Abstract
Objective
To determine whether neutrophils contribute to amyotrophic lateral sclerosis (ALS) progression, we tested the association of baseline neutrophil count on ALS survival, whether the effect was sex specific, and whether neutrophils accumulate in the spinal cord.
Methods
A prospective cohort study was conducted between June 22, 2011, and October 30, 2019. Blood leukocytes were isolated from ALS participants and neutrophil levels assessed by flow cytometry. Participant survival outcomes were analyzed by groups (<2 × 106, 2–4 × 106, and >4 × 106 neutrophils/mL) with adjustments for relevant ALS covariates and by sex. Neutrophil levels were assessed from CNS tissue from a subset of participants.
Results
A total of 269 participants with ALS within 2 years of an ALS diagnosis were included. Participants with baseline neutrophil counts over 4 × 106/mL had a 2.1 times higher mortality rate than those with a neutrophil count lower than 2 × 106/mL (95% CI: 1.3–3.5, p = 0.004) when adjusting for age, sex, and other covariates. This effect was more pronounced in females, with a hazard ratio of 3.8 (95% CI: 1.8–8.2, p = 0.001) in the >4 × 106/mL vs <2 × 106/mL group. Furthermore, ALS participants (n = 8) had increased neutrophils in cervical (p = 0.049) and thoracic (p = 0.022) spinal cord segments compared with control participants (n = 8).
Conclusions
Higher neutrophil counts early in ALS associate with a shorter survival in female participants. Furthermore, neutrophils accumulate in ALS spinal cord supporting a pathophysiologic correlate. These data justify the consideration of immunity and sex for personalized therapeutic development in ALS.
Classification of Evidence
This study provides Class III evidence that in female participants with ALS, higher baseline neutrophil counts are associated with shorter survival.
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease1 with incompletely understood mechanisms and treatment options. The immune system is a therapeutic target in ALS. Early attempts to treat ALS using global immune suppression were ineffective or exacerbated disease.2,3 However, an increasing body of literature suggests that specific immune cell types may have beneficial or detrimental effects on disease outcomes4,5; therefore, global immune suppression would suppress beneficial cell function in parallel with destructive function. In response, the next generation of immune-based ALS therapeutic trials is more precise, targeting specific cell populations or immune pathways.
We used our existing immunophenotyping pipeline to explore the potential impact of sex and immunity on ALS survival. Previously, we have shown an association between neutrophil levels and ALS progression,6,7 findings supported by Choi et al.8 who report a connection between the ratio of neutrophils to lymphocytes and ALS survival. We therefore examined total neutrophil levels and how differences in peripheral neutrophil levels associate with survival in men and women, as there are significant differences in ALS rates between sexes.9 As it is still unclear whether peripheral neutrophil changes actively contribute to ALS progression or are simply a marker of disease, we also compared neutrophil levels in postmortem CNS tissue from ALS and control participants to determine whether neutrophils accumulate in the CNS during disease. We also examined whether cellular accumulation is affected by sex.
Methods
Survival Study Participants
All patients seen at the University of Michigan Pranger ALS clinic were invited to participate in this study. Participants provided written informed consent, and the study was approved by the institutional review board. Study protocols were previously published.6,7,10,11 Participants with sampling within 2 years of diagnosis were included; furthermore, 2 participants with a prolonged symptom onset to diagnosis interval (9.8 and 15.8 years) were also excluded. Flow cytometry data were obtained between June 22, 2011, and October 20, 2019.
Postmortem Tissue Participants
Persons with ALS seen at the University of Michigan Pranger ALS clinic and control volunteers (no neurologic disease, Alzheimer disease, probable Alzheimer, or possible Parkinson disease) consented to donate postmortem CNS tissue to the University of Michigan Brain Bank.
Enrichment of Leukocytes From Peripheral Blood
Study participants provided blood during clinical encounters. Following peripheral venipuncture, blood was collected into a BD Vacutainer sodium heparin tube (BD Biosciences, San Jose, CA), placed at 4°C, transferred to the laboratory on ice, and processed within 3 hours of collection. One milliliter of whole blood was split into 2 tubes and lysed with red blood cell lysing buffer (0.8% NH4Cl, 0.098% KHCO3, 0.1 mM EDTA, and 13.8 mM HEPES) using gentle rocking for 12 minutes. Cells were then pelleted, washed twice with flow buffer (phosphate-buffered saline [PBS], 4% fetal bovine serum [FBS], and 0.1% sodium azide), and counted using a hemocytometer (Hausser Scientific, Horsham, PA).
Isolation of Leukocytes From Human CNS Tissue
Postmortem tissue was collected from ALS and control participants between February 27, 2019, and January 1, 2020. After death postmortem, the spinal cord was divided into cervical, thoracic, and lumbar sections and transferred for laboratory analysis, at which point the immunologist (B.J.M.) was blinded to the diagnosis. Spinal cord tissue was physically dissociated using surgical scissors and enzymatically dissociated with collagenase for 90 minutes with gentle mixing at 15-minute intervals in RPMI-1640 medium (supplemented with 5% FBS [both Thermo Fisher Scientific, Waltham, MA], 50 μg/mL penicillin, 100 μg/mL streptomycin, and 20 mg/mL Clostridium histolyticum collagenase [all 3 from Sigma-Aldrich, St. Louis, MO]). The resulting suspension was placed on a 70-μm cell strainer (Corning, Corning, NY) over a 50-mL conical tube (Corning) and dissociated further by grinding with a sterile 3-mL syringe plunger (BD Biosciences), resuspended in 30% stock isotonic Percoll (90% Percoll [GE Healthcare, Chicago, IL] and 10% 10X Hanks' balanced salt solution without Ca2+ or Mg2+ [Thermo Fisher Scientific]), layered on the top of 70% stock Percoll, and centrifuged at 500g for 30 minutes. After removal of neuronal debris, the resulting interface was collected, washed, resuspended, and counted using a hemocytometer before analysis by flow cytometry.
Flow Cytometry
Human leukocyte suspensions were plated in round bottom 96-well plates (Corning) at ≤106 cells/25 μL flow buffer and blocked using 10 μg/mL human TruStain FcX blocking solution (BioLegend, San Diego, CA). Cells were stained for 30 minutes in the dark at 4°C in a 50 μL final volume using a cocktail of fluorescently labeled antibodies against cell surface markers suspended in flow buffer (1X PBS, 2% FBS, and 1% sodium azide). After staining, cells were washed with 200 μL flow buffer, pelleted, resuspended in 185 μL of BD stabilizing fixative (BD Biosciences), and transferred for analysis to polystyrene tubes (12 × 75 mm) (Becton Dickinson, Franklin Lakes, NJ). Cells were analyzed on a BD FACSCanto or LSRFortessa flow cytometer with FACSDiva software (BD Biosciences) and analyzed by FlowJo (FlowJo, Ashland, OR). Fluorophore-conjugated antibodies were CD45-BV421 (BioLegend, catalogue #304032), CD16-PE (BioLegend, catalogue # 302056), CD11b-PerCP-Cy/5.5 (BioLegend, catalogue #301328), control IgG-PE-Cy7 (BioLegend, catalogue # 400125), and CD15-PE-Cy7 (BD, catalogue # 560827). Peripheral neutrophil levels were gated as previously described7; CNS neutrophils were gated using CD45, CD11b, and CD15. A nonspecific IgG control was used for CD15 staining to subtract nonspecific events.
Survival Analysis
Descriptive statistics were produced for demographics and ALS disease characteristics. Study population differences were compared between male and female participants and participants with differing neutrophil counts by analysis of variance tests and chi-square tests.
Cox proportional hazards models assessed the association between neutrophil levels and ALS survival endpoints, defined as the time from diagnosis to death. Associations were adjusted for potential confounders, e.g., age, sex, onset segment, El Escorial criteria at diagnosis, and time from symptom onset to diagnosis. A delay in blood sample collection (median duration of 7 months) occurred in some participants, potentially leading to a selection bias if ignored. To account for this gap, a left-truncated survival analysis was performed.12 To determine a functional form of continuous neutrophil counts, we first fit penalized spline regression models and found a nonlinear effect of neutrophils. Continuous neutrophil counts were categorized into 3 groups (neutrophil counts: <2, 2–4, and >4 × 106 neutrophils/mL of blood) based on the nonlinear effect of neutrophils identified by penalized spline regression (figure 1). Neutrophil grouping was also confirmed by a sensitivity analysis in which the recategorized neutrophil variables into one-unit-change bins were examined, and bins of similar effect sizes were combined. Linearity of continuous adjustment factors (age and time from symptom onset to diagnosis) was checked, and no violation was found for their linearity assumption. The proportional hazards assumptions were checked using global and individual Schoenfeld tests with graphical assessment of the rescaled Schoenfeld residuals over time, and there were no concerning violations. Estimated, covariate-adjusted survival curves were plotted based on the Cox proportional hazards model using the survminer R package following published methods.13,14 To perform this method, the data are replicated 6 times (once for each sex by the neutrophil group), and each replicated data set serves as a pseudopopulation where sex by neutrophil groupings are assigned to be the same, keeping all other covariates unchanged. This ensures that plotted survival differences are due to the association with neutrophils and not a result of imbalances in other clinical factors such as sex, age, and onset segment. Analyses were performed with R version 3.6.2.
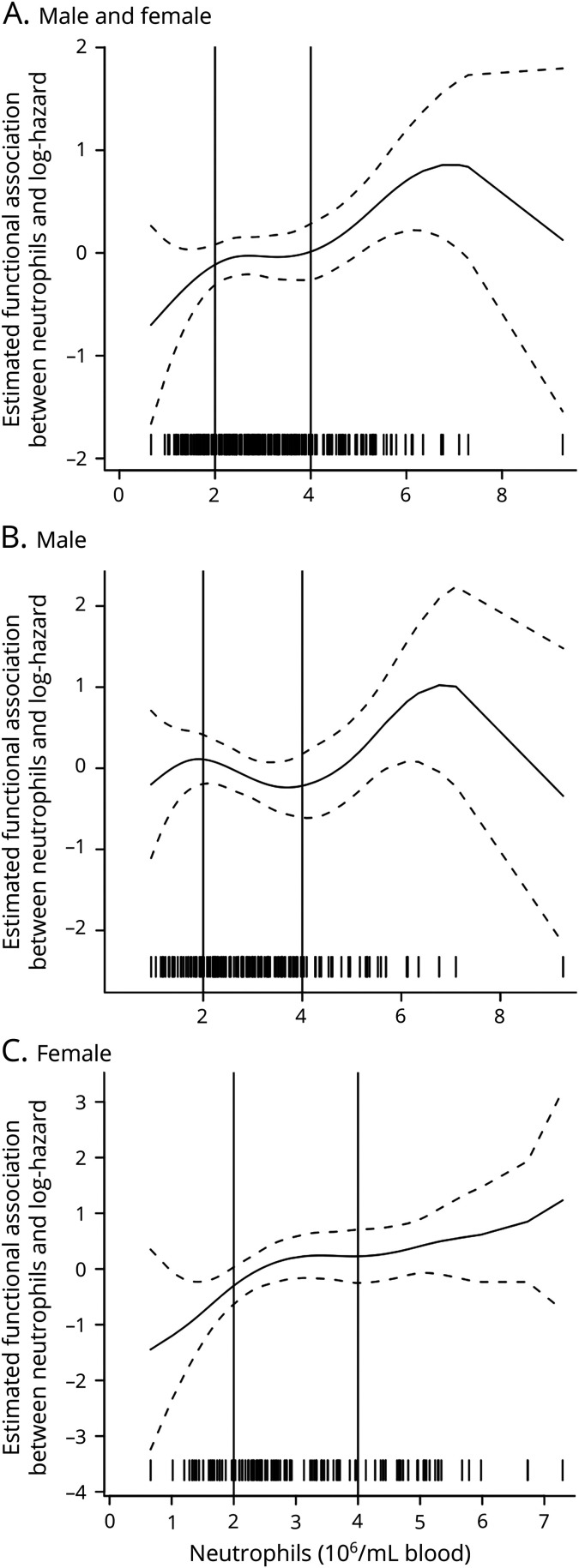
Figure 1. Estimated Functional Form of the Association Between Neutrophils and Survival.
Plots show the estimated functional form of the association between neutrophils and survival (the larger the value on the y axis, the higher the hazard) for (A) all participants (model 1), (B) male participants (model 2), and (C) female participants (model 2). (A) Model 1 shows an overall flat line between the 2 × 106/mL and 4 × 106/mL neutrophil counts with an upward sloping line on either side, therefore justifying the cutoffs at <2 × 106/ml, 2–4 × 106/mL, and >4 × 106/mL. Similar trends in the functional form of neutrophils and survival were observed for males (B) and females (C). Each tick mark at the bottom of the graph represents a single participant sample. As illustrated, the decreased risk for the highest neutrophil count is driven by 1 participant.
CNS Infiltration Analysis
Neutrophil level differences were compared using Prism (GraphPad, San Diego, CA). The Shapiro-Wilk test assessed the normality of distribution. One or more data sets did not adhere to Gaussian distribution in each tissue section; Mann-Whitney was therefore used to assess significance.
Data Availability
Deidentified data will be shared on request from a qualified investigator.
Results
Survival Analysis
To examine the impact of sex on immune-based therapies in ALS, we used flow cytometry to measure neutrophil levels in the peripheral blood of 271 study participants who had provided samples within 2 years of initial diagnosis. Two participants were excluded for missing covariates, one with uncertain onset segment and the other with uncertain onset date (see table 1 for population demographics). Thus, this analysis included 269 participants with a median age of 67.6 years, 45% female, a median diagnostic delay (time from symptom onset to diagnosis) of 1.02 years, median time from symptom onset to flow cytometry of 1.75 years, and a median Revised ALS Functional Rating Scale (ALSFRS-R) of 33. The median time to death was 1.5 years with a median time to censoring of 1.59 years, indicating that observation time was longer in the censored participants compared with those who died. These data indicate the participants were still early in their ALS disease course with preserved functional abilities at study entry. Furthermore, male and female groups were comparable in terms of baseline neutrophil value, age, family history of ALS, race, El Escorial criteria, ALSFRS-R, time from symptom onset to diagnosis, and time from diagnosis and symptom onset to neutrophil collection; the only significant difference was onset segment favoring bulbar onset in females (table 1). When divided by neutrophil levels and sex, there were no significant differences in age, family history of ALS, race, El Escorial criteria, time from symptom onset to diagnosis, and time from diagnosis and symptom onset to neutrophil collection. There was a significant difference in baseline ALSFRS-R scores that were lower in the higher neutrophil group and in onset segment, with females showing a higher proportion of bulbar onset and males showing a higher proportion of cervical onset (table 2). Two separate statistical survival models were constructed to associate peripheral neutrophil levels with participant survival and sex.
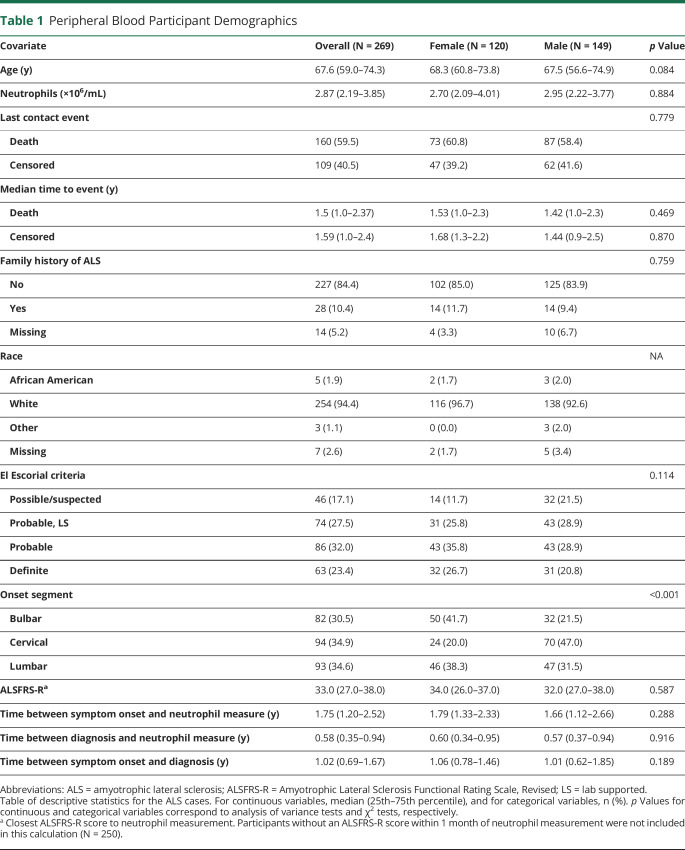
Table 1.
Peripheral Blood Participant Demographics
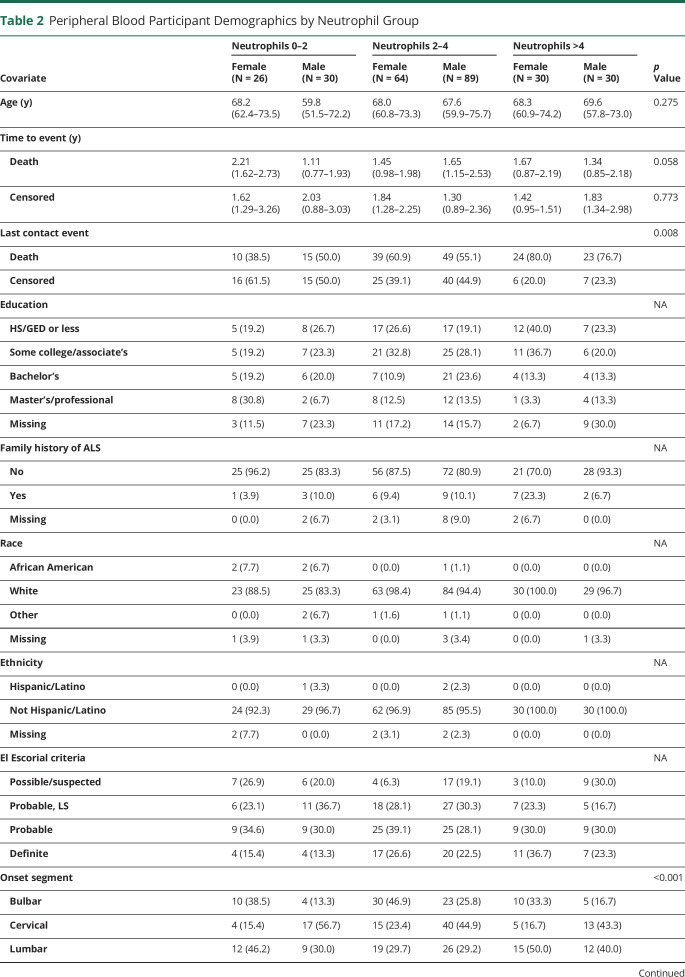
Table 2.
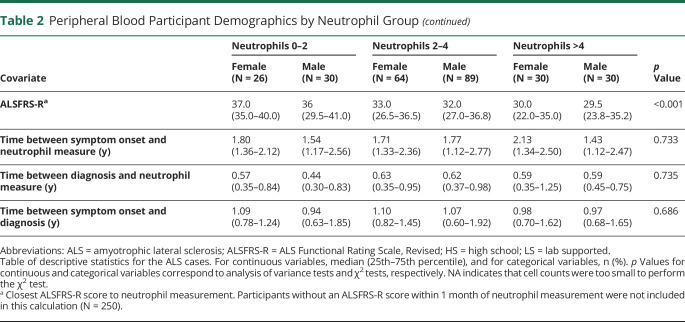
Peripheral Blood Participant Demographics by Neutrophil Group
In the first survival model (model 1), data from male and female study participants were combined and adjusted for potential confounders such as sex, age, onset segment, El Escorial criteria at diagnosis, and time from symptom onset to diagnosis. Study participants were grouped based on peripheral neutrophil levels: <2 × 106 neutrophils/mL of blood, 2–4 × 106/mL, and >4 × 106/mL for survival analysis. In the second survival model (model 2), participant data were similarly grouped based on peripheral neutrophil levels, and data were adjusted based on potential cofounders. Rather than adjusting for sex, participants were stratified into male and female groups.
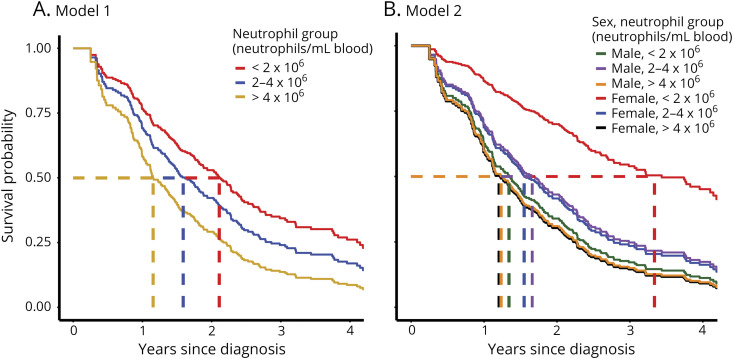
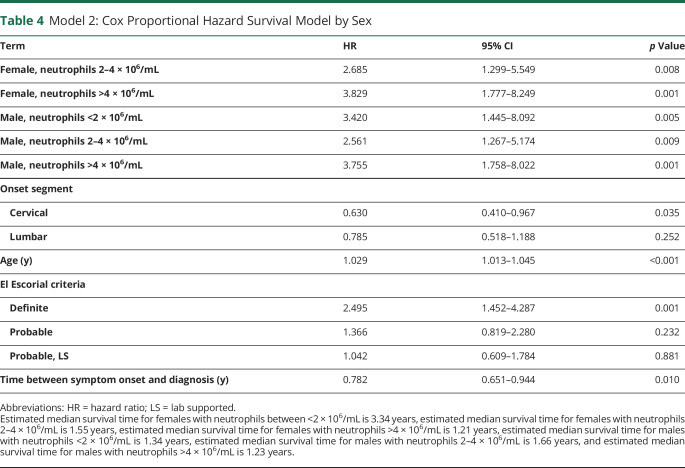
After constructing each model and adjusting for covariates, we examined the association between peripheral neutrophil levels and ALS survival before and after stratifying for sex. In model 1 (combined male and female participants), neutrophil levels were inversely associated with survival: participants with low peripheral neutrophils had the longest survival (median 2.11 years), followed by participants with moderate neutrophil levels (median 1.59 years) and participants with high neutrophil levels (median 1.15 years) (figure 2A). The mortality rate of participants with high neutrophil levels was 2.11 times higher than that of participants with low neutrophil levels (p = 0.004; table 3), when adjusting for sex, age, onset segment and other covariates. There was a trend toward reduced survival in participants with a moderate number of peripheral neutrophils compared with low neutrophils not reaching statistical significance. These data indicate that increasing levels of peripheral neutrophils are associated with reduced survival in ALS. Although as we show below, this association was likely driven by sex.
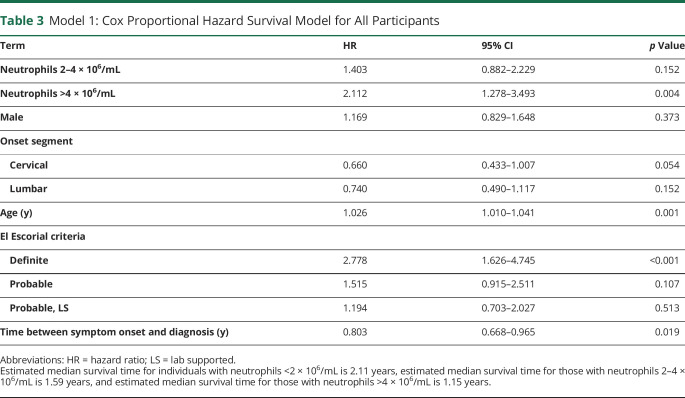
Figure 2. Neutrophil Impact on ALS Survival in Male and Female Participants.
Estimated survival curves using 2 separate models for analysis. (A) In model 1, survival curves were adjusted for age, sex, onset segment, El Escorial criteria at diagnosis, and time between symptom onset and diagnosis. Study ALS participants were categorized into 3 neutrophil groups (<2, 2–4, and >4 × 106 neutrophils/mL of blood). Survival curves were generated by creating a pseudopopulation with identical population characteristics as the study participants for each neutrophil group and calculating their expected survival based on model 1 (table 3). Dashed lines indicate the median survival for each neutrophil group. (B) In model 2, female and male survival curves were displayed separately after adjusting for age, onset segment, El Escorial criteria at diagnosis, and time between symptom onset and diagnosis were estimated from the interaction model. Male and female data sets were categorized into 3 neutrophil groups per sex (<2, 2–4, and >4 × 106 neutrophils/mL of blood). Survival curves were generated by creating a pseudopopulation with identical population characteristics as the study participants for each neutrophil and sex group and calculating their expected survival based on model 2 (table 4). Dashed lines indicate the median survival for each neutrophil and sex group. ALS = amyotrophic lateral sclerosis.
Table 3.
Model 1: Cox Proportional Hazard Survival Model for All Participants
Survival Analysis by Sex
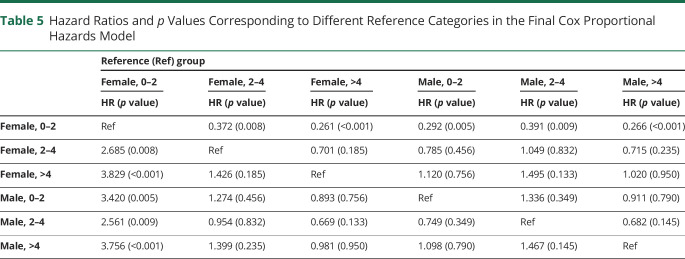
Next, we used the second statistical model where the neutrophil-survival association was stratified by sex. In model 2, median survival times for those with >4 × 106 neutrophils/mL were 1.23 years in males and 1.21 years in females (figure 2B). Median survival times for those with >2–4 × 106 neutrophils/mL were 1.66 years in males and 1.55 years in females. However, the absolute difference in median survival times was most pronounced in those with <2 × 106 neutrophils/mL resulting in 1.34 years in males and 3.34 years in females The 5 of 6 neutrophil and sex groups had a statistically significant higher mortality rate when compared with the one <2 × 106/mL neutrophil female group (table 4) when adjusting for age, onset segment, and other covariates. Similar associations were not seen when comparing the hazard ratio and p value with each of the other neutrophil and sex groups acting as the reference category (table 5). These data suggest that low neutrophil levels have a different impact in males and females.
Table 4.
Model 2: Cox Proportional Hazard Survival Model by Sex
Table 5.
Hazard Ratios and p Values Corresponding to Different Reference Categories in the Final Cox Proportional Hazards Model
As effect modification by sex was of particular interest, a likelihood ratio test comparing the marginal (model 1) and interaction (model 2) models assessed how well the data set fit each statistical model. The likelihood ratio test yielded a p value of 0.026, indicating that the sex-based interaction model (model 2) significantly fit the data better. Importantly, these models showed no violation of proportional hazards with the global Schoenfeld test, yielding p values of 0.29 and 0.14 for the marginal and interaction models, respectively. Thus, both models are valid, but model 2—which stratifies by sex—is more accurate.
CNS Neutrophil Levels in ALS
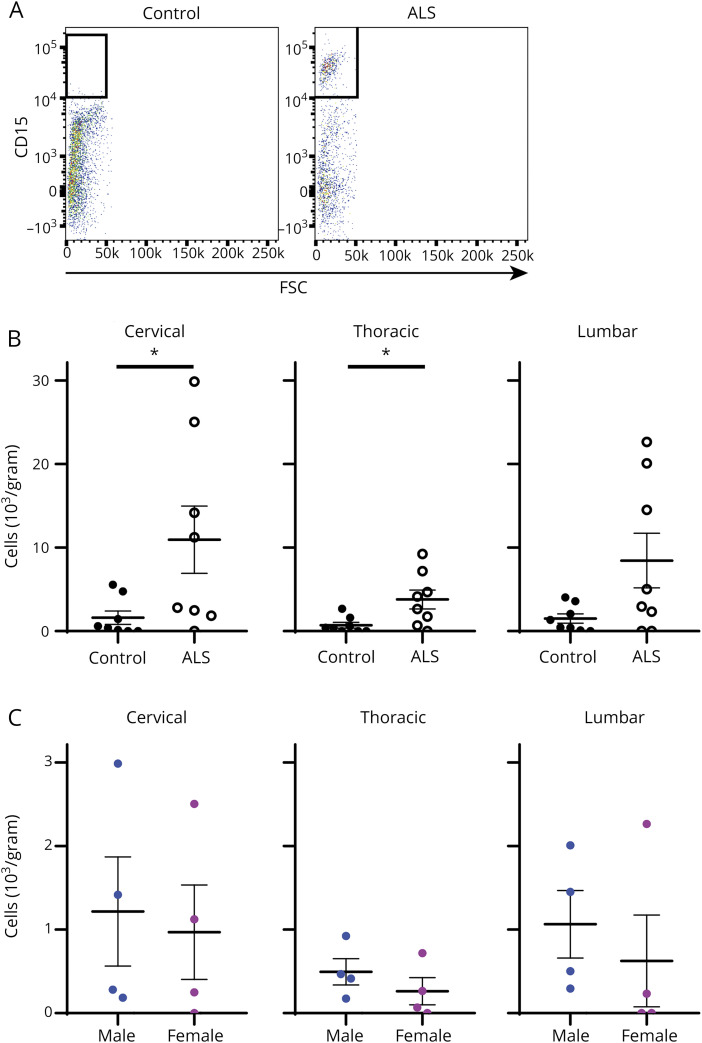
Finally, we examined whether neutrophils accumulate in the CNS during ALS and whether CNS neutrophil levels were affected by sex. We measured neutrophil levels in CNS postmortem tissue of 8 control and 8 ALS participants. Mean death age for cases and controls was 72.5 and 85.6 years, respectively. ALS cases were 50% male and controls were 100% male. Final neuropathologic diagnosis in all cases was ALS and, in controls, was Alzheimer 12.5%, probable Alzheimer 50%, possible Parkinson 12.5%, and no diagnosis 25%. We previously found that neutrophils accumulate in the spinal cord of ALS mice,15 so in human participants we examined neutrophil levels in 3 distinct spinal cord sections (cervical, thoracic, and lumbar). When assessed using flow cytometry, we detected increased neutrophil levels in the spinal cord of ALS compared with control participants (figure 3A). When quantified, mean neutrophil counts were significantly increased in the cervical (p = 0.049) and thoracic (p = 0.022) sections of ALS participants compared with control participants (figure 3B). However, significant differences in neutrophil levels between ALS male and female participants were not observed (figure 3C), indicating that neutrophils accumulate in the CNS of ALS participants without a clear difference between sexes.
Figure 3. Accumulation of Neutrophils in the CNS During ALS.
(A) Spinal cord tissue from control and ALS participants (cervical sections, collected postmortem) was analyzed for neutrophils using flow cytometry. (B) Total neutrophils were quantified in postmortem tissue of the cervical, thoracic, and lumbar spinal cord sections from control and ALS participants. Mean neutrophil counts were: cervical cord, ALS = 10.9 × 103/g, control = 1.6 × 103/g, p = 0.049; thoracic cord, ALS = 3.8 × 103/g, control = 0.7 × 103/g, p = 0.002; and lumbar cord, ALS = 8.4 × 103/g, control = 1.5 × 103/g, p = 0.194. (C) Neutrophil accumulation in the CNS of male and female ALS study participants is compared in the cervical, thoracic, and lumbar sections of the spinal cord. Mean neutrophil counts were: cervical cord, males 12.2 × 103/g, females 9.7 × 103/g, p = 0.686; thoracic cord, males 4.9 × 103/g, females 2.6 × 103/g, p = 0.343; lumbar cord, males 10.6 × 103/g, females 6.2 × 103/g, p = 0.314. *p < 0.05. ALS = amyotrophic lateral sclerosis; FSC = forward scatter.
Discussion
We demonstrate that the immune system has differing effects in male and female ALS participants and that sex should be accounted for during the development of ALS therapeutics and early phase clinical trials, particularly those focused on the immune system. Our analysis was performed by associating peripheral neutrophil levels with participant survival. Two separate survival models examined the potential role of neutrophils in ALS and to determine whether sex affects immunity in ALS. When data from male and female study participants were combined, we found increasing levels of peripheral neutrophils were associated with increased mortality. However, when stratified by sex, we found that neutrophil levels had drastically different associations with ALS mortality. These observations are of particular importance given the recent interest in ALS immune factors and the growing number of clinical trials targeting the immune system.
Our first statistical model combined data from male and female participants to elucidate the overall impact of neutrophils in ALS, to set a baseline of comparison for the second statistical model, and to ensure our analysis was consistent with previously published studies. When participants were grouped by peripheral neutrophil levels, we found that increasing peripheral neutrophils were associated with increased mortality in ALS. These data are consistent with our previous studies that have linked neutrophil levels to disease progression,6 linked neutrophil levels to disease progression rates,7 and have demonstrated CNS accumulation in an ALS mouse model.15 They are also highly consistent with a recent study, which found that a high ratio of neutrophils to lymphocytes was associated with reduced survival in ALS.8 Furthermore, they are consistent with another recent study showing that higher neutrophil levels associate with a faster disease progression as measured by the change in ALSFRS-R.16 Although to date these studies have been correlative, other studies have demonstrated a central role for neutrophils in neurologic damage. Neutrophils are some of the first responders to neurologic damage,17 and although they can facilitate repair in the spinal cord, they are equally capable of inducing damage.18 For example, in MS, neutrophils facilitate further damage by activating other immune cell types,19 and targeting neutrophils attenuates disease.19,20 The accumulation of neutrophils in the CNS supports their involvement in disease progression, although the underlying mechanism or mechanisms driving neutrophil accumulation in the CNS of ALS participants remain unclear. Multiple proinflammatory cytokines are upregulated in the plasma of ALS participants,21–23 and these cytokines could be increasing neutrophil activity and trafficking. In addition, other immune factors linked with ALS can also affect neutrophil function. Leukotriene B4, platelet-activating factor, and C5a, for example, have all been associated with ALS24–27 and can enhance the activity, trafficking, and survival of neutrophils.28,29
However, although our data and previous studies suggest neutrophils may directly contribute to increased mortality during ALS, our second statistical model suggests that the role of neutrophils is complicated by sex. When the neutrophil-survival association was stratified by sex, we found that low peripheral neutrophil levels had completely different effects in males and females. In female participants, low peripheral neutrophil levels are associated with a longer median survival. By contrast, low neutrophil levels in male participants did not associate with increased survival. The mechanism for this discrepancy is currently not clear, although several possibilities exist. The first is the direct effects of sex hormones on cellular activity: males and females display altered immune activation states in multiple cell types,30–32 including neutrophils,33–35 because of the immunomodulatory effects of male and female hormones.36 A second possibility is that sex-based differences contribute to altered neutrophil levels or altered CNS infiltration. This is unlikely, however, as we saw no differences in peripheral or spinal cord neutrophil levels between male and female ALS participants. A third possibility is that sex fundamentally alters the immune environment within the CNS. Previous data support this last possibility. In rodent models, for example, the microglia of healthy males and females have different transcriptomic signatures,37 and hormone levels create different immune environments during sexual development.38,39 The ability of neutrophils to initiate tissue repair or drive further damage has also been linked to the local microenvironment.40
Although the underlying mechanism remains to be determined, our data demonstrate that sex should be considered when examining the role of the immune system in ALS. Although few ALS studies have specifically examined the effect of sex on disease, others have detected immune discrepancies as well. For instance, 1 study examining the role of fractalkine receptor function in the microglia of ALS mice found that knockout of the receptor accelerated disease progression only in male mice.41 Another study showed that axonal sprouting varies between male and female SOD1G37R mice.42 A more recent prospective study found that prediagnostic eosinophil levels associated with ALS risk differently in males and females.43 Finally, low-density lipoprotein–related receptor protein 4 antibodies are more commonly detected in female vs male ALS participants.44 Combined with our observations, these studies suggest that multiple immune cell types, not just neutrophils, are affected by sex during ALS. We contend that any ALS study addressing immunity should account for sex.
This is particularly important because the ALS field becomes increasingly interested in immune-based therapies. A recent phase I trial, for instance, isolated regulatory T cells through leukapheresis and expanded the cell population ex vivo before reintroducing them into the trial participant.45 In addition, the upcoming HEALEY ALS Platform Trial will include immunomodulatory drugs targeting complement or myeloperoxidase.46 Despite this new focus on immunity in ALS, sex-specific treatments are frequently not addressed, and sex was not specified as an important variable in the most recent ALS clinical trial guideline revisions47 despite higher proportions of ALS in men than in women, particularly in younger study participants.9 Our data suggest that separate immune mechanisms may exist in men and women and that future immune-based ALS studies should account for sex.
These findings should not be surprising given that autoimmune diseases, such as systemic lupus erythematosus and MS, disproportionately affect females.36 Furthermore, there are inherent differences in neutrophil activity between males and females,33–35 and neutrophil extracellular traps differ between males and females with relapsing remitting MS.48 Overall, sex and immune system differences are well understood in non-neurologic disease and MS, and our data suggest the same may be true for ALS.
Our study does have limitations. It is primarily correlative, as it did not use any intervention to assess the impact of neutrophils on disease progression. Similarly, the accumulation of neutrophils in the CNS could be a byproduct of neuronal damage, rather than a contributing factor. Peripheral neutrophil levels were not measured in participants until they visited the ALS clinic, meaning participants in the earliest stages of observable disease who were yet to be diagnosed were missed; however, the immune system may also be involved before observable symptoms. When participants were divided by neutrophil count (table 2), the group with the highest neutrophils did have a lower ALSFRS-R score. Importantly, there were no differences in (1) time from symptom onset to neutrophil measure; (2) time between diagnosis and neutrophil measure; and (3) time between symptom onset and diagnosis. Therefore, we believe these ALSFRS-R differences by subgroup reflect the severity of this group as opposed to capturing these participants later in disease. All 8 control participants who donated CNS tissue were male and older, on average, than ALS participants, meaning that innate immune differences between the sexes or based on age were not accounted for in the CNS analysis. Although a strength of our study is the use of an inclusive clinic population, in contrast to a clinical trial population that largely excludes patients,49 replication of our findings in other cohorts is needed to address generalizability.
In summary, the heterogeneous nature of ALS complicates the identification of therapeutic targets and the outcomes of early phase clinical studies.50 However, despite the well-established differences in ALS rates between males and females,9 few studies take sex into consideration as an independent variable. Our current data indicate that not only do differences in immunity exist between males and females during ALS but also that these differences link to different disease outcomes. Our observations suggest that immunomodulatory therapies for the treatment of ALS should be assessed separately by sex in both preclinical and early phase studies to better account for patient-specific immune signatures. Such a shift moves us closer to more personalized therapeutics for the treatment of ALS.
Acknowledgment
Blake Swihart, BA, MA, Jayna Duell, BS, RN, Amanda Williams, BS, Thomas Dent, BS, Adam Patterson, BS, Crystal Pacut, BS, Matthew Perkins, MD, Joshua Famie, BS, Caroline Piecuch, BS, Faye Mendelson, BS, Shayna Mason, Maegan Tabbey, BS, and Carey Backus, BA, University of Michigan, provided technical support. Stacey Sakowski Jacoby, PhD, and Masha G. Savelieff, PhD, University of Michigan, provided editing assistance. These contributors receive a salary for their work but were not directly compensated for this specific project.
Glossary
- ALS
amyotrophic lateral sclerosis
- ALSFRS-R
ALS Functional Rating Scale, Revised
- FBS
fetal bovine serum
- PBS
phosphate-buffered saline
Appendix. Authors

Contributor Information
Benjamin J. Murdock, Email: murdockb@med.umich.edu.
Jonathan Boss, Email: bossjona@umich.edu.
Sehee Kim, Email: seheek@umich.edu.
Eva L. Feldman, Email: efeldman@med.umich.edu.
Study Funding
This study was supported by R21 NS102960, the University of Michigan NeuroNetwork for Emerging Therapies, the Robert Nederlander Program, the A. Alfred Taubman Medical Research Institute, the University of Michigan MCubed program, contract N021258-00 from Target ALS, contract NS102960 from the NIH, contract 200-2013-56856 from the Centers for Disease Control and Prevention/Agency for Toxic Substances and Disease Registry, the NIH (NIH K23ES027221), and contract N021258 from the CReATe Consortium (the CReATe consortium [U54NS092091] is part of the Rare Diseases Clinical Research Network [RDCRN], an initiative of the Office of Rare Diseases Research [ORDR], and the National Center for Advancing Translational Sciences [NCATS]. CReATe is funded through a collaboration between the NCATS and the NINDS).
Disclosure
No authors declare conflicts of interest relevant to this manuscript. Go to Neurology.org/NN for full disclosures.
References
- 1.Goutman SA. Diagnosis and clinical management of amyotrophic lateral sclerosis and other motor neuron disorders. Continuum (Minneap Minn) 2017;23:1332–1359. [DOI] [PubMed] [Google Scholar]
- 2.Gordon PH, Moore DH, Miller RG, et al. Efficacy of minocycline in patients with amyotrophic lateral sclerosis: a phase III randomised trial. Lancet Neurol 2007;6:1045–1053. [DOI] [PubMed] [Google Scholar]
- 3.Meininger V, Asselain B, Guillet P, et al. Pentoxifylline in ALS: a double-blind, randomized, multicenter, placebo-controlled trial. Neurology 2006;66:88–92. [DOI] [PubMed] [Google Scholar]
- 4.Murdock BJ, Bender DE, Segal BM, Feldman EL. The dual roles of immunity in ALS: injury overrides protection. Neurobiol Dis 2015;77:1–12. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Beers DR, Appel SH. Immune-mediated mechanisms in the pathoprogression of amyotrophic lateral sclerosis. J Neuroimmune Pharmacol 2013;8:888–899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murdock BJ, Bender DE, Kashlan SR, et al. Increased ratio of circulating neutrophils to monocytes in amyotrophic lateral sclerosis. Neurol Neuroimmunol Neuroinflamm 2016;3:e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Murdock BJ, Zhou T, Kashlan SR, Little RJ, Goutman SA, Feldman EL. Correlation of peripheral immunity with rapid amyotrophic lateral sclerosis progression. JAMA Neurol 2017;74:1446–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi SJ, Hong YH, Kim SM, Shin JY, Suh YJ, Sung JJ. High neutrophil-to-lymphocyte ratio predicts short survival duration in amyotrophic lateral sclerosis. Sci Rep 2020;10:428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manjaly ZR, Scott KM, Abhinav K, et al. The sex ratio in amyotrophic lateral sclerosis: a population based study. Amyotroph Lateral Scler 2010;11:439–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goutman SA, Boss J, Patterson A, Mukherjee B, Batterman S, Feldman EL. High plasma concentrations of organic pollutants negatively impact survival in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 2019;90:907–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su FC, Goutman SA, Chernyak S, et al. Association of environmental toxins with amyotrophic lateral sclerosis. JAMA Neurol 2016;73:803–811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wu F, Kim S, Qin J, Saran R, Li Y. A pairwise likelihood augmented Cox estimator for left-truncated data. Biometrics 2018;74:100–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alimujiang A, Wiensch A, Boss J, et al. Association between life purpose and mortality among US adults older than 50 years. JAMA Netw Open 2019;2:e194270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Therneau TM, Crowson CS, Atkinson EJ. Adjusted survival curves. 2015. [Google Scholar]
- 15.Figueroa-Romero C, Guo K, Murdock BJ, et al. Temporal evolution of the microbiome, immune system and epigenome with disease progression in ALS mice. Dis Model Mech 2019;13:dmm041947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Woodruff TM, McCombe PA, Henderson RD, et al. Monocytes and neutrophils are associated with clinical features in amyotrophic lateral sclerosis. Brain Commun 2020;2:fcaa013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim CF, Moalem-Taylor G. Detailed characterization of neuro-immune responses following neuropathic injury in mice. Brain Res 2011;1405:95–108. [DOI] [PubMed] [Google Scholar]
- 18.Neirinckx V, Coste C, Franzen R, Gothot A, Rogister B, Wislet S. Neutrophil contribution to spinal cord injury and repair. J Neuroinflammation 2014;11:150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Steinbach K, Piedavent M, Bauer S, Neumann JT, Friese MA. Neutrophils amplify autoimmune central nervous system infiltrates by maturing local APCs. J Immunol 2013;191:4531–4539. [DOI] [PubMed] [Google Scholar]
- 20.Khaw YM, Cunningham C, Tierney A, Sivaguru M, Inoue M. Neutrophil-selective deletion of Cxcr2 protects against CNS neurodegeneration in a mouse model of multiple sclerosis. J Neuroinflammation 2020;17:49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aebischer J, Moumen A, Sazdovitch V, Seilhean D, Meininger V, Raoul C. Elevated levels of IFNgamma and LIGHT in the spinal cord of patients with sporadic amyotrophic lateral sclerosis. Eur J Neurol 2012;19:752–759, e745–e756. [DOI] [PubMed] [Google Scholar]
- 22.Cereda C, Baiocchi C, Bongioanni P, et al. TNF and sTNFR1/2 plasma levels in ALS patients. J Neuroimmunol 2008;194:123–131. [DOI] [PubMed] [Google Scholar]
- 23.Rentzos M, Rombos A, Nikolaou C, et al. Interleukin-15 and interleukin-12 are elevated in serum and cerebrospinal fluid of patients with amyotrophic lateral sclerosis. Eur Neurol 2010;63:285–290. [DOI] [PubMed] [Google Scholar]
- 24.Malaspina A, Puentes F, Amor S. Disease origin and progression in amyotrophic lateral sclerosis: an immunology perspective. Int Immunol 2015;27:117–129. [DOI] [PubMed] [Google Scholar]
- 25.Woodruff TM, Costantini KJ, Crane JW, et al. The complement factor C5a contributes to pathology in a rat model of amyotrophic lateral sclerosis. J Immunol 2008;181:8727–8734. [DOI] [PubMed] [Google Scholar]
- 26.Lee JD, Levin SC, Willis EF, Li R, Woodruff TM, Noakes PG. Complement components are upregulated and correlate with disease progression in the TDP-43(Q331K) mouse model of amyotrophic lateral sclerosis. J Neuroinflammation 2018;15:171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Briones MRS, Snyder AM, Ferreira RC, Neely EB, Connor JR, Broach JR. A possible role for platelet-activating factor receptor in amyotrophic lateral sclerosis treatment. Front Neurol 2018;9:39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Webster RO, Hong SR, Johnston RB Jr, Henson PM. Biologial effects of the human complement fragments C5a and C5ades Arg on neutrophil function. Immunopharmacology 1980;2:201–219. [DOI] [PubMed] [Google Scholar]
- 29.Khreiss T, Jozsef L, Chan JS, Filep JG. Activation of extracellular signal-regulated kinase couples platelet-activating factor-induced adhesion and delayed apoptosis of human neutrophils. Cell Signal 2004;16:801–810. [DOI] [PubMed] [Google Scholar]
- 30.Hou J, Zheng WF. Effect of sex hormones on NK and ADCC activity of mice. Int J Immunopharmacol 1988;10:15–22. [DOI] [PubMed] [Google Scholar]
- 31.Rettew JA, Huet-Hudson YM, Marriott I. Testosterone reduces macrophage expression in the mouse of toll-like receptor 4, a trigger for inflammation and innate immunity. Biol Reprod 2008;78:432–437. [DOI] [PubMed] [Google Scholar]
- 32.Potluri T, Fink AL, Sylvia KE, et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. NPJ Vaccin 2019;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Buyon JP, Korchak HM, Rutherford LE, Ganguly M, Weissmann G. Female hormones reduce neutrophil responsiveness in vitro. Arthritis Rheum 1984;27:623–630. [DOI] [PubMed] [Google Scholar]
- 34.Miller AP, Feng W, Xing D, et al. Estrogen modulates inflammatory mediator expression and neutrophil chemotaxis in injured arteries. Circulation 2004;110:1664–1669. [DOI] [PubMed] [Google Scholar]
- 35.Marin DP, Bolin AP, dos Santos Rde C, Curi R, Otton R. Testosterone suppresses oxidative stress in human neutrophils. Cell Biochem Funct 2010;28:394–402. [DOI] [PubMed] [Google Scholar]
- 36.Klein SL, Flanagan KL. Sex differences in immune responses. Nat Rev Immunol 2016;16:626–638. [DOI] [PubMed] [Google Scholar]
- 37.Villa A, Gelosa P, Castiglioni L, et al. Sex-specific features of microglia from adult mice. Cell Rep 2018;23:3501–3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lenz KM, Nugent BM, Haliyur R, McCarthy MM. Microglia are essential to masculinization of brain and behavior. J Neurosci 2013;33:2761–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenz KM, Wright CL, Martin RC, McCarthy MM. Prostaglandin E(2) regulates AMPA receptor phosphorylation and promotes membrane insertion in preoptic area neurons and glia during sexual differentiation. PLoS One 2011;6:e18500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butterfield TA, Best TM, Merrick MA. The dual roles of neutrophils and macrophages in inflammation: a critical balance between tissue damage and repair. J Athl Train 2006;41:457–465. [PMC free article] [PubMed] [Google Scholar]
- 41.Cardona AE, Pioro EP, Sasse ME, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci 2006;9:917–924. [DOI] [PubMed] [Google Scholar]
- 42.Martineau E, Di Polo A, Vande Velde C, Robitaille R. Sex-specific differences in motor-unit remodeling in a mouse model of ALS. eNeuro 2020;7:ENEURO.0388-19.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yazdani S, Mariosa D, Hammar N, et al. Peripheral immune biomarkers and neurodegenerative diseases: a prospective cohort study with 20 years of follow-up. Ann Neurol 2019;86:913–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rivner MH, Liu S, Quarles B, et al. Agrin and low-density lipoprotein-related receptor protein 4 antibodies in amyotrophic lateral sclerosis patients. Muscle Nerve 2017;55:430–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thonhoff JR, Beers DR, Zhao W, et al. Expanded autologous regulatory T-lymphocyte infusions in ALS: a phase I, first-in-human study. Neurol Neuroimmunol Neuroinflamm 2018;5:e465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.HEALEY ALS Platform Trial—Master Protocol. Available at: ClinicalTrials.gov/show/NCT04297683. Accessed May 19, 2020. [Google Scholar]
- 47.van den Berg LH, Sorenson E, Gronseth G, et al. Revised Airlie House consensus guidelines for design and implementation of ALS clinical trials. Neurology 2019;92:e1610–e1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tillack K, Naegele M, Haueis C, et al. Gender differences in circulating levels of neutrophil extracellular traps in serum of multiple sclerosis patients. J Neuroimmunol 2013;261:108–119. [DOI] [PubMed] [Google Scholar]
- 49.van Eijk RPA, Westeneng HJ, Nikolakopoulos S, et al. Refining eligibility criteria for amyotrophic lateral sclerosis clinical trials. Neurology 2019;92:e451–e460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Goyal NA, Berry JD, Windebank A, et al. Addressing heterogeneity in amyotrophic lateral sclerosis CLINICAL TRIALS. Muscle Nerve 2020;62:156–166. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Deidentified data will be shared on request from a qualified investigator.