen Biotech COVID-19 vaccine EUA, guidance has been suggested that, for patients in whom a second COVID-19 mRNA vaccine dose is contraindicated, this viral vector vaccine may be administered with a precaution under the supervision of a health care professional capable of managing anaphylaxis.33 In this setting, referral to an allergist-immunologist should be considered and the minimum interval for administration after the COVID-19 mRNA vaccine would be 28 days.5 The Janssen Biotech vaccine is based on a recombinant replication incompetent human adenovirus serotype (Ad26) vector that encodes the SARS-CoV-2 viral spike glycoprotein.34 At this time, the incremental safety and efficacy of administration of the Janssen Biotech vaccine (which contains polysorbate 80 as an excipient) given to an individual who has only received a single COVID-19 mRNA dose are unknown, but the vaccine represents a patient-preference option to consider if it is available.34 However, relying on this approach may have unintended consequences in regard to both population equity (ie, some patients may receive 1.5 vaccine doses while others receive none) and access in resource-constrained settings with limited COVID-19 vaccine supply (ie, the Janssen Biotech vaccine may not be available to every patient at the time vaccination is needed).
Caveats of Risk-Stratification Approaches
Uncertainty presently characterizes many aspects of the COVID-19 vaccines and vaccine reactions, which has been further heightened by the emergence of several variants in which the amount of protection offered by a single vaccine dose would be even less certain.1 , 26 The benefit of history-guided risk stratification that may also use excipient skin testing and/or vaccine rechallenge with informed consent and shared decision making is currently unknown.6 , 7 Although polyethylene glycol is the only excipient in the COVID-19 mRNA vaccines that is shared and can be readily tested, polyethylene glycol prick and intradermal skin testing has uncertain sensitivity, specificity, and positive and negative predictive values.6 , 7 In addition, getting patients seen and assessed by an allergist-immunologist available to perform this testing within the allowable 6-week time period may be challenging in some areas and could delay vaccination.6 , 7 It is possible that risk stratification may not be beneficial until the prevalence of vaccine anaphylaxis reaches a much higher threshold than exists in the general population.6 , 7 , 23, 24, 25 It is still unclear what is triggering reported COVID-19 mRNA vaccine reactions and through what mechanism they occur. Reactions may be non–immunoglobulin E–mediated resulting from direct complement or mast cell activation (complement activation–related pseudoallergy), which renders it very difficult to predict in whom a second-dose reaction may result.7 , 35, 36, 37, 38, 39 We can be somewhat reassured that most of the reports of anaphylaxis to date have come from first-dose reactions and we are not seeing a more significant signal of anaphylaxis occurring for the first time on the second dose or mild first-dose reactions leading to more severe second-dose reactions. These and other questions will hopefully be answered as we continue to expand our knowledge base regarding COVID-19 and vaccination-related immunity.
Opportunities For Shared Decision Making And Learning
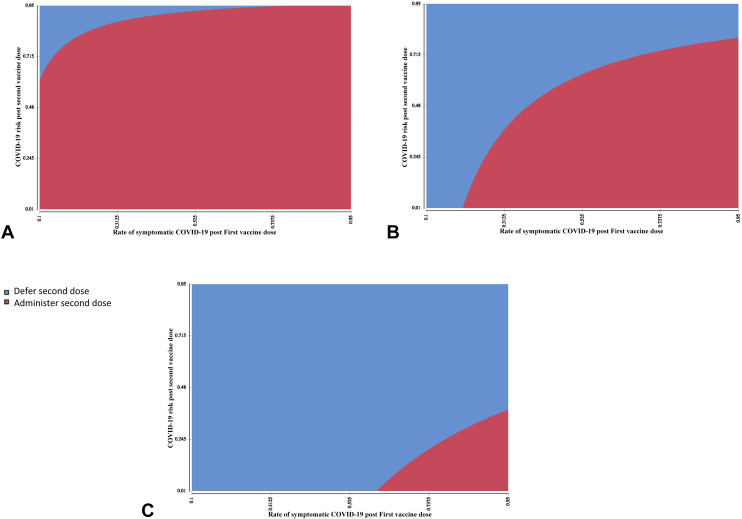
Uncertainties regarding COVID-19 vaccination will likely remain a reality of life in 2021 and beyond. Allergists-immunologists must continue to partner with patients and multidisciplinary health care providers to discuss the knowns and the unknowns and weigh the benefits and risks of approaches to vaccination. The ultimate goals of optimizing individual and population health outcomes need to be prioritized. As we enter these discussions, we must remember the 3 conversations of effective shared decision-making—team talk to outline patient goals and preferences, option talk to clarify choices, and decision talk to reach preference-based decisions.8 In this model, an allergist-immunologist's primary role is to provide expert advice regarding the existing evidence (evolving and uncertain as it may be), while acknowledging that patients are the experts of their personal contexts and what matters most to them.8 This process could be facilitated on a public health level through development of validated patient-decision aids—tools that at present are not available in the context of subsequent dose decisions for patients with prior COVID-19 vaccine reactions.40 It is highly likely that the vast majority of individuals without prior COVID-19 vaccine anaphylaxis who have concerns about second-dose safety can safely receive the second dose and routinely withholding it would be a disservice not only to their health but also to the larger population.6 , 7 Assuming that high durable immunity of a certain threshold can indeed be obtained after a single vaccination, a model of COVID-19 vaccination that considers harms and benefits of deferring or foregoing a second vaccine dose in 2021 could be useful in the individual decision-making paradigm. A shared decision for second-dose–deferral might be appropriate in the rare patient with a greatly elevated relative risk of second-dose anaphylaxis where first-dose protection is high and there is a low risk of COVID-19 related morbidity (Figure 1 ). This makes it imperative to determine what constitutes durable first-dose protection of a COVID-19 mRNA vaccine intended to be given as a 2-dose series as well as to understand the value of the second (or any additional) dose. Vaccination is crucial to ensure both patient and population health—a key consideration as we begin to appreciate the daunting task of fighting a pandemic virus that is actively mutating. Dr. Anthony Fauci noted that “viruses cannot mutate if they cannot replicate,” and so we are in a race against time to preempt COVID-19 variants.41 The dawning realization that COVID-19 is likely to become endemic also necessitates an approach to revaccination of patients with a prior reaction to any COVID-19 vaccine. In the case of the COVID-19 mRNA vaccines, even if a second dose is deferred this year,1 because annual COVID-19 vaccinations will likely be the norm, decisions will need to be made about future administration of a COVID-19 vaccine even if it is not a COVID-19 mRNA vaccine.34 , 42 Given that multiple additional vaccines will likely be granted EUA in the United States over the next few months,34 such as the adenoviral vector and protein subunit SARS-CoV-2 vaccines, an additional consideration for the future will be revaccination with a different COVID-19 vaccine construct, although at present such an approach would also be characterized by some degree of uncertainty.
Figure 1.
Cost-effectiveness of 2021 COVID-19 second-vaccine–dose deferral across anaphylaxis second-dose risk. Rates of symptomatic COVID-19 post first vaccine dose are shown on the x axis with rates of residual COVID-19 risk after a second dose on the y axis, across rates of second-dose vaccine anaphylaxis: (A) 1%, (B) 5%, and (C) 15%.
While allergists-immunologists worldwide begin to gain first-hand experience with the clinical assessment and management of individuals who have CDC-defined allergy contraindications or precautions to COVID-19 vaccination, it becomes helpful to have shared platforms for communicating patient assessments and outcomes. There are notable limitations to the CDC's passive surveillance system for detecting and confirming anaphylaxis. Sharing allergist-immunologist clinical assessment and vaccination outcomes can enrich our clinical knowledge base. Current platforms for clinical data sharing have emerged, including the COVID-19 Vaccine Allergy Case Registry,43 a Health Insurance Portability and Accountability Act (HIPAA)–compliant U.S.-based registry for documenting hypersensitivity reactions related to COVID-19 vaccination, developed by the U.S. Drug Allergy Registry consortium,44 and the Global COVID-19 and Vaccine-Associated Anaphylaxis Surveillance Project, an international anaphylaxis registry supported by the World Allergy Organization.45
Conclusions
As of March 23, 2021, 123,924,888 confirmed COVID-19 cases had resulted in 2,727,640 deaths around the world.46 Thus far, the risk of anaphylaxis-related death due to COVID-19 immunization appears to be exceptionally rare, with over 458 million doses given to date.28 , 47 , 48 In the months to come, the continued emergence of SARS-CoV-2 variants may pose challenges to vaccination strategies.26 There will also be the benefit of additional COVID-19 vaccines being granted EUA in the United States.34 As we continue to provide each patient the right care at the right time, new discoveries will continue to allow insights that will shape and optimize our practice. While we are accumulating this evidence base, our ultimate goal must be to avoid unnecessary vaccine hesitancy and deferral and to optimize both vaccine acceptance and efficacy, while safely vaccinating the world.
Footnotes
No funding has been received for this study.
Conflicts of interest: M. Shaker is a member of the Joint Taskforce on Allergy Practice Parameters; has a family member who is CEO of Altrix Medical; serves on the Editorial Board of the Journal of Food Allergy and the Annals of Allergy, Asthma, and Immunology. E. Phillips reports grants from the National Institutes of Health (grant nos. P50GM115305, R01HG010863, R01AI152183, R21AI139021, and U01AI154659) and from the National Health and Medical Research Council of Australia; receives royalties from Uptodate and consulting fees from Janssen Vertex, Regeneron, and Biocryst; is codirector of IIID Pty Ltd, which holds a patent for HLA-B∗57:01 testing for abacavir hypersensitivity; and has a patent pending for Detection of Human Leukocyte Antigen-A∗32:01 in connection with Diagnosing Drug Reaction with Eosinophilia and Systemic Symptoms without any financial remuneration and not directly related to the submitted work. E. M. Abrams is on the Steering Committee of Food Allergy Canada's National Food Allergy Action Plan; is a collaborator with the Institute for Health metrics and Evaluation; and has received moderator/speaker fees from AstraZeneca, GSK, and Sanofi. J. Oppenheimer performs research/adjudication for AZ, GSK, Sanofi, Novartis; is a consultant for GSK, AZ, Sanofi; is an associate editor for Annals of Allergy Asthma Immunology and AllergyWatch; is a section editor for Current Opinion of Allergy; receives royalties from Up to Date; is a board liaison for ABIM for ABAI. T. K. V. Leek has served on advisory boards and received honoraria from Aralez, Bausch Health, and Pfizer. D. P. Mack is a member of the Board of Directors for the Canadian Society of Allergy and Clinical Immunology; serves on the Editorial Board of the Journal of Food Allergy; has provided consultation and speaker services for Pfizer, Aimmune, Merck, Covis, and Pediapharm; and has been part of an advisory board for Pfizer and Bausch Health. M. Greenhawt has served as a consultant for the Canadian Transportation Agency, Thermo Fisher, Intrommune, and Aimmune Therapeutics; is a member of physician/medical advisory boards for Aimmune Therapeutics, DBV Technologies, Sanofi/Genzyme, Genentech, Nutricia, Kaleo Pharmaceutical, Nestle, Acquestive, Allergy Therapeutics, Pfizer, US World Meds, Allergenis, Aravax, and Monsanto; is a member of the scientific advisory council for the National Peanut Board; has received honorarium for lectures from Thermo Fisher, Aimmune, DBV, Before Brands, multiple state allergy societies, the American College of Allergy Asthma and Immunology, the European Academy of Allergy and Clinical Immunology; is an associate editor for the Annals of Allergy, Asthma, and Immunology; and is a member of the Joint Task Force on Allergy Practice Parameters.
The rest of the authors declare that they have no relevant conflicts of interest.
References
- 1.Liu A. How important is the second dose of the COVID-19 mRNA vaccine? J Allergy Clin Immunol Pract. 2021 doi: 10.1016/j.jaip.2021.02.061. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadire S.R., Wachter R.M. Delayed second dose versus standard regimen for Covid-19 vaccination. N Engl J Med. 2021;384:e28. doi: 10.1056/NEJMclde2101987. [DOI] [PubMed] [Google Scholar]
- 3.Amit S., Regev-Yochay G., Afek A., Kreiss Y., Leshem E. Early rate reductions of SARS-CoV-2 infection and COVID-19 in BNT162b2 vaccine recipients. Lancet. 2021;397:875–877. doi: 10.1016/S0140-6736(21)00448-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skowronski D.M., De Serres G. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2021;384:1576–1577. doi: 10.1056/NEJMc2036242. [DOI] [PubMed] [Google Scholar]
- 5.U.S. Centers for Disease Control and Prevention Interim clinical considerations for use of mRNA COVID-19 vaccines currently authorized in the United States. https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fcovid-19%2Finfo-by-product%2Fpfizer%2Fclinical-considerations.html Available from:
- 6.Greenhawt M., Abrams E.M., Oppenheimer J., Vander Leek T.K., Mack D.P., Singer A.G., et al. The COVID-19 pandemic in 2021: avoiding overdiagnosis of anaphylaxis risk while safely vaccinating the world. J Allergy Clin Immunol Pract. 2021;9:1438–1441. doi: 10.1016/j.jaip.2021.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Banerji A., Wickner P.G., Saff R., Stone C.A., Jr., Robinson L.B., Long A.A., et al. mRNA vaccines to prevent COVID-19 disease and reported allergic reactions: current evidence and suggested approach. J Allergy Clin Immunol Pract. 2021;9:1423–1437. doi: 10.1016/j.jaip.2020.12.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abrams E.M., Shaker M., Oppenheimer J., Davis R.S., Bukstein D.A., Greenhawt M. The challenges and opportunities for shared decision making highlighted by COVID-19. J Allergy Clin Immunol Pract. 2020;8:2474–2480.e1. doi: 10.1016/j.jaip.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shaker M., Hsu Blatman K., Abrams E.M. Engaging patient partners in state-of-the-art allergy care: finding balance when discussing risk. Ann Allergy Asthma Immunol. 2020;125:252–261. doi: 10.1016/j.anai.2020.01.029. [DOI] [PubMed] [Google Scholar]
- 10.Abrams E.M., Greenhawt M. Risk communication during COVID-19. J Allergy Clin Immunol Pract. 2020;8:1791–1794. doi: 10.1016/j.jaip.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan N., Barda N., Kepten E., Miron O., Perchik S., Katz M.A., et al. BNT162b2 mRNA Covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abrams E.M., Singer A.G., Shaker M., Greenhawt M. What the COVID-19 pandemic can teach us about resource stewardship and quality in health care. J Allergy Clin Immunol Pract. 2021;9:608–612. doi: 10.1016/j.jaip.2020.11.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Shaker M., Stukus D., Chan E.S., Fleischer D.M., Spergel J.M., Greenhawt M. "To screen or not to screen": comparing the health and economic benefits of early peanut introduction strategies in five countries. Allergy. 2018;73:1707–1714. doi: 10.1111/all.13446. [DOI] [PubMed] [Google Scholar]
- 17.Abrams E.M., Ben-Shoshan M. Delabeling penicillin allergy: is skin testing required at all? J Allergy Clin Immunol Pract. 2019;7:1377. doi: 10.1016/j.jaip.2018.11.052. [DOI] [PubMed] [Google Scholar]
- 18.Greenhawt M., Turner P.J., Kelso J.M. Administration of influenza vaccines to egg allergic recipients: a practice parameter update 2017. Ann Allergy Asthma Immunol. 2018;120:49–52. doi: 10.1016/j.anai.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 19.Blumenthal K.G., Li Y., Banerji A., Yun B.J., Long A.A., Walensky R.P. The cost of penicillin allergy evaluation. J Allergy Clin Immunol Pract. 2018;6:1019–1027.e2. doi: 10.1016/j.jaip.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Blumenthal K.G., Ryan E.E., Li Y., Lee H., Kuhlen J.L., Shenoy E.S. The impact of a reported penicillin allergy on surgical site infection risk. Clin Infect Dis. 2018;66:329–336. doi: 10.1093/cid/cix794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vaccines and Related Biological Products Advisory Committee meeting December 17, 2020. FDA briefing document: Moderna COVID-19 vaccine. https://www.fda.gov/media/144434/download Available from: [DOI] [PubMed]
- 22.Prendecki M., Clark C., Brown J., Cox A.L., Gleeson S., Buckian M., et al. Effect of previous SARS-CoV-2 infection on humoral and T-cell responses to single-dose BNT162b2 vaccine. Lancet. 2021;397:1178–1181. doi: 10.1016/S0140-6736(21)00502-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shaker M., Abrams E.M., Greenhawt M. A cost-effectiveness evaluation of hospitalizations, fatalities, and economic outcomes associated with universal versus anaphylaxis risk-stratified COVID-19 vaccination strategies. J Allergy Clin Immunol Pract. 2021;9:S2213–S2298. doi: 10.1016/j.jaip.2021.02.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.CDC COVID-19 Response Team Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Pfizer-BioNTech COVID-19 vaccine—United States, December 14-23. MMWR Morb Mortal Wkly Rep. 2021;70:46–51. doi: 10.15585/mmwr.mm7002e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.CDC COVID-19 Response Team Food and Drug Administration Allergic reactions including anaphylaxis after receipt of the first dose of Moderna COVID-19 vaccine—United States, December 21, 2020–January 10, 2021. MMWR Morb Mortal Wkly Rep. 2021;70:125–129. doi: 10.15585/mmwr.mm7004e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu K., Werner A.P., Koch M., Choi A., Narayanan E., Steward-Jones G.B.E., et al. Serum neutralizing activity elicited by mRNA-1273 vaccine—preliminary report. N Engl J Med. 2021;384:1468–1470. doi: 10.1056/NEJMc2102179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shaker M., Greenhawt M. A primer on cost-effectiveness in the allergy clinic. Ann Allergy Asthma Immunol. 2019;123:120–128.e1. doi: 10.1016/j.anai.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 28.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020–January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kelso J.M., Greenhawt M.J., Li J.T., Nicklas R.A., Bernstein D.I., Blessing-Moore J., et al. Adverse reactions to vaccines practice parameter 2012 update. J Allergy Clin Immunol. 2012;130:25–43. doi: 10.1016/j.jaci.2012.04.003. [DOI] [PubMed] [Google Scholar]
- 30.Canadian Society of Allergy and Clinical Immunology Statement of Pfizer/BioNTech Vaccine Anaphylaxis. https://csaci.ca/wp-content/uploads/2021/01/COVID-19-VaccineTesting-AdministrationGuidance-JAN5.pdf Available from:
- 31.Klimek L., Jutel M., Akdis C.A., Bousquet J., Akdis M., Torres-Jaen M., et al. ARIA-EAACI statement on severe allergic reactions to COVID-19 vaccines—an EAACI-ARIA position paper [published online ahead of print December 30, 2020] Allergy. [DOI] [PubMed]
- 32.Murphy K.R., Patel N.C., Ein D., Hudelson M., Kodoth S., Marshall G.D., Jr., et al. Insights from American College of Allergy, Asthma, and Immunology COVID-19 Vaccine Task Force: allergic reactions to mRNA SARS-CoV-2 vaccines. Ann Allergy Asthma Immunol. 2021;126:318–320. doi: 10.1016/j.anai.2021.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.ACIP COVID-19 Vaccines Work Group Clinical considerations for use of COVID-19 VAccines. March 1, 2021. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2021-02/28-03-01/03-COVID-MacNeil.pdf Available from:
- 34.U.S. Food and Drug Administration Janssen Biotech Emergency Use Authorization. February 27, 2021. https://www.fda.gov/media/146303/download Available from:
- 35.Hamad I., Hunter A.C., Szebeni J., Moghimi S.M. Poly(ethylene glycol)s generate complement activation products in human serum through increased alternative pathway turnover and a MASP-2-dependent process. Mol Immunol. 2008;46:225–232. doi: 10.1016/j.molimm.2008.08.276. [DOI] [PubMed] [Google Scholar]
- 36.Lund J.M., Alexopoulou L., Sato A., Karow M., Adams N.C., Gale N.W., et al. Recognition of single-stranded RNA viruses by Toll-like receptor 7. Proc Natl Acad Sci U S A. 2004;101:5598–5603. doi: 10.1073/pnas.0400937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kulka M., Alexopoulou L., Flavell R.A., Metcalfe D.D. Activation of mast cells by double-stranded RNA: evidence for activation through Toll-like receptor 3. J Allergy Clin Immunol. 2004;114:174–182. doi: 10.1016/j.jaci.2004.03.049. [DOI] [PubMed] [Google Scholar]
- 38.Turner P.J., Ansotegui I.J., Campbell D.E., Cardona V., Ebisawa M., El-Gamal Y., et al. COVID-19 vaccine-associated anaphylaxis: a statement of the World Allergy Organization Anaphylaxis Committee. World Allergy Organ J. 2021;14:100517. doi: 10.1016/j.waojou.2021.100517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Szebeni J., Muggia F., Gabizon A., Barenholz Y. Activation of complement by therapeutic liposomes and other lipid excipient-based therapeutic products: prediction and prevention. Adv Drug Deliv Rev. 2011;63:1020–1030. doi: 10.1016/j.addr.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 40.International Patient Decision Aid Standards (IPDAS) Collaboration. http://ipdas.ohri.ca Available from: [DOI] [PMC free article] [PubMed]
- 41.Maxouris C., Yan H. Fauci urges vaccinations to stop new virus strains: “viruses cannot mutate if they don’t replicate.” CNN. February 8, 2021. https://www.cnn.com/2021/02/01/health/us-coronavirus-monday/index.html Available from:
- 42.Lovelace B., Jr. J&J CEO says people may need annual COVID vaccine shots for the next several years. CNBC. February 9, 2021. https://www.cnbc.com/2021/02/09/covid-vaccine-jj-ceo-says-people-may-get-annual-shots-for-the-next-several-years.html Available from:
- 43.Mass General Allergy Research COVID-19 Vaccine Allergy Case Registry. https://allergyresearch.massgeneral.org/ Available from:
- 44.Blumenthal K.G., Harkness T., Phillips E.J., Ramsey A., Banerji A., Samarakoon U., et al. Patient characteristics and concerns about drug allergy: a report from the United States Drug Allergy Registry. J Allergy Clin Immunol Pract. 2020;8:2958–2967. doi: 10.1016/j.jaip.2020.08.018. [DOI] [PubMed] [Google Scholar]
- 45.World Allergy Organization COVID-19 Registry. https://www.worldallergy.org/resources/covid-19 Available from:
- 46.Johns Hopkins University Coronavirus Resource Center. https://coronavirus.jhu.edu Available from:
- 47.Our World in Data. Coronavirus (COVID-19) vaccinations. https://ourworldindata.org/covid-vaccinations Available from:
- 48.Bloomberg More than 458 million shots given: Covid-19 Tracker. https://www.bloomberg.com/graphics/covid-vaccine-tracker-global-distribution/ Available from: