Abstract
This review article discusses the preclinical evidence and clinical trials testing the use of a peptide agonist of the glucagon-like peptide (GLP) receptor that promotes insulin secretion in the animal models of and patient with Parkinson's disease (PD). In particular, we focus on the therapeutic effects of the GLP receptor agonist exendin-4, also called exenatide, in PD. The ultimate goal of this article is to provide a critical assessment of the laboratory and clinical data toward guiding the translation of exendin-4 as a clinically relevant therapeutic for PD.
Keywords: Exenatide, exendin-4, glucagon-like peptide-1, Parkinson's disease
Introduction
Parkinson's disease (PD) is a neurodegenerative pathology characterized by the affection and loss of dopaminergic neurons located at the substantia nigra. Dopamine (DA) is a neurotransmitter whose key function is to modulate normal movement.
DA is an important neurotransmitter as it modulates normal movement. DA is released from the A9 neurons of the substantia nigra pars compacta (SNc). DA-ergic neurons correlate with medium spiny neurons (MSNs) inside the dorsal striatum, which is composed of the caudate and putamen. There are two classic striatopallidal pathways [Figure 1]. DA inhibits the indirect GPe (external segment of the globe pallidus) through receptors (D2R) expressed by MSN; therefore, the indirect GPi (internal segment of the globe in primates or entopeduncular nucleus in rodents) is activated by means of D1R that it expresses MSN.[1,2] These converging pathways cause the activation of GPe and the suppression of all neural activity in the subthalamic nucleus (STN) and GPi, which will regulate and potentiate the neurolatalamic activity and help movement.[3] In neurodegenerative pathologies, such as PD, the decrease in dopaminergic innervation to the putamen and the caudate lead to hyperactivity in the GABA-ergic inputs to the GPe, and subsequently suppress the inhibitory outputs of the Gpe to the STN,[4] activates STN and GPi neurons, and reduces neural activation in the thalamus. DA denervation favors the activation of GPi neurons through the direct striatopallidal pathway. Injuring the STN oGPi favors a functional improvement in rats injured with 6-OHDA,[5] monkeys that were treated with 1-methyl-4-phenyl- 1,2,3,6-tetrahydropyridine (MPTP)[6] and PD patients.[7] However, L-DOPA or DA agonists have the ability to overstimulate DA receptors, both in the indirect and direct pathways in the injured brain, causing the neural firing in the STN and GPi to be reduced and it is at this time that they activate the thalamus and hence an increase in involuntary movements in monkeys treated with MPTP[8] and patients with PD.[9]
Figure 1.
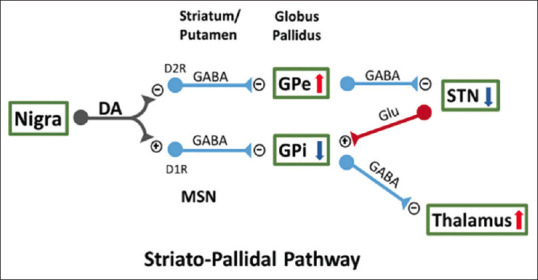
There are two striatopallidal pathways of the internal segment of the globe (GPi) and that of the external segment of the pale globe (GPe) and these regulate neural activity in STN and the thalamus, in the same way movement. MSN: Medium spiny neurons, STN: Subthalamic nucleus
Exendin-4 in Parkinson 's Disease Animal Models
Accumulating evidence indicates that some molecules such as endogenous incretin glucagon-like peptide-1 (GLP-1) as well as exendin-4, a long-acting GLP-1 receptor (GLP-1R) agonist which has been approved for the treatment of type 2 diabetes mellitus,[10,11] have a protective effect against tyrosine hydroxylase immunoreactivity (THir) in primary pontomesencephalic neurons from 6-OHDA lesioning. Similarly, administration of exendin 4 into the lateral ventricle has also been reported to be effective in mitigating the loss of THir neurons, preserve DA levels in the SNc, and improving the behavioral function of mice receiving MPTP.[12] This GLP-1R-mediated protection effect has been widely found across animal models of PD.[13,14,15] It is important to highlight that clinical studies have demonstrated that patients that took exendin-4 for 1 year had better motor skills rather than those taking placebo.[16] These observations denote that activation of the GLP-1R can reduce the progression of DA degeneration.
Unfortunately, there are some limitations of GLP-1R agonists like GLP-1 for clinical use, specifically their relatively short half-life and as peptide-based drugs, its limited brain uptake.[17] A N-terminal of GLP-1 amino acid change prevents its breakdown by dipeptidyl peptidase-4 (DPP4) extending its half-life from 1.5 min to 2.4 h.[10] This duplicates the daily clinical formulation Byetta, which is considered the short-acting drug version. In contrast, it has been shown that sustained release technology to exendin-4 allows a continuous release of the same peptide present in Byetta over weeks to months after a single acute subcutaneous administration, resulting in the long-acting formulations of PT320 (1 or 2 weeks) and Bydureon (1-week administration). This technology gives the opportunity to maintain brain target concentration by maintaining steady-state plasma levels of the peptide, optimizing by consequence of the beneficial potential of the drug treatment.[18] Despite the limitations of exendin-4, its solid safety and efficacy profiles in PD animal models lay the foundation of its clinical application for PD patients.
Exendin-4 in Parkinson 's Disease Patients
Two randomized controlled trials in PD patients tested the therapeutic effects of exendin-4, which was more routinely called exenatide in the clinic. The first clinical trial entailed a double-blind randomized study of self-administered exenatide or placebo in 62 participants over 48 weeks of treatment then followed by a 12-week washout interval.[16] The second clinical trial involved a single-blinded randomized study over a 12-month period of self-administered exenatide versus no treatment in 45 participants, who were subsequently monitored for 14 months and 24 months after 2 months and 12 months of exenatide cessation.[19] Performance bias was detected in one trial.[19]
When comparing the primary outcomes between exenatide versus placebo, an analysis of the first clinical trial[20] revealed low-certainty evidence to support that exenatide improves motor deficit based on the Movement Disorder Society-Unified PD Rating Scale (MDS-UPDRS) Part III during the off-stage. Further examination indicated low-certainty evidence to indicate that exenatide minimal effects on health-related quality of life (HRQoL) based on the PD Questionnaire (PDQ)-39 Summary Index, the EuroQol scale measuring health status in five dimensions, or the EQ5D Visual Analog Scale. Although serious adverse events (SAEs) were detected, they were independent of exenatide treatment. Furthermore, there is low-certainty evidence suggesting that exenatide affords minimal weight loss.
The analysis of exenatide versus no treatment based on primary outcomes at 14 and 24 months[20] revealed very low-certainty to good evidence to support that exenatide ameliorates motor dysfunction based on MDS-UPDRS Part III off medication. Similarly, the data were inconclusive in demonstrating that exenatide improves HRQoL based on the PDQ-39 SI. In addition, there is minimal evidence to suggest that exenatide results in SAEs or weight loss.
Based on the results of the two clinical trials, there are modest motor improvements in exenatide-treated PD patients. Interestingly, even with exenatide withdrawal, the motor improvement persisted suggesting that exenatide may modify the disease progression. Although SAEs were observed, they were likely independent of exenatide. Unfortunately, the results are not conclusive for attenuation of HRQoL, nonmotor symptoms, activities of daily living, and psychological manifestations in exenatide-treated PD patients.
Conclusion
In conclusion, solid preclinical data reveal that GLP-1R agonists, like exendin-4, are effective in animal models of PD, with encouraging data in PD patients. That exendin-4's serum levels are maintained in the brain, coupled with DA modulation, provide insights on the mechanistic action towards neuroprotection against the neurodegeneration associated with PD. These laboratory observations stand as the basis for advancing the use of exendin-4 in PD patients.
Conflicts of interest
Prof. Cesario V. Borlongan is Associate Editor of Brain Circulation.
References
- 1.Durieux PF, Schiffmann SN, de Kerchove DA. Targeting neuronal populations of the striatum. Front Neuroanat. 2011;5:40. doi: 10.3389/fnana.2011.00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerfen CR, Surmeier DJ. Modulation of striatal projection systems by dopamine. Annu Rev Neurosci. 2011;34:441–66. doi: 10.1146/annurev-neuro-061010-113641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nambu A, Tokuno H, Takada M. Functional significance of the cortico-subthalamo-pallidal 'hyperdirect' pathway. Neurosci Res. 2002;43:111–7. doi: 10.1016/s0168-0102(02)00027-5. [DOI] [PubMed] [Google Scholar]
- 4.Petri D, Pum M, Vesper J, Huston JP, Schnitzler A. GABAA-receptor activation in the subthalamic nucleus compensates behavioral asymmetries in the hemiparkinsonian rat. Behav Brain Res. 2013;252:58–67. doi: 10.1016/j.bbr.2013.05.044. [DOI] [PubMed] [Google Scholar]
- 5.Touchon JC, Moore C, Frederickson J, Meshul CK. Lesion of subthalamic or motor thalamic nucleus in 6-hydroxydopamine treated rats: Effects on striatal glutamate and apomorphine-induced contralateral rotations. Synapse. 2004;51:287–98. doi: 10.1002/syn.10306. [DOI] [PubMed] [Google Scholar]
- 6.Bergman H, Wichmann T, DeLong MR. Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science. 1990;249:1436–8. doi: 10.1126/science.2402638. [DOI] [PubMed] [Google Scholar]
- 7.Baron MS, Vitek JL, Bakay RA, Green J, McDonald WM, Cole SA, et al. Treatment of advanced Parkinson's disease by unilateral posterior GPi pallidotomy: 4-year results of a pilot study. Mov Disord. 2000;15:230–7. doi: 10.1002/1531-8257(200003)15:2<230::aid-mds1005>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 8.Papa SM, Desimone R, Fiorani M, Oldfield EH. Internal globus pallidus discharge is nearly suppressed during levodopa-induced dyskinesias. Ann Neurol. 1999;46:732–8. doi: 10.1002/1531-8249(199911)46:5<732::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 9.Merello M, Balej J, Delfino M, Cammarota A, Betti O, Leiguarda R. Apomorphine induces changes in GPi spontaneous outflow in patients with Parkinson's disease. Mov Disord. 1999;14:45–9. doi: 10.1002/1531-8257(199901)14:1<45::aid-mds1009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 10.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like peptide-1. Cell Metab. 2018;27:740–56. doi: 10.1016/j.cmet.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Gentilella R, Pechtner V, Corcos A, Consoli A. Glucagon-like peptide-1 receptor agonists in type 2 diabetes treatment: Are they all the same? Diabetes Metab Res Rev. 2019;35:e3070. doi: 10.1002/dmrr.3070. [DOI] [PubMed] [Google Scholar]
- 12.Li Y, Perry T, Kindy MS, Harvey BK, Tweedie D, Holloway HW, et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc Natl Acad Sci U S A. 2009;106:1285–90. doi: 10.1073/pnas.0806720106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim DS, Choi HI, Wang Y, Luo Y, Hoffer BJ, Greig NH. A new treatment strategy for Parkinson's disease through the gut-brain axis: The glucagon-like peptide-1 receptor pathway. Cell Transplant. 2017;26:1560–71. doi: 10.1177/0963689717721234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Athauda D, Foltynie T. Protective effects of the GLP-1 mimetic exendin-4 in Parkinson's disease. Neuropharmacology. 2018;136:260–70. doi: 10.1016/j.neuropharm.2017.09.023. [DOI] [PubMed] [Google Scholar]
- 15.Hölscher C. Brain insulin resistance: Role in neurodegenerative disease and potential for targeting. Expert Opin Investig Drugs. 2020;29:333–48. doi: 10.1080/13543784.2020.1738383. [DOI] [PubMed] [Google Scholar]
- 16.Athauda D, Maclagan K, Skene SS, Bajwa-Joseph M, Letchford D, Chowdhury K, et al. Exenatide once weekly versus placebo in Parkinson's disease: A randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:1664–75. doi: 10.1016/S0140-6736(17)31585-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Glotfelty EJ, Olson L, Karlsson TE, Li Y, Greig NH. Glucagon-like peptide-1 (GLP-1)-based receptor agonists as a treatment for Parkinson's disease. Expert Opin Investig Drugs. 2020;29:595–602. doi: 10.1080/13543784.2020.1764534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li Y, Vaughan KL, Tweedie D, Jung J, Kim HK, Choi HI, et al. Pharmacokinetics of Exenatide in nonhuman primates following its administration in the form of sustained-release PT320 and Bydureon. Sci Rep. 2019;9:17208. doi: 10.1038/s41598-019-53356-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aviles-Olmos I, Dickson J, Kefalopoulou Z, Djamshidian A, Ell P, Soderlund T, et al. Exenatide and the treatment of patients with Parkinson's disease. J Clin Invest. 2013;123:2730–6. doi: 10.1172/JCI68295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mulvaney CA, Duarte GS, Handley J, Evans DJ, Menon S, Wyse R, et al. GLP-1 receptor agonists for Parkinson's disease. Cochrane Database Syst Rev. 2020;7:CD012990. doi: 10.1002/14651858.CD012990.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]


