Dear Editor,
We still do not have effective therapy to combat SARS-CoV-2 except vaccination. Antimicrobial photodynamic therapy (aPDT) might be a good choice for the prevention and treatment of SARS-CoV-2 because it may only target the infection area using photosensitizers with a specific wavelength. “Hypocrellin B” is a substance used traditionally in Chinese medicine and as a photosensitizer which is used in China to treat many diseases. Its photodynamic action and research progress of "hypocrellin B" will be discussed. Why do we apply PDT with "hypocrellin B" as a photosensitizer against SARS-CoV-2? Is this a possible choice for the treatment of patients with SARS-CoV-2?
1. Background
Hypocrellin B (HB) is a natural pigment of perylquinone derivatives isolated from the traditional Chinese herb Hypocrella bambuase [1]. It has significant anti-tumor and anti-viral properties as well as a strong photodynamic effect on malignant tumor, and human immunodeficiency virus type I (HIV-I) [2].
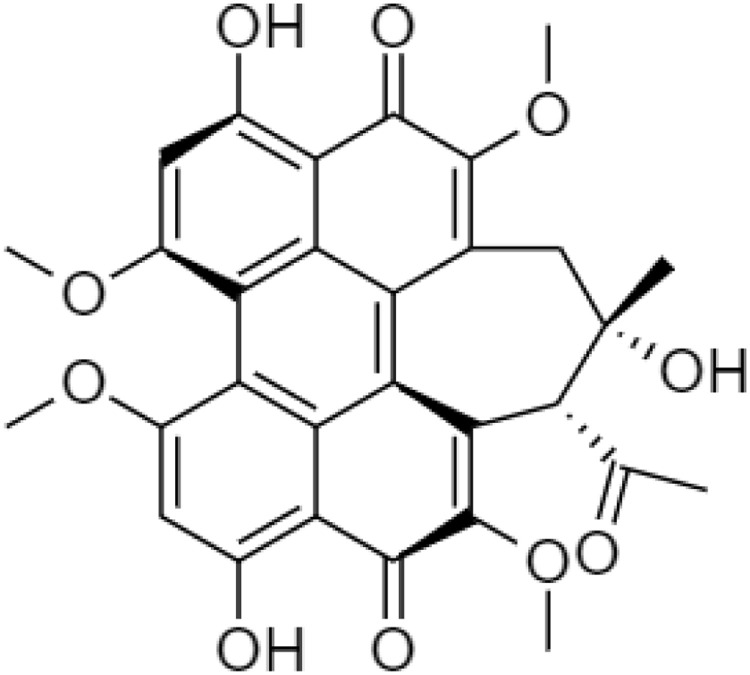
In general, HB consists of aromatic rings, phenolic hydroxyl groups, carbonyl groups, side-rings, and methoxyl groups (Fig. 1 ). It has two major fluorescence peaks including the neutral mono-molecule at ∼610 nm, an excited proton at ∼660 nm, and the zwitterions between ∼610 nm to 660 nm because the perylene quinonoid pigments have three visible bands start from the transition of π–π* conjugated systems and intramolecular proton transfer [3].
Fig. 1.
Chemical structure of Hypocrellin B (HB).
The target infection sites of SARS-CoV-2 are the nasal and oral cavity, nasopharynx and bronchial tree. There are inflamed cells in the infected sites, and, photosensitizers generally accumulate in inflamed sites. The light used in PDT can be directed through an optical fiber which is a very thin glass strand and placed close to the initial infection sites to deliver the amount of light into the nasal, oral cavity, nasopharynx, and bronchial tree for the treatment of SARS-CoV-2.
2. Photodynamic action of hypocrellin B (HB)
Upon light irradiation of HB, produces reactive oxygen species (ROS), including singlet oxygen (1O2), superoxide (O2 −), hydroxyl radical (•OH), and hydrogen peroxide (H2O2). Singlet oxygen (1O2) is generated by the energy transfer from the excited state triplet of HB to ground state oxygen, while superoxide (O2 −) is generated by the monovalent anion radical of HB when ground state oxygen is reduced. Hydrogen peroxide (H2O2) is formed while ground state oxygen is reduced by its divalent anion after photosensitization by HB [4].
HB activated upon interaction with a specific wavelength of light selectively initiate necrosis and apoptosis to the target cells or specific area. HB is non-toxic in dark and could be absorbed by cells within the body but it would be gone in normal and healthy cells after 1–3 days [5]. PDT causes acute inflammation leading to immune stimulation and increasing the number of antigens to T cells in an immune system [6]. Hypocrellin-mediated PDT has been an approved and effective treatment for esophageal cancer, nasopharyngeal cancer, lung cancer, and ovarian cancer which re-established the chemosensitivity and radiosensitivity of human cells as hypocrellin contributed to the phototoxicity of cells [7].
3. Research progress of hypocrellin B (HB)
In 2004, Zhou N.K. et al. reported the effect of hypocrellins B-photodynamic therapy (HB-PDT) on lung cancer cell line A549. It induced apoptosis and the IC50 of HB-PDT was 33.82 ng/mL [8]. In 2005, Shang L.Q. et al. also indicated that HB-PDT could induce apoptosis of the esophageal cancer cells with a high IC50 value which was 34.16 ng/mL [9]. Jiang Y. et al. investigated the apoptosis of breast cancer cells after HB-PDT. It down-regulated the HER2 gene expression and inhibited breast cancer cell growth [10]. 2014 afterward, Jiang Y. et al. also identified the effect of HB-PDT on apoptosis, adhesion, and migration of cancer cells in vitro. They found that HB-PDT increased the early apoptotic and late apoptotic (necrotic) rates to 16.40 % and 24.67 % respectively, and inhibited adhesion and migration of human ovarian cancer HO-8910 cells [11]. These results proved that the singlet oxygen (1O2) and superoxide (O2 −) were the activated reactive oxygen species (ROS) for cell death induced by hypocrellins photosensitization [12].
Emerging evidence had shown that nano-system combined with hypocrellins B could significantly improve their solubility, bioavailability, and enhance their photodynamic efficacy [13]. Bai D.Q. et al. prepared HB-loaded nano-photosensitizer with an absorption range between 400−700 nm for the treatment of nasopharyngeal carcinoma cells. It increased the apoptotic rate of CNE2 cells to 34.32 ± 1.94 % after PDT [14]. Zhao Li. et al. also prepared a poly butyl-cyanoacrylate nanoparticle (PBCA-NP) entrapped with HB to PDT in ovarian cancer. The drug encapsulation efficiency was reached 92.7 % and drug-loading content was 15 % for PBCA-NP. It controlled drug release and tumor targeting as well as the efficacy of PDT which prolonged the half-life of HB from 9.45 to 12.99 h for the treatment of ovarian cancer [15]. Chang J.E. et al. developed HB encapsulated hyaluronic acid-ceramide nanoparticles (HB-NPs) and HB-(paclitaxel)-encapsulated hyaluronic acid-ceramide nanoparticles (HB-P-NPs) to study the anticancer efficacy of PDT on lung cancer. The results showed that it increased the phototoxicity and only 7.52 ± 0.38 % of the A549 cells survived in the treatment of HB-P-NPs with PDT [16].
4. Antimicrobial photodynamic therapy (aPDT) for SARS-CoV-2
Recently, Kipshidze N. et al. suggested the usage of PDT to treat COVID-19 based on the unique features of acute respiratory distress syndrome (ARDS) and the homology modeling and molecular docking. As SARS-CoV-2 binds to the heme groups in hemoglobin, leading to severe hypoxia. Heme is composed of a ring-like organic compound known as a porphyrin to which an iron atom is attached. Hemoglobin is the blood travel agent between the lungs and the tissues. It could use the injection of porphyrin-based photosensitizers either systemically or locally into the lungs through the pulmonary artery using micro-catheters with the application of PDT. This activated the porphyrin-based photosensitizers in a specific absorption wavelength from 450 to 800 nm to cause photosensitization to form the ROS. It reduced SARS-CoV-2 loading and destroyed its binding on the protein-membrane from the upper respiratory tract, prevent damage to the lung [17].
Moghissi K. et al. considered the usage of methylene Blue (MB)-mediated PDT topically within the airway to prevent the spread, and treat SARS-CoV-2. MB is a photosensitizer activated by light from 650 − 660 nm and delivered through the cricothyroid membrane using a fine catheter. The MB-mediated PDT could be tried in the treatment of early and advanced broncho-pulmonary infection [18].
Dias L. D. et al. reported the application of PDT for the treatment of infections in the respiratory tract [19]. It was an efficient therapy to decrease the microbial load (viral and bacterial) in the respiratory tract through the generation of ROS to promote the damage of viruses targets such as nucleic acids (DNA or RNA) [20].
5. Conclusion
The above information suggests that HB is a possible candidate as a photosensitizer in the application of PDT for the treatment of SARS-CoV-2. Growing evidence has shown that HB is a non-toxic and natural photosensitizer. HB-mediated PDT has been studied in esophageal cancer, nasopharyngeal cancer, lung cancer, and ovarian cancer. Its photosensitization is similar to the porphyrin or methylene blue via ROS to decrease the loading of SARS-CoV-2 and destroy the SARS-CoV-2 binding on the protein-membrane of an upper respiratory tract. Meanwhile, HB might be further developed into a nano-system to get a better PDT efficacy and improve its solubility and bioavailability. However, much more work need be done in human clinical trials of HB-aPDT for SARS-CoV-2.
Funding/support
The authors received no funding source/grants or other materials support for this work.
Declaration of Competing Interest
The authors have no conflicts of interest to disclose.
References
- 1.Hudson J.B., Zhou J., Chen J., Harris L., Yip L., Towers G.H. Hypocrellin, from Hypocrella bambuase, is phototoxic to human immunodeficiency virus. Photochem. Photobiol. 1994;60(3):253–255. doi: 10.1111/j.1751-1097.1994.tb05100.x. [DOI] [PubMed] [Google Scholar]
- 2.Ma G., Khan S.I., Jacob M.R., Tekwani B.L., Li Z., Pasco D.S., Walker L.A., Khan I.A. Antimicrobial and antileishmanial activities of hypocrellins A and B. Antimicrob. Agents Chemother. 2004;48:4450–4452. doi: 10.1128/AAC.48.11.4450-4452.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diwu Z.J., Jiang L.J., Zhang M.H. The effects of environments on the fluorescence spectra of hypocrellin A and B. Acta Phys. Chim. Sin. 1989;5 [Google Scholar]
- 4.Zhen J.D., Lown J.W.J.P. Hypocrellins and their use in photosensitization. Photobiology. 1990;52(3):609–616. doi: 10.1111/j.1751-1097.1990.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 5.Kwiatkowski S., Knap B., Przystupski D., Saczko J., Kędzierska E., Knap-Czop K., Kotlińska J., Michel O., Kotowski K., Kulbacka J. Photodynamic therapy - mechanisms, photosensitizers and combinations. Biomed. Pharmacother. 2018;106:1098–1107. doi: 10.1016/j.biopha.2018.07.049. [DOI] [PubMed] [Google Scholar]
- 6.Castano A.P., Mroz P., Hamblin M.R. Photodynamic therapy and anti-tumour immunity. Nat. Rev. Cancer. 2006;6(7):535–545. doi: 10.1038/nrc1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mansoori B., Mohammadi A., Amin Doustvandi M., Mohammadnejad F., Kamari F., Gjerstorff M.F., Baradaran B., Hamblin M.R. Photodynamic therapy for cancer: role of natural products. Photodiagnosis Photodyn. Ther. 2019;26:395–404. doi: 10.1016/j.pdpdt.2019.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou N.K. Comparative study on the killing effect between hypocrellins B-photodynamic therapy and hematoporphyrin derivative-photodynamic therapy on human lung cancer cells. Med. j. Chin. Peoples Liberation Army. 2004;29(12):1076–1078. [Google Scholar]
- 9.Shang L.Q., Zhou N.K., Gu Y., Liu F.G., Zeng J. Comparative study on killing effect to esophageal Cancer cell line between hypocrellins B-photodynamic therapy and hematoporphyrin derivative-photodynamic therapy. Chin. J. Cancer Prevent.Treat. 2005;12(15):1139–1142. [Google Scholar]
- 10.Jiang Y., Xia X., Leung A.W., Xiang J., Xu C. Apoptosis of breast cancer cells induced by hypocrellin B under light-emitting diode irradiation. Photodiagn. Photodyn. Ther. 2012;9(4):337–343. doi: 10.1016/j.pdpdt.2012.03.009. [DOI] [PubMed] [Google Scholar]
- 11.Jiang Y., Leung A.W., Wang X., Zhang H., Xu C. Effect of photodynamic therapy with hypocrellin B on apoptosis, adhesion, and migration of cancer cells. Int. J. Radiat. Biol. 2014;90(7):575–579. doi: 10.3109/09553002.2014.906765. [DOI] [PubMed] [Google Scholar]
- 12.Weishaupt K.R., Gomer C.J., Dougherty T.J. Identification of singlet oxygen as the cytotoxic agent in photoinactivation of a murine tumor. Cancer Res. 1976;36:2326–2329. [PubMed] [Google Scholar]
- 13.Gomes A.J., Lunardi C.N., Tedesco A.C. Characterization of biodegradable poly (D, L-lactide-co-glycolide) nanoparticles loaded with bacteriochlorophyll-a for photodynamic therapy. Photomed. Laser Surg. 2007;25:428–435. doi: 10.1089/pho.2007.2089. [DOI] [PubMed] [Google Scholar]
- 14.Bai D.Q., Yow C.M.N., Tan Y., Chu E.S.M., Xu C.S. Photodynamic action of LED activated nanoscale photosensitizer in nasopharyngeal carcinoma cells. Laser Phys. 2010;20:544–550. [Google Scholar]
- 15.Li Z., Sun L., Lu Z., Su X., Yang Q., Qu X., Li L., Kong B. Enhanced effect of photodynamic therapy in ovarian cancer using a nanoparticle drug delivery system. Int. J. Oncol. 2015;47:1070–1076. doi: 10.3892/ijo.2015.3079. [DOI] [PubMed] [Google Scholar]
- 16.Chang J.E., Cho H.J., Jheon S. Anticancer efficacy of photodynamic therapy with lung cancer-targeted nanoparticles. Cancer Res. 2016;118 doi: 10.3791/54865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kipshidze N., Yeo N., Kipshidze N. Photodynamic therapy for COVID-19. Nat. Photonics. 2020;14:651–652. [Google Scholar]
- 18.Moghissi K., Dixon K., Gibbins S. Does PDT have potential in the treatment of COVID 19 patients? Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dias L.D., Blanco K.C., Bagnato V.S. COVID-19: beyond the virus. The use of photodynamic therapy for the treatment of infections in the respiratory tract. Photodiagn. Photodyn. Ther. 2020;31 doi: 10.1016/j.pdpdt.2020.101804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wiehe A., O’Brien J.M., Senge M.O. Trends and targets in antiviral phototherapy. Photochem. Photobiol. Sci. 2019;18(11):2565–2612. doi: 10.1039/c9pp00211a. [DOI] [PubMed] [Google Scholar]