Abstract
Objective
Investigate the value of including cerebellar degeneration-related protein 2-like (CDR2L) as a marker in commercial diagnostic tests for anti-Yo–associated paraneoplastic cerebellar degeneration (PCD).
Methods
We included sera and CSF samples from 24 patients with suspected PCD (6 of whom had PCD with underlying gynecologic or breast cancer), who were positive for Yo antibodies using the commercially available, paraneoplastic neurologic syndromes (PNS) 14 Line Assay from Ravo Diagnostika. The samples were further evaluated using the EUROLINE PNS 12 Ag Line Assay and a cell-based assay (CBA) from Euroimmun. For confirmation of positive lineblot results, we used indirect immunofluorescence of rat cerebellar sections. We also tested all samples in 2 assays developed in-house: a CBA for CDR2L and a Western blot analysis using recombinant cerebellar degeneration-related protein 2 (CDR2) and CDR2L proteins.
Results
In PNS 14 and PNS 12 Ag Line Assays, anti-CDR2 reactivity was observed for 24 (100%) and 20 (83%) of the 24 samples, respectively. Thirteen of 24 subjects (54%) were also positive using the Euroimmun CBA. Rat cerebellar immunofluorescence was the best confirmatory test. In our in-house CBA for CDR2L and Western blot for CDR2 and CDR2L, only the 6 patients with confirmed PCD reacted with CDR2L.
Conclusions
Commercially available tests for Yo antibody detection have low specificity for PCD because these assays use CDR2 as antigen. By adding a test for CDR2L, which is the major Yo antigen, the accuracy of PCD diagnosis greatly improved.
Classification of Evidence
This study provides Class III evidence that a CBA for CDR2L accurately identifies patients with PCD.
Paraneoplastic neurologic syndromes (PNS) are rare, immune-mediated diseases triggered by cancer that differ in clinical features, prognosis, and associated onconeural antibodies.1–4 Paraneoplastic cerebellar degeneration (PCD) is one of the most common of these syndromes, observed in individuals with gynecologic or breast cancer. These patients usually have Yo antibodies in serum and CSF.5–7 Evidence suggests that anti-Yo targets 2 intracellular antigens, cerebellar degeneration-related protein 2 (CDR2) and CDR2-like (CDR2L), expressed in the nucleus and cytoplasm of Purkinje neurons in the cerebellum, respectively.8–10 The interaction between anti-Yo and CDR proteins is believed to mediate Purkinje neuron dysfunction and death, leaving the patients in a severely disabled state.11,12
Onconeural antibodies identified in the sera or CSF of patients are key diagnostic biomarkers for PCD.3 Commercial line assays are available, but the diagnostic value of these tests has been questioned.1,13 The specificity of anti-Yo is low, with less than 10% confirmation rate.1 Because anti-Yo is associated with most PCD cases related to gynecologic and breast cancer, improved diagnostic tests for anti-Yo is important to ensure a correct clinical diagnosis and prevent unnecessary tests or inappropriate treatment.
Our recent studies have suggested that CDR2L, which shares 50% sequence homology with CDR2, is likely the main target of anti-Yo.12 We postulate that the low specificity for detecting Yo antibodies seen with commercial immunoassays is that they use CDR2 as the Yo-antigen. Here, we assessed the value of including CDR2L as a diagnostic marker to increase the specificity for Yo antibody detection.
Methods
Patients
We performed a retrospective analysis of 9,527 sera and CSF samples from patients screened for onconeural antibodies at the Neurological Research Laboratory, Haukeland University Hospital, Bergen, from 2017 to 2020. We included the 24 patients with serum and/or CSF Yo reactive bands detected by the commercial PNS 14 Line Assay from Ravo Diagnostika (Freiburg im Breisgau, Germany) in the study. Positive samples were also tested with EUROLINE PNS 12 Ag and cell-based assay (CBA) from Euroimmun (Lübeck, Germany) and were further explored by indirect immunofluorescence of rat cerebellar section. Samples were also tested in 2 assays for CDR2L developed in-house.
Standard Protocol Approvals, Registration, and Patient Consents
Patient records for the 24 included cases were obtained and anonymized before the study. PCD was diagnosed according to the established criteria.2 The study was approved by The Regional Committee for Health and Medical Research Ethics in Norway, REK #123524.
Commercial Line Immunoassays for Anti-CDR2 Detection
The PNS 14 Line Assay (Ravo Diagnostika, #PNS14-003) includes 14 different antigens for PNS: GAD65, HuD, Yo, Ri, CV2/CRMP5, amphiphysin, Ma1, Ma2, SOX1, Tr/DNER, Zic4, titin, recoverin, and Protein Kinase C γ. The EUROLINE PNS 12 Ag (Euroimmun, #DL1111-1601-7-G) includes 12 different antigens for PNS: amphiphysin, CV2/CRMP5, Ma2, Ri, Yo, Hu, recoverin, SOX1, titin, Zic4, GAD65, and Tr/DNER. Serum and CSF samples from 24 patients were analyzed in both immunoassays following the manufacturer's instructions. Two independent investigators graded band intensities from + to +++, compared to a positive control sample (+++).
The serum and CSF from the 24 patients were also tested for anti-Yo using a commercial CBA (Purkinje Cell Mosaic 1; Euroimmun, #FA1113-1005-1) consisting of BIOCHIP Mosaics with 4 positions (Yo/CDR2-, Tr/DNER-, ITPR1-, and CARP-transfected human embryonic kidney 293 [HEK293] cells), positive and negative controls. Briefly, aliquots of 30 µL serum (diluted 1:100) or of CSF (diluted 1:1) were applied to each reaction field on the BIOCHIP slide. After incubation (30 minutes, room temperature), the slide was washed with phosphate-buffered saline containing 0.2% Tween 20 (PBS-Tween 20; 5 minutes, room temperature), followed by incubation with goat anti-human IgG secondary antibody conjugated to Alexa Fluor 488 (Thermo Fisher Scientific, Waltham, MA, 1:500, #A-11013, 30 minutes, room temperature). The slide was rinsed with PBS-Tween 20 and mounted on a glass coverslip. The cutoff for Yo/CDR2 was set to 1:100, as advised by the manufacturer. Two independent investigators evaluated the results.
Indirect Immunofluorescence
All procedures were performed according to the NIH Guidelines for the Care and Use of Laboratory Animals Norway (FOTS 20135149/20157494/20170001). Wistar Hannover GLAST rats were anesthetized and transcardially perfused with ice-cold 4% paraformaldehyde (PFA) in PBS. The brains were postfixed (24 hours, 4°C), incubated with 18% sucrose in PBS (72 hours, 4°C), snap-frozen, and cut on a cryostat to 10-μm parasagittal sections.14 Heat-induced antigen retrieval was performed with a pressure cooker in Diva Decloaker buffer solution (Biocare Medical, Pacheco, CA; #DV2004MX). Sections were blocked in bovine serum albumin (BSA) and Triton X-100 in PBS (2 hours, room temperature), followed by incubation with patient samples (1:500 in blocking solution, overnight, 4°C). Finally, the sections were rinsed with PBS, incubated with secondary antibody (Alexa Fluor 488 goat anti-human IgG, 1:100, 90 minutes, room temperature), rinsed, and mounted with ProLong Diamond Antifade Mountant with 4’,6-diamidino-2-phenylindole (DAPI) (Thermo Fisher Scientific, #P36962).8
CBA for Anti-CDR2L Detection
HEK293 cells were cultured in 8-well Nunc Lab-Tec II Chamber Slide System (Thermo Fisher Scientific, #154534) in Eagle Minimum Essential Medium supplemented with 10% fetal bovine serum and penicillin/streptomycin (37°C, 5% CO2). Cells were transfected with a plasmid for expression of Myc-DDK-tagged CDR2L (Origene, Rockville, MD; #RC206909) using Lipofectamine 3000 Reagent (Invitrogen, Carlsbad, CA; #L3000008). At 48 hours after transfection, coverslips were washed with PBS and fixed with 4% PFA/4% glucose in PBS (20 minutes, room temperature). Demembranation with 0.1% triton X-100 in PBS (7 minutes, room temperature) was followed by blocking with 10% Sea Block blocking buffer (Thermo Fisher Scientific, #37527) in PBS (1 hour, room temperature). Coverslips were incubated with the serum (1:10 and 1:100) or CSF (1:10 and 1:100), mouse anti-DYKDDDDK tag or FLAG tag (DDK) (Origene, #TA50011-100, 1:1,000), anti-CDR2L (Protein Technology, Pencroft Way, Manchester, UK; #14563-1-AP), or anti-CDR2 (Sigma-Aldrich, St. Louis, MO; #HPA023870) in blocking solution (1 hour, room temperature). Finally, coverslips were washed with PBS, incubated with secondary antibody (Alexa Fluor 488 goat anti-human, Alexa Fluor 488 goat anti-rabbit, or Alexa Fluor 594 goat-anti-mouse, Thermo Fisher Scientific, #A-11013, #A-11008, #A-11005, respectively, 1 hour, room temperature) and mounted using ProLong Diamond Antifade Mountant with DAPI.
Western Blot for Anti-CDR2 and Anti-CDR2L Detection
The transcription/translation-coupled reticulocyte lysate system (Promega, Madison, WI; #L4610) was used for cell-free protein expression of CDR2L and CDR2. Purified plasmids encoding the 2 proteins (2.0 μg; Origene, RC204900 [CDR2] and #RC206909 [CDR2L]) were incubated with the transcription/translation lysate, T7 RNA polymerase promoter, reaction buffer, recombinant RNasin ribonuclease inhibitor (Promega, #N2511), and amino acid mixture (30°C, 1.5 hours). A negative control without plasmid was included in each experiment. The reaction products were evaluated by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis, followed by Western blot analysis.
The reticulocyte extract was denatured in Laemmli buffer (Bio-Rad, Hercules, CA; #1610747, 95°C, 5 minutes) and 2.5% β-mercaptoethanol subjected to SDS-polyacrylamide gel electrophoresis separation on a 10% TGX gel (Bio-Rad, #456-1035) and transferred to a polyvinylidene difluoride membrane using the Trans-Blot Turbo Transfer kit (Bio-Rad, #170-4274). The blots were blocked in 5% dry milk (Bio-Rad, #170-6404) dissolved in 1x Tris-buffered saline with 0.1% Tween 20 (TBS-Tween 20) and incubated with serum or CSF sample diluted in 3% BSA in TBS-Tween 20 (1:250/1:100, 4°C, overnight). Antibody fixation was visualized using horseradish peroxidase anti-human IgG (Dako, Carpinteria, CA; #P0214, 1 hour, room temperature).
Imaging
Rat cerebellar sections and CBAs were imaged on a Leica Leitz DM RBE fluorescence microscope with CoolLED pE-300-W LED illumination. Images were evaluated by 2 independent investigators. ImageJ was used for background subtraction of microscopy images and evaluation of Western blot results.
Data Availability
Data related to the current article are available from the corresponding authors on reasonable request.
Results
Between 2017 and 2020, 24 of 9,527 tested serum or CSF samples (0.25%) from patients with suspected PNS showed a reactive band for Yo antibodies using the PNS 14 Line Assay from Ravo Diagnostika and 20 (83%) showed a reactive band using EUROLINE PNS 12 Ag from Euroimmun (table). Thirteen of the 24 patients (54%) had a confirmed positive CBA CDR2 assay, whereas only 8 stained Purkinje cells in the immunofluorescence assay (table).
Table.
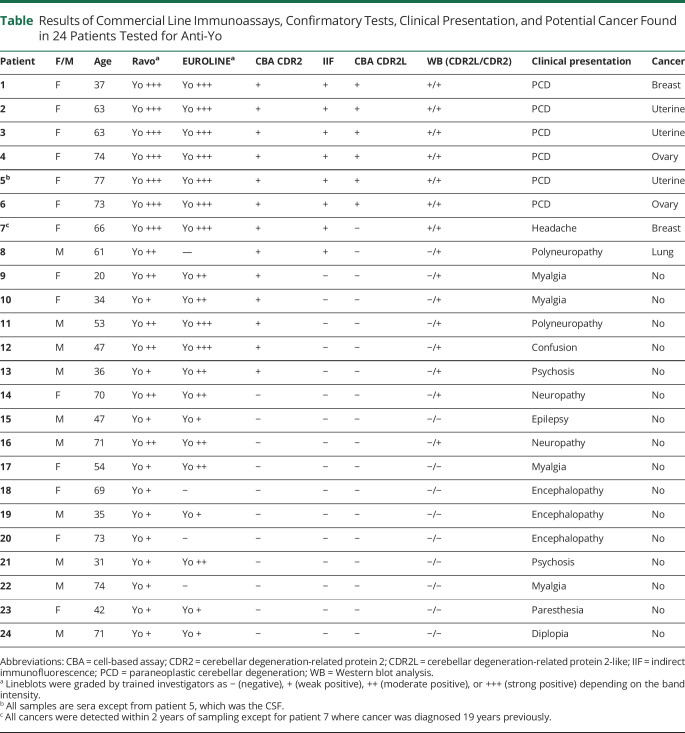
Results of Commercial Line Immunoassays, Confirmatory Tests, Clinical Presentation, and Potential Cancer Found in 24 Patients Tested for Anti-Yo
Using staining of rat cerebellar sections as a confirmatory test, we found that Yo positive sera from 6 PCD patients showed granular, cytoplasmic staining in Purkinje neurons (figure 1A). In the group of 18 nonconfirmed PCD cases, serum samples from 2 patients (7 and 8) stained Purkinje neurons but with no granular cytoplasmic staining; these patients were therefore interpreted as anti-Yo negative (figure 1B). The remaining 16 serum samples were negative (figure 1C). The commercial line immunoassays alone yielded a high number of false positive results (18/24 [75%] for the Ravo assay and 16/24 [67%] for the Euroimmun assay). Even when combined with the CBA CDR2 (Euroimmun), the false positive rate was high at 7 of 24 (29%). The best-established method for Yo antibody confirmation was careful interpretation of immunofluorescent staining of Purkinje neurons in rat cerebellar sections.
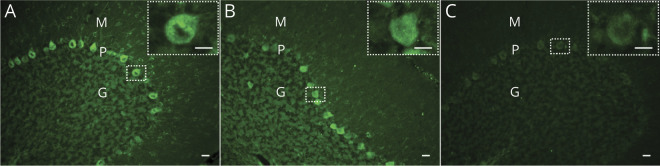
Figure 1. Representative Images of Rat Cerebellar Sections Incubated With Patient Samples.
(A) Sera from confirmed paraneoplastic cerebellar degeneration (PCD) cases (cerebellar degeneration-related protein 2-like [CDR2L]+/cerebellar degeneration-related protein 2 [CDR2]+, patients 1–6) show granular, cytoplasmic staining of Purkinje neurons. (B) Sera from the 2 cases without PCD but with previously detected cancer (CDR2L−/CDR2+, patients 7 and 8) stain the cytoplasm of Purkinje neurons, but no granular staining is observed. (C) Sera from the remaining cases without PCD and without cancer (CDR2L−/CDR2+, patient 9–13) do not stain Purkinje neurons of rat cerebellar sections. CDR2L/CDR2 testing is based on line blots and cell-based assays. G = granular layer; M = molecular layer; P = Purkinje neuron layer. Scale bar = 20 μm; zoom in scale bar = 10 μm.
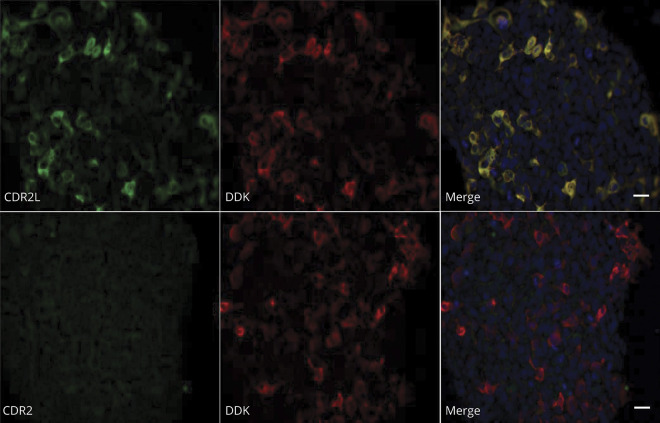
Because we recently showed that the major target for Yo-antibodies is not CDR2, but CDR2L,12 we developed an assay based on HEK293 cells transfected with a plasmid for expression of Myc-DDK-tagged CDR2L and stained these cells with patient sera or CSF. To evaluate the specificity of our in-house CDR2L CBA, HEK293 cells that express Myc-DDK-tagged CDR2L were stained with anti-DDK, anti-CDR2L, or anti-CDR2 (figure 2). There was complete overlap between CDR2L and DDK cytoplasmic staining. The absence of CDR2 antibody staining confirmed that there was no cross-reactivity between CDR2 and CDR2L antibodies.
Figure 2. No Cross-Reactivity Is Observed Between CDR2 Antibodies and CDR2L in Human Embryonic Kidney 293 Cells That Express Myc-DDK-Tagged CDR2L.
Upper row: cells stained with anti-CDR2L (green) and anti-DDK (red). Lower row: cells stained with anti-CDR2 (no reaction), and anti-DDK (red). Nuclei are stained with DAPI. Scale bar = 20 μm. CDR2 = cerebellar degeneration-related protein 2; CDR2L = cerebellar degeneration-related protein 2-like.
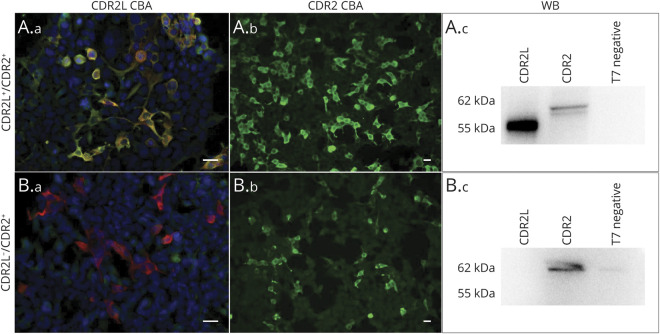
Samples from the 6 confirmed PCD cases stained both CDR2L-transfected cells and commercial CBA for CDR2 (figure 3, A.a and A.b). However, the samples from the 7 patients with CBA CDR2-positive staining, but no PCD, did not show CBA staining for CDR2L (figure 3, B.a and B.b). These results were confirmed by Western blot analysis of recombinant CDR2 (62 kDa) and CDR2L (55 kDa) proteins (figure 3, A.c and B.c) with the exception of the sample from one patient without PCD (patient7) who tested positive for CDR2L in Western blot but not in CBA. We also found that Western blot analysis with CDR2 was negative for 9 and 6 patients who were weak-to-moderate positive on the Ravo and Euroimmun assays, respectively (table).
Figure 3. Representative Images of Patient Sera (1:100) Double Positive for CDR2L and CDR2 (A.a–A.c), and Single Positive for CDR2 (B.a–B.c) in Indirect Immunofluorescence of CDR2L Transfected Human Embryonic Kidney 293 Cells (A.a, B.a), Commercial CBA for CDR2 (A.b, B.b), and WB (A.c, B.c).
A negative control containing reticulocyte lysate without recombinant protein was included in each experiment. Anti-CDR2/CDR2L, green; anti-DDK, red; merge, yellow. Scale bar = 20 μm. CBA = cell-based assay; CDR2 = cerebellar degeneration-related protein 2; CDR2L = cerebellar degeneration-related protein 2-like; WB = Western blot analysis.
Discussion
Commercial line immunoassays enable simple and rapid detection of onconeural antibodies in patients with suspected PNS. In this study, we evaluated the diagnostic accuracy of Yo antibody testing by commercial line immunoassays and routine confirmatory tests. We found an approximate 70% false positivity rate using commercial assays alone, which is in line with recent studies.1,13 The discrepancy between the 2 commercial assays is most likely related to the nature of the antigens: Both use recombinant CDR2 proteins, but the sequence length, and therefore protein structure, and the cell lines in which the recombinant CDR2 is produced differ. Band intensities were graded from + to +++ compared to a positive control. Overall, we observed that samples with intense reactive bands on the line immunoassays were more likely to be from patients with PCD than those with weaker reactive bands, as was also reported recently.13
The number of false positive tests for PCD was reduced by combining the results from the 2 line immunoassays with a CBA for CDR2. In agreement with another study,1 we found several men among the CDR2-positive but PCD-negative patients, supporting the hypothesis that CDR2 is not the natural Yo antigen.
In our hands, the best confirmatory test among the established techniques were rat cerebellar immunofluorescence. However, many clinical laboratories are not equipped to perform indirect immunofluorescence assays, and Purkinje cell staining can be difficult to interpret because only granular cytoplasmic staining is characteristic of anti-Yo.8,12 This pattern probably represents ribosomal staining because it has been shown recently that CDR2L interacts with the ribosomal subunit protein rpS6.10 Sera from 2 of our patients without PCD, but with previous cancer, also stained the rat Purkinje cell cytoplasm, but the cytoplasmic staining was not granular. The specific target for this staining is unknown, but such false positive anti-Yo staining must be interpreted with caution because it is unrelated to PCD.
In our cohort of 24 patients, all positive for Yo antibodies based on the commercial line immunoassays, only 6 had PCD. This means that routine testing using only line immunoassays must be performed with care and must be confirmed to prevent misdiagnosis, unnecessary testing, and incorrect treatment. Some laboratories use immunohistochemistry for initial screening, which may avoid false positive results based on commercial line immunoassays alone. However, these analyses are laborious and require skilled personnel to interpret the binding patterns.
We have previously shown that Yo antibodies bind both endogenous and recombinant CDR2L but only recombinant CDR2.12 These findings imply that there are independent antibody responses to CDR2L and CDR2, supported by the fact that the most highly enriched regions of CDR2L are the most divergent regions between the 2 proteins.15 Because CDR2 and CDR2L share common epitopes, this probably explains the frequent detection of false positive results, which are CDR2 restricted. This is supported by our recent findings that PCD-related Yo antibodies bind only endogenous CDR2L not endogenous CDR2.12
We hypothesized that the specificity of the routine commercial tests could be increased by including CDR2L as a target protein. In the present study, we developed 2 techniques for detection of CDR2L: a CBA consisting of HEK293 cells that express Myc-DDK-tagged CDR2L and a Western blot–based analysis of recombinant CDR2 and CDR2L proteins. Our CDR2L CBA identified all 6 patients with PCD and was negative for the 18 nonconfirmed cases. Western blot analysis with recombinant CDR2L identified the 6 PCD patients and one patient with no PCD but with a previous diagnosis of breast cancer. The apparent mismatch between our CBA and Western blot assays is unclear but may represent a differently expressed epitope of CDR2L detected by each of the 2 assays. Interestingly, patients with weak-to-moderate positive commercial line immunoassays were also found negative by the CDR2 Western blot analysis, again suggesting differences in the epitopes detected.
Although our study cohort is small, our data demonstrate that detection of CDR2L adds an important dimension to the diagnostic accuracy of PCD testing. Currently, we do not know whether testing for CDR2L antibodies alone would be sufficient for diagnosis of PCD because our cohort were selected based on anti-CDR2 positivity. This question will require larger patient cohorts including PCD patients who test negative in commercial line immunoassays and patients who have PNS caused by other onconeural antibodies.
In conclusion, our results underline the importance of confirmatory tests when interpreting results from the currently commercially available anti-Yo detection assays. The high proportion of false positive results appears to be due to the use of CDR2 as antigen. Therefore, all positive samples tested by commercial line immunoassays must be confirmed by immunofluorescence or immunohistochemistry. However, our results support the thesis that CDR2L is the major Yo antigen, and we suggest that CDR2L should be included in the commercially available line immunoassays and CBAs for Yo antibody detection.
Acknowledgment
The authors thank Torbjørn Kråkenes for technical help and Laurence Bindoff for valuable discussion.
Glossary
- BSA
bovine serum albumin
- CBA
cell-based assay
- CDR2
cerebellar degeneration-related protein 2
- CDR2L
cerebellar degeneration-related protein 2-like
- HEK293
human embryonic kidney 293
- PCD
paraneoplastic cerebellar degeneration
- PFA
paraformaldehyde
- PNS
paraneoplastic neurologic syndromes
- SDS
sodium dodecyl sulfate
Appendix. Authors
Study Funding
The study was funded by Helse Vest.
Disclosure
I. Herdlevær, M. Haugen, K. Mazengia, C. Totland, and C. Vedeler report no disclosures relevant to the manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Déchelotte B, Muñiz-Castrillo S, Joubert B, et al. Diagnostic yield of commercial immunodots to diagnose paraneoplastic neurologic syndromes. Neurol Neuroimmunol Neuroinflamm 2020;7:e701. doi: 10.1212/NXI.0000000000000701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graus F, Delattre JY, Antoine JC, et al. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry 2004;75:1135–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yshii L, Bost C, Liblau R. Immunological bases of paraneoplastic cerebellar degeneration and therapeutic implications. Front Immunol 2020;11:991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dalmau J, Rosenfeld MR. Paraneoplastic syndromes of the CNS. Lancet Neurol 2008;7:327–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Storstein A, Monstad SE, Haugen M, et al. Onconeural antibodies: improved detection and clinical correlations. J Neuroimmunol 2011;232:166–170. [DOI] [PubMed] [Google Scholar]
- 6.O'Brien TJ, Pasaliaris B, D'Apice A, Byrne E. Anti-Yo positive paraneoplastic cerebellar degeneration: a report of three cases and review of the literature. J Clin Neurosci 1995;2:316–320. [DOI] [PubMed] [Google Scholar]
- 7.Venkatraman A, Opal P. Paraneoplastic cerebellar degeneration with anti-Yo antibodies—a review. Ann Clin Transl Neurol 2016;3:655–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raspotnig M, Haugen M, Thorsteinsdottir M, et al. Cerebellar degeneration-related proteins 2 and 2-like are present in ovarian cancer in patients with and without Yo antibodies. Cancer Immunol Immunother 2017;66:1463–1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Totland C, Aarskog NK, Eichler TW, et al. CDR2 antigen and Yo antibodies. Cancer Immunol Immunother 2011;60:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herdlevær I, Kråkenes T, Schubert M, Vedeler CA. Localization of CDR2L and CDR2 in paraneoplastic cerebellar degeneration. Ann Clin Translational Neurol 2020;7:2231–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Storstein A, Krossnes BK, Vedeler CA. Morphological and immunohistochemical characterization of paraneoplastic cerebellar degeneration associated with Yo antibodies. Acta Neurol Scand 2009;120:64–67. [DOI] [PubMed] [Google Scholar]
- 12.Krakenes T, Herdlevaer I, Raspotnig M, Haugen M, Schubert M, Vedeler CA. CDR2L is the major Yo antibody target in paraneoplastic cerebellar degeneration. Ann Neurol 2019;86:316–321. [DOI] [PubMed] [Google Scholar]
- 13.Ruiz-García R, Martínez-Hernández E, Saiz A, Dalmau J, Graus F. The diagnostic value of onconeural antibodies depends on how they are tested. Front Immunol 2020;11:1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schubert M, Panja D, Haugen M, Bramham CR, Vedeler CA. Paraneoplastic CDR2 and CDR2L antibodies affect Purkinje cell calcium homeostasis. Acta Neuropathol 2014;128:835–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.O'Donovan B, Mandel-Brehm C, Vazquez SE, et al. High resolution epitope mapping of anti-Hu and anti-Yo autoimmunity by programmable phage display. Brain Commun 2020;2:fcaa059. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data related to the current article are available from the corresponding authors on reasonable request.