Abstract
Objective
To present observations on administration of natalizumab to 18 patients with the comorbid MS and psoriasis, who represented a full subset of patients with such comorbidity within the patient records available.
Methods
A retrospective analysis of patient records was performed. Patient histories were gathered and included date of diagnosis of MS and psoriasis, MS disease-modifying therapies (DMTs), Expanded Disability Status Scale (EDSS), reason for DMT switch, and effects on MS and psoriasis status.
Results
On initiation of natalizumab, all 18 patients had a complete cessation of MS disease activity (within 2–8 months) with significant patient-reported improvement of psoriasis (within 1–5 months). This improvement was independent of previous MS therapy and led to 15 of 18 patients needing no additional treatment for MS and psoriasis (remaining 3 patients continued to use topical treatments for psoriasis).
Conclusions
In this cohort of 18 patients with comorbid MS and psoriasis, beneficial results on both diseases were observed after initiation of therapy with natalizumab.
Natalizumab is a humanized monoclonal antibody that is approved for the treatment of relapsing forms of MS.1 Its mechanism of action is associated with modulation of lymphocytes trafficking through biological barriers. Current literature on MS and comorbid psoriasis discuss both detrimental and beneficial associations with natalizumab.2–5
In 2010, we reported at the American Academy of Neurology that our first 4 cases showed improvement of psoriasis with natalizumab treatment.6 In the present report, we have extended these observations to a total of 18 patients with MS—who represent the entire cohort of patients with such comorbidities from 3 different neurology practices—and were followed with regular intervals over a period from 2004 through 2020.
Methods
The Institutional Review Board found that this research meets requirements for a waiver of consent form under 45 CFR 46.116(f) [2018 Requirements] 45 CFR 46.116(d) [pre-2018 Requirements]. Retrospective data were gathered including date of diagnosis of MS and psoriasis, MS disease-modifying therapies (DMTs), MS activity, Expanded Disability Status Scale (EDSS), reason for DMT switch, concomitant medications, and ongoing data on MS and psoriasis status. To minimize potential bias, clinical observations were obtained from 3 independent neurology clinics.
Every case of comorbid MS and psoriasis was collected, provided that the patient was on natalizumab treatment for MS; this amounted to 18 cases. In all 18 cases, the MS was diagnosed based on concurrent McDonalds criteria. Sixteen of 18 patients had CSF analysis positive for several unique oligoclonal bands (2 patients declined the lumbar puncture procedure). Psoriasis diagnosis was confirmed by treating dermatologists. Psoriasis status was evaluated using the Subject Global Impression scale (grades: much worse, worse, somewhat worse, no change, somewhat better, better, and much better); only “better” and “much better” counted as improvement. No tumor necrosis factor inhibitors were used in the observed cases. All psoriasis cases observed were plaque skin psoriasis. Two cases also had upper extremity nail involvement with cuticle atrophy.
Statistical analysis was performed with Kruskal-Wallis nonparametric analysis of variance using Graph pad InStat software.
Data Availability
Anonymized data will be shared by request from any qualified investigator.
Results
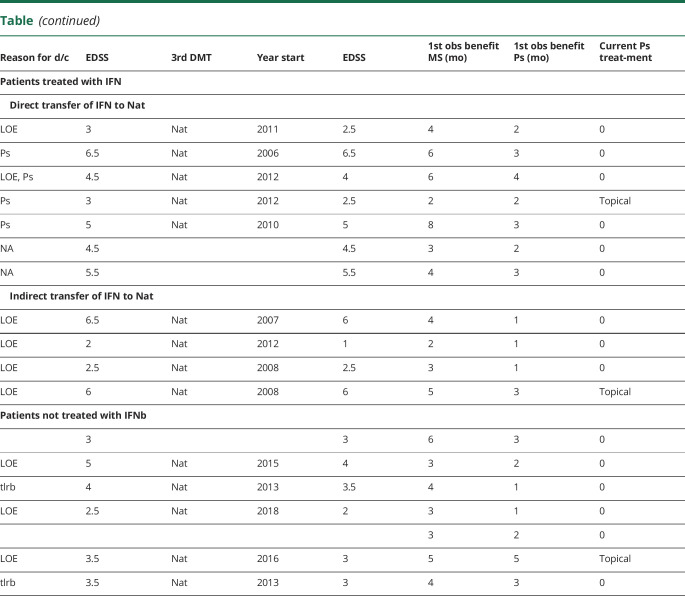
As demonstrated by the data presented in table, interferon-beta (IFNb) treatment was used by 11 patients; in 10 of 11 cases, it coincided either with worsening of psoriasis or with psoriasis developed de novo while on the treatment. IFNb treatment was associated with new MS activity in 3 of 11 patients. According to our data, glatiramer acetate treatment was not associated with psoriasis worsening. Three patients were started on dimethyl fumarate (DMF) but discontinued because of tolerability issues or lack of efficacy for MS. Two patients who discontinued DMF for tolerability reasons were not on DMF for a sufficient amount of time to determine any effects on psoriasis. One DMF patient was on therapy for approximately 2 years and had incomplete control on psoriasis.
Table.
Summary of Patient Information
All 18 patients at different points were started on natalizumab. One of the patients actually had natalizumab treatment initiated twice—as a first-line DMT and as his 3rd DMT.
In all 18 cases, natalizumab use was associated with patient-reported improvement of the comorbid psoriasis; 16 of 18 patients at the time of last evaluation (2017–2020) were not needed to be on systemic/immunosuppressive therapy for psoriasis. Most cases, positive clinical effects attributed to natalizumab were reported first in the clinical presentation of skin condition before any effects on their MS.
We examined whether the treatment regimens resulted in any significant difference in response time for benefit for either MS or Ps (see last 3 columns in table). There was no significant difference between any of the 3 treatment paradigms (direct transition from IFN to Nat, indirect transfer from IFN to Nat, or no IFN before Nat) for the time for benefit for MS or Ps (data not shown). Furthermore, there was no significant difference between any of these treatment paradigms and the lag in time between MS benefit and beginning of Ps benefit.
Discussion
Given still limited published data, no definite conclusion on direct relationship between natalizumab treatment and psoriasis condition can be drawn. Moreover, no clinical recommendations can be made. However, these observations provide additional data for better understanding of treatment responses of patients having comorbid MS and psoriasis.
Comorbidity of psoriasis in a fraction of patients with MS was recognized several decades ago,7,8 and a more recent report suggested that psoriasis comorbidity may influence progression of MS.9 There is also similar immunologic connections of psoriasis and MS, with involvement of Th17 cells in both diseases.10,11 Although both diseases share common genetic risk factors to influence susceptibility to autoimmune disease, the association of MS with other immune-mediated diseases such as psoriasis has recently been recognized to be due predominantly to surveillance bias or similar influence by environmental factors in both diseases.12,13
Psoriasis is a dermatological chronic immune-mediated disease. The cellular component of the psoriatic lesion tissue includes epidermal infiltration with lymphocytes, including Th1 and Th17, activated T cells, innate lymphocytes, and dendritic cells, causing keratinocyte proliferation.14,15 Natalizumab blocks immune cell entry to the CNS including the entry of T cells and B cells.16 Natalizumab also has been studied for the treatment of the Crohn's disease because it was shown to modulate adhesion between the leukocytes and endothelial cells.17 Natalizumab may exert its putative benefit in psoriasis by blocking immune cell infiltration into the skin in a similar manner.
In spite of this relatively close association between MS and psoriasis and higher incidence of psoriasis in MS population compared with the general population, it is well known that at least some MS DMT not only show no efficacy in psoriasis but also may exacerbate the condition. Two hypotheses could be proposed to explain how treatment with natalizumab was associated with a potential improvement of the psoriasis: (1) transition of patients to natalizumab may alleviate exacerbation of psoriasis resulting from an iatrogenic previous DMT or (2) there may be beneficial effects from the natalizumab toward psoriasis.
It is well known, for example, that IFNb treatment has been suggested to potentially trigger onset of psoriasis in patients with MS. In our population, 5 of the 11 patients who received IFNb were subsequently diagnosed with psoriasis after initiation of IFNb therapy. It has been postulated that IFNb may induce the IL 17-driven conditions such as psoriasis because IL-17 enhances keratinocyte proliferation while inhibiting keratinocyte its differentiation; in the meantime, keratinocytes induce further Th17 cell recruitment and IL-17 production.15
Regarding potential improvement of psoriasis after discontinuation of IFNb, 4 of the 11 IFNb patients received another DMT before initiation of natalizumab. Notably, of the remaining 7 IFNb patients who transitioned directly from IFNb to natalizumab, 2 of those patients had lag times between therapies of 2–3 years. These results indicate that mere transition from IFNb may not totally account for the improvement of psoriasis observed with natalizumab treatment and suggest that natalizumab may actually exert some beneficial effect toward psoriasis. The possible interaction of other MS therapies with psoriasis (and vice versa) has not yet been investigated. Of note, an effective psoriasis treatment with a monoclonal antibody efalizumab that blocks trafficking through inhibition of LFA-1/ICAM-1 interaction was withdrawn because of risk for progressive multifocal leukoencephalopathy.18 In light of this example where an effective drug for psoriasis blocks trafficking, it would seem quite plausible that another drug for MS, such as natalizumab that also blocks trafficking, might also be effective in psoriasis. This also raises the question as to whether other MS drugs that interfere with trafficking might also have efficacy in psoriasis.
Preclinical studies showed that Th1 cells preferentially infiltrate the spinal cord via an α4 integrin-mediated mechanism, whereas the entry of Th17 cells into the brain parenchyma occurs in the absence of α4 integrins but is dependent on the expression of αLβ2.19 If these preclinical findings are applicable to psoriasis, it emphasizes that α4 integrin blockade does provide benefit in psoriasis when accompanied by MS.
There has been one case reported of a patient who developed disseminated psoriasis over the course of six infusions with natalizumab.3 The direct link of natalizumab was not established in this case, but those authors noted that psoriatic (and not rheumatic) arthritis patients can harbor anti-integrin antibodies and that natalizumab might similarly alter laminin function to promote dermal changes seen in psoriasis. However, there are not any subsequent reports of natalizumab-associated exacerbation of psoriasis, suggesting that if this was the cause, then the incidence would be more wide-spread. In our case, we have seen just the opposite in that natalizumab was associated with clinical improvement of psoriasis. It would be potentially more plausible that natalizumab may potentially block the deleterious effects of patient associated anti integrin antibodies. More studies would be needed to test this possibility.
This observational study was limited because only a small number of patients were observed and were not compared against any control group. Further randomized studies will enable neurologists to obtain definitive answers to the optimal therapy when this comorbidity arises.
Acknowledgment
Evan Riddle, a Biogen employee, contributed to the development of this publication. Sergey Grando, Professor of Dermatology, University of California Irvine, and Daniel Arkfeld, Professor of Rheumatology, University of Southern California, contributed to the methodology and results discussion.
Glossary
- DMF
dimethyl fumarate
- DMT
disease-modifying therapy
- EDSS
Expanded Disability Status Scale
- IFNb
interferon-beta
Appendix. Authors
Contributor Information
Aida Yakupova, Email: aidayakupova@yandex.ru.
Jonathan Eskenazi, Email: jeskena@hotmail.com.
Noel G. Carlson, Email: noel.carlson@va.gov.
Lawrence Steinman, Email: aidayakupova@yandex.ru.
Study Funding
There was no funding provided for this study. Partial effort for Noel Carlson was provided by the Department of Veterans Affairs. The authors report no targeted funding.
Disclosure
R. Berkovich received past research support from Biogen, Novartis, Sanofi, Mallinckrodt and Teva and she consulted for Alexion, Bayer, Biogen, Celgene, Genetec, Mallinckrodt, Novartis, Sanofi. A. Yakupova and J. Eskenazi have no financial relationships to disclose. N.G. Carlson received past research support from Biogen outside the scope of this work. L. Steinman has consulted with Roche, Novartis, Tolerion, Atreca, TG Therapeutics, and Atara Biopharma and all these consultations are outside the scope of this work. Go to Neurology.org/NN for full disclosures.
References
- 1.Polman CH, O'Connor PW, Havrdova E, et al. ; AFFIRM Investigators. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med 2006;354:899–910. [DOI] [PubMed] [Google Scholar]
- 2.Lambrianides S, Kinnis E, Leonidou E, Pantzaris M. Does natalizumab induce or aggravate psoriasis? A case study and review of literature. Case Rep Neurol 2018. 10:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Millán-Pascual J, Turpín-Fenoll L, Del Saz-Saucedo P, Rueda-Medina I, Navarro-Muñoz S. Psoriasis during natalizumab treatment for multiple sclerosis. J Neurol 2012;259:2758–2760. [DOI] [PubMed] [Google Scholar]
- 4.Clark SJ, Wang Q, Mao-Draayer Y. Switching from natalizumab to fingolimod: case report and review of literature. J Immunol Clin Res 2016;3:1030. [Google Scholar]
- 5.Vacchiano V, Foschi M, Sabattini L, Scandellari C, Lugaresi A. Arthritic psoriasis during natalizumab treatment: a case report and review of the literature. Neurol Sci 2018;39:181–183. [DOI] [PubMed] [Google Scholar]
- 6.Berkovich RR. Four cases of comorbid multiple sclerosis and psoriasis: sustained remission of both conditions while on natalizumab. Presented at American Academy of Neurology 62nd Meeting; Toronto, Canada; April 10–17, 2010;Abstract: P06.163.
- 7.Cendrowski W. Multiple sclerosis and psoriasis. Wiad Lek 1989;42:575–578. [PubMed] [Google Scholar]
- 8.Midgard R, Grønning M, Riise T, Kvåle G, Nyland H. Multiple sclerosis and chronic inflammatory diseases. A case‐control study. Acta Neurol Scand 1996;93:322–328. [DOI] [PubMed] [Google Scholar]
- 9.Miron G, Gurevich M, Baum S, Achiron A, Barzilai A. Psoriasis comorbidity affects multiple sclerosis neurological progression: a retrospective case–control analysis. J Eur Acad Dermatol Venereol 2017;12:2055–2061. [DOI] [PubMed] [Google Scholar]
- 10.Kwok T, Jing Loo W, Guenther L. Psoriasis and multiple sclerosis: is there a link? J Cutan Med Surg 2010;14:151–155. [DOI] [PubMed] [Google Scholar]
- 11.Axtell RC, Raman C, Steinman L. Interferon-β exacerbates Th17-mediated inflammatory disease. Trends Immunol 2011;32:272–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roshanisefat H, Bahmanyar S, Hillert J, Olsson T, Montgomery S. Shared genetic factors may not explain the raised risk of comorbid inflammatory diseases in multiple sclerosis. Mult Scler 2012;18:1430–1436. [DOI] [PubMed] [Google Scholar]
- 13.Olafsson S, Stridh P, Bos SDet al. Fourteen sequence variants that associate with multiple sclerosis discovered by meta-analysis informed by genetic correlations. NPJ Genom Med 2017;2:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weller RB, Hunter HJ, Mann MW. Clinical Dermatology. Chichester, UK: John Wiley & Sons, Ltd; 2014. [Google Scholar]
- 15.Deng Y, Chang C, Lu Q. The inflammatory response in psoriasis: a comprehensive review. Clin Rev Allergy Immunol 2016;50:377–389. [DOI] [PubMed] [Google Scholar]
- 16.Stuve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006; 59 743–747. [DOI] [PubMed] [Google Scholar]
- 17.Guagnozzi D, Caprilli R, Natalizumab in the treatment of Chron's disease. Biologics 2008;2:275–284. [PMC free article] [PubMed] [Google Scholar]
- 18.Pugashetti R, Koo JJ. Efalizumab discontinuation: a practical strategy. Dermatolog Treat 2009;20:132–136. [DOI] [PubMed] [Google Scholar]
- 19.Rothhammer V, Heink S, Petermann F, et al. Th17 lymphocytes traffic to the central nervous system independently of α4 integrin expression during EAE. J Exp Med 2011;208:2465–2476. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Anonymized data will be shared by request from any qualified investigator.