Abstract
Background:
Men with azoospermia undergoing a surgical sperm retrieval are anxious about the well-being of the baby. It is therefore important to study the perinatal outcomes in this group compared to the ejaculate sample group.
Aim:
The aim of the study was to compare the perinatal outcomes between ejaculate and surgical sperm retrieval (SSR) groups in couples undergoing intracytoplasmic sperm injection for male factor.
Study Setting and Design:
This was a retrospective cohort study conducted in a university-level infertility unit.
Materials and Methods:
It is a retrospective cohort study analysis of 628 assisted reproductive technique (ART) cycles with male factor and combined (male and female) factor infertility over a period of 5 years (January 2011–December 2015). All women who underwent a fresh embryo ART cycle were followed up. The study population included the ejaculate and SSR groups. The perinatal outcomes of these two groups were compared. The congenital anomaly risks among the two groups were also analyzed.
Statistical Analysis:
Chi-square test, Fisher's exact test and Logistic regression
Results:
A total of 628 ART cycles were included in the current study, of which 478 cycles used ejaculate sperm, while SSR was done in 150 cycles. The analysis was restricted to singletons, and the risk of preterm birth was 22.9% in the ejaculate group, 5.9% in the epididymal group, and 12% in the testicular group (epididymal vs. ejaculate odds ratio [OR], 0.21; 95% confidence interval [CI]: 0.02–1.66) (testicular vs. ejaculate OR, 0.46; 95% CI: 0.12–1.65). The risk of low birth weight was 23.7% in the ejaculate group, 11.8% in the epididymal group, and 20.0% in the testicular group (epididymal vs. ejaculate OR, 0.42; 95% CI: 0.09–1.9) (testicular vs. ejaculate OR, 0.80; 95% CI: 0.27–2.3). The incidence of congenital anomalies was 7.3% in the ejaculate group, 0 in the epididymal group, and 3.5% in the testicular group (epididymal vs. ejaculate OR, 0.28; 95% CI: 0.01–5.2) (testicular vs. ejaculate OR, 0.63; 95% CI: 0.10–3.7) which was not significantly different.
Conclusion:
The current study showed no significant differences in the risk of adverse perinatal outcomes in the ejaculate group versus the surgically retrieved sperm groups.
KEYWORDS: Congenital anomaly, ejaculate, perinatal outcomes, surgical sperm retrieval
INTRODUCTION
Worldwide, about one in six couples in the reproductive age group are infertile.[1] Male factor alone contributes to about 20% of infertile couples and adds to another 30%–40% in combination with female factors.[2] About 10%–15% of infertile men are diagnosed with azoospermia.[3]
The causes of azoospermia are broadly classified as obstructive or nonobstructive. In obstructive azoospermia (OA), spermatogenesis is normal and the causes can be congenital or acquired. In non-OA (NOA), spermatogenesis is either absent or severely impaired. Surgical correction of OA is not always possible. The pregnancy rates following surgical correction of OA range from 22% to 40%.[4,5] Surgical sperm retrieval (SSR) followed by intracytoplasmic sperm injection (ICSI) is the main line of treatment for couples with azoospermia who are keen on using their own gametes during assisted reproduction treatment.[6] Most men with OA and approximately half of the men with NOA still have residual spermatogenesis, which allows for surgical retrieval of spermatozoa.[7] In men with OA, percutaneous epididymal sperm aspiration (PESA) or microsurgical epididymal sperm aspiration is used to retrieve sperms from the epididymis. In men with NOA, testicular sperm aspiration (TESA) or testicular sperm extraction (TESE) or micro-TESE can be done to extract sperms from the testis.[6]
Several studies have compared the reproductive outcomes following SSR in men with epididymal versus testicular source.[8,9,10] Very few studies have reported the perinatal and neonatal outcomes following SSR in azoospermic men. Furthermore, there is always a concern regarding the health of the offspring when spermatozoa from azoospermic men are used for ICSI. A study by Deng et al. showed no difference in the perinatal outcomes between epididymal, testicular, and ejaculate sources of sperms.[11] A retrospective study by Kawasss et al. on the perinatal and neonatal outcomes in ejaculate versus surgically retrieved sperms showed no statistically significant difference between ejaculate versus epididymal sperm and ejaculate versus testicular sperm.[12] In view of the limited and inconsistent data regarding perinatal outcomes, we planned the current study to evaluate the perinatal and neonatal outcomes in couples undergoing SSR followed by ICSI and compared them to ejaculate sperm in nonazoospermic infertile males.
MATERIALS AND METHODS
The current study was a retrospective cohort study, conducted in a university-level infertility unit. Ethics committe approval was taken for the study. Anonymized data were obtained from hospital electronic records, and a total of 628 cycles were analyzed. This was with prior informed consent from couples for the use of anonymised data. Couples with male factor infertility and combination (male and female factors) who underwent assisted reproductive technique (ART)-ICSI fresh transfer cycle were included in the analysis. Couples with female factor infertility, frozen-thawed cycles, and cycles with the mild stimulation protocol were excluded. The study period was between January 2011 and December 2015. Informed consent was obtained from patients for the use of patient data for research purpose.
Diagnostic semen analysis was done based on the existing WHO criteria, and azoospermia was confirmed by two observers in both fresh and centrifuged samples. The azoospermia men were classified into obstructive or nonobstructive based on the clinical examination, blood tests (follicle-stimulating hormone), and genetic tests (peripheral blood karyotyping and Y chromosomal microdeletions were done). Diagnostic SSR was planned before ART.
Assisted reproductive technique protocol and surgical sperm retrieval procedure
The various ART protocols used in our unit include GnRH antagonist (flexible), long GnRH agonist (luteal phase), short flare protocol, and ultralong protocol. Controlled ovarian hyperstimulation was done with recombinant gonadotropins (Recagon, Merck Sharp and Dohme, New Jersey, USA, and Gonal-F, Merck Serono, Switzerland). The starting dose range was between 100 IU and 450 IU. Monitoring was done with ultrasonography, and when at least three follicles reached 17–20 mm size, transvaginal oocyte retrieval (TVOR) was planned 35 h after human chorionic gonadotropin trigger. On the day of TVOR depending on the severity of the male factor, either an ejaculate sample or SSR sample was collected and ICSI was done.
PESA was done when there was OA with epididymal distension. In PESA, a 20G needle is introduced into the epididymis under the cord block and the aspirate is checked for spermatozoa. In OA with failed PESA, and in men with NOA, TESA was done. In TESA, an 18G needle is introduced into the testis under the cord block and the testicular tissue aspirated. The tissue is then processed in the laboratory and sperms isolated for ICSI. The embryo transfer was done at either cleavage stage (day 2, 3, or 4) or blastocyst stage (day 5), and between one to three embryos were transferred depending on the age, number of cycles, and stage of embryos. Luteal support was initiated on the day of oocyte retrieval with progesterone vaginal suppository 400 mg (Naturogest, Zydus Healthcare Limited, India) twice daily and parenteral progesterone (Gestone, Ferring Pharmaceuticals, Switzerland) 100 mg twice weekly, until the pregnancy test (18 days after oocyte retrieval). Once the pregnancy test is positive, transvaginal sonography (TVS) was done after 2 weeks to check the fetal viability, the number of gestational sacs, and the location of the sac. After another TVS at 8-week period of gestation, the woman was referred to the obstetrics department. The pregnancy outcome and perinatal outcomes were followed up through electronic records of the hospital and by electronic communication.
The primary outcome was live birth rate, defined as a fetus showing any sign of life, beyond 22-week gestational age. The live birth rate was expressed per embryo transfer. The secondary outcomes were clinical pregnancy rate, defined as pregnancy (diagnosed by ultrasonographic visualization of one or more gestational sacs) expressed per embryo transfer. The miscarriage rate was defined as the spontaneous loss of a pregnancy before 22 completed weeks of gestational age. The miscarriage rate was expressed as miscarriage per clinical pregnancy. Preterm birth (PTB) was defined as a birth that takes place after 28 weeks and before 37 completed weeks of gestational age. Extreme PTB was defined as a birth that takes place after 22 but before 28 completed weeks of gestational age. Low birth weight (LBW) defines as a birth weight less than 2500 g. Very LBW (VLBW) is a birth weight less than 1500 g. Congenital anomaly was defined as structural or functional disorders that occur during intrauterine life and can be identified prenatally, at birth, or later in life.[13]
Statistical analysis
In view of retrospective nature of the study, sample size calculation was not done. Continuous variables are expressed as means ± standard deviation and median with interquartile range and compared with Student's t-test or Mann–Whitney U-test. Categorical variables are summarized as frequency and percentage. Pearson Chi-square and Fisher's exact test were used to find out the association between categorical variables. The relationship of study factors to the outcome was assessed using logistic regression and penalized logistic regression. The odds ratio (OR) with a 95% confidence interval (CI) was reported. Analyses were performed using SAS software (SAS® Institute Inc., USA, version 9.2).
RESULTS
A total of 2264 ART cycles were performed during the study period, out of which 628 cycles were included in the current study [Figure 1]. Among the included cycles, ejaculate sperm was used in 478 cycles and SSR was done in 150 cycles.
Figure 1.
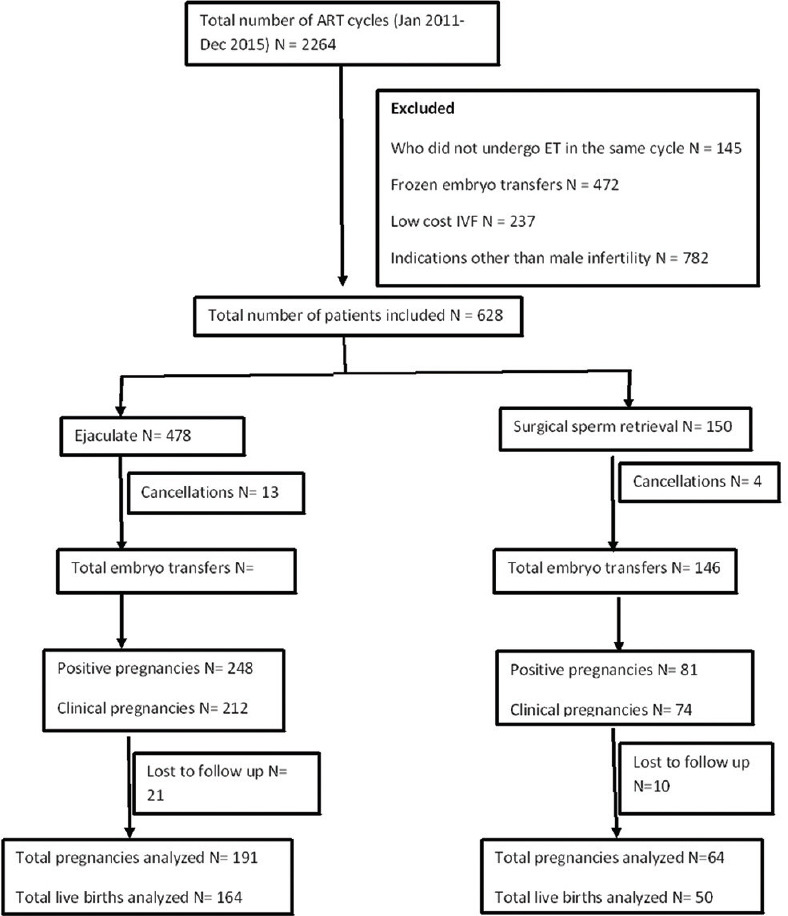
Flowchart of patient recruitment, allotment, and follow-up
The baseline characteristics of the included patients are shown in Table 1. The female age was significantly lower in the SSR group. Couples with male factor alone were higher in the SSR group. There was no statistical difference in clinical pregnancies per embryo transfer (45.6% vs. 50.6%, P = 0.15) or live birth rates/ET – 36.9% vs 36.7% per embryo transfer (35.0% vs. 34.9%, P = 0.97) [Table 2]. There was no statistical difference in the mode of delivery or congenital anomalies in both the groups. The most common anomalies seen were cardiac (patent ductus arteriosus and ventricular septal defect) and urogenital anomalies (inguinal hernias and hydrocele). The gestational age at delivery was lower in the ejaculate group compared to the SSR group (36.3 ± 2.3 weeks vs. 37.3 ± 1.3 weeks, P ≤ 0.001). Overall, the incidence of PTB (including multiple births) was 31.4% in the ejaculate group and 17.1% in the SSR group (P = 0.02) [Table 2].
Table 1.
Baseline characteristics of study participants
| Ejaculate (n=478), n (%) | Surgical sperm retrieval (n=150), n (%) | P | |
|---|---|---|---|
| Female age* (years) | 31.58±4.5 | 30.59±4.9 | 0.03 |
| Male age* (years) | 37.54±5.2 | 37.68±5.6 | 0.77 |
| Female BMI* | 25.53±4.2 | 24.50±3.5 | 0.004 |
| Total dose of gonadotropins* | 2323.21±1121.03 | 2133.47±1127.97 | 0.07 |
| Oocytes retrieved* | 8.00±4.15 | 8.69±4.47 | 0.09 |
| Infertility | |||
| Primary | 383 (80.1) | 128 (85.3) | 0.15 |
| Secondary | 95 (19.9) | 22 (14.7) | |
| ART cycle number | |||
| 1 | 310 (64.9) | 104 (69.3) | 0.31 |
| >1 | 168 (35.1) | 46 (30.6) | |
| Protocol | |||
| Long protocol | 93 (19.5) | 31 (20.7) | 0.40 |
| Short protocol | 42 (8.8) | 11 (7.3) | |
| Antagonist | 329 (68.8) | 107 (71.3) | |
| Ultralong protocol | 14 (2.9) | 1 (0.7) | |
| Cycle cancellations | 13 (2.7) | 4 (2.7) | 1.0 |
| Fertilization failure | 12 (2.5) | 2 (1.3) | 0.53 |
| Indication for IVF | |||
| Male factor | 295 (61.7) | 124 (82.7) | <0.001 |
| Combination (male + female) | 183 (38.3) | 26 (17.3) | |
| Number of embryos transferred# | 2 (2, 3) | 2 (2, 3) | 0.42 |
| Day of embryo transfer | |||
| Cleavage | 407 (87.5) | 131 (89.7) | 0.47 |
| Blastocyst | 58 (12.5) | 15 (10.3) | |
| Total embryo transfers | 465 (97.3) | 146 (97.3) | 1.00 |
| Total pregnancies/embryo transfer | 248/465 (53.3) | 81/146 (55.5) | 0.65 |
| Mean birth weight | 2.53±0.61 | 2.77±0.46 | 0.01 |
*Mean±SD, #Median (IQR), BMI=Body mass index, ART=Assisted reproductive technique, IVF=In vitro fertilization, SD=Standard deviation, IQR=Interquartile range
Table 2.
Details regarding clinical pregnancies
| Ejaculate (n=465), n (%) | Surgical sperm retrieval (n=146), n (%) | P | |
|---|---|---|---|
| Clinical pregnancies/embryo transfer | 212/465 (45.6) | 74/146 (50.6) | 0.15 |
| Gestational sac number | |||
| Single | 136 (64.2) | 49 (66.2) | 0.71 |
| Multiple | 76 (35.8) | 25 (33.7) | |
| Miscarriage* | 24/191 (12.5) | 10/64 (15.6) | 0.53 |
| Ectopic pregnancy* | 3/191 (1.5) | 4/64 (6.25) | 0.04 |
| Preterm* | 60/191 (31.4) | 11/64 (17.1) | 0.02 |
| Total live births/embryo transfer* | 164/444 (36.9) | 50/136 (36.7) | 0.97 |
| Mode of delivery* | |||
| Normal vaginal delivery | 31 (18.4) | 13 (27.4) | 0.16 |
| LSCS | 124 (76.1) | 34 (66.7) | 0.18 |
| Instrumental delivery | 9 (5.5) | 3 (5.8) | 0.92 |
| Period of gestation* | 36.3±2.3 | 37.3±1.3 | <0.001 |
| Anomalies* | 12/164 (7.4) | 1/50 (2.0) | 0.16 |
*For those who were available for follow-up. LSCS=Lower-segment cesarean section
When the analysis was done for only singleton live births [Table 3], the risk of PTB was 22.9% in the ejaculate group, 5.9% in the PESA group, and 12% in the TESA group (PESA vs. ejaculate OR, 0.21; 95% CI: 0.02–1.66) (TESA vs. ejaculate OR, 0.46; 95% CI: 0.12–1.65). The risk of LBW was 23.7% in the ejaculate group, 11.8% in the PESA group, and 20.0% in the TESA group (PESA vs. ejaculate OR, 0.42; 95% CI: 0.09–1.9) (TESA vs. ejaculate OR, 0.80; 95% CI: 0.27–2.3). Therefore, there was no statistical difference in PTB or LBW for singletons in all the three groups. There were 12/164 anomalies in the ejaculate group, 0 in the PESA group, and 1/29 in the TESA group (PESA vs. ejaculate OR, 0.28; 95% CI: 0.01–5.2) (TESA vs. ejaculate OR, 0.63; 95% CI: 0.10–3.7).
Table 3.
Subgroup analysis (ejaculate vs. epididymal sperms vs. testicular sperms)
| Group 1 Ejaculate (n=465), n (%) |
Group 2 Epididymal sperm retrieval (n=57), n (%) |
Group3 Testicular sperm retrieval (n=89), n (%) |
OR (95% CI) |
||
|---|---|---|---|---|---|
| Group 2 versus Group 1 | Group 3 versus Group 1 | ||||
| Positive pregnancies per embryo transfer | 248/465 (53.3) | 35/57 (61.4) | 46/89 (51.6) | 1.39 (0.79-2.44) | 0.93 (0.59-1.47) |
| Clinical pregnancies per embryo transfer | 212/465 (45.5) | 32/57 (56.1) | 42/89 (47.1) | 1.86 (0.54-6.39) | 1.83 (0.62-5.41) |
| Live birth per embryo transfer | 164/444 (36.9) | 21/52 (40.3) | 29/83 (34.9) | 0.86 (0.48-1.55) | 1.11 (0.68-1.81) |
| Preterm birth (<37 weeks) singletons* | 27/118 (22.9) | 1/17 (5.9) | 3/25 (12.0) | 0.21 (0.02-1.66) | 0.46 (0.12-1.65) |
| Low birth weight (<2.5 kg) singletons* | 28/118 (23.7) | 2/17 (11.8) | 5/25 (20.0) | 0.42 (0.09-1.90) | 0.80 (0.27-2.30) |
| Anomalies** | 12/164 (7.3) | 0 | 1/29 (3.47) | 0.28 (0.01-5.2) | 0.63 (0.10-3.7) |
*Among 214 live births, 160 singletons were analyzed for the PTB and LBW, **Penalized logistic regression. PTB=Preterm birth, LBW=Low birth weight, OR=Odds ratio, CI=Confidence interval
A subgroup analysis in the SSR groups (PESA vs. TESA) [Table 4] showed lower pregnancies (61.4% vs. 51.6%; OR, 0.67; 95% CI: 0.34–1.32) and clinical pregnancies (56.1% vs. 47.1%; OR, 0.98; 95% CI: 0.20–4.71) in the TESA group. However, this was not statistically significant. The risk of PTB (5.9% vs. 12.0%; OR, 2.18; 95% CI: 0.20–22.94) and LBW (11.8% vs. 20.0%; OR, 1.87; 95% CI: 0.31–11.02) for singletons was also higher in the TESA group. This was also not statistically significant among both the groups.
Table 4.
Subgroup analysis in patients with surgical sperm retrieval
| Epididymal sperm retrieval (n=57), n (%) | Testicular sperm retrieval (n=89), n (%) | OR (95% CI) | |
|---|---|---|---|
| Positive pregnancies per embryo transfer | 35/57 (61.4) | 46/89 (51.6) | 0.67 (0.34-1.32) |
| Clinical pregnancies per embryo transfer | 32/57 (56.1) | 42/89 (47.1) | 0.98 (0.20-4.71) |
| Live birth per embryo transfer | 21/52 (40.3) | 29/83 (34.9) | 1.28 (0.63-2.62) |
| Preterm birth (<37 weeks) singletons* | 1/17 (5.9) | 3/25 (12.0) | 2.18 (0.20-22.94) |
| Early preterm birth* | - | - | - |
| Low birth weight (<2.5 kg) singletons* | 2/17 (11.8) | 5/25 (20.0) | 1.87 (0.31-11.02) |
| Very low birth weight* | - | - | - |
*Among the 50 live births in SSR, 42 singletons were analyzed. SSR=Surgical sperm retrieval, OR=Odds ratio, CI=Confidence interval
A subgroup analysis in the SSR groups ([OA] vs. NOA) [Table 5] showed lower clinical pregnancies (55.8% vs. 37.7%; OR 2.08; 95% CI: 1.02–4.26) and live births (40.3% vs. 20.8%; OR, 2.56; 95% CI: 1.10–5.94) in the NOA group, and it was statistically significant. The risk of PTB (11.8% vs. 16.7%; OR, 0.66; 95% CI: 0.04–9.02) and LBW (23.5% vs. 33.3%; OR, 0.61; 95% CI: 0.08–4.70) for singletons was also higher in the TESA group, however, this was not statistically significant among both the groups.
Table 5.
Subgroup analysis in patients with surgical sperm retrieval
| Obstructive azoospermia (n=77), n (%) | Nonobstructive azoospermia (n=54), n (%) | OR (95% CI) | |
|---|---|---|---|
| Clinical pregnancies per embryo transfer | 43/77 (55.8) | 20/53 (37.7) | 2.08 (1.02-4.26) |
| Live birth per embryo transfer | 29/72 (40.3) | 10/48 (20.8) | 2.56 (1.10-5.94) |
| Preterm birth (<37 weeks) singletons* | 2/17 (11.8) | 1/6 (16.7) | 0.66 (0.04-9.02) |
| Early preterm birth* | - | - | - |
| Low birth weight (<2.5 kg) singletons* | 4/17 (23.5) | 2/6 (33.3) | 0.61 (0.08-4.70) |
| Very low birth weight* | - | - | - |
Among the 146 cases of surgical sperm retrieval, 15 underwent TESA on TVOR day due to ejaculation failure and were excluded from analysis. TESA=Testicular sperm aspiration, TVOR=Transvaginal oocyte retrieval, OR=Odds ratio, CI=Confidence interval, *analyzed for singletons. Among 29 live births, 23 singletons were analyzed
DISCUSSION
The current study did not show any significantly increased risk of PTB and LBW following the use of ejaculate sperm compared to surgically retrieved sperm. There was no significant difference in the risk of congenital anomalies among the two groups. When the outcomes were compared between the epididymal and testicular sperm groups, though a trend toward a higher risk of PTB and LBW was noted in the testicular sperm group, it did not reach statistical significance. The comparison between OA and NOA showed significantly higher live birth rates in the OA group while the PTB and LBW showed a no statistical significance analyzed among the singletons.
A study by Deng et al. evaluated the neonatal outcomes after ICSI in 2512 cycles with testicular (n = 148), epididymal (n = 1031), and ejaculate sperm (n = 1333). For LBW, they observed no significant difference between testicular sperm versus ejaculate and epididymal sperm versus ejaculate. For PTB, the comparison between testicular sperm versus ejaculate and epididymal sperm versus ejaculate also did not show any significant difference. The congenital anomaly incidence among the three groups was 0 in the testicular group, 1.7% in the epididymal group, and 2.3% in the ejaculate group which showed no statistically significant difference.[11] The study by Kawass et al., which looked at the trends and perinatal outcomes in male factor ART (n = 347,078 cycles), showed no significant difference in the LBW between epididymal sperm versus ejaculate (adjusted risk ratio [aRR], 1.01; 95% CI [1.00–1.02]) and testicular versus ejaculate sperms (aRR, 1.00; 95% CI [0.99–1.01]) which is in agreement with our study. For PTB also, there was no significant difference between epididymal versus ejaculate sperm (aRR, 1.01; 95% CI [1.00–1.02]) and testicular source versus ejaculate (aRR, 1.00; 95% CI [0.99–1.01]), similar to our study.[12] A 10-year study in China from 2006 to 2016 (n = 10,520) assessed the neonatal outcomes in children born after ICSI from epididymal and testicular spermatozoa and compared them with ejaculate sperm. No differences were noted in the perinatal outcomes (P > 0.05). The congenital anomalies among the three groups also showed no difference (P > 0.05), which is similar to our study.[14] Few more retrospective studies which looked at the neonatal and perinatal outcomes in nonejaculate sperm also found no differences in the outcomes and congenital malformations.[15,16,17]
A population-based cohort study in Denmark from 1995 to 2009 compared the perinatal outcomes of the SSR group with three groups, i.e., the ejaculate ICSI, conventional in vitro fertilization (IVF), and naturally conceived (NC) groups. The observations of this study were similar to our study, with no differences in the LBW and PTB between the SSR group and the ejaculate ICSI group. The congenital malformation risk also did not show any difference when the SSR group was compared with the ejaculate ICSI group, which is in agreement with our study, but the risk of the cardiac defect was higher when the SSR group was compared with the conventional IVF and NC groups.[18]
The perinatal outcomes are influenced not only by the source of the sperm but also by the etiology of azoospermia. The testicular source of sperm in OA may have a different prognosis compared to the testicular source in NOA. The comparison of OA versus NOA in the current study is similar to the systematic review by Esteves and Agarwal, which showed significantly higher live births in the OA group, while the perinatal outcomes showed no significant difference between the groups. However, the PTB (17.9% vs. 9.4%; P = 0.10) and LBW (10.5% vs. 9.4%; P = 0.34) in this study were higher in the OA group in comparison to NOA and ejaculate which is in disagreement with the current study.[19] Another study by Jefferys et al. showed that the live birth rates and perinatal outcomes were not significantly different in both the OA and NOA groups, not in agreement with the current study in terms of live births.[20]
The current study is one of the few studies which compared the perinatal outcomes and congenital anomaly risk in the azoospermia population with male factor and combined factor infertility. The main concern for the couples undergoing IVF is the health of the baby. The testicular source is more likely to be associated with immature sperms and sometimes with certain genetic abnormalities (Y chromosome microdeletions) which can be transmitted to the offspring, and the information on perinatal outcomes can be used to counsel the couples accordingly.[21,22] The epididymal sperms are usually mature sperms, but they are more prone to oxidative stress and may ultimately affect the embryo quality and are thought to subsequently affect perinatal outcomes.[23]
The limitation of our study is its retrospective nature, and hence, the results need to be interpreted with caution. The small sample size was due to the exclusion of multiple births and restricting the analysis to singletons as higher-order pregnancies are inherently associated with adverse perinatal outcomes. In view of the lower incidence of azoospermia, an adequately powered study with a larger sample size would avoid a possible type II error. The available perinatal outcomes were restricted to LBW/PTB due to limitations in the data collection following birth raising the possibility of detection bias. There were no women who had early preterm or VLBW, and hence, these outcomes could not be analyzed. Some women were lost to follow-up, following referral to the obstetrics unit which could have led to attrition bias.
CONCLUSION
The current study showed no significant differences in the risk of adverse perinatal outcomes in the ejaculate group versus the surgically retrieved sperm groups. The risk of congenital anomalies was also not significantly different among the groups. When comparing the source of sperm, i.e., epididymal versus testicular, though PTB and LBW babies in singletons showed a trend toward higher risk in the testicular sperm group, it did not reach significance. The men with azoospermia planned for SSR can be counseled and reassured accordingly as the source of sperm does not affect the perinatal outcomes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Greil AL, McQuillan J, Lowry M, Shreffler KM. Infertility treatment and fertility-specific distress: A longitudinal analysis of a population-based sample of U.S. women. Soc Sci Med. 2011;73:87–94. doi: 10.1016/j.socscimed.2011.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thonneau P, Marchand S, Tallec A, Ferial ML, Ducot B, Lansac J, et al. Incidence and main causes of infertility in a resident population (1,850,000) of three French regions (1988-1989) Hum Reprod. 1991;6:811–6. doi: 10.1093/oxfordjournals.humrep.a137433. [DOI] [PubMed] [Google Scholar]
- 3.Jarow JP, Espeland MA, Lipshultz LI. Evaluation of the azoospermic patient. J Urol. 1989;142:62–5. doi: 10.1016/s0022-5347(17)38662-7. [DOI] [PubMed] [Google Scholar]
- 4.Jee SH, Hong YK. One-layer vasovasostomy: Microsurgical versus loupe-assisted. Fertil Steril. 2010;94:2308–11. doi: 10.1016/j.fertnstert.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 5.Kavoussi PK. Validation of robot-assisted vasectomy reversal. Asian J Androl. 2015;17:245–7. doi: 10.4103/1008-682X.142141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Int Braz J Urol. 2011;37:570–83. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 7.Esteves SC, Miyaoka R, Agarwal A. Sperm retrieval techniques for assisted reproduction. Nat Rev Dis Prim. 2019;5:1–21. doi: 10.1590/s1677-55382011000500002. [DOI] [PubMed] [Google Scholar]
- 8.Aboulghar MA, Mansour RT, Serour GI, Fahmy I, Kamal A, Tawab NA, et al. Fertilization and pregnancy rates after intracytoplasmic sperm injection using ejaculate semen and surgically retrieved sperm. Fertil Steril. 1997;68:108–11. doi: 10.1016/s0015-0282(97)81484-7. [DOI] [PubMed] [Google Scholar]
- 9.Ghazzawi IM, Sarraf MG, Taher MR, Khalifa FA. Comparison of the fertilizing capability of spermatozoa from ejaculates, epididymal aspirates and testicular biopsies using intracytoplasmic sperm injection. Hum Reprod. 1998;13:348–52. doi: 10.1093/humrep/13.2.348. [DOI] [PubMed] [Google Scholar]
- 10.Ubaldi F, Nagy ZP, Rienzi L, Tesarik J, Anniballo R, Franco G, et al. Reproductive capacity of spermatozoa from men with testicular failure. Hum Reprod. 1999;14:2796–800. doi: 10.1093/humrep/14.11.2796. [DOI] [PubMed] [Google Scholar]
- 11.Deng T, Guo N, Gu L, Yuan X, Du Y, Hua X, et al. Pregnancy and neonatal outcomes after ICSI with testicular, epididymal, or ejaculated sperm: Analysis of 2,512 cycles during an 8-year period. Int J Clin Exp Med. 2019;12:10712–20. [Google Scholar]
- 12.Kawwass JF, Chang J, Boulet SL, Nangia A, Mehta A, Kissin DM. Surgically acquired sperm use for assisted reproductive technology: Trends and perinatal outcomes, USA, 2004-2015. J Assist Reprod Genet. 2018;35:1229–37. doi: 10.1007/s10815-018-1178-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, et al. The international glossary on infertility and fertility care, 2017. Fertil Steril. 2017;108:393–406. doi: 10.1016/j.fertnstert.2017.06.005. [DOI] [PubMed] [Google Scholar]
- 14.Jin L, Li Z, Gu L, Huang B. Neonatal outcome of children born after ICSI with epididymal or testicular sperm: A 10-year study in China. Sci Rep. 2020;10:5145. doi: 10.1038/s41598-020-62102-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Belva F, de Schrijver F, Tournaye H, Liebaers I, Devroey P, Haentjens P, et al. Neonatal outcome of 724 children born after ICSI using non-ejaculated sperm. Hum Reprod. 2011;26:1752–8. doi: 10.1093/humrep/der121. [DOI] [PubMed] [Google Scholar]
- 16.Yh G, Rn D, Yc Su, Li J, Zhang YJ, Sun YP. Follow-up of children born after intracytoplasmic sperm injection with epididymal and testicular spermatozoa. Chin Med J (Engl) 2013;126:2129–33. [PubMed] [Google Scholar]
- 17.Wennerholm UB, Bergh C, Hamberger L, Westlander G, Wikland M, Wood M. Obstetric outcome of pregnancies following ICSI, classified according to sperm origin and quality. Hum Reprod. 2000;15:1189–94. doi: 10.1093/humrep/15.5.1189. [DOI] [PubMed] [Google Scholar]
- 18.Fedder J, Loft A, Parner ET, Rasmussen S, Pinborg A. Neonatal outcome and congenital malformations in children born after ICSI with testicular or epididymal sperm: A controlled national cohort study. Hum Reprod. 2013;28:230–40. doi: 10.1093/humrep/des377. [DOI] [PubMed] [Google Scholar]
- 19.Esteves SC, Agarwal A. Reproductive outcomes, including neonatal data, following sperm injection in men with obstructive and nonobstructive azoospermia: Case series and systematic review. Clinics. 2013;68:141–9. doi: 10.6061/clinics/2013(Sup01)16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jefferys AE, Griffith H, Wilson P, Gordon UD. Cohort study of perinatal outcomes of children born following surgical sperm recovery. Hum Fertil. 2016;19:207–11. doi: 10.1080/14647273.2016.1218071. [DOI] [PubMed] [Google Scholar]
- 21.Woldringh GH, Besselink DE, Tillema AH, Hendriks JC, Kremer JA. Karyotyping, congenital anomalies and follow-up of children after intracytoplasmic sperm injection with non-ejaculated sperm: A systematic review. Hum Reprod Update. 2010;16:12–9. doi: 10.1093/humupd/dmp030. [DOI] [PubMed] [Google Scholar]
- 22.Silber SJ, Devroey P, Tournaye H, van Steirteghem AC. Fertilizing capacity of epididymal and testicular sperm using intracytoplasmic sperm injection (ICSI) Reprod Fertil Dev. 1995;7:281–92. doi: 10.1071/rd9950281. [DOI] [PubMed] [Google Scholar]
- 23.Silber SJ. The use of epididymal sperm for the treatment of male infertility. Baillieres Clin Obstet Gynaecol. 1997;11:739–52. doi: 10.1016/s0950-3552(97)80010-7. [DOI] [PubMed] [Google Scholar]


