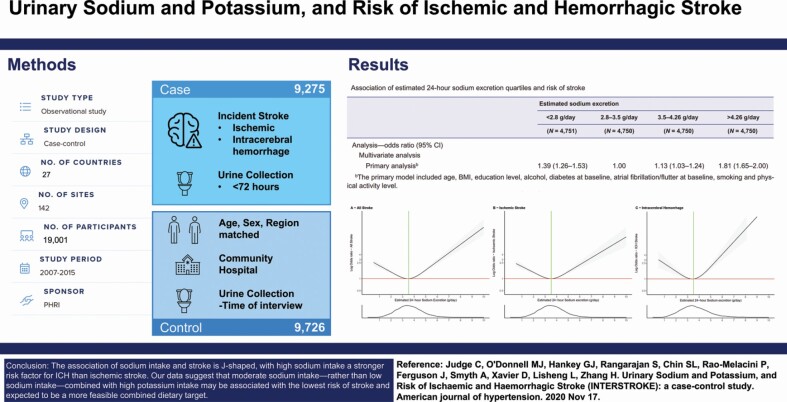
Abstract
BACKGROUND
Although low sodium intake (<2 g/day) and high potassium intake (>3.5 g/day) are proposed as public health interventions to reduce stroke risk, there is uncertainty about the benefit and feasibility of this combined recommendation on prevention of stroke.
METHODS
We obtained random urine samples from 9,275 cases of acute first stroke and 9,726 matched controls from 27 countries and estimated the 24-hour sodium and potassium excretion, a surrogate for intake, using the Tanaka formula. Using multivariable conditional logistic regression, we determined the associations of estimated 24-hour urinary sodium and potassium excretion with stroke and its subtypes.
RESULTS
Compared with an estimated urinary sodium excretion of 2.8–3.5 g/day (reference), higher (>4.26 g/day) (odds ratio [OR] 1.81; 95% confidence interval [CI], 1.65–2.00) and lower (<2.8 g/day) sodium excretion (OR 1.39; 95% CI, 1.26–1.53) were significantly associated with increased risk of stroke. The stroke risk associated with the highest quartile of sodium intake (sodium excretion >4.26 g/day) was significantly greater (P < 0.001) for intracerebral hemorrhage (ICH) (OR 2.38; 95% CI, 1.93–2.92) than for ischemic stroke (OR 1.67; 95% CI, 1.50–1.87). Urinary potassium was inversely and linearly associated with risk of stroke, and stronger for ischemic stroke than ICH (P = 0.026). In an analysis of combined sodium and potassium excretion, the combination of high potassium intake (>1.58 g/day) and moderate sodium intake (2.8–3.5 g/day) was associated with the lowest risk of stroke.
CONCLUSIONS
The association of sodium intake and stroke is J-shaped, with high sodium intake a stronger risk factor for ICH than ischemic stroke. Our data suggest that moderate sodium intake—rather than low sodium intake—combined with high potassium intake may be associated with the lowest risk of stroke and expected to be a more feasible combined dietary target.
Keywords: blood pressure, hypertension, intracerebral hemorrhage, ischemic stroke, potassium, sodium, stroke
Graphical Abstract
Graphical Abstract.
Hypertension is the key modifiable risk factor for stroke and increasing sodium intake is positively associated with blood pressure.1,2 Reduction of sodium intake, to low intake levels of 2 g/day or lower, has been proposed to be an effective population-level intervention to reduce blood pressure, and inferentially, reduce the burden of stroke.3–5 However, despite the modest positive association between sodium intake and blood pressure,6 the pattern of association of sodium intake with cardiovascular disease is consistently J-shaped in a number of large epidemiologic studies,7–9 despite using different methods to estimate sodium intake (24-hour urine collection, early morning fasting samples, or random nonfasting urine samples). For stroke, individual studies report an inconsistent relationship between sodium intake and stroke, with different epidemiologic studies reporting a linear association, a curvilinear relationship, or J-shaped association.10 In addition, the association of high sodium intake with stroke persists in most observational studies, after adjusting for blood pressure, suggesting mechanisms other than blood pressure may also mediate the increased cardiovascular risk.7
Considerable public health efforts and resources are being invested in targeting low sodium intake (<2 g/day),11 although there is controversy about whether low sodium intake represents the optimal target for cardiovascular prevention. Moreover, the feasibility of a combined target of low sodium and high potassium intake is challenged because only a very small proportion of the population consume this joint electrolyte target12,13; sodium and potassium intake usually correlate positively with each other indicating that targeting low sodium intake is more likely to be associated with reductions in potassium intake among free-living individuals, and vice versa.14 Increased potassium intake appears to be an important target for stroke prevention, with meta-analyses reporting a linear reduction in stroke risk associated with increased potassium intake.15 Studies also suggest that the adverse cardiovascular effects of high sodium intake may be mitigated with high potassium intake.12,16,17 Therefore, evaluating the association of sodium intake with stroke necessarily requires a combined analysis of the relationship of both electrolytes with stroke risk overall, and within stroke subtypes.17
INTERSTROKE was a standardized international case–control study that included participants from 30 countries.1 The unique aspects of this observational study are the breadth of the international population included, the standardized measurement of vascular risk factors (including diet) and the valid determination of primary stroke subtype (ischemic or intracerebral hemorrhage [ICH]) using neuroimaging.
In this paper, we report the individual, and joint, associations of estimated sodium and potassium excretion (surrogates for intake) with stroke and its subtypes.
METHODS
Study design and participants
INTERSTROKE is a large, international case–control study of risk factors for first stroke. 13,462 stroke patients and 13,483 matched controls were recruited between 11 January 2007 and 8 August 2015. For the current analyses, we include 9,275 cases and 9,726 controls with urinary measures of sodium and potassium (8,761 matched pairs for conditional analysis). Each case was matched for sex and age (±5 years) with controls (Supplementary Table S1 online). Cases were patients with first acute stroke, either ischemic or ICH, with confirmation by computed tomography or magnetic resonance imaging brain imaging. Patients with stroke were enrolled within 5 days of symptom onset and within 72 hours of hospital admission. Stroke severity was measured using the modified Rankin scale at the time of recruitment and at 1-month follow-up. The study was approved by the ethics committees in all participating centers. Written informed consent was obtained from participants or their proxy.
Measurement of risk factors
Standardized questionnaires were used to collect data on demographics, lifestyle stroke risk factors, and characteristics of acute stroke from all cases and controls (Supplementary Table S2 online). Physical measurements of weight, height, waist and hip circumferences, heart rate, and blood pressure were recorded in a standardized manner. In cases, blood pressure and heart rate were measured at 3 time-points: at admission, the next morning, and at the time of interview. A modified Rankin scale score was collected at 3 time-points for cases: preadmission, time of interview and at 1-month follow-up (either in person or by phone), and 1 time-point for controls (time of interview). Ischemic stroke subtype was based on clinical assessment (baseline and 1-month), neuroimaging (baseline), and results of tests to determine etiology (ultrasound of carotids, cardiac imaging, and cardiac monitoring). Hypertension was defined as a composite of self-reported hypertension and a blood pressure reading of greater than 140/90 mm Hg at recruitment. Diabetes mellitus was defined as self-reported diabetes or a HbA1c of greater than 6.5% at recruitment.
Blood and urine collection and analysis
Nonfasting blood and urine samples were taken from cases within 72 hours of recruitment and controls (at the time of interview), frozen at −20° to −70° and shipped to core laboratories (Hamilton-Canada, Beijing-China, Bangalore-India, and Istanbul-Turkey). Several formulae exist for estimation of 24-hour sodium and potassium excretion from spot urinary sodium/potassium measurements.18–20 These formulae have been validated against 24-hour urine collections21 and serve as a valid measure of mean population sodium and potassium intake.22 The Tanaka formula was used to estimate 24-hour urine sodium and potassium excretion18 and is reported to be associated with the least biased estimate for casual (nonfasting) urine samples in an international population.21
Statistical analysis
We calculated the correlation between urinary sodium and potassium excretion using Pearson’s correlation coefficient, and of sodium and potassium excretion with blood pressure using an intraclass correlation coefficient in controls (excluding those with known hypertension or taking diuretics). We used multivariable conditional logistic regression to evaluate the association of sodium and potassium excretion with stroke, employing restricted cubic-spline plots to explore the pattern of association.23 For analysis of categories of estimated sodium excretion, we set the reference group as the second quartile (2.8–3.5 g/day), as this was identified as the lowest risk category on initial univariate analyses, and consistent with the range of lowest risk on cubic splines. Similarly, we set the first quartile (<1.34 g/day) as the reference group for estimated potassium excretion.
We adjusted for covariates in 4 sequential models. Model 1 was adjusted for age and body mass index. Model 2 (the primary model) was additionally adjusted for education level (none-reference, 1–8 years, 9–12 years, Trade School, College/University), alcohol intake (never-reference, former, current), diabetes, atrial fibrillation or flutter, smoking (never-reference, former, current), and physical activity level (strenuous-reference, moderate, mild, mainly sedentary). Model 3 included all the variables in model 2 and added, estimated excretion of potassium (Tanaka) and the alternative healthy eating index dietary score as an overall measure of diet quality. Model 4 included hypertension status, mean systolic blood pressure, mean diastolic blood pressure, and medications which modify sodium excretion, which was a model to explore variables potentially along the causal pathway mediating the association of sodium and potassium intake with stroke. Model 4 was reproduced separately with the 3 components of the mean blood pressure variable: time of admission, the morning after admission, and during the interview. We examined the consistency of these associations by performing analyses in subgroups using our primary model (conditional analysis) based on key characteristics that might modify the association between sodium, potassium, and stroke (ethnicity, body mass index, sex, age, hypertension, and diuretic therapy), using the Wald test to assess statistical interactions. We excluded small subgroups (<500 participants).
We performed an analysis of the combined effects of sodium and potassium excretion, in which we generated 8 categories (4 × 2) by sodium excretion quartile (<2.8, 2.8–3.5, 3.5–4.26, and >4.26 g/day) and potassium excretion above and below median (1.58 g/day). We completed a sensitivity analysis in which we excluded patients (cases) with a modified Rankin score greater than 2, as such patients may more likely not consume their usual diets and may receive cointerventions (e.g., intravenous fluids and enteric feeding due to their disability). Given that time from hospital admission to sample collection may also affect the classification of intake categories, we completed an analysis that excluded participants with samples collected greater than 48 hours after admission. All analyses were performed using R version 3.5.3 (Great Truth).
Role of the funding source
The study funders had no role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication.
RESULTS
INTERSTROKE participants
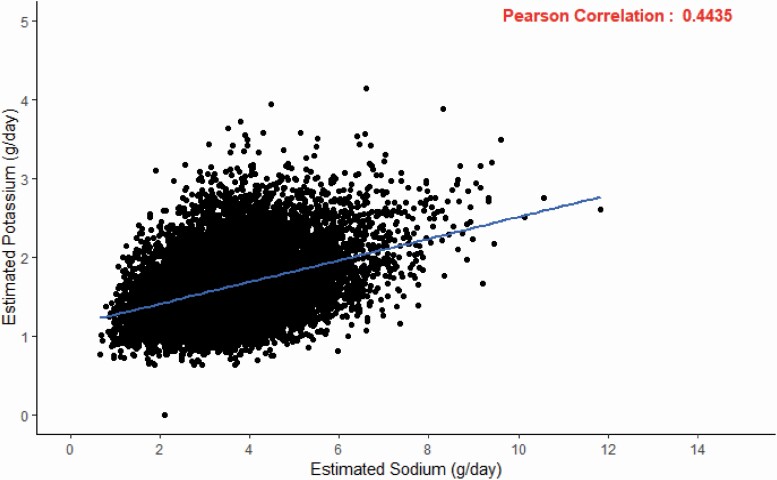
Between 11 January 2007 and 8 August 2015, the INTERTSROKE study enrolled 9,275 cases of acute first stroke and 9,726 matched controls (8,761 matched pairs for conditional analysis) who also had urinary samples collected. Table 1 and Supplementary Table S3 online (sodium) and Supplementary Table S4 online (potassium) outline the characteristics of patients including comorbidities, stroke type, stroke severity, and blood pressure by quartiles of sodium and potassium excretion. The mean time between stroke onset and collection of urine sample was 2.08 ± 1.27 days and the mean time between hospitalization and collection of urine sample was 1.51 ± 1.04 days. Figure 1 reports the scatterplot and a statistically significant correlation (R = 0.4435, P < 0.0001) between estimated urinary sodium and potassium excretion for cases and controls in the INTERSTROKE population. The mean 24-hour sodium excretion per day were 3.69 g for cases and 3.54 g for control (P < 0.001) (Table 1). The mean 24-hour potassium excretion per day were 1.57 g for cases and 1.68 g for controls (P < 0.001) (Supplementary Table S4 online).
Table 1.
Characteristics of the study participants at baseline, according to estimated sodium excretion (conditional analysis)
| Characteristic | Case | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Estimated sodium excretion | ||||||||||
| All | <2.8 g/day | 2.8–3.5 g/day | 3.5–4.3 g/day | >4.3 g/day | All | <2.8 g/day | 2.8–3.5 g/day | 3.5–4.3 g/day | >4.3 g/day | |
| (N = 8,761) | (N = 2,214) | (N = 1,911) | (N = 2,065) | (N = 2,571) | (N = 8,767) | (N = 2,075) | (N = 2,481) | (N = 2,352) | (N = 1,859) | |
| Estimated excretion, g/day | ||||||||||
| Sodium | 3.69 ± 1.28 | 2.23 ± 0.44 | 3.19 ± 0.20 | 3.88 ± 0.21 | 5.18 ± 1.02 | 3.54 ± 1.04 | 2.32 ± 0.41 | 3.19 ± 0.20 | 3.88 ± 0.21 | 4.93 ± 0.93 |
| Potassium | 1.58 ± 0.38 | 1.43 ± 0.30 | 1.49 ± 0.33 | 1.56 ± 0.34 | 1.78 ± 0.42 | 1.68 ± 0.42 | 1.48 ± 0.35 | 1.62 ± 0.39 | 1.75 ± 0.40 | 1.88 ± 0.44 |
| Age, year | 62.9 ± 13.4 | 63.9 ± 13.7 | 63.0 ± 13.4 | 62.5 ± 13.2 | 62.3 ± 13.2 | 62.1 ± 13.2 | 63.7 ± 13.5 | 62.0 ± 13.2 | 61.3 ± 12.9 | 61.5 ± 13.0 |
| Female sex, no. (%) | 3,574 (40.8) | 959 (26.8) | 770 (21.5) | 797 (22.3) | 1,048 (29.3) | 3,580 (40.8) | 925 (25.8) | 978 (27.3) | 929 (25.9) | 748 (20.9) |
| Geographic region, no. (%) | ||||||||||
| Western Europe/North America | 1,544 (17.6) | 567 (36.7) | 394 (25.5) | 302 (19.6) | 281 (18.2) | 1,544 (17.6) | 381 (24.7) | 439 (28.4) | 447 (29.0) | 277 (17.9) |
| Eastern/Central Europe/ Middle East | 1,079 (12.3) | 206 (19.1) | 213 (19.7) | 290 (26.9) | 370 (34.3) | 1,079 (12.3) | 211 (19.6) | 287 (26.6) | 322 (29.8) | 259 (24.0) |
| Africa | 587 (6.70) | 278 (47.4) | 126 (21.5) | 90 (15.3) | 93 (15.8) | 587 (6.70) | 213 (36.3) | 192 (32.7) | 118 (20.1) | 64 (10.9) |
| China | 3,891 (44.4) | 728 (18.7) | 836 (21.5) | 1,053 (27.1) | 1,274 (32.7) | 3,891 (44.4) | 832 (21.4) | 1,089 (28.0) | 1,051 (27.0) | 919 (23.6) |
| South East Asia | 615 (7.02) | 155 (25.2) | 123 (20.0) | 125 (20.3) | 212 (34.5) | 615 (7.01) | 204 (33.2) | 214 (34.8) | 126 (20.5) | 71 (11.5) |
| South America | 1,045 (11.9) | 280 (26.8) | 219 (21.0) | 205 (19.6) | 341 (32.6) | 1,051 (12.0) | 234 (22.3) | 260 (24.7) | 288 (27.4) | 269 (25.6) |
| Stroke type, no. (%) | ||||||||||
| Ischemic | 6,805 (77.7) | 1,710 (77.4) | 1,537 (81.0) | 1,646 (80.0) | 1,912 (74.7) | — | — | — | — | — |
| ICH | 1,919 (21.9) | 499 (22.6) | 361 (19.0) | 411 (20.0) | 648 (25.3) | — | — | — | — | — |
| Hypertension, no. (%) | 5,243 (59.8) | 1,363 (61.6) | 1,134 (59.3) | 1,192 (57.7) | 1,554 (60.4) | 3,299 (37.6) | 974 (46.9) | 998 (40.2) | 988 (42.0) | 856 (46.0) |
| Blood pressure, mm Hg | ||||||||||
| Systolic | 148 ± 21.2 | 149 ± 22.0 | 147 ± 20.8 | 148 ± 20.5 | 149 ± 21.3 | 133 ± 18.5 | 133 ± 19.1 | 133 ± 18.3 | 133 ± 17.8 | 134 ± 18.7 |
| Diastolic | 86.5 ± 12.3 | 87.0 ± 13.2 | 85.4 ± 12.1 | 86.5 ± 11.8 | 86.9 ± 12.0 | 80.1 ± 10.7 | 79.3 ± 11.2 | 79.8 ± 10.5 | 80.3 ± 10.3 | 81.1 ± 10.6 |
| Cholesterol, mmol/l | ||||||||||
| HDL | 1.15 ± 0.35 | 1.18 ± 0.37 | 1.16 ± 0.37 | 1.14 ± 0.34 | 1.12 ± 0.33 | 1.22 ± 0.37 | 1.22 ± 0.38 | 1.23 ± 0.38 | 1.21 ± 0.35 | 1.20 ± 0.37 |
| LDL | 2.97 ± 1.01 | 3.03 ± 1.08 | 2.96 ± 0.98 | 2.99 ± 0.99 | 2.91 ± 0.98 | 2.98 ± 0.96 | 2.98 ± 1.01 | 3.04 ± 0.97 | 2.97 ± 0.94 | 2.90 ± 0.92 |
| Diabetes mellitus, no. (%) | 1,486 (17.0) | 355 (16.0) | 315 (16.5) | 359 (17.4) | 457 (17.8) | 1,108 (12.6) | 283 (13.6) | 304 (12.3) | 303 (12.9) | 218 (11.7) |
| AFIB/atrial flutter, no. (%) | 936 (10.7) | 301 (13.6) | 210 (11.0) | 170 (8.23) | 255 (9.92) | 270 (3.08) | 74 (3.57) | 69 (2.78) | 61 (2.59) | 66 (3.55) |
| Diuretic preadmission, no. (%) | 1,132 (12.9) | 298 (13.5) | 260 (13.6) | 229 (11.1) | 345 (13.4) | 782 (8.92) | 194 (9.35) | 185 (7.46) | 204 (8.68) | 199 (10.7) |
| Diuretic in hospital, no. (%) | 1,994 (22.8) | 537 (24.3) | 418 (21.9) | 441 (21.4) | 598 (23.3) | 352 (12.8) | 73 (11.3) | 80 (10.2) | 95 (13.7) | 104 (16.3) |
| Current smoker, no. (%) | 2,623 (29.9) | 610 (27.6) | 591 (30.9) | 666 (32.3) | 756 (29.4) | 1,850 (21.1) | 429 (20.7) | 522 (21.0) | 510 (21.7) | 389 (20.9) |
Abbreviations: AFIB, atrial fibrillation; HDL, high-density lipoprotein; ICH, intracerebral hemorrhage; LDL, low-density lipoprotein.
Figure 1.
Scatterplot of estimated urinary sodium and potassium excretion.
Estimated sodium excretion (quartiles) and blood pressure
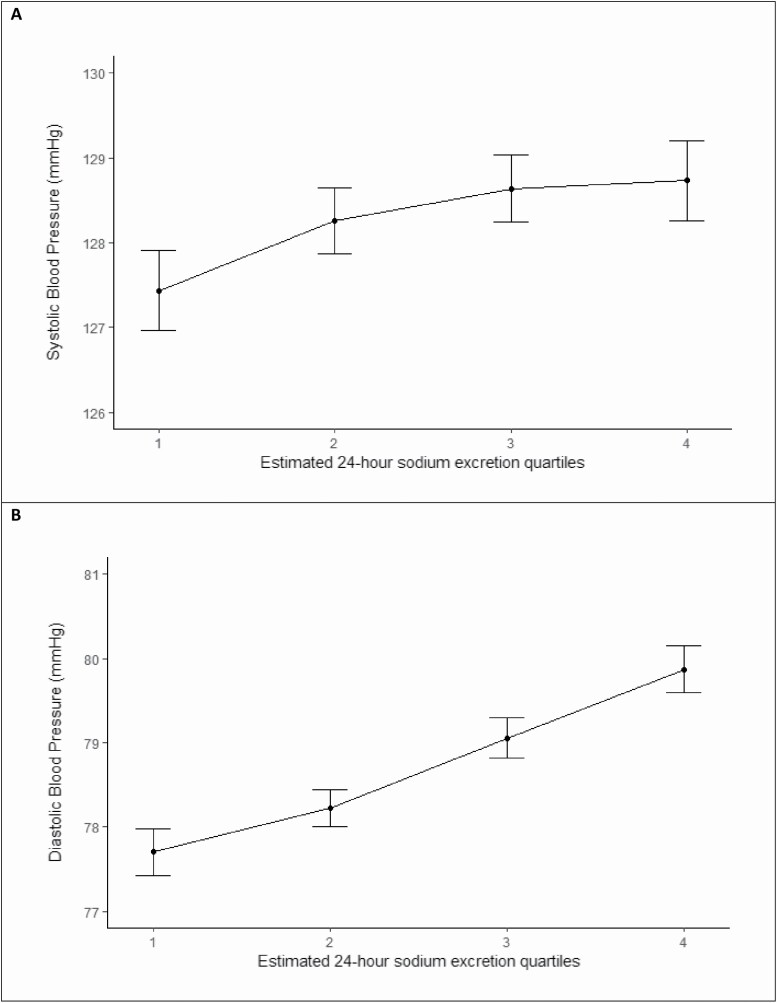
Figure 2 reports the association of urinary sodium excretion and blood pressure among controls not receiving antihypertensive therapy or diuretics and indicates a graded increase in blood pressure with increasing sodium intake. For each 1-g increment in estimated sodium excretion, there was an increment of 1.01 mm Hg in systolic blood pressure (P < 0.001) and an increment of 0.48 mm Hg in diastolic blood pressure (P < 0.001).
Figure 2.
Mean systolic and diastolic blood pressure by sodium quartile (controls excluding baseline hypertension and prehospital diuretic use).
Estimated sodium excretion (quartiles) and risk of stroke and stroke subtypes
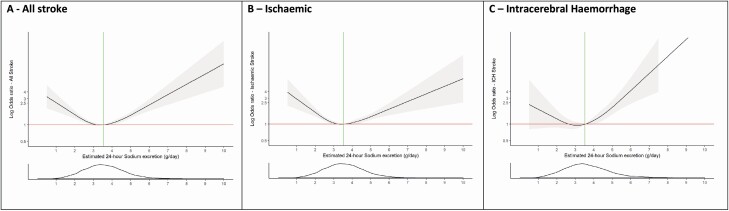
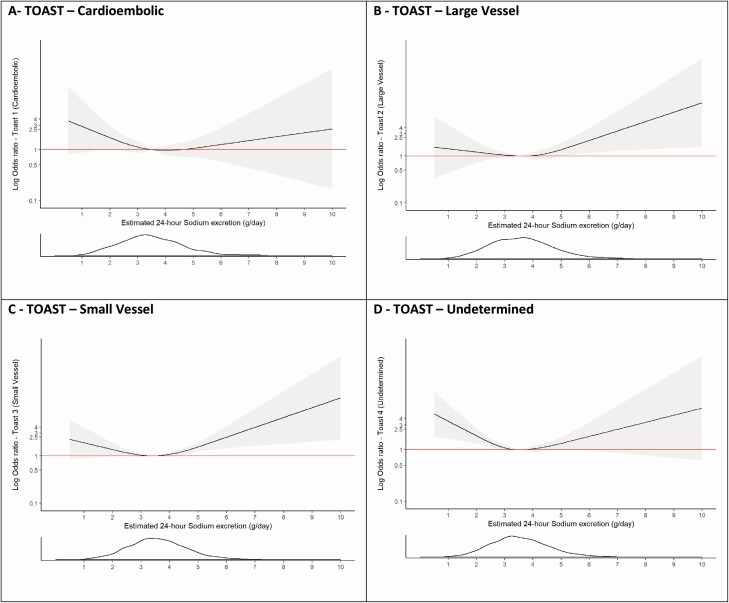
Compared with Q2 (sodium excretion of 2.8–3.5 g/day [reference]), Q1 (odds ratio [OR] 1.39; 95% confidence interval [CI], 1.26–1.53, sodium excretion <2.8 g/day), and Q4 (OR 1.81; 95% CI, 1.65–2.00, sodium excretion >4.26 g/day) were associated with significant increases in the risk of all stroke (Table 2). The highest quartile (Q4 >4.26 g/day) was more strongly associated with ICH (OR 2.38; 95% CI, 1.93–2.92) than ischemic stroke (OR 1.67; 95% CI, 1.50–1.87) (P < 0.001). Sodium excretion <2.8 g/day was significantly associated with both ischemic stroke (OR 1.36; 95% CI, 1.22–1.52) and ICH (OR 1.62; 95% CI, 1.32–1.99) (Figure 3 and Supplementary Tables S5 and S6 online). The association of high sodium excretion (>4.26 g/day) and stroke remained significant after adjustment for blood pressure and prior history of hypertension (OR 2.35; 95% CI, 2.08–2.65). Within ischemic stroke subtypes, the association of high sodium intake was significant for small vessel and large vessel ischemic stroke, but not significant for cardioembolic stroke (Figure 4).
Table 2.
Association of estimated 24-hour sodium excretion quartiles and risk of stroke
| Estimated sodium excretion | ||||
|---|---|---|---|---|
| <2.8 g/day | 2.8–3.5 g/day | 3.5–4.26 g/day | >4.26 g/day | |
| (N = 4,751) | (N = 4,750) | (N = 4,750) | (N = 4,750) | |
| Analysis—odds ratio (95% CI) | ||||
| Univariate analysisa | 1.40 (1.28–1.52) | 1.00 | 1.15 (1.06–1.25) | 1.84 (1.69–2.01) |
| Multivariate analysis | ||||
| Analysis including age and BMI | 1.41 (1.29–1.54) | 1.00 | 1.14 (1.05–1.25) | 1.86 (1.70–2.03) |
| Primary analysisb | 1.39 (1.26–1.53) | 1.00 | 1.13 (1.03–1.24) | 1.81 (1.65–2.00) |
| Analysis including dietary score and potassiumc | 1.24 (1.12–1.37) | 1.00 | 1.28 (1.16–1.41) | 2.49 (2.24–2.77) |
| Analysis including HTN and medications which modify sodium excretiond | 1.16 (1.03–1.30) | 1.00 | 1.22 (1.09–1.36) | 2.35 (2.08–2.65) |
| Sensitivity analysis | ||||
| Primary analysis excluding MRC >2 | 1.37 (1.18–1.58) | 1.00 | 1.08 (0.95–1.23) | 1.64 (1.42–1.88) |
| Primary analysis excluding urine collection >48 hours | 1.28 (1.12–1.47) | 1.00 | 1.18 (1.03–1.34) | 1.91 (1.67–2.18) |
Urine collection from time of stroke onset to time of urine collection. Abbreviations: ACE, angiotensin-converting enzyme inhibitors (ACE inhibitors); BMI, body mass index; CI, confidence interval; HTN, hypertension; MRC, modified Rankin scale.
aThe univariate analysis was performed using the logistic regression model.
bThe primary model included age, BMI, education level, alcohol, diabetes at baseline, atrial fibrillation/flutter at baseline, smoking and physical activity level.
cDietary score was the alternative healthy eating index (AHEI).
dHypertension variables hypertension status, systolic blood pressure, and diastolic blood pressure. We adjusted for prehospital ACE inhibitor, angiotensin receptor blocker, and diuretic use.
Figure 3.
Association of estimated 24-hour sodium excretion (Tanaka) with risk of stroke and pathological stroke subtypes. Panel a shows a restricted cubic spline of the association between estimated 24-hour sodium excretion and risk of all stroke. Panel b shows a restricted cubic spline of the association between estimated 24-hour sodium excretion and risk of ischemic stroke. Panel c shows a restricted cubic spline of the association between estimated 24-hour sodium excretion and risk of intracerebral hemorrhage. All plots were adjusted for age, BMI, education level, alcohol intake, diabetes at baseline, atrial fibrillation/flutter at baseline, smoking, and physical activity level. The gray ribbons indicate 95% confidence interval. The green lines represent the median value for each population. The distribution of the exposure (sodium excretion) is plotted below each spline. Abbreviation: BMI, body mass index.
Figure 4.
Association of estimated sodium excretion (Tanaka) and risk of ischemic stroke subtypes (TOAST classification). Panel a shows a restricted cubic spline of the association between estimated 24-hour sodium excretion and cardioembolic stroke (TOAST 1). Panel b shows a restricted cubic spline of the association between estimated 24-hour sodium excretion and large vessel stroke (TOAST 2). Panel c shows a restricted cubic spline of the association between estimated 24-hour sodium excretion and small vessel stroke (TOAST 3). Panel d shows a restricted cubic spline of the association between estimated 24-hour sodium excretion and stroke of undetermined cause (TOAST 4). All plots were adjusted for age, BMI, education level, alcohol intake, diabetes at baseline, atrial fibrillation/flutter at baseline, smoking, and physical activity level. The gray ribbons indicate 95% confidence interval. The distribution of the exposure (potassium excretion) is plotted below each spline. Abbreviation: BMI, body mass index.
Estimated potassium excretion and risk of stroke and stroke subtypes
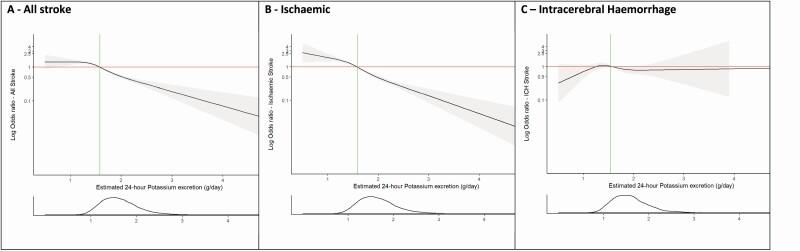
Compared with Q1 (potassium excretion of <1.34 g/day [reference]), Q2 (OR 0.83; 95% CI, 0.76–0.92, potassium excretion 1.34–1.58 g/day), Q3 (OR 0.68; 95% CI, 0.62–0.75, potassium excretion 1.58–1.86 g/day), and Q4 (OR 0.46; 95% CI, 0.41–0.51, potassium excretion >1.86 g/day) were all associated with a significant lower risk of all stroke, which was largely related to the association of potassium excretion with ischemic stroke and there was no significant association with ICH (Table 3, Figure 5).
Table 3.
Association of estimated 24-hour potassium excretion quartiles and risk of stroke
| Estimated potassium excretion quartiles | ||||
|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
| <1.34 g/day | 1.34–1.58 g/day | 1.58–1.86 g/day | >1.86 g/day | |
| (N = 4,817) | (N = 4,816) | (N = 4,817) | (N = 4,816) | |
| Analysis—odds ratio (95% CI) | ||||
| Univariate analysisa | 1.00 | 0.80 (0.73–0.87) | 0.67 (0.61–0.73) | 0.43 (0.39–0.47) |
| Multivariate analysis | ||||
| Analysis including age and BMI | 1.00 | 0.80 (0.73–0.88) | 0.64 (0.59–0.71) | 0.42 (0.38–0.46) |
| Primary analysisb | 1.00 | 0.83 (0.76–0.92) | 0.68 (0.62–0.75) | 0.46 (0.41–0.51) |
| Analysis including dietary score and sodiumc | 1.00 | 0.75 (0.68–0.83) | 0.56 (0.51–0.63) | 0.33 (0.29–0.37) |
| Analysis including HTN and medications which modify potassium excretiond | 1.00 | 0.76 (0.68–0.86) | 0.57 (0.51–0.65) | 0.33 (0.29–0.38) |
| Sensitivity analysis | ||||
| Primary analysis excluding MRC >2 | 1.00 | 0.70 (0.60–0.81) | 0.48 (0.41–0.56) | 0.24 (0.20–0.28) |
| Primary analysis excluding urine collection >48 hours | 1.00 | 0.87 (0.76–0.99) | 0.78 (0.68–0.89) | 0.67 (0.58–0.77) |
Urine collection from time of stroke onset to time of urine collection. Abbreviations: ACE, angiotensin-converting enzyme inhibitors (ACE inhibitors); BMI, body mass index; CI, confidence interval; HTN, hypertension; MRC, modified Rankin scale.
aThe univariate analysis was performed using the logistic regression model.
bThe primary model included age, BMI, education level, alcohol, diabetes at baseline, atrial fibrillation/flutter at baseline, smoking, and physical activity level.
cDietary score was the alternative healthy eating index (AHEI).
dHypertension variables hypertension status, systolic blood pressure, and diastolic blood pressure. We adjusted for prehospital ACE inhibitor, angiotensin receptor blocker, and diuretic use.
Figure 5.
Association of estimated 24-hour potassium excretion (Tanaka) with risk of stroke and pathological stroke subtypes. Panel a shows a restricted cubic spline of the association between estimated 24-hour potassium excretion and risk of all stroke. Panel b shows a restricted cubic spline of the association between estimated 24-hour potassium excretion and risk of ischemic stroke. Panel c shows a restricted cubic spline of the association between estimated 24-hour potassium excretion and risk of intracerebral hemorrhage. All plots were adjusted for age, BMI, education level, alcohol intake, diabetes at baseline, atrial fibrillation/flutter at baseline, smoking, and physical activity level. The gray ribbons indicate 95% confidence interval. The green lines represent the median value for each population. The distribution of the exposure (sodium excretion) is plotted below each spline. Abbreviation: BMI, body mass index.
Joint urinary sodium and potassium excretion and risk of stroke
For all stroke, compared with a joint reference category of moderate sodium excretion (2.8–3.5 g/day) and high potassium intake (≥1.58 g/day) (lowest risk category), all other categories were associated with an increased risk of stroke, with sodium excretion >4.26 g/day and potassium excretion <1.58 g/day reporting the highest magnitude of risk (OR 4.17; 95% CI, 3.51–4.96) (Table 4, Supplementary Figures S1 and S2 online). The magnitude of association is reduced for both low (<2.8 g/day) and high (>4.26 g/day) sodium excretion when potassium excretion is high (≥1.58 g/day) (P < 0.001 for interaction) (Table 4).
Table 4.
Association of joint urinary sodium and potassium excretion with stroke
| Joint association of urinary sodium and potassium excretion with stroke | |||||
|---|---|---|---|---|---|
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | ||
| <2.8 g/day | 2.8–3.5 g/day | 3.5–4.26 g/day | >4.26 g/day | ||
| Potassium less than the median (<1.58 g/day) | ORJoint 2.10 (1.89–2.50) | ORJoint 1.94 (1.69–2.24) | ORJoint 2.62 (2.26–3.05) | ORJoint 4.17 (3.51–4.96) | P for interaction |
| (n = 3,176) | (n = 2,600) | (n = 2,217) | (n = 1,471) | <0.001 | |
| Potassium greater than or equal to the median (≥1.58 g/day) | ORJoint 1.69 (1.44–1.98) | Ref 1.0 | ORJoint 1.10 (0.95–1.26) | ORJoint 2.26 (1.97–2.59) | |
| (n = 1,575) | (n = 2,149) | (n = 2,530) | (n = 3,278) |
The primary model included age, BMI, education level, alcohol, diabetes at baseline, atrial fibrillation/flutter at baseline, smoking, and physical activity level. Abbreviations: BMI, body mass index; OR, odds ratio.
Subgroup and sensitivity analysis of the associations between sodium and potassium excretion and risk of stroke
There was a significantly increased risk of stroke for Q4 (>4.26 g/day) vs. Q2 (2.8–3.5 g/day) in participants with a body mass index less than or equal to 30 (OR 1.92; 95% CI, 1.72–2.15) compared with participants with a body mass index greater than 30 (OR 1.35; 95% CI, 0.81–2.26) (P = 0.009 for interaction). The association of high sodium excretion with stroke (>4.26 g/day) vs. Q2 (2.8–3.5 g/day) was significant for European (OR 1.34; 95% CI, 1.11–1.63), Chinese (OR 1.85; 95% CI, 1.59–2.14), Other Asian (OR 10.83 (6.35–18.48), Latin American (OR 1.61; 95% CI, 1.23–2.13), Black African (OR 1.95; 95% CI, 1.10–3.47), and “Other” African ethnicity (OR 1.96; 95% CI, 1.12–3.43). The association of low sodium excretion with stroke (<2.8 g/day) vs. Q2 (2.8–3.5 g/day) was significant for European (OR 1.57; 95% CI, 1.30–1.89), Arab (OR 2.51; 95% CI, 1.13–5.56), Latin American (OR 1.40; 95% CI, 1.06–1.87), Black African (OR 2.21; 95% CI, 1.37–3.55), and Other ethnicity (OR 2.21; 95% CI, 1.43–3.41) (P < 0.001 for interaction). The associations for both high sodium excretion (OR 1.46; 95% CI, 0.77–2.76) and low sodium excretion (OR 1.55; 95% CI, 0.71–3.41) compared with Q2 (2.8–3.5 g/day) were nonsignificant for participants with diuretic use at baseline (P = 0.1505 for interaction). Sex, age, or baseline hypertension status did not alter the association significantly between both high and low estimated sodium excretion and stroke (Supplementary Table S7 online).
The exclusion of cases with modified Rankin scale greater than 2, and the exclusion of cases with urine collected greater than 48 hours after symptom onset did not materially alter findings (Tables 2 and 3).
We repeated all analyses with the Kawasaki formula, urinary sodium/creatinine ratio, and urinary sodium, which revealed consistent patterns of association, but, as expected, different thresholds of sodium and potassium excretion (g/day) were associated with stroke risk (Supplementary Figures S3–S6 online).
DISCUSSION
In this large, international, case–control study, we report an overall J-shaped association between sodium intake and stroke risk, with the lowest risk at moderate sodium intake (2.8–3.5 g/day), employing estimated 24-hour urinary excretion of sodium as a surrogate for intake. The association of high sodium intake was stronger for ICH compared with ischemic stroke and within ischemic stroke subtypes, was significant for small vessel and large vessel ischemic stroke, but not significant for cardioembolic stroke. The association between estimated potassium excretion and risk of ischemic stroke was inverse and linear, but not significant for ICH. The magnitude of association for both low (<2.8 g/day) and high (>4.26 g/day) sodium excretion was diminished in those with high potassium intake (≥1.58 g/day) (P < 0.001 for interaction). In our analysis of combined categories of sodium and potassium intake, the category of highest potassium intake (≥1.58 g/day) and moderate sodium intake (2.8–3.5 g/day) was associated with the lowest risk of stroke.
Most national and international guidelines recommend low sodium intake in the entire population, for stroke prevention (e.g., WHO recommend an intake of <2.0 g/day). Primarily, the target of low sodium is based on the short-term Phase IIa DASH-Sodium trial which reported a blood pressure reduction when reducing sodium intake to less than 1.5 g/day by providing all meals to the participants,24 the longer-term trials (TONE and TOHP-2) which achieved mean sodium intakes of 2.3 and 3.11 g/day (despite targeting a sodium intake of 1.8 g/day or lower) through intense dietary counseling.25,26 While a target of <2.0 g/day can be achieved in a highly controlled environment, we report that a very low proportion of the population consume a low sodium intake, and an even lower proportion consume a combined low sodium and high potassium intake. Our findings are consistent with other epidemiologic studies, and support the contention that a lower limit of sodium intake exists among free-living populations due to neurohormonal control mechanisms that autoregulate the consumption of sodium.27 Activation of the renin–angiotensin–aldosterone system occurs when sodium intake falls below approximately 3.0 g/day. An analysis of the HOPE study reported a positive association between higher quintiles of plasma renin activity and cardiovascular outcomes including stroke,28 and consistently, the relative risk for the highest quintile of plasma renin activity was 1.43, identical to our estimate for the stroke risk associated with sodium excretion <2.8 g/day. In addition, we report a different pattern of association between sodium intake and blood pressure (positive and monotonic) compared with the pattern of association with stroke risk (J-shaped). These patterns have also been reported in several recent large cohort studies,7,29 and challenge assumptions that underpin current guidelines (i.e., that all reductions in blood pressure will reduce stroke, regardless of baseline sodium intake level).9 Our findings do however, support public health interventions to reduce sodium intake among populations with high sodium intake and support transitioning populations from high to moderate sodium intake in order to reduce stroke.30 Our data suggest that the risk associated with a higher sodium intake may be greater in regions outside of Europe and North America.
Our data also provide important insights into the anticipated effects of reducing high sodium intake on patterns of stroke and its subtypes; reducing high sodium intake is likely to have a greater effect on reducing ICH than ischemic stroke, but is nevertheless expected to also reduce ischemic stroke, and thereby the global burden of all stroke. In ecological studies of stroke incidence in China, for example, population-level reductions in high sodium intake parallel reductions in stroke incidence, and are more marked for ICH than ischemic stroke.31 In the INTERSTROKE study, about 40% of the control population were consuming higher sodium intake in a range associated with stroke risk, supporting a targeted-population approach to sodium reduction, rather than a population-wide approach.
Our analyses also suggest that increases in potassium intake may be of comparable, or greater, importance to stroke prevention than reductions in sodium intake.12,16,17 This finding is consistent with reports that high potassium intake is a marker of a healthy diet, i.e., rich in fruit and vegetables.32
A recent analysis of the PURE cohort study reported that the combination of moderate sodium intake and high potassium intake was associated with the lowest cardiovascular risk, and our analyses are further evidence that such a combined target may be optimal.12 A cluster randomized controlled trial reported significant reductions in cardiovascular risk with potassium salt substitution.33 Potassium salt substitution not only increased potassium intake but also reduced high sodium intake (but not to low intake levels).33 An ongoing large cluster randomized controlled trial in China is currently evaluating potassium salt substitutes for prevention of stroke.34 Our analyses, and those of other studies, raise major concerns about the feasibility of increasing potassium intake, while simultaneously achieving low sodium intake. They suggest that populations should target moderate sodium intake and high potassium intake as the optimal balance, as the former is expected to make the latter more achievable.
Measurement of sodium and potassium intake is a major challenge, and there is no “gold” standard for estimating usual sodium and potassium intake.35 The reference standard,36 repeated 24-hour urinary collections, is impractical in large epidemiologic studies and would invariably lead to the exclusion of a substantial proportion of participants and a biased sample. In our study, the mean intake of sodium in the control group was 3.54 g/day, which is close to the mean intake reported by the Global Burden of Diseases Nutrition and Chronic Diseases Expert Group (3.95 g/day) and the PURE study (4.9 g/day).7,37 In the PURE study, a fasting morning urine sample was collected and the Kawasaki formula was used to estimate sodium excretion. In contrast, we collected a random urine sample and used the Tanaka formula to estimate sodium excretion. A validation study of 1,083 participants from the PURE study showed a similar differences between mean sodium estimated using the Kawasaki equation and Tanaka equation.21 Importantly, however, irrespective of the method we employed in INTERSTROKE, which included urinary sodium/creatinine ratio, Tanaka formula or Kawasaki formula, the J-shaped pattern of association were consistent among all analyses, and median intake levels are associated with lowest cardiovascular risk. Collectively, despite the study-by-study variation in methods of estimating sodium intake, there is a remarkable consistency in findings from large epidemiologic studies that the optimal range of sodium intake resides within a range between 2.7 and 5.0 g/day. This is also the range identified in a meta-analysis of prospective cohort studies published before 2014,9 by Graudal et al., and consistent with prospective cohort studies reported since then, including PURE,12 CRIC,38 and PREVEND39 studies.
The case–control design has inherent limitations, including sampling bias (selection of cases and controls) and measurement bias (recall bias). Sensitivity analysis by control type (community vs. hospital) did not alter our findings and estimated urinary sodium excretion is an objective lab measurement and not susceptible to recall bias. Another limitation is the potential acute effects of stroke on excretion of sodium intake, particularly the change in oral intake and use of intravenous fluids in those with severe stroke. To address this issue, we performed several sensitivity analyses by excluding patients with a modified Rankin score greater than 2 as these patients are likely have received intravenous fluids and enteric feeding due to their disability. Excluding these patients did not materially alter our findings. In addition, increasing time from admission to urinary sample measurement may reduce the correlation of usual (prestroke) diet with urinary estimate. Confining the analyses to those with early urine collections (<48 hours) did not alter conclusions.
A key strength of the INTERSTROKE study was the availability of neuroimaging to classify all cases of stroke. INTERSTROKE is the only large study which reliably examines whether the associations of sodium and potassium differs between ischemic stroke, ischemic stroke subtypes, and ICH. Obtaining such information from cohort studies is impractical. The diverse international population included in our results are widely generalizable.
In conclusion, an estimated sodium excretion <2.8 and >4.26 g/day are both associated with an increased risk of stroke (reference 2.8–3.5 g/day). An estimated potassium excretion of greater than 1.34 g/day was associated with a reduced risk of stroke (reference <1.34 g/day). In the absence of large randomized controlled trials, the collective information from observational data on over 300,000 individuals suggests that the optimal intake of sodium is a moderate level combined with high potassium intake.
Supplementary Material
Contributor Information
Conor Judge, Department of Medicine, NUI Galway, Galway, Ireland; Department of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada; Wellcome Trust Health Research Board Irish Clinical Academic Training (ICAT), Dublin, Ireland.
Martin J O’Donnell, Department of Medicine, NUI Galway, Galway, Ireland; Department of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
Graeme J Hankey, School of Medicine and Pharmacology, Faculty of Health and Medical Sciences, University of Western Australia, Perth, Western Australia, Australia.
Sumathy Rangarajan, Department of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
Siu Lim Chin, Department of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
Purnima Rao-Melacini, Department of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
John Ferguson, Department of Medicine, NUI Galway, Galway, Ireland.
Andrew Smyth, Department of Medicine, NUI Galway, Galway, Ireland.
Denis Xavier, Department of Medicine, St John’s Medical College and Research Institute, Bangalore, India.
Liu Lisheng, Department of Medicine, National Center of Cardiovascular Disease, Beijing, China.
Hongye Zhang, Department of Medicine, Beijing Hypertension League Institute, Beijing, China.
Patricio Lopez-Jaramillo, Department of Medicine, Instituto de Investigaciones MASIRA, Universidad de Santander, Bucaramanga, Colombia.
Albertino Damasceno, Faculty of Medicine, Eduardo Mondlane University, Maputo, Mozambique.
Peter Langhorne, Department of Medicine, Glasgow Royal Infirmary, University of Glasgow, Glasgow, Scotland, UK.
Annika Rosengren, Department of Molecular and Clinical Medicine, University of Gothenburg and Region Västra Götaland, Sahlgrenska University Hospital, Gothenburg, Sweden.
Antonio L Dans, College of Medicine, University of Philippines, Manila, Philippines.
Ahmed Elsayed, Department of Surgery, Al Shaab Teaching Hospital, Khartoum, Sudan.
Alvaro Avezum, Department of Medicine, International Research Center, Hospital Alemão Oswaldo Cruz, São Paulo, Brazil.
Charles Mondo, Department of Medicine, Kiruddu National Referral Hospital, Kampala, Uganda.
Danuta Ryglewicz, Military Institute of Aviation Medicine, Warsaw, Poland.
Anna Czlonkowska, Department of Medicine, Military Institute of Aviation Medicine, Warsaw, Poland.
Nana Pogosova, Department of Medicine, National Medical Research Center of Cardiology, Moscow, Russia.
Christian Weimar, Department of Neurology, University Hospital, Essen, Germany.
Rafael Diaz, Department of Medicine, Estudios Clínicos Latino America (ECLA), Instituto Cardiovascular de Rosario (ICR), Rosario, Argentina.
Khalid Yusoff, Department of Medicine, Universiti Teknologi MARA, Selayang, Selangor and UCSI University, Kuala Lumpur, Malaysia.
Afzalhussein Yusufali, Department of Medicine, Hatta Hospital, Dubai Health Authority/Dubai Medical College, Dubai, UAE.
Aytekin Oguz, Department of Internal Medicine, Istanbul Medeniyet University, Istanbul, Turkey.
Xingyu Wang, Department of Medicine, Beijing Hypertension League Institute, Beijing, China.
Fernando Lanas, Faculty of Medicine, Universidad de La Frontera, Temuco, Chile.
Okechukwu S Ogah, Department of Medicine, University College Hospital, Ibadan, Nigeria.
Adesola Ogunniyi, Department of Medicine, University College Hospital, Ibadan, Oyo State, Nigeria.
Helle K Iversen, Department of Neurology, Rigshospitalet, University of Copenhagen, Denmark.
German Malaga, School of Medicine, Universidad Peruana Cayetano Heredia, Lima, Peru.
Zvonko Rumboldt, Department of Medicine, University of Split, Split, Croatia.
Shahram Oveisgharan, Department of Medicine, Rush Alzheimer Disease Research Center in Chicago, Chicago, Illinois, USA.
Fawaz Al Hussain, Department of Medicine, King Saud University, Riyadh, Saudi Arabia.
Salim Yusuf, Department of Medicine, Population Health Research Institute, McMaster University and Hamilton Health Sciences, Hamilton, Ontario, Canada.
FUNDING
The INTERSTROKE study is funded by the Canadian Institutes of Health Research, Heart and Stroke Foundation of Canada, Canadian Stroke Network, Swedish Research Council, Swedish Heart and Lung Foundation, The Health & Medical Care Committee of the Regional Executive Board, Region Västra Götaland, and through unrestricted grants from several pharmaceutical companies with major contributions from Astra Zeneca, Boehringer Ingelheim (Canada), Pfizer (Canada), MERCK, Sharp and Dohme, Swedish Heart and Lung Foundation, UK Chest, and UK Heart and Stroke. C.J. was funded by Wellcome Trust—Health Research Board Irish Clinical Academic Training (ICAT). M.O.D. was funded by European Research Council COSIP Grant 640580.
AUTHORS’ CONTRIBUTIONS
M.J.O’D. and S.Y. designed the study. C.J. and M.J.O’D. planned analyses and wrote the first draft of the report. C.J. and J.F. did the statistical analyses. All authors contributed to the collection of data, discussions and interpretation of the data, and to the writing of the report. All authors had full access to data and reviewed and approved the drafts of the report.
ETHICS COMMITTEE APPROVAL
The study was approved by the ethics committees in all participating centers.
DISCLOSURE
G.J.H. reports personal fees from Bayer and Medscape, outside of the submitted work. All other authors declare no competing interests.
REFERENCES
- 1. O’Donnell MJ, Chin SL, Rangarajan S, Xavier D, Liu L, Zhang H, Rao-Melacini P, Zhang X, Pais P, Agapay S, Lopez-Jaramillo P, Damasceno A, Langhorne P, McQueen MJ, Rosengren A, Dehghan M, Hankey GJ, Dans AL, Elsayed A, Avezum A, Mondo C, Diener HC, Ryglewicz D, Czlonkowska A, Pogosova N, Weimar C, Iqbal R, Diaz R, Yusoff K, Yusufali A, Oguz A, Wang X, Penaherrera E, Lanas F, Ogah OS, Ogunniyi A, Iversen HK, Malaga G, Rumboldt Z, Oveisgharan S, Al Hussain F, Magazi D, Nilanont Y, Ferguson J, Pare G, Yusuf S; INTERSTROKE investigators . Global and regional effects of potentially modifiable risk factors associated with acute stroke in 32 countries (INTERSTROKE): a case-control study. Lancet 2016; 388:761–775. [DOI] [PubMed] [Google Scholar]
- 2. Mente A, O’Donnell MJ, Rangarajan S, McQueen MJ, Poirier P, Wielgosz A, Morrison H, Li W, Wang X, Di C, Mony P, Devanath A, Rosengren A, Oguz A, Zatonska K, Yusufali AH, Lopez-Jaramillo P, Avezum A, Ismail N, Lanas F, Puoane T, Diaz R, Kelishadi R, Iqbal R, Yusuf R, Chifamba J, Khatib R, Teo K, Yusuf S; PURE Investigators . Association of urinary sodium and potassium excretion with blood pressure. N Engl J Med 2014; 371:601–611. [DOI] [PubMed] [Google Scholar]
- 3. World Health Organization (WHO). Guideline: Sodium Intake for Adults and Children [Internet]. Geneva, 2012. [cited 19 November 2018]. http://www.who.int/nutrition/publications/guidelines/sodium_intake_printversion.pdf [Google Scholar]
- 4. Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD; American Heart Association Strategic Planning Task Force and Statistics Committee . Defining and setting national goals for cardiovascular health promotion and disease reduction: the American Heart Association’s strategic Impact Goal through 2020 and beyond. Circulation 2010; 121:586–613. [DOI] [PubMed] [Google Scholar]
- 5. Cao J, Eshak ES, Liu K, Gero K, Liu Z, Yu C. Age-period-cohort analysis of stroke mortality attributable to high sodium intake in China and Japan. Stroke 2019; 50:1648–1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. He FJ, Li J, Macgregor GA. Effect of longer-term modest salt reduction on blood pressure. Cochrane Database Syst Rev 2013. Art. No. CD004937 (doi: 10.1002/14651858.CD004937.pub2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. O’Donnell M, Mente A, Rangarajan S, McQueen MJ, Wang X, Liu L, Yan H, Lee SF, Mony P, Devanath A, Rosengren A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Yusoff K, Iqbal R, Ilow R, Mohammadifard N, Gulec S, Yusufali AH, Kruger L, Yusuf R, Chifamba J, Kabali C, Dagenais G, Lear SA, Teo K, Yusuf S; PURE Investigators . Urinary sodium and potassium excretion, mortality, and cardiovascular events. N Engl J Med 2014; 371:612–623. [DOI] [PubMed] [Google Scholar]
- 8. Cogswell ME, Mugavero K, Bowman BA, Frieden TR. Dietary sodium and cardiovascular disease risk—measurement matters. N Engl J Med 2016; 375:580–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Graudal N, Jürgens G, Baslund B, Alderman MH. Compared with usual sodium intake, low- and excessive-sodium diets are associated with increased mortality: a meta-analysis. Am J Hypertens 2014; 27:1129–1137. [DOI] [PubMed] [Google Scholar]
- 10. Zhu Y, Zhang J, Li Z, Liu Y, Fan X, Zhang Y, Zhang Y. Association of sodium intake and major cardiovascular outcomes: a dose-response meta-analysis of prospective cohort studies. BMC Cardiovasc Disord 2018; 18:192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Collins M, Mason H, O’Flaherty M, Guzman-Castillo M, Critchley J, Capewell S. An economic evaluation of salt reduction policies to reduce coronary heart disease in England: a policy modeling study. Value Health 2014; 17:517–524. [DOI] [PubMed] [Google Scholar]
- 12. O’Donnell M, Mente A, Rangarajan S, McQueen MJ, O’Leary N, Yin L, Liu X, Swaminathan S, Khatib R, Rosengren A, Ferguson J, Smyth A, Lopez-Jaramillo P, Diaz R, Avezum A, Lanas F, Ismail N, Yusoff K, Dans A, Iqbal R, Szuba A, Mohammadifard N, Oguz A, Yusufali AH, Alhabib KF, Kruger IM, Yusuf R, Chifamba J, Yeates K, Dagenais G, Wielgosz A, Lear SA, Teo K, Yusuf S; PURE Investigators . Joint association of urinary sodium and potassium excretion with cardiovascular events and mortality: prospective cohort study. BMJ 2019; 364:l772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Drewnowski A, Rehm CD, Maillot M, Mendoza A, Monsivais P. The feasibility of meeting the WHO guidelines for sodium and potassium: a cross-national comparison study. BMJ Open 2015; 5:e006625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ, Hu FB. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med 2011; 171:1183–1191. [DOI] [PubMed] [Google Scholar]
- 15. Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ 2013; 346:f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Appel LJ, Moore TJ, Obarzanek E, Vollmer WM, Svetkey LP, Sacks FM, Bray GA, Vogt TM, Cutler JA, Windhauser MM, Lin PH, Karanja N. A clinical trial of the effects of dietary patterns on blood pressure. DASH Collaborative Research Group. N Engl J Med 1997; 336:1117–1124. [DOI] [PubMed] [Google Scholar]
- 17. Cook NR, Obarzanek E, Cutler JA, Buring JE, Rexrode KM, Kumanyika SK, Appel LJ, Whelton PK; Trials of Hypertension Prevention Collaborative Research Group . Joint effects of sodium and potassium intake on subsequent cardiovascular disease: the Trials of Hypertension Prevention follow-up study. Arch Intern Med 2009; 169:32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tanaka T, Okamura T, Miura K, Kadowaki T, Ueshima H, Nakagawa H, Hashimoto T. A simple method to estimate populational 24-h urinary sodium and potassium excretion using a casual urine specimen. J Hum Hypertens 2002; 16:97–103. [DOI] [PubMed] [Google Scholar]
- 19. Kawasaki T, Itoh K, Uezono K, Sasaki H. A simple method for estimating 24 h urinary sodium and potassium excretion from second morning voiding urine specimen in adults. Clin Exp Pharmacol Physiol 1993; 20:7–14. [DOI] [PubMed] [Google Scholar]
- 20. Brown IJ, Dyer AR, Chan Q, Cogswell ME, Ueshima H, Stamler J, Elliott P; INTERSALT Co-Operative Research Group . Estimating 24-hour urinary sodium excretion from casual urinary sodium concentrations in Western populations: the INTERSALT study. Am J Epidemiol 2013; 177:1180–1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mente A, O’Donnell MJ, Dagenais G, Wielgosz A, Lear SA, McQueen MJ, Jiang Y, Xingyu W, Jian B, Calik KB, Akalin AA, Mony P, Devanath A, Yusufali AH, Lopez-Jaramillo P, Avezum A Jr, Yusoff K, Rosengren A, Kruger L, Orlandini A, Rangarajan S, Teo K, Yusuf S. Validation and comparison of three formulae to estimate sodium and potassium excretion from a single morning fasting urine compared to 24-h measures in 11 countries. J Hypertens 2014; 32:1005–1014; discussion 1015. [DOI] [PubMed] [Google Scholar]
- 22. O’Brien E. Salt—too much or too little? Lancet 2016; 388:439–440. [DOI] [PubMed] [Google Scholar]
- 23. Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis, 2nd edn. Springer: Cham, Heidelberg, New York, 2015, p. 582 (Springer series in statistics). [Google Scholar]
- 24. Sacks FM, Svetkey LP, Vollmer WM, Appel LJ, Bray GA, Harsha D, Obarzanek E, Conlin PR, Miller ER III, Simons-Morton DG, Karanja N, Lin PH; DASH-Sodium Collaborative Research Group . Effects on blood pressure of reduced dietary sodium and the Dietary Approaches to Stop Hypertension (DASH) diet. DASH-Sodium Collaborative Research Group. N Engl J Med 2001; 344:3–10. [DOI] [PubMed] [Google Scholar]
- 25. Whelton PK, Appel LJ, Espeland MA, Applegate WB, Ettinger WH Jr, Kostis JB, Kumanyika S, Lacy CR, Johnson KC, Folmar S, Cutler JA. Sodium reduction and weight loss in the treatment of hypertension in older persons: a randomized controlled trial of nonpharmacologic interventions in the elderly (TONE). TONE Collaborative Research Group. JAMA 1998; 279:839–846. [DOI] [PubMed] [Google Scholar]
- 26. The Trials of Hypertension Prevention Collaborative Research Group. Effects of weight loss and sodium reduction intervention on blood pressure and hypertension incidence in overweight people with high-normal blood pressure: the trials of hypertension prevention, phase II. Arch Intern Med 1997; 157:657. [PubMed] [Google Scholar]
- 27. Lowell BB. New neuroscience of homeostasis and drives for food, water, and salt. N Engl J Med 2019; 380:459–471. [DOI] [PubMed] [Google Scholar]
- 28. Verma S, Gupta M, Holmes DT, Xu L, Teoh H, Gupta S, Yusuf S, Lonn EM. Plasma renin activity predicts cardiovascular mortality in the Heart Outcomes Prevention Evaluation (HOPE) study. Eur Heart J 2011; 32:2135–2142. [DOI] [PubMed] [Google Scholar]
- 29. Stolarz-Skrzypek K, Kuznetsova T, Thijs L, Tikhonoff V, Seidlerová J, Richart T, Jin Y, Olszanecka A, Malyutina S, Casiglia E, Filipovský J, Kawecka-Jaszcz K, Nikitin Y, Staessen JA; European Project on Genes in Hypertension (EPOGH) Investigators . Fatal and nonfatal outcomes, incidence of hypertension, and blood pressure changes in relation to urinary sodium excretion. JAMA 2011; 305:1777–1785. [DOI] [PubMed] [Google Scholar]
- 30. Hyseni L, Elliot-Green A, Lloyd-Williams F, Kypridemos C, O’Flaherty M, McGill R, Orton L, Bromley H, Cappuccio FP, Capewell S. Systematic review of dietary salt reduction policies: evidence for an effectiveness hierarchy? PLoS One 2017; 12:e0177535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li Y, Huang Z, Jin C, Xing A, Liu Y, Huangfu C, Lichtenstein AH, Tucker KL, Wu S, Gao X. Longitudinal change of perceived salt intake and stroke risk in a Chinese population. Stroke 2018; 49:1332–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mente A, Irvine EJ, Honey RJ, Logan AG. Urinary potassium is a clinically useful test to detect a poor quality diet. J Nutr 2009; 139:743–749. [DOI] [PubMed] [Google Scholar]
- 33. Chang HY, Hu YW, Yue CS, Wen YW, Yeh WT, Hsu LS, Tsai SY, Pan WH. Effect of potassium-enriched salt on cardiovascular mortality and medical expenses of elderly men. Am J Clin Nutr 2006; 83:1289–1296. [DOI] [PubMed] [Google Scholar]
- 34. Neal B, Tian M, Li N, Elliott P, Yan LL, Labarthe DR, Huang L, Yin X, Hao Z, Stepien S, Shi J, Feng X, Zhang J, Zhang Y, Zhang R, Wu Y. Rationale, design, and baseline characteristics of the Salt Substitute and Stroke Study (SSaSS)—a large-scale cluster randomized controlled trial. Am Heart J 2017; 188:109–117. [DOI] [PubMed] [Google Scholar]
- 35. Versi E. “Gold standard” is an appropriate term. BMJ 1992; 305:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. McLean RM. Measuring population sodium intake: a review of methods. Nutrients 2014; 6:4651–4662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Mozaffarian D, Fahimi S, Singh GM, Micha R, Khatibzadeh S, Engell RE, Lim S, Danaei G, Ezzati M, Powles J; Global Burden of Diseases Nutrition and Chronic Diseases Expert Group . Global sodium consumption and death from cardiovascular causes. N Engl J Med 2014; 371:624–634. [DOI] [PubMed] [Google Scholar]
- 38. Mills KT, Chen J, Yang W, Appel LJ, Kusek JW, Alper A, Delafontaine P, Keane MG, Mohler E, Ojo A, Rahman M, Ricardo AC, Soliman EZ, Steigerwalt S, Townsend R, He J; Chronic Renal Insufficiency Cohort (CRIC) Study Investigators . Sodium excretion and the risk of cardiovascular disease in patients with chronic kidney disease. JAMA 2016; 315:2200–2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kieneker LM, Eisenga MF, Gansevoort RT, de Boer RA, Navis G, Dullaart RPF, Joosten MM, Bakker SJL. Association of low urinary sodium excretion with increased risk of stroke. Mayo Clin Proc 2018; 93:1803–1809. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.