Abstract
Amyotrophic lateral sclerosis (ALS) stands as a neurodegenerative disorder characterized by the rapid progression of motor neuron loss in the brain and spinal cord. Unfortunately, treatment options for ALS are limited, and therefore, novel therapies that prevent further motor neuron degeneration are of dire need. In ALS, the infiltration of pathological elements from the blood to the central nervous system (CNS) compartment that spur motor neuron damage may be prevented via restoration of the impaired blood-CNS-barrier. Transplantation of human bone marrow endothelial progenitor cells (hBM-EPCs) demonstrated therapeutic promise in a mouse model of ALS due to their capacity to mitigate the altered blood-CNS-barrier by restoring endothelial cell (EC) integrity. Remarkably, the hBM-EPCs can release angiogenic factors that endogenously ameliorate impaired ECs. In addition, these cells may produce extracellular vesicles (EVs) that carry a wide range of vesicular factors, which aid in alleviating EC damage. In an in vitro study, hBM-EPC-derived EVs were effectively uptaken by the mouse brain endothelial cells (mBECs) and cell damage was significantly attenuated. Interestingly, the incorporation of EVs into mBECs was inhibited via β1 integrin hindrance. This review explores preclinical studies of the therapeutic potential of hBM-EPCs, specifically via hBM-EPC-derived EVs, for the repair of the damaged blood-CNS-barrier in ALS as a novel treatment approach.
Keywords: Neurodegenerative disorder, ALS, blood-CNS-barrier, stem cell, extracellular vesicle, transplantation
Introduction
Previous research has demonstrated that extracellular vesicles (EVs) play a crucial role in regulating communication between cells in a variety of physiological and pathological circumstances and compose of microvesicles, apoptotic bodies, and exosomes.[1,2,3,4,5,6,7] Microvesicles are 50–1,000 nm in size, exosomes are 40–120 nm, and apoptotic bodies are 500–2,000 nm.[2,8] EVs are not only distinguished by constituent's sizes, but also by inner contents and biogenesis pathways.[2,8,9] Importantly, EVs are largely involved in mediating cross-talk between cells by transporting a variety of biomolecules (e.g., proteins, lipids, peptides, mRNA, microRNA) among cells. As a result, EVs contribute to stem cell plasticity,[1,10] immune system responses,[11,12,13,14] and angiogenesis.[15,16] In addition, EVs may play a role in the transport of therapeutic factors from stem cells[17] that bear regenerative capacity, promote angiogenesis, attenuate inflammation, and inhibit apoptosis.[18]
Due to the capacity of nanovesicles to traverse the blood-brain barrier and their low tendency to evoke an immune response, nanoparticle-based therapies have arisen as potential deliverers of therapeutics for the treatment of neurodegenerative illnesses.[2,4,19] Notably, human bone marrow (hBM) mesenchymal stromal cells (MSC)-derived EVs demonstrated rehabilitative capacity in animal models of lung injury induced by either LPS[20] or ischemia-reperfusion,[21] as well as of kidney damage[22] and sepsis.[23] In myocardial ischemia/reperfusion injury mice, exosomes derived from MSCs diminished infarct volume.[24] Following treatment with MSC-isolated exosomes, healing of femur fracture in mice accelerated.[25] In immunodeficient SCID mice, endothelial cell (EC) viability, proliferation, and angiogenesis were promoted by human endothelial progenitor cell (EPC)-derived microvesicle administration.[16] Another study using SCID mice explored the efficacy of human pancreatic islet xenotransplantation in conjunction with microvesicles harvested from EPCs in ameliorating immune deficiency.[15] Notably, this combined therapy enhanced angiogenesis and upregulated the production and release of insulin in treated mice.[15] Thus, EVs may serve as effective transporters for the therapeutic delivery of various cargo proteins in the treatment of numerous diseases. Nonetheless, the mechanism underlying EV cargo delivery warrants further investigation.[17] This review discusses the efficacy of EVs in treating amyotrophic lateral sclerosis (ALS), specifically EVs secreted by hBM-EPCs, and the mechanisms behind the therapeutic actions of these vesicles.
Preclinical Studies Supporting Human Bone Marrow-Endothelial Progenitor Cells Therapy in Amyotrophic Lateral Sclerosis
In contemplating the role EVs in hBM-EPC therapy, a brief introduction on the status of hBM-EPC therapy for ALS will provide a better appreciation of the scientific merit of EV contribution to regenerative medicine. In ALS studies of regenerative stem cell therapy targeting the impaired blood-central nervous system (CNS)-barrier, one of the pathogenic disease mechanisms, may serve as a potent therapeutic strategy for this aggressive neurodegenerative malady.[17,26,27,28] When hBM-EPCs were delivered intravenously to symptomatic G93A SOD1 mutant mice (an ALS model), the blood-CNS-barrier was significantly restored potentially due to the replacement of damaged ECs with “healthy” transplanted cells.[29] Barrier functionality in gray matter horns and white matter columns in the spinal cord, as well as in gray and white matter in the cerebral motor cortex/brainstem was substantially ameliorated following extensive hBM-EPC engraftment.[29] This improvement of barrier structure and function in the SOD1 mutant mice was observed through a significant reduction in capillary permeability and bolstering of perivascular astrocyte end-feet function.[29] Therefore, the manifestations of disease in these mice were greatly mitigated via blood-CNS-barrier restoration, resulting in augmented motor neuron viability in the spinal cord.[29]
A subsequent in vitro study examined the mechanism underlying hBM-EPC-mediated blood-CNS-barrier repair.[30] Cultured hBM-EPCs were observed in a normogenic environment at various time points.[30] Notably, the cells demonstrated a steady secretion of VEGF-A and angiogenin-1, as well as structural changes in cytoskeletal F-actin filaments, and immunoexpression of zonula occludens 1 and occludin.[30] Furthermore, these results indicate that hBM-EPCs may provide endogenous endothelium repair behind the rehabilitative capacity of these cells along with cell replacement of damaged ECs.[30] In a similar investigation, human peripheral blood-derived EPCs promoted angiogenesis and renewal of impaired brain tissue injury via secretion of biomolecules.[31] On account of hBM-EPCs' capacity to replace damaged ECs and also bolster EC functionality, the efficacy of both of these reparative mechanisms in alleviating ALS-induced blood-CNS-barrier injury should be examined.
The Therapeutic Potential of Human Bone Marrow-Endothelial Progenitor Cells-Derived Extracellular Vesicles in Amyotrophic Lateral Sclerosis
Recently, the preclinical study has explored hBM-EPC-derived EVs as cell-free therapeutics for blood-CNS-barrier restoration in conditions mirroring ALS.[17] An in vitro investigation found that hBM-EPC-derived EVs served as nanosized vesicles and after adding to culture media at concentration of 1 μg/ml, were effectively integrated into mouse brain endothelial cells (mBECs) and ameliorated ALS-induced injury of mBECs following exposure to plasma obtained from symptomatic ALS mice.[17] The total protein content and size of the EVs were noted at specific culture time points during normogenic conditioning.[17] Indeed, EVs are heterogeneous in size and content; however, these vesicles primarily consist of exosomes and microvesicles. Interestingly, the apoptotic bodies in EVs were noted upon vesicle isolation from the hBM-EPC cultures at 5 DIV.[17] The appearance of these EV constituents may be explained by the natural variations in cellular nuclei and cytosol shapes that ensue with apoptosis.[17] Of note, another in vitro study found morphological variations in hBM-EPCs, as the cells transformed from more rounded at 24 h to more elongated at 72 h.[30]
The hBM-EPCs were subject to ALS plasma derived from symptomatic G93A SOD1 mice in culture. It has been shown that G93A SOD1 mutant mice during disease progression contain high levels of the unfavorable humoral factor, as well as heightened concentrations of pro-inflammatory type 1 cytokines and comparatively lower levels of anti-inflammatory type II cytokines.[32] As exposure to ALS mouse plasma grew, cell death accompanied by morphological changes in hBM-EPCs was also increased.[17] Notably, 97 cytokines in the blood plasma of G93A SOD1 mutant mice over the course of disease were delineated, and an upregulated concentration of multiple cytokines correlated with increased mortality rate for mice.[33] However, these biomolecules may not be effective as a prognostic tool due to disparities in levels of expression.[33]
As a diagnostic marker for ALS, peripheral blood levels of particular cytokines may be useful.[34] Plasma extracted from ALS patients exhibited upregulated concentrations of pro-inflammatory cytokines tumor necrosis factor-alpha (TNF-α), TNF receptor 1, interleukin-6 (IL-6), and IL-1β.[35,36,37] Through the trans-signaling pathway, humoral IL-6 may contribute to inflammation of ECs.[38] Furthermore, the heightened levels of inflammatory cytokines associated with ALS, inducing neuroinflammation, may exacerbate transplanted cell viability in the bloodstream.[17] In addition, ALS engenders deleterious humoral conditions with greater levels of injurious factors in the blood circulation, which may also negatively affect the functionality of endogenous ECs.[17] Nevertheless, further investigation into the correlation between cytokine levels and their impact on ECs in ALS at various stages of the disease are warranted. An in vitro study utilizing mBECs examines this point. Upon 3% ALS mouse plasma treatment, cell survival of mBECs substantially decreased in vitro.[17] However, adding 1 μg/ml of hBM-EPC-derived EVs to culture media greatly mitigated cell deterioration and warped morphology.[17] Conversely, at an EV dose of 5 μg/ml, cell viability plummeted, indicating an EV level toxic to ECs. Furthermore, before clinical trials, extensive preclinical investigation is necessary to elucidate optimal dosage and timing of EV treatment.[17]
MSCs have also risen as a potential source of EVs for cell-free therapeutics.[21,22,23,24,25,39,40,41,42] Human BM-EPC-derived microvesicles have demonstrated therapeutic potency in animal models, specifically via the augmentation of angiogenesis.[15,16] The microvesicles were incorporated in ECs and showed the ability to carry angiogenic factors to regions of brain injury[15] Importantly, the microvesicles mirrored the activity of the hBM-EPCs from which they were harvested and were critical for intercellular communication.[6,43] Therefore, these EVs have been coined “nanosized extracellular organelles.”[3]
Potential Mechanisms Underlying Human Bone Marrow-Endothelial Progenitor Cells-Derived Extracellular Vesicles-Induced Neuroprotection in Amyotrophic Lateral Sclerosis
Preclinical studies have also explored the mechanisms behind EV uptake by cells, as well as mitigation of EC impairment spurred by ALS. Remarkably, hBM-EPCs secrete protein and lipid-based therapeutic factors that upregulate neuronal progenitor cell survival and differentiation in vitro.[44] Moreover, EVs harvested from hBM-EPCs may serve as potent carriers for therapeutic biomolecules in neurodegenerative disorders. The cellular and molecular links between neuroprotection to EC restoration remain an outstanding issue that requires further investigations. In mBEC culture, following the addition of hBM-EPC-derived EVs, the vesicles were found ubiquitously throughout the cytosol and cellular projections.[17] Therefore, the therapeutic effects of these EVs may be due to their capacity to carry a wide range of biomolecules to various cell compartments, thereby aiding the preservation of cellular function.[17] Nonetheless, a multi-omic investigation is warranted to delineate the types of biomolecules involved with the rehabilitative capacity of these EVs.[17] In general, EVs vary significantly in structure, content, and biological mechanisms.[2,45,46]
Notably, the following biomarkers have been found on the surface of exosomes: CD9, CD63, CD81, tetraspanins, and flotillin.[47,48,49,50] With respect to microvesicles, integrins, selectins, and CD40 have been identified.[16,51] Finally, annexin V, C2b, and thrombospondin have been delineated in apoptotic bodies.[52,53,54] To more effectively distinguish EV subtypes, a ratio comparing the number of a particular biomarker with the others in the vesicles should be determined.[17] Additionally, proteomic studies and analysis of RNA in EVs are key factors to delineate the molecular cargo of EVs and may aid in elucidating the EV mechanisms in ALS.[17,54,55,56,57] Since EVs are enclosed in a heterogeneous phospholipid bilayer, investigation into how components of the membrane factor into EV structure, function, and stability is warranted.[7] Regarding hBM-EPC-derived EVs specifically, delineating the composition of the lipid membrane may provide a molecular understanding of cellular fusion and fission for these vesicles.[58] Moreover, in-depth investigations that aim to identify the protein and lipid composition of EVs may serve as a robust area of future research.[59]
In addition to further elucidating the molecular heterogeneity of EVs isolated from hBM-EPCs, the mechanisms behind the incorporation of these vesicles into cells must be more extensively evaluated. Indeed, EVs may be uptaken by cells via membrane fusion, endocytosis, phagocytosis, and macropinocytosis.[5] The particular pathways that drive EV incorporation are influenced by the membrane composition of the target cell and the vesicle, indicating the importance of identifying the proteins and/or glycoproteins involved in EV internalization.[5] While EVs can be internalized into target cells via endolysosomal mechanisms or budding, endocytosis seems to be the most prominent pathway.[2,5,8] Nevertheless, it is also important to assess the fusion mechanism where the EV lipid membrane fuses directly with the membrane of target cells.[17,60] Future studies should investigate the mechanisms of EV uptake into target cells despite hindering or blocking by ligands or receptor-mediated incorporation.[2,5,12] Notably, microvesicle uptake by human EPCs was attenuated via the inhibition of α4 integrin and β1 integrin (CD29).[16] In vitro, preconditioning hBM-EPC-derived EVs with anti-CD29 inhibiting antibodies resulted in reduced uptake of EVs by mBECs.[17] Consequently, cell death was significantly exacerbated following exposure to 3% ALS mouse plasma compared to the effect of EV treatment.[17] Moreover, cell adhesion molecules may play a critical role in the uptake of EVs by receiving cells.[17]
Conclusion
In summary, EVs isolated from hBM-EPCs demonstrate potential therapeutic promise in ALS. Animal models of ALS showed substantial EC damage, leading to impairment of blood-CNS-barrier integrity. The effects of hBM-EPC-derived EVs culminated in ameliorated EC deterioration in vitro [Figure 1], most likely due to EV uptake into ECs and the release of therapeutic factors from the vesicles. This effect likely results from hBM-EPC transplantation Nonetheless, the specific biomolecules secreted by these EVs have not been fully illuminated. Additionally, the safety and efficacy of these vesicles need to be investigated in vivo to further elucidate the therapeutic potency of hBM-EPC-derived EVs in ALS. This review paper is limited to the potential contribution of EVs derived from hBM-EPCs in the observed functional recovery in ALS following hBM-EPC transplantation. Other mechanisms of action, including but not limited to hBM-EPC differentiation and integration into the host brain tissue, equally warrant consideration in advancing hBM-EPC transplantation for ALS.
Figure 1.
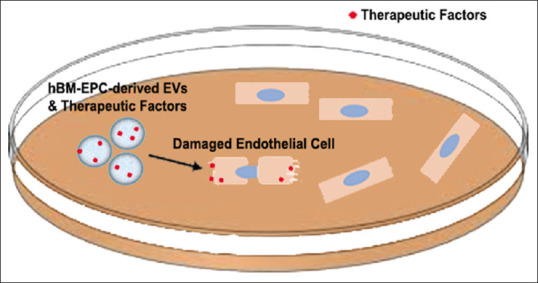
Extracellular vesicle-mediated repair of damaged endothelial cells. Schematic diagram shows how extracellular vesicles derived from human bone marrow endothelial progenitor cells are secreted and uptaken by damaged endothelial cells in an in vitro setting
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgment
SGD is supported by the NIH, NINDS (1R01NS090962) grant.
The figure was created using Adobe Photoshop.
References
- 1.Bakhshandeh B, Kamaleddin MA, Aalishah K. A comprehensive review on exosomes and microvesicles as epigenetic factors. Curr Stem Cell Res Ther. 2017;12:31–6. doi: 10.2174/1574888x11666160709211528. [DOI] [PubMed] [Google Scholar]
- 2.EL Andaloussi S, Mäger I, Breakefield XO, Wood MJ. Extracellular vesicles: Biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–57. doi: 10.1038/nrd3978. [DOI] [PubMed] [Google Scholar]
- 3.Gho YS, Lee C. Emergent properties of extracellular vesicles: A holistic approach to decode the complexity of intercellular communication networks. Mol Biosyst. 2017;13:1291–6. doi: 10.1039/c7mb00146k. [DOI] [PubMed] [Google Scholar]
- 4.Iraci N, Leonardi T, Gessler F, Vega B, Pluchino S. Focus on extracellular vesicles: Physiological role and signalling properties of extracellular membrane vesicles. Int J Mol Sci. 2016;17:171. doi: 10.3390/ijms17020171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mulcahy LA, Pink RC, Carter DR. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014:3. doi: 10.3402/jev.v3.24641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tetta C, Ghigo E, Silengo L, Deregibus MC, Camussi G. Extracellular vesicles as an emerging mechanism of cell-to-cell communication. Endocrine. 2013;44:11–9. doi: 10.1007/s12020-012-9839-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoon YJ, Kim OY, Gho YS. Extracellular vesicles as emerging intercellular communicasomes. BMB Rep. 2014;47:531–9. doi: 10.5483/BMBRep.2014.47.10.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–89. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 9.Gould SJ, Raposo G. As we wait: Coping with an imperfect nomenclature for extracellular vesicles. J Extracell Vesicles. 2013:2. doi: 10.3402/jev.v2i0.20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee Y, El Andaloussi S, Wood MJ. Exosomes and microvesicles: Extracellular vesicles for genetic information transfer and gene therapy. Hum Mol Genet. 2012;21:R125–34. doi: 10.1093/hmg/dds317. [DOI] [PubMed] [Google Scholar]
- 11.Bobrie A, Colombo M, Raposo G, Théry C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic. 2011;12:1659–68. doi: 10.1111/j.1600-0854.2011.01225.x. [DOI] [PubMed] [Google Scholar]
- 12.Chaput N, Théry C. Exosomes: immune properties and potential clinical implementations. Semin Immunopathol. 2011;33:419–40. doi: 10.1007/s00281-010-0233-9. [DOI] [PubMed] [Google Scholar]
- 13.Robbins PD, Morelli AE. Regulation of immune responses by extracellular vesicles. Nat Rev Immunol. 2014;14:195–208. doi: 10.1038/nri3622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Théry C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nat Rev Immunol. 2009;9:581–93. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 15.Cantaluppi V, Biancone L, Figliolini F, Beltramo S, Medica D, Deregibus MC, et al. Microvesicles derived from endothelial progenitor cells enhance neoangiogenesis of human pancreatic islets. Cell Transplant. 2012;21:1305–20. doi: 10.3727/096368911X627534. [DOI] [PubMed] [Google Scholar]
- 16.Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, et al. Endothelial progenitor cell derived microvesicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–8. doi: 10.1182/blood-2007-03-078709. [DOI] [PubMed] [Google Scholar]
- 17.Garbuzova-Davis S, Willing AE, Ehrhart J, Wang L, Sanberg PR, Borlongan CV. Cell-free extracellular vesicles derived from human bone marrow endothelial progenitor cells as potential therapeutics for microvascular endothelium restoration in ALS. Neuromolecular Med. 2020;22:503–16. doi: 10.1007/s12017-020-08607-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suárez-Meade P, Carvajal HG, Yasuhara T, Tajiri N, Date I, Borlongan CV. Regenerative medicine for central nervous system disorders: Role of therapeutic molecules in stem cell therapy. Brain Circ. 2015;1:125–32. [Google Scholar]
- 19.Das CK, Jena BC, Banerjee I, Das S, Parekh A, Bhutia SK, et al. Exosome as a novel shuttle for delivery of therapeutics across biological barriers. Mol Pharm. 2019;16:24–40. doi: 10.1021/acs.molpharmaceut.8b00901. [DOI] [PubMed] [Google Scholar]
- 20.Morrison TJ, Jackson MV, Cunningham EK, Kissenpfennig A, McAuley DF, O'Kane CM, et al. Mesenchymal stromal cells modulate macrophages in clinically relevant lung injury models by extracellular vesicle mitochondrial transfer. Am J Respir Crit Care Med. 2017;196:1275–86. doi: 10.1164/rccm.201701-0170OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stone ML, Zhao Y, Robert Smith J, Weiss ML, Kron IL, Laubach VE, et al. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir Res. 2017;18:212. doi: 10.1186/s12931-017-0704-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aghajani Nargesi A, Lerman LO, Eirin A. Mesenchymal stem cell-derived extracellular vesicles for kidney repair: Current status and looming challenges. Stem Cell Res Ther. 2017;8:273. doi: 10.1186/s13287-017-0727-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng G, Huang R, Qiu G, Ge M, Wang J, Shu Q, et al. Mesenchymal stromal cell-derived extracellular vesicles: Regenerative and immunomodulatory effects and potential applications in sepsis. Cell Tissue Res. 2018;374:1–5. doi: 10.1007/s00441-018-2871-5. [DOI] [PubMed] [Google Scholar]
- 24.Lai RC, Arslan F, Lee MM, Sze NS, Choo AK, Chen TS, et al. Exosome secreted by MSC reduces myocardial ischemia/reperfusion injury. Stem Cell Res. 2010;4:214–22. doi: 10.1016/j.scr.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med. 2016;5:1620–30. doi: 10.5966/sctm.2015-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Eve DJ, Steiner G, Mahendrasah A, Sanberg PR, Kurien C, Thomson A, et al. Reduction of microhemorrhages in the spinal cord of symptomatic ALS mice after intravenous human bone marrow stem cell transplantation accompanies repair of the blood-spinal cord barrier. Oncotarget. 2018;9:10621–34. doi: 10.18632/oncotarget.24360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garbuzova-Davis S, Kurien C, Thomson A, Falco D, Ahmad S, Staffetti J, et al. Endothelial and astrocytic support by human bone marrow stem cell grafts into symptomatic ALS mice towards blood-spinal cord barrier repair. Sci Rep. 2017;7:884. doi: 10.1038/s41598-017-00993-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garbuzova-Davis S, Haller E, Navarro S, Besong TE, Boccio KJ, Hailu S, et al. Transplantation of human bone marrow stem cells into symptomatic ALS mice enhances structural and functional blood-spinal cord barrier repair. Exp Neurol. 2018;310:33–47. doi: 10.1016/j.expneurol.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garbuzova-Davis S, Kurien C, Haller E, Eve DJ, Navarro S, Steiner G, et al. Human bone marrow endothelial progenitor cell transplantation into symptomatic ALS mice delays disease progression and increases motor neuron survival by repairing blood-spinal cord barrier. Sci Rep. 2019;9:5280. doi: 10.1038/s41598-019-41747-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Garbuzova-Davis S, Ehrhart J, Mustafa H, Llauget A, Boccio KJ, Sanberg PR, et al. Phenotypic characteristics of human bone marrow-derived endothelial progenitor cells in vitro support cell effectiveness for repair of the blood-spinal cord barrier in ALS. Brain Res. 2019;1724:146428. doi: 10.1016/j.brainres.2019.146428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbich C, Aicher A, Heeschen C, Dernbach E, Hofmann WK, Zeiher AM, et al. Soluble factors released by endothelial progenitor cells promote migration of endothelial cells and cardiac resident progenitor cells. J Mol Cell Cardiol. 2005;39:733–42. doi: 10.1016/j.yjmcc.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Jeyachandran A, Mertens B, McKissick EA, Mitchell CS. Type I Vs. Type II cytokine levels as a function of SOD1 G93A mouse amyotrophic lateral sclerosis disease progression. Front Cell Neurosci. 2015;9:462. doi: 10.3389/fncel.2015.00462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Moreno-Martínez L, de la Torre M, Toivonen JM, Zaragoza P, García-Redondo A, Calvo AC, et al. Circulating cytokines could not be good prognostic biomarkers in a mouse model of amyotrophic lateral sclerosis. Front Immunol. 2019;10:801. doi: 10.3389/fimmu.2019.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moreno-Martinez L, Calvo AC, Muñoz MJ, Osta R. Are circulating cytokines reliable biomarkers for amyotrophic lateral sclerosis? Int J Mol Sci. 2019;20:2759. doi: 10.3390/ijms20112759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehrhart J, Smith AJ, Kuzmin-Nichols N, Zesiewicz TA, Jahan I, Shytle RD, et al. Humoral factors in ALS patients during disease progression. J Neuroinflammation. 2015;12:127. doi: 10.1186/s12974-015-0350-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hu Y, Cao C, Qiu XY, Yu Y, Yuan J, Zhao Y, et al. Increased peripheral blood inflammatory cytokine levels in amyotrophic lateral sclerosis: A meta-analysis study. Sci Rep. 2017;7:9094. doi: 10.1038/s41598-017-09097-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lam L, Chin L, Halder RC, Sagong B, Famenini S, Sayre J, et al. Epigenetic changes in T-cell and monocyte signatures and production of neurotoxic cytokines in ALS patients. FASEB J. 2016;30:3461–73. doi: 10.1096/fj.201600259RR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Garbuzova-Davis S, Ehrhart J, Sanberg PR, Borlongan CV. Potential role of humoral IL-6 cytokine in mediating pro-inflammatory endothelial cell response in amyotrophic lateral sclerosis. Int J Mol Sci. 2018;19:423. doi: 10.3390/ijms19020423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dabrowska S, Andrzejewska A, Lukomska B, Janowski M. Neuroinflammation as a target for treatment of stroke using mesenchymal stem cells and extracellular vesicles. J Neuroinflammation. 2019;16:178. doi: 10.1186/s12974-019-1571-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dabrowska S, Andrzejewska A, Strzemecki D, Muraca M, Janowski M, Lukomska B. Human bone marrow mesenchymal stem cell-derived extracellular vesicles attenuate neuroinflammation evoked by focal brain injury in rats. J Neuroinflammation. 2019;16:216. doi: 10.1186/s12974-019-1602-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiu G, Zheng G, Ge M, Wang J, Huang R, Shu Q, et al. Mesenchymal stem cell-derived extracellular vesicles affect disease outcomes via transfer of microRNAs. Stem Cell Res Ther. 2018;9:320. doi: 10.1186/s13287-018-1069-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shojaati G, Khandaker I, Funderburgh ML, Mann MM, Basu R, Stolz DB, et al. Mesenchymal stem cells reduce corneal fibrosis and inflammation via extracellular vesicle-mediated delivery of miRNA. Stem Cells Transl Med. 2019;8:1192–201. doi: 10.1002/sctm.18-0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camussi G, Deregibus MC, Bruno S, Cantaluppi V, Biancone L. Exosomes/microvesicles as a mechanism of cell-to-cell communication. Kidney Int. 2010;78:838–48. doi: 10.1038/ki.2010.278. [DOI] [PubMed] [Google Scholar]
- 44.Sadanandan N, Di Santo S, Widmer HR. Another win for endothelial progenitor cells: Endothelial progenitor cell-derived conditioned medium promotes proliferation and exerts neuroprotection in cultured neuronal progenitor cells. Brain Circ. 2019;5:106–11. doi: 10.4103/bc.bc_41_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Akers JC, Gonda D, Kim R, Carter BS, Chen CC. Biogenesis of extracellular vesicles (EV): Exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol. 2013;113:1–1. doi: 10.1007/s11060-013-1084-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.György B, Szabó TG, Pásztói M, Pál Z, Misják P, Aradi B, et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol Life Sci. 2011;68:2667–88. doi: 10.1007/s00018-011-0689-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kalluri R, LeBleu VS. The biology, function, and biomedical applications of exosomes. Science. 2020;367:eaau6977. doi: 10.1126/science.aau6977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kowal J, Arras G, Colombo M, Jouve M, Morath JP, Primdal-Bengtson B, et al. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc Natl Acad Sci U S A. 2016;113:E968–77. doi: 10.1073/pnas.1521230113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rana S, Zöller M. Exosome target cell selection and the importance of exosomal tetraspanins: A hypothesis. Biochem Soc Trans. 2011;39:559–62. doi: 10.1042/BST0390559. [DOI] [PubMed] [Google Scholar]
- 50.Raposo G, Stoorvogel W. Extracellular vesicles: Exosomes, microvesicles, and friends. J Cell Biol. 2013;200:373–83. doi: 10.1083/jcb.201211138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cocucci E, Racchetti G, Meldolesi J. Shedding microvesicles: Artefacts no more. Trends Cell Biol. 2009;19:43–51. doi: 10.1016/j.tcb.2008.11.003. [DOI] [PubMed] [Google Scholar]
- 52.Igami K, Uchiumi T, Ueda S, Kamioka K, Setoyama D, Gotoh K, et al. Characterization and function of medium and large extracellular vesicles from plasma and urine by surface antigens and Annexin V. Peer J Anal Chem. 2020;2:e4. [Google Scholar]
- 53.van Engeland M, Nieland LJ, Ramaekers FC, Schutte B, Reutelingsperger CP. Annexin V-affinity assay: A review on an apoptosis detection system based on phosphatidylserine exposure. Cytometry. 1998;31:1–9. doi: 10.1002/(sici)1097-0320(19980101)31:1<1::aid-cyto1>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 54.Choi DS, Kim DK, Kim YK, Gho YS. Proteomics of extracellular vesicles: Exosomes and ectosomes. Mass Spectrom Rev. 2015;34:474–90. doi: 10.1002/mas.21420. [DOI] [PubMed] [Google Scholar]
- 55.Hill AF, Pegtel DM, Lambertz U, Leonardi T, O'Driscoll L, Pluchino S, et al. ISEV position paper: Extracellular vesicle RNA analysis and bioinformatics. J Extracell Vesicles. 2013:2. doi: 10.3402/jev.v2i0.22859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim KM, Abdelmohsen K, Mustapic M, Kapogiannis D, Gorospe M. RNA in extracellular vesicles. Wiley Interdiscip Rev RNA. 2017:8. doi: 10.1002/wrna.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mateescu B, Kowal EJ, van Balkom BW, Bartel S, Bhattacharyya SN, Buzás EI, et al. Obstacles and opportunities in the functional analysis of extracellular vesicle RNA – An ISEV position paper. J Extracell Vesicles. 2017;6:1286095. doi: 10.1080/20013078.2017.1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chernomordik LV, Kozlov MM. Protein-lipid interplay in fusion and fission of biological membranes. Annu Rev Biochem. 2003;72:175–207. doi: 10.1146/annurev.biochem.72.121801.161504. [DOI] [PubMed] [Google Scholar]
- 59.Osteikoetxea X, Balogh A, Szabó-Taylor K, Németh A, Szabó TG, Pálóczi K, et al. Improved characterization of EV preparations based on protein to lipid ratio and lipid properties. PLoS One. 2015;10:e0121184. doi: 10.1371/journal.pone.0121184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Subra C, Laulagnier K, Perret B, Record M. Exosome lipidomics unravels lipid sorting at the level of multivesicular bodies. Biochimie. 2007;89:205–12. doi: 10.1016/j.biochi.2006.10.014. [DOI] [PubMed] [Google Scholar]


