Abstract
Here, we present two approaches to map DNA double-strand breaks (DSBs) and single-strand breaks (SSBs) in the genome of human cells. We named these methods respectively DSB-Seq and SSB-Seq. We tested the DSB and SSB-Seq in HCT1116, human colon cancer cells, and validated the results using the topoisomerase 2 (Top2)-poisoning agent etoposide (ETO). These methods are powerful tools for the direct detection of the physiological and pathological “breakome” of the DNA in human cells.
Keywords: DNA damage, Double-strand breaks (DSBs), Single-strand breaks (SSBs), Topoisomerase 2 (Top2), Etoposide (ETO)
1. Introduction
The cellular genome is constantly exposed to extracellular and intracellular DNA damaging agents that compromise DNA integrity and threaten genomic stability (Fig. 1). As a result, cellular mechanisms to cope with DNA damage developed early in evolution and are conserved in current animal and plant species. Understanding the sensitivity of the genome to various DNA insults is instrumental to implement effective preventive and treatment strategies of diseases. Most of the techniques developed to study DNA breaks are based on their indirect detection, by examining the localization of proteins involved in the repair of DNA damage such as the phosphorylated histone variant γ-H2AX [1] or the replication protein A (RPA), or by detecting the single-stranded DNA that transiently accumulates at DSB sites [2]. However, despite past work having provided comprehensive information on the pathways involved in detecting and repairing DNA breakage, our knowledge of how the genome breaks in response to various agents is incomplete and would be advanced by improved technology to detect DNA breaks.
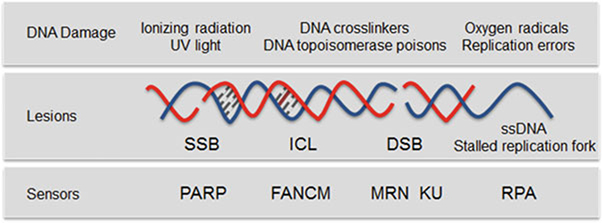
Fig. 1.
Types of DNA damage. Exogenous and endogenous DNA damaging agents generate various types of lesions including SSBs and DSBs. PARP predominantly acts as a sensor of SSB [17]. RPA binds to regions of single-stranded DNA (ssDNA) that are exposed to stalled replication forks or after DSB resection [18]. The multifunctional MRN complex and KU detect DSBs, FANCM is required for the DNA interstrand crosslink (ICL)-induced checkpoint response [19]. FANCM = Fanconi anemia complementation group M; ICL = interstrand crosslink; MRN = MRE11-RAD50-NBS1 complex; PARP = poly(ADP-ribose) polymerase; RPA = replication protein A
Several assays to detect DSBs were developed during recent years that either rely on the mapping of chromosomal translocation by using a “bait” DSB introduced into the genome [3–5] or by capturing the double-strand broken ends with oligodeoxynucleotides or integrase-defective lentiviral vectors. The junctions between the genomic DNA and the transfected DNA correspond to the location of the DSBs and can successively be sequenced [6, 7].
While these methods provide high resolution of DSB and low background, they are limited to the detection of only translocated breaks or integrated DNA. Consequently, they cannot be used to study highly dynamic genome instability events. Additionally, due to the necessity to introduce a “bait” DSB into the genome or double-stranded DNA into the nuclei of living cells, the use of these methods is now restricted to well-established cell lines, not primary cells or tissues. Real-time capture of genome damaging events is provided by recent methods based on direct in situ labeling of DSBs [8, 9]. However, the methods were not implemented to identify SSBs and so they do not allow a direct comparison between DSBs and SSBs in the same genome context [10].
Here, we present two comprehensive experimental and computational approaches to map DSBs and SSBs across the genome of human cells [11]. These are based on the direct labeling of breaks with two independent strategies. The approaches could be extended to any cell lines and tissue and even on previously isolated genomic DNA.
To detect SSBs (SSB-Seq, Fig. 2a) the high molecular weight genomic DNA isolated form HCT116 cells is subjected to nick translation. Nick translation is a tagging technique in which DNA polymerase I is used to replace some of the nucleotides of a DNA sequence with their labeled analogues—digoxigenin-modified nucleotides—creating a tagged DNA fragment that can be immuno-precipitated with anti-digoxigenin antibody and sequenced [12]. To increase the resolution of mapping, we restricted the digoxigenin-labeling to a small patch of DNA by including in the reaction dideoxynucleotides (ddNTP), to inhibit excessive chain elongation by DNA polymerase I. As a control for the labeling, samples were also nick-translated without digoxigenin-labeled nucleotides.
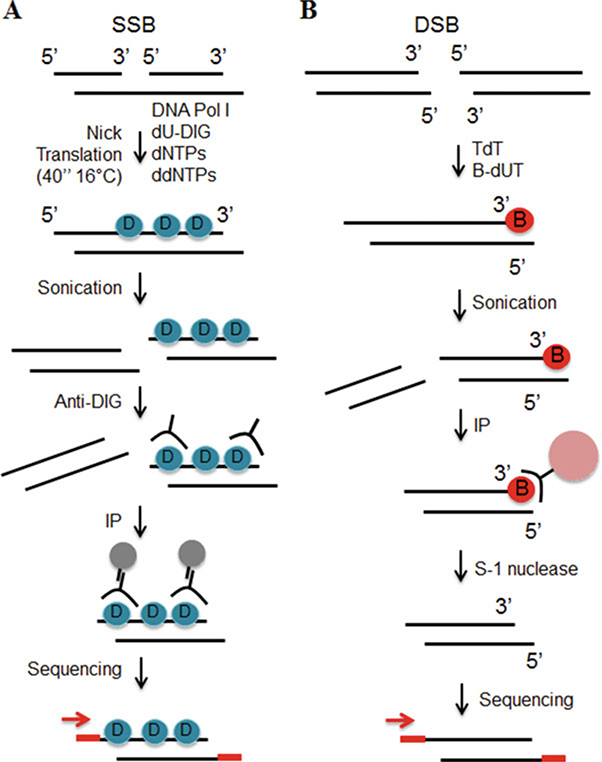
Fig. 2.
DNA breaks mapping workflow. (a) SSBs are labeled during nick translation using nucleotides covalently linked to digoxigenin (blue circle). The DNA is subsequently purified, sonicated and incubated with anti-digoxigenin antibody (anti-DIG). The immuno-precipitated DNA is sequenced. (b) 3′ tails of DSBs are ligated to biotinylated nucleotides (red circle). After sonication the labeled fragments are captured on streptavidin beads (pink circle). Tails are removed from released fragments and DNA is sequenced
To map DSBs (DSB-Seq, Fig. 2b) the double-stranded DNA ends were 3′-end tailed with terminal deoxynucleotidyl transferase (TdT), a DNA polymerase I that catalyzes the addition of nucleotides to the 3′ terminus of a DNA molecule. In the presence of TdT, biotinylated nucleotides were added to the region of DSB and after fragmentation, the biotinylated DNA was streptavidin-selected. In parallel, 3′-tailing was performed in the absence of biotinylated nucleotides, which constituted our negative control for labeling and selection. To remove the biotinylated-tails, samples were treated with S-1 nuclease and the resulting DNA was purified and sequenced (this technique was modified from our previous work [13]).
Our methods provide maps of the DNA break landscape, in various cell types and tissues, and in different experimental conditions.
2. Materials
2.1. Reagents
Proteinase K (Solution in water, 20 mg/ml).
Phenol, Tris saturated.
Phenol:Chloroform:Isoamyl Alcohol 25:24:1.
Ethanol 100%.
Ammonium acetate 7.5 M.
RNase, DNase-free (500 μg/ml).
SDS 10%.
dATP, dGTP, dCTP, dTTP.
Digoxigenin-11-dUTP.
ddATP, ddGTP, ddCTP, ddTTP.
E. coli DNA polymerase I.
EDTA 0.5 M, (pH 8.0).
Anti-digoxigenin antibody.
Protein G-Sepharose beads.
Terminal transferase (TdT).
Biotin-16-dUTP.
TTP.
Streptavidin-coated beads.
S-1 nuclease.
Klenow (exo-).
T4 DNA ligase.
Agarose (agarose gels are prepared in TAE buffer).
2% precast agarose gel (e.g., E-Gel, Invitrogen).
Illumina adapter (Adaptor oligo mix).
Illumina primers (Fw: 5′-aca ctc ttt ccc tac acg acg c-3′/Rv: 5′-caa gca gaa gac ggc ata cga gc-3′).
2.2. Buffers
Lysis buffer: 10 mM Tris–HCl (pH 8.0), 100 mM EDTA (pH 8.0), 0.5% SDS.
TE buffer: 10 mM Tris–HCl (pH 8.0), 1 mM EDTA (pH 8.0).
PBS buffer: 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 2 mM KHPO4 (pH 7.2).
NP-40 buffer: 20 mM Tris–HCl (pH 8.0), 137 mM NaCl, 10% Glycerol, 1% NP-40, 2 mM EDTA (pH 8.0).
TdT reaction buffer: 50 mM Potassium acetate, 20 mM Tris-acetate, 10 mM magnesium acetate (pH 7.9).
DSB washing buffer: 10 mM Tris–HCl (pH 7.5), 1 mM EDTA (pH 8.0), and 2.0 M NaCl.
DSB elution buffer: 10 mM Tris–HCl (pH 7.5), 1 mM EDTA (pH 8.0), 1 M NaCl, and 2 M β-mercaptoethanol.
TAE buffer: 40 mM Tris (pH 7.6), 20 mM acetic acid, 1 mM EDTA (pH 8.0).
End repair buffer: 33 mM Tris-acetate (pH 7.0), 66 mM potassium acetate, 10 mM magnesium acetate, 0.5 mM DTT.
2.3. Equipment
Gel electrophoresis apparatus.
Spectrophotometer.
Thermoblock (a heating and cooling unit).
Sonicator.
Rocker.
E-Gel Precast Agarose Electrophoresis System (Invitrogen).
Genome sequencer.
2.4. Kits
QIAquick PCR Purification Kit (Qiagen).
Epicentre DNA END-Repair kit.
MinElute gel extraction kit (Qiagen).
3. Methods
3.1. Purification of High Molecular Weight DNA
Wash cells (1 × 108) twice with ice-cold PBS (see Note 1).
Lyse cells with 10 ml of lysis buffer (see Note 2).
Collect lysate by scraping and transfer the suspension in a 50 ml conical tube.
Digest the sample overnight with proteinase K (200 μg/ml) at 52 °C.
Purify DNA twice with phenol (1:1 v/v) and once with phenol-chloroform (1:1 v/v) (see Note 3).
Precipitate DNA with 2 volumes of ethanol 100% in the presence of 2 M ammonium acetate (see Note 4).
Centrifuge 4,400 × ℊ for 30 min. Remove the supernatant, add ethanol 70%, mix by inverting the sample, centrifuge 4,400 × ℊ for 25 min.
Air-dry the pellet (see Note 5).
Add to the pellet 500 μl of TE buffer and incubate it for 12 h at room temperature with gentle rotation (see Note 6).
Incubate the sample with 5 μg of pancreatic RNase for 1 h at 37°.
Adjust the sample to 0.5% SDS and incubate for 1 h at 55 °C with proteinase K (200 μg/ml).
Bring the volume to 10 ml with TE buffer and extract the DNA twice with phenol-chloroform (1:1 v/v) (see Note 5).
Precipitate the DNA with 2 volumes of ethanol 100% in the presence of 2 M ammonium acetate (see Note 6).
Air-dry the pellet (see Note 5).
Add to the pellet 1 ml of TE buffer and incubate it for 12 h at room temperature with gentle rotation.
Run 10 μl of DNA on a 0.6% agarose gel (see Note 7).
Determine DNA concentration with a spectrophotometer (see Note 8).
3.2. SSB-Seq
In a final volume of 1.5 ml, incubate with gentle mixing 500 μg of DNA for 40 s at 16 °C with a mixture of 200 μM of dATP, dGTP, dCTP and 20 μM of digoxigenin-11-dUTP, 117 μM of ddATP, ddGTP, ddCTP and 1000 units of E. coli DNA polymerase I. As a negative control for labeling, incubate 500 μg of DNA with the same reagents except digoxigenin-11-dUTP that is substituted with 20 μM of dTTP (see Note 9).
Stop the reaction with 50 μM EDTA.
Extract DNA with phenol-chloroform (1:1 v/v).
Precipitate DNA in the presence of 2 volumes of 100% ethanol and 2 M ammonium acetate.
Centrifuge 16,000 × ℊ for 30 min.
Air-dry pellet.
Add 1 ml of TE and mix by vortexing.
To help resuspension, incubate the sample at 45 °C for 15 min, mix by vortexing.
Precipitate DNA in the presence of 2 volumes of 100% ethanol and 2 M ammonium acetate.
Centrifuge 16,000 × ℊ for 30 min. Remove the supernatant. Add 70% ethanol. Centrifuge 16,000 × ℊ for 15 min (see Note 10). Remove the supernatant.
Air-dry pellet.
Add to the pellet 300 μl of TE buffer.
Shear the DNA by sonication to an average fragment size of 250 bp (see Note 11).
Incubate the sample with 10 μg of anti-digoxigenin antibody at 4 °C, overnight with gentle rotation.
To recover the immuno-complexes add 60 μl of Protein G-Sepharose beads (see Note 12) and incubate for 4 h at 4 °C.
Wash the beads once with PBS buffer, three times with NP-40 buffer; twice with TE buffer; and finally add to the beads 200 μl of TE buffer (see Note 13).
Adjust the sample to 0.5% SDS and digest with proteinase K (200 μg/ml) at 65 °C overnight.
Purify DNA using QIAquick PCR Purification Kit according to the manufacturer’s instructions and quantify the eluate (see Note 14).
3.3. DSB-Seq
Incubate 500 μg of DNA in 3 ml of TdT buffer with 24,000 U TdT, 0.5 mM dCTP and 5 mM CoCl2 at 37 °C for 5 min.
Add 0.02 mM Biotin-16-dUTP and incubate at 37 °C for 30 min. As a control of labeling, incubate 500 μg of DNA with the same reagents substituting TTP for Biotin-16-dUTP.
Stop the reaction by adding EDTA to a final concentration of 20 μM.
Extract the sample with phenol-chloroform (1:1 v/v).
Precipitate DNA in the presence of 2 volumes of 100% ethanol and 2 M ammonium acetate.
Centrifuge 16,000 × ℊ for 30 min.
Air-dry pellet.
Add 1 ml of TE and mix by vortexing.
To help resuspension, incubate the sample at 45 °C for 15 min, mix by vortexing.
Precipitate DNA in the presence of 2 volumes of 100% ethanol and 2 M ammonium acetate.
Centrifuge 16,000 × ℊ for 30 min. Remove the supernatant. Add ethanol 70%. Centrifuge 16,000 × ℊ for 15 min (see Note 15). Remove the supernatant.
Air-dry the pellet and dissolve it in 200 ml of TE buffer.
Sonicate the biotinylated DNA to generate 200–400 bp DNA fragments (see Note 11).
Capture the biotinylated fragments by mixing the DNA with 200 μl of streptavidin-coated beads (see Note 16).
Incubate for 3 h at room temperature with agitation.
Wash the beads four times with DSB washing buffer, incubating the sample at 50 °C (see Note 17).
Wash the beads four times with DSB washing buffer, incubating the sample at room temperature (see Note 18).
To disrupt biotin-streptavidin complexes incubate the sample in 200 μl DSB elution buffer at 75 °C for 4 h (see Note 19).
Purify free DNA fragments with a QIAquick PCR Purification Kit.
To remove the biotinylated tails from DNA, incubate the sample with 30 U of S-1 nuclease in 110 μl of recommended buffer for 30 min at 37 °C.
Purify DNA with a QIAquick PCR Purification Kit.
Quantify the recovered DNA.
3.4. Template Preparation for Sequencing Analysis
The DNA recovered from the immuno-precipitation/biotin-step-tavidin selection as well as 10 μl of the Input DNA—genomic DNA not subjected to immuno-precipitation—will be subjected to library preparation and sequencing (see Note 20).
To generate blunt-ended DNA, incubate the DNA for 45 min at room temperature in the 25 μl reaction with a mixture of End repair buffer, 0.25 mM of each dNTPs, 1 mM ATP, and 1 μl End-Repair Enzyme mix (T4 DNA polymerase + T4 PNK) (see Note 21).
Purify the DNA with a MinElute Reaction Cleanup Kit (see Note 22).
In the 25 μl reaction, treat the blunt-ended DNA with 15 units of Klenow(exo-) for 30 min at 37 °C in the presence of 0.2 mM dATP to generate a protruding 3’A base used for adaptor ligation.
Purify the DNA with a MinElute Reaction Cleanup Kit.
In the 20 μl reaction, ligate Illumina adapter to the end of DNA fragments by incubating with 0.1 μl Adaptor oligo mix and 1000 units of T4 DNA ligase at room temperature for 30 min.
Purify DNA using MinElute Reaction Cleanup Kit.
To size-select the adapter ligated DNA, run the sample through 2% E-Gel electrophoresis.
Excise the gel slice, around the 200–400 bp region.
Purify DNA from the gel using the MinElute gel extraction kit and elute in a final volume of 12 μl elution buffer.
Amplify the DNA for 18 cycles using Illumina primers (Fw: 5′-aca ctc ttt ccc tac acg acg c-3′/Rv: 5′-caa gca gaa gac ggc ata cga gc-3′) according to the following protocol: 98 °C for 30 s; 65 °C for 30 s; 72 °C for 30 s.
Run the PCR product through 2.5% agarose gel and excise the gel slice around 220 bps–500 bps.
Purify the DNA from the gel using MinElute gel extraction kit.
The purified DNA is used directly for cluster generation and sequencing analysis using the Illumina Genome Analyzer following the manufacturer’s protocols.
3.5. Processing of Sequencing Data
Process sequencing data from SSB-Seq and DSB-Seq protocols using Illumina Analysis Pipeline (image analysis and base calling).
Check quality of high-throughput sequencing data with the FastQC software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/).
Align short sequencing reads of length 36 bp to the reference human genome using the Bowtie 2 tool (version 2.2.2) with default parameters [14].
Remove redundant reads from the datasets, to minimize potential PCR bias, using Samtools package (http://www.htslib.org/doc/samtools.html).
Generate a read density visualization of the aligned sequencing data that can be viewed in most genome browsers (e.g., UCSC Genome Browser http://genome.ucsc.edu/cgi-bin/hgGateway). For wiggle track format (wig), extend the reads to the average length of the genomic fragments, count the number of reads at each position in the genome, and normalize the library size to 1 million reads [15]. For faster upload and display of wiggle file it can be compressed to bigWig format with the wigToBigWig tool (http://hgdownload.cse.ucsc.edu/admin/exe/).
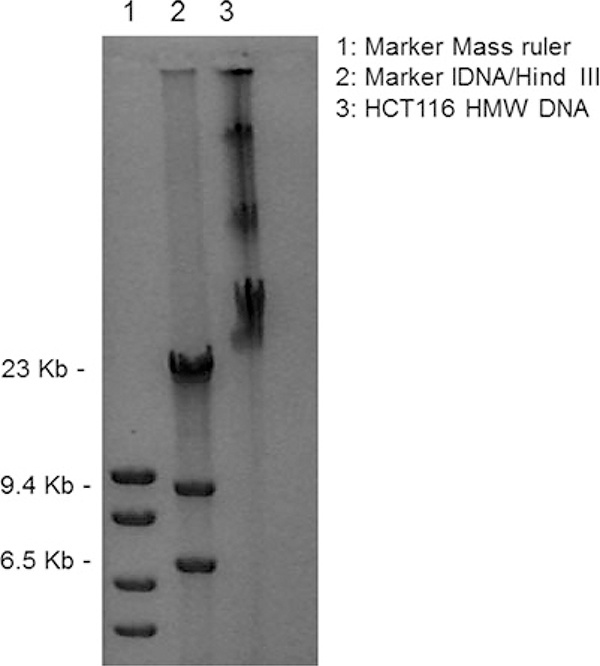
Fig. 3.
Representative example of High Molecular Weight (HMW) DNA after the purification steps described in Subheading 3.1. In lanes 1 and 2 we run two different markers. The numbers on the left refer to the molecular weight of the marker in lane 2
Footnotes
This protocol is designed for adherent cells. If suspension cells are used, centrifuge cells 140 × ℊ for 4 min, at 4 °C.
Lysis buffer should be kept at room temperature.
In general, during the steps preceding nick-translation DNA should be handled very gently. After the addition of phenol or phenol-chloroform mix the phases by inverting the tube for a few minutes. The aqueous phase should be purified from the organic phase with a 25 ml pipette, sucking the liquid very slowly. Do not vortex the sample.
Mix by gently inverting the sample for 3 min until a white puff forms.
The sample should not be completely dry, otherwise resuspension will be difficult.
Do not resuspend the pellet by pipetting.
This step is performed to check the quality of DNA, which should run around 23 kb or higher molecular weight. The sample is viscous; therefore, the tip should be cut before transferring the aliquot for gel electrophoresis. See Fig. 3 as a representative example.
1 × 108 HCT116 cells give approximately 1 mg of DNA. The 260/280 ratio should be around 1.8 and 1.9 and the 260/230 ratio between 2.0 and 2.2.
It is important to control the time of nick translation. Thus, it is suggested to incubate one sample at the time.
The double precipitation with ammonium acetate is necessary to remove free digoxigenin-11-dUTP.
Check the DNA fragment sizes by running a 1% agarose gel. In our procedure sonication was performed with an ultrasonic sonicator (Bioruptor, Diagenode) at medium power, by pulsing 30 times for 30 s and incubating on ice for 30 s between each pulse.
To prepare the beads, mix the slurry, take 60 μl of beads per immuno-precipitation, wash the beads three times with ice-cold PBS buffer. Perform each wash by adding 900 μl of ice-cold PBS buffer. Incubate the beads for 10 min by rocking at 4 °C. Centrifuge the sample 4 min at 1500 × ℊ, 4 °C. Remove the supernatant.
Perform each wash by adding 900 μl of ice-cold washing buffer. Incubate the immuno-complex for 10 min by rocking, centrifuge the sample for 4 min at 1500 × ℊ, 4 °C. Remove the supernatant.
This protocol is suitable for mapping SSBs with a free 3′ end. SSBs generated during topoisomerase 1 catalytic cycle will be labeled only after the treatment of DNA with tyrosyl-DNA phosphodiesterase 1 (TDP1) [16].
The double precipitation with ammonium acetate is necessary to remove free Biotin-16-dUTP.
Available from Invitrogen (Dynabeads kilobase BINDER Kit, Dynal). To prepare the beads, use the magnet to separate beads from the supernatant. Add to the beads 200 μl of binding buffer (provided with the beads). Mix for 5 min. Remove the supernatant. Repeat the wash.
To perform each wash, add 900 μl of washing solution. Incubate at 50° for 5 min with agitation. Use the magnet to separate the beads from the washing buffer. Add a new washing solution.
To perform each wash, add 900 μl of the washing solution. Incubate at room temperature for 5 min, with agitation. Use the magnet to separate the beads from the washing buffer. Add a new washing solution.
For a better yield, add 100 μl of DSB elution buffer, incubate at 75 °C for 2 h, use the magnet to separate the supernatant from the beads. Keep the supernatant. Add to the beads 100 μl of new DSB elution buffer, incubate at 75 °C for 2 h, use the magnet to separate the supernatant from the beads. Pool the supernatants together.
The Input DNA is sonicated.
The Epicentre DNA END-Repair kit is available at Epicentre Biotechnologies.
This kit is available at QIAGEN.
References
- 1.Iacovoni JS et al. (2010) High-resolution profiling of gammaH2AX around DNA double strand breaks in the mammalian genome. EMBO J 29(8):1446–1457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blitzblau HG, Hochwagen A (2011) Genome-wide detection of meiotic DNA double-strand break hotspots using single-stranded DNA. Methods Mol Biol 745:47–63 [DOI] [PubMed] [Google Scholar]
- 3.Hu J et al. (2016) Detecting DNA double-stranded breaks in mammalian genomes by linear amplification-mediated high-throughput genome-wide translocation sequencing. Nat Protoc 11(5):853–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Klein IA et al. (2011) Translocation-capture sequencing reveals the extent and nature of chromosomal rearrangements in B lymphocytes. Cell 147(1):95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chiarle R et al. (2011) Genome-wide translocation sequencing reveals mechanisms of chromosome breaks and rearrangements in B cells. Cell 147(1):107–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tsai SQ et al. (2015) GUIDE-seq enables genome-wide profiling of off-target cleavage by CRISPR-Cas nucleases. Nat Biotechnol 33 (2):187–197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang XL et al. (2015) Unbiased detection of off-target cleavage by CRISPR-Cas9 and TALENs using integrase-defective lentiviral vectors. Nat Biotechnol 33(2):175–178 [DOI] [PubMed] [Google Scholar]
- 8.Crosetto N et al. (2013) Nucleotide-resolution DNA double-strand break mapping by next-generation sequencing. Nat Methods 10 (4):361–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Canela A et al. (2016) DNA breaks and end resection measured genome-wide by end sequencing. Mol Cell 63(5):898–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aguilera A, Garcia-Muse T (2013) Causes of genome instability. Annu Rev Genet 47:1–32 [DOI] [PubMed] [Google Scholar]
- 11.Baranello L et al. (2014) DNA break mapping reveals topoisomerase II activity genome-wide. Int J Mol Sci 15(7):13111–13122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rigby PW et al. (1977) Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol 113(1):237–251 [DOI] [PubMed] [Google Scholar]
- 13.Kouzine F et al. (2013) Global regulation of promoter melting in naive lymphocytes. Cell 153(5):988–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Langmead B, Salzberg SL (2012) Fast gapped-read alignment with bowtie 2. Nat Methods 9 (4):357–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bardet AF et al. (2012) A computational pipeline for comparative ChIP-seq analyses. Nat Protoc 7(1):45–61 [DOI] [PubMed] [Google Scholar]
- 16.Caldecott KW (2008) Single-strand break repair and genetic disease. Nat Rev Genet 9 (8):619–631 [DOI] [PubMed] [Google Scholar]
- 17.Barnes DE, Lindahl T (2004) Repair and genetic consequences of endogenous DNA base damage in mammalian cells. Annu Rev Genet 38:445–476 [DOI] [PubMed] [Google Scholar]
- 18.Sartori AA et al. (2007) Human CtIP promotes DNA end resection. Nature 450(7169):509–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Symington LS, Gautier J (2011) Double-strand break end resection and repair pathway choice. Annu Rev Genet 45:247–271 [DOI] [PubMed] [Google Scholar]