Abstract
This review discusses the potential of major histocompatibility complex (MHC) Class II constructs as stroke therapeutics. We focus on the delivery of MHC Class II construct, DRmQ, as a safe and effective treatment for ischemic stroke. DRmQ was observed to attenuate behavioral deficits and decrease microglia activation and proinflammatory cytokines, illustrating its ability to mitigate the secondary cell death following stroke. Similar anti-neuroinflammation treatments, such as transplantation of mesenchymal stem cells and mitochondrial transfers, are briefly discussed to provide further support that sequestration of inflammation stands as a robust therapeutic target for stroke.
Keywords: Cell death, cytokines, DRmQ, mesenchymal stem cell, mitochondrial transfer, stem cell therapy, stroke therapy
Expanding Immune Response
Stroke continues to be one of the leading causes of death around the world,[1,2] and neuroinflammation remains a primary cause of the secondary cell death that occurs after an ischemic event.[3,4] It is more difficult to develop a stroke treatment due to the limited therapeutic window that accompanies a stroke.[5,6,7] Many therapeutic treatments aim at improving the immune response, specifically weakening neuroinflammation.[8,9] Stroke is associated with both central and peripheral inflammatory responses, and the spleen contributes a major role in secondary cell death.[10] Partial major histocompatibility complex (MHC) Class II constructs can limit early immune response, therefore, sequestering ischemic-induced inflammation.[11,12] MHC Class II constructs are a viable stroke treatment because of their neuroantigen-specific modulation of T-cells as well as CD74 signaling.[11,12,15]
Major Histocompatibility Complex Class II Constructs
MHC Class II constructs can potentially mitigate cell death in traumatic brain injury (TBI) and stroke.[12] DRhQ and DRmQ represent advanced MHC Class II constructs that have shown safety and efficacy in preclinical models of TBI and stroke. DRhQ was created for clinical development while DRmQ was designed as a preclinical therapeutic for demonstrating proof-of-concept efficacy in animal models of autoimmune encephalomyelitis.[13,14,15] Using Stroke Therapy Academic Industry Roundtable (STAIR) guidelines, we tested DRmQ in a stroke model in rats.[16] Two different species of stroke model were used to analyze the clinical importance of MHC Class II constructs. That study observed the anti-inflammatory effects of DRmQ in rat stroke models using behavioral and histological assays.[17]
Major Histocompatibility Complex Class II constructs as Stroke Therapeutics
A potent stroke treatment entails the inhibition of neuroantigen-specific T-cells, cytotoxic monocytes, and macrophages, a combination which could provide potential therapeutic benefits to stroke patients. Indeed, attenuation of histological and behavioral deficits was seen in stroke animals that were administered partial MHC Class II constructs subcutaneously.[17] Adult Sprague-Dawley rats that had been exposed to a stroke model and a mouse partial MHC Class II construct (i.e., DRmQ) were analyzed.[17] A decrease in stroke-induced motor deficits, neuroinflammation, infarcts, and peri-infarct cell loss was observed.[17] Tumor necrosis factor (TNF)-α and interleukin-6 upregulation was also altered in the spleen of DRmQ-treated stroke animals providing further evidence of potential regulation of peripheral inflammation by MHC Class II constructs.[17] These results suggest that the use of partial MHC Class II constructs as a viable treatment for stroke may halt central and peripheral inflammation responses reducing the neuroinflammation that results in secondary cell death after stroke [Figure 1].
Figure 1.
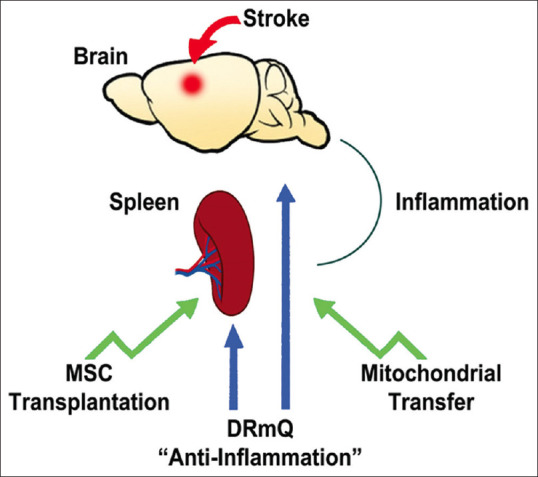
This demonstrates the anti-inflammation-based therapeutic stroke targets. DRmQ robustly sequesters central and peripheral inflammation leading to a reduction in stroke-induced behavioral and histological deficits. These therapeutic effects may also be accomplished by similar anti-neuroinflammation strategies, such as stem cell transplantation and mitochondrial transfer
Behavioral Improvements in DRmQ-Treated Stroke Animals
Several behavioral tests were conducted to compare the motor skills in the DRmQ-treated mice and the mice that were not treated with DRmQ.[17] The DRmQ-treated group showed improved features in all of the motor tests that were administered. The DRmQ-treated animals also featured a smaller cerebral infarct area and improved cell survival when compared to the control mice.[17] Reduced proinflammatory cytokines in the peri-infarct area were also observed. Finally, the spleen was analyzed to determine the effects of DRmQ on peripheral inflammatory response. Results showed a higher spleen weight in DRmQ-treated stroke animals suggesting a suppression of the splenic inflammatory response and reduced splenic atrophy.[17]
Normalized Inflammatory Responses in DRmQ-Treated Stroke Animals
DRmQ treatment was seen to improve stroke-induced inflammatory markers.[17] There was a large decrease in the expression of the proinflammatory cytokine TNF-α and Ionized calcium-binding adaptor protein-1-activated microglia in the brain. This decrease in microglia activation and proinflammatory cytokines potentially reflects the ability of DRmQ to reduce secondary cell death in stroke.[17] DRmQ was also observed to reduce inflammation and splenic atrophy in the spleen. Overall, these results demonstrate DRmQ's ability to suppress the inflammatory response by reducing proinflammatory cytokines.
Since neuroinflammation is a primary cause of secondary cell death in stroke, targeting inflammation is essential when developing treatments for stroke.[10,11,13,18,19,20,21,22] MHC Class II constructs regulate the immune response in stroke and other neurovascular diseases.[10,12,15] In stroke, treatment with a MHC Class II construct decreased the infarct size in both genders of mice after middle cerebral artery occlusion (MCAO) by inhibiting activated microglia and infiltrating monocytes.[8] MHC Class II construct administration also displayed similar results in the distal MCAO stroke model by decreasing infarct size and reducing proinflammatory cytokines.[8,12,23]
Anti-Neuroinflammation-Based Treatments
Parallel studies demonstrating the potential of anti-neuroinflammation-based treatments, along the lines of producing similar therapeutic effects as MHC Class II constructs, can be appreciated in mesenchymal stem cell (MSC) transplantation and mitochondrial transfer, as discussed below.
Stem Cell Therapy
MSCs are multipotent stem cells located in the bone marrow alongside connective tissue.[24,25,26] MSCs are very easily obtained and manipulated which makes them an appealing cell source for the treatment of stroke.[27,28,29] Allogeneic MSCs from a donor can be transplanted without the need for immunosuppression. These characteristics of MSCs make them a viable candidate for ischemic stroke treatments.[30] Similar to the neurotherapeutic effects produced by MHC Class II constructs in suppressing stroke-induced inflammation, stem cell therapy has the ability to reduce the deleterious inflammatory responses that follow ischemic events.[30,31,32,33]
Mitochondrial Transfer
Following an ischemic event, one secondary cell death event that contributes to neuroinflammation is the lack of adenosine triphosphate and nutrients, which indicates the importance of the role of mitochondria in stroke pathology.[34] The penumbral neurovascular unit degenerates rapidly due to mitochondrial dysfunction and inflammation requiring an effective treatment for stroke. Mitochondrial transfer via stem cell transplantation has been shown as a viable option in restoring mitochondrial function after stroke.[34] Notably, stem cell-mediated mitochondria transfers promote cell survival coincident with dampened inflammation in stroke animals, thus serving as an effective strategy in reducing detrimental inflammation in ischemic stroke.[34]
Conclusion
This review discusses the therapeutic effects of DRmQ following STAIR criteria as well as providing evidence to support the importance of the peripheral component in stroke treatment and pathology.[16,17] DRmQ improved peripheral inflammation from the spleen which is a critical therapeutic target in neuroprotection during ischemia.[35,36] The ability of DRmQ to modulate the central and peripheral inflammatory response contributes to its potential therapeutic effects in treating stroke.
Further research needs to determine whether the therapeutic effects of DRmQ could extend into the chronic phase of stroke. Although no harmful effects were observed from DRmQ treatment, it is important to remain cautious of any adverse effects when testing the long-term effect of DRmQ. Correct dosage and administration information also needs to be researched to optimize the effects of the treatment.
Similar anti-neuroinflammation-based treatments, such as stem cell therapy and mitochondrial transfer, resemble the therapeutic benefits of MHC Class II constructs in dampening inflammation after stroke. Altogether, these preclinical data advance the use of targeting inflammation as a potential treatment for stroke and other neurological disorders characterized by a harmful inflammatory response.
Financial support and sponsorship
Biomedical Laboratory Research and Development Merit Review Award 2I01 BX000226 (AAV), Senior Research Career Scientist Award 1IK6BX004209 (AAV), the National Institute of Allergy and Infectious Diseases award 2R42AI122574 (AAV), and the NIH R21AI148409 award (HO).
Conflicts of interest
Prof. Cesario V. Borlongan is Associate Editor of Brain Circulation.
Acknowledgment
All figures were created with Biorender.com.
References
- 1.Nishino H, Borlongan CV. Restoration of function by neural transplantation in the ischemic brain. Prog Brain Res. 2000;127:461–76. doi: 10.1016/s0079-6123(00)27022-2. [DOI] [PubMed] [Google Scholar]
- 2.Hara K, Yasuhara T, Maki M, Matsukawa N, Masuda T, Yu SJ, et al. Neural progenitor NT2N cell lines from teratocarcinoma for transplantation therapy in stroke. Prog Neurobiol. 2008;85:318–34. doi: 10.1016/j.pneurobio.2008.04.005. [DOI] [PubMed] [Google Scholar]
- 3.Borlongan CV. Cell therapy for stroke: Remaining issues to address before embarking on clinical trials. Stroke. 2009;40:S146–8. doi: 10.1161/STROKEAHA.108.533091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blecharz-Lang KG, Wagner J, Fries A, Nieminen-Kelhä M, Rösner J, Schneider UC, et al. Interleukin 6-mediated endothelial barrier disturbances can be attenuated by Blockade of the IL6 receptor expressed in brain microvascular endothelial cells. Transl Stroke Res. 2018;9:631–42. doi: 10.1007/s12975-018-0614-2. [DOI] [PubMed] [Google Scholar]
- 5.Simon R, Meller R, Yang T, Pearson A, Wilson G. Enhancing base excision repair of mitochondrial DNA to reduce ischemic injury following reperfusion. Transl Stroke Res. 2019;10:664–71. doi: 10.1007/s12975-018-0680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navarro-Oviedo M, Roncal C, Salicio A, Belzunce M, Rabal O, Toledo E, et al. MMP10 promotes efficient thrombolysis after is-chemic stroke in mice with induced diabetes. Transl Stroke Res. 2018;10:389–401. doi: 10.1007/s12975-018-0652-9. [DOI] [PubMed] [Google Scholar]
- 7.Griemert EV, Recarte Pelz K, Engelhard K, Schäfer MK, Thal SC. PAI-1 but not PAI-2 gene deficiency attenuates ischemic brain in-jury after experimental stroke. Transl Stroke Res. 2018;10:372–80. doi: 10.1007/s12975-018-0644-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Benedek G, Vandenbark AA, Alkayed NJ, Offner H. Partial MHC Class II constructs as novel immunomodulatory therapy for stroke. Neurochem Int. 2017;107:138–47. doi: 10.1016/j.neuint.2016.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tan Q, Guo P, Zhou J, Zhang J, Zhang B, Lan C, et al. Targeting neutrophil extracellular traps enhanced tPA fibrinolysis for experi-mental intracerebral hemorrhage. Transl Res. 2019;211:139–146. doi: 10.1016/j.trsl.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Liu Z, Ren H, Zhang L, Gao S, Ren L, et al. DRα1-MOG-35-55 treatment reduces lesion volumes and improves neurological deficits after traumatic brain injury. Metab Brain Dis. 2017;32:1395–402. doi: 10.1007/s11011-017-9991-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dotson AL, Chen Y, Zhu W, Libal N, Alkayed NJ, Offner H. Partial MHC constructs treat thromboembolic ischemic stroke characterized by early immune expansion. Transl Stroke Res. 2016;7:70–8. doi: 10.1007/s12975-015-0436-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandenbark AA, Meza-Romero R, Benedek G, Offner H. A novel neurotherapeutic for multiple sclerosis, ischemic injury, methamphetamine addiction, and traumatic brain injury. J Neuroinflammation. 2019;16:14. doi: 10.1186/s12974-018-1393-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang J, Ye Q, Xu J, Benedek G, Zhang H, Yang Y, et al. DRα1-MOG-35-55 reduces permanent ischemic brain injury. Transl Stroke Res. 2017;8:284–93. doi: 10.1007/s12975-016-0514-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Meza-Romero R, Benedek G, Gerstner G, Kent G, Nguyen H, Offner H, et al. Increased CD74 binding and EAE treatment efficacy of a modified DRα1 molecular construct. Metab Brain Dis. 2019;34:153–64. doi: 10.1007/s11011-018-0331-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benedek G, Zhu W, Libal N, Casper A, Yu X, Meza-Romero R, et al. A novel HLA-DRα1-MOG-35-55 construct treats experimental stroke. Metab Brain Dis. 2014;29:37–45. doi: 10.1007/s11011-013-9440-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saver JL, Albers GW, Dunn B, Johnston KC, Fisher M STAIR VI Consortium. Stroke Therapy Academic Industry Roundtable (STAIR) recommendations for extended window acute stroke therapy trials. Stroke. 2009;40:2594–600. doi: 10.1161/STROKEAHA.109.552554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee JY, Castelli V, Bonsack B, Coats AB, Navarro-Torres L, Garcia-Sanchez J, et al. A novel partial MHC Class II construct, DRmQ, inhibits central and peripheral inflammatory responses to promote neuroprotection in experimental stroke. Transl Stroke Res. 2020;11:831–6. doi: 10.1007/s12975-019-00756-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu W, Libal NL, Casper A, Bodhankar S, Offner H, Alkayed NJ. Recombinant T cell receptor ligand treatment improves neurological outcome in the presence of tissue plasminogen activator in experimental ischemic stroke. Transl Stroke Res. 2014;5:612–7. doi: 10.1007/s12975-014-0348-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu K, Lee JY, Kaneko Y, Tuazon JP, Vale F, van Loveren H, et al. Human stem cells transplanted into the rat stroke brain migrate to the spleen via lymphatic and inflammation pathways. Haematologica. 2019;104:1062–73. doi: 10.3324/haematol.2018.206581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pan J, Palmateer J, Schallert T, Hart M, Pandya A, Vandenbark AA, et al. Novel humanized recombinant T cell receptor ligands protect the female brain after experimental stroke. Transl Stroke Res. 2014;5:577–85. doi: 10.1007/s12975-014-0345-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Seifert HA, Offner H. The splenic response to stroke: From rodents to stroke subjects. J Neuroinflammation. 2018;15:195. doi: 10.1186/s12974-018-1239-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seifert HA, Benedek G, Nguyen H, Gerstner G, Zhang Y, Kent G, et al. Antibiotics protect against EAE by increasing regulatory and anti-inflammatory cells. Metab Brain Dis. 2018;33:1599–607. doi: 10.1007/s11011-018-0266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu W, Casper A, Libal NL, Murphy SJ, Bodhankar S, Offner H, et al. Preclinical evaluation of recombinant T cell receptor ligand RTL1000 as a therapeutic agent in ischemic stroke. Transl Stroke Res. 2015;6:60–8. doi: 10.1007/s12975-014-0373-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pittenger MF, Martin BJ. Mesenchymal stem cells and their potential as cardiac therapeutics. Circ Res. 2004;95:9–20. doi: 10.1161/01.RES.0000135902.99383.6f. [DOI] [PubMed] [Google Scholar]
- 25.Walczak P, Zhang J, Gilad AA, Kedziorek DA, Ruiz-Cabello J, Young RG, et al. Dual-modality monitoring of targeted intraarterial delivery of mesenchymal stem cells after transient ischemia. Stroke. 2008;39:1569–74. doi: 10.1161/STROKEAHA.107.502047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uccelli A, Moretta L, Pistoia V. Immunoregulatory function of mesenchymal stem cells. Eur J Immunol. 2006;36:2566–73. doi: 10.1002/eji.200636416. [DOI] [PubMed] [Google Scholar]
- 27.Ishikawa H, Tajiri N, Shinozuka K, Vasconcellos J, Kaneko Y, Lee HJ, et al. Vasculogenesis in experimental stroke after human cerebral endothelial cell transplantation. Stroke. 2013;44:3473–81. doi: 10.1161/STROKEAHA.113.001943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Borlongan CV, Tajima Y, Trojanowski JQ, Lee VM, Sanberg PR. Cerebral ischemia and CNS transplantation: Differential effects of grafted fetal rat striatal cells and human neurons derived from a clonal cell line. Neuroreport. 1998;9:3703–9. doi: 10.1097/00001756-199811160-00025. [DOI] [PubMed] [Google Scholar]
- 29.Bhatia R, Hare JM. Mesenchymal stem cells: Future source for reparative medicine. Congest Heart Fail. 2005;11:87–91. doi: 10.1111/j.1527-5299.2005.03618.x. [DOI] [PubMed] [Google Scholar]
- 30.Watanabe M, Yavagal DR. Intra-arterial delivery of mesenchymal stem cells. Brain Circ. 2016;2:114–7. doi: 10.4103/2394-8108.192522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu X, Ye R, Yan T, Yu SP, Wei L, Xu G, et al. Cell based therapies for ischemic stroke: From basic science to bedside. Prog Neurobiol. 2014;115:92–115. doi: 10.1016/j.pneurobio.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chen J, Li Y, Wang L, Lu M, Zhang X, Chopp M. Therapeutic benefit of intracerebral transplantation of bone marrow stromal cells after cerebral ischemia in rats. J Neurol Sci. 2001;189:49–57. doi: 10.1016/s0022-510x(01)00557-3. [DOI] [PubMed] [Google Scholar]
- 33.Blum A, Balkan W, Hare JM. Advances in cell-based therapy for peripheral vascular disease. Atherosclerosis. 2012;223:269–77. doi: 10.1016/j.atherosclerosis.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Russo E, Nguyen H, Lippert T, Tuazon J, Borlongan CV, Napoli E. Mitochondrial targeting as a novel therapy for stroke. Brain Circ. 2018;4:84–94. doi: 10.4103/bc.bc_14_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nguyen H, Zarriello S, Coats A, Nelson C, Kingsbury C, Gorsky A, et al. Stem cell therapy for neurological disorders: A focus on aging. Neurobiol Dis. 2019;126:85–104. doi: 10.1016/j.nbd.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stonesifer C, Corey S, Ghanekar S, Diamandis Z, Acosta SA, Borlongan CV. Stem cell therapy for abrogating stroke-induced neuroinflammation and relevant secondary cell death mechanisms. Prog Neurobiol. 2017;158:94–131. doi: 10.1016/j.pneurobio.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]


