Abstract
To date the locus with the most robust human genetic association to COVID-19 susceptibility is 3p21.31. Here, we integrate genome-scale CRISPR loss-of-function screens and eQTLs in diverse cell types and tissues to pinpoint genes underlying COVID-19 risk. Our findings identify SLC6A20 and CXCR6 as putative causal genes that mediate COVID-19 risk and highlight the usefulness of this integrative approach to bridge the divide between correlational and causal studies of human biology.
Keywords: COVID-19, SARS-CoV-2, GWAS, eQTL, CRISPR
Background
COVID-19, which is caused by SARS-CoV-2 (Severe Acute Respiratory Syndrome-Coronavirus-2) infection, results in diverse individual disease courses from asymptotic carriers to severe disease with respiratory failure [1, 2]. A growing body of evidence suggests also a notable role of genetic factors in COVID-19 susceptibility and severity [3–5], and several studies have identified a robust association at chromosome 3p21.31 locus. However, the functional mechanisms of this association are unclear. The locus includes multiple protein-coding genes, for example, LIMD1, SACM1L, SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, and XCR1, many of which have potentially relevant role in the pathophysiology of COVID-19. As genetic variants have been shown to often exert their effect on complex traits or disease via cis-regulation of gene expression [6, 7], expression quantitative trait locus (eQTL) mapping could serve as a mean to pinpoint candidate genes for trait or diseases of interest.
We previously performed a genome-scale CRISPR loss-of-function screen to identify genes required for SARS-CoV-2 viral infection in human lung epithelial-like cells expressing ACE2 (A549ACE2) [8]. In this work, we present an integrative approach using the results of the CRISPR screen and eQTLs in various cell types and tissues from the eQTL Catalogue [9] and Genotype Tissue Expression (GTEx) [7] v8 data release to pinpoint genes underlying COVID-19 risk in the chr3 locus. We show that genes enriched in the in vitro CRISPR screen contribute to COVID-19 risk in humans, and highlight SLC6A20 and CXCR6 as the putative causal genes in the chr3 COVID-19 risk locus.
Results and Discussion
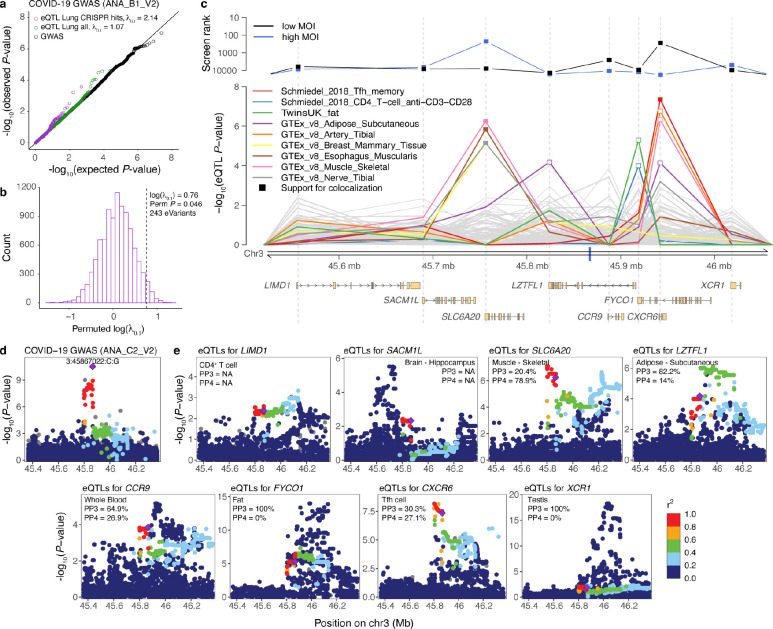
We sought to determine to what extent the top-ranked genes from the CRISPR screen — whose loss reduces SARS-CoV-2 infection — mediate disease risk in humans by leveraging data from COVID-19 genome-wide association study (GWAS). We hypothesized that genetic regulatory variants for genes pinpointed by the CRISPR screen would show an increased signal for genetic association for COVID-19 in the human population. To this end, we analyzed if eQTLs from lung tissue [7] for these genes would be enriched for overall association signal in data from the COVID-19 Host Genetic Initiative [4] with six COVID-19-related GWAS (release 3) with different definition of cases and controls. Indeed, we observed inflation of the GWAS association signal for variants that are cis-eQTLs for top-ranked CRISPR hit genes in the hospitalized vs not hospitalized COVID-19 GWAS, compared to all lung eQTLs (λ0.1 = 2.14, permutation P-value = 0.046, Fig. 1a–b). We did not see a significant signal for the other five GWAS, which could be explained by the low power of these early GWAS data (Additional file 1: Figure S1). Nevertheless, our results establish the link between genes enriched in the in vitro CRISPR screen and the genetic component of COVID-19 risk in humans.
Fig. 1. Genetic regulatory effects of CRISPR hit genes and prioritization of genes in the 3p21.31 cluster associated with COVID-19 GWAS.
a, Q-Q plot showing the expected and observed P-value distribution of hospitalized vs not hospitalized COVID-19 GWAS (ANA_B1_V2) for three sets of variants: all variants tested in GWAS (black), variants that are lead eQTLs in GTEx Lung (green), and variants that are lead eQTLs in GTEx Lung for the top-ranked genes enriched in at least one CRISPR screen (purple). Inflation estimate λ0.1 measures the inflation of test statistics relative to the chi-square quantile function of 0.1. b, Histogram of the permuted log(λ0.1) to test the significance of the inflation of variants that are eQTLs for CRISPR screen hit genes (n = 243) in Lung in ANA_B1_V2 COVID-19 GWAS. c, Prioritization of genes in the 3p21.31 locus associated with COVID-19 vs population (ANA_C2_V2) GWAS. The top panel shows the ranking of the genes in the locus according to the second-best guide RNA score in the low MOI (black) and high MOI (blue) pooled CRISPR screens. The middle panel shows the eQTL P-values for the lead GWAS variant 3:45867022:C:G (denoted as a blue tick on the x-axis) in different cell types and tissues from the eQTL Catalogue and GTEx v8, 110 eQTL data sets in total. Highlighted are nine cell types/tissues, where the eQTL P-value for the lead GWAS variant is < 10−4 for at least one gene in the region. Filled square denotes suggestive support for colocalization between the GWAS and eQTL signal (posterior probability for one shared causal variant (PP4) > 0.25). The bottom panel depicts the transcripts of the eight protein-coding genes in the locus. Ranks and eQTL P-values are aligned to match the start of the gene which is shown as a grey dashed line across the panels. d-e, Regional association plots of the COVID-19 vs population (ANA-C2-V2) GWAS (d) and cis-eQTLs for the eight genes (e) from the associated locus in the cell type/tissue where the lead GWAS variant has the lowest eQTL P-value. Purple diamond denotes the lead GWAS variant, and the data points are colored based on the (weighted average) LD between the lead GWAS variant and other variants in the region in the respective study population. PP3 and PP4 - posterior probability for two different variants or one shared causal variant in coloc, respectively.
To elucidate which genes in the chromosome 3 locus might mediate the genetic association, we first observed that Solute Carrier Family 6 Member 20 (SLC6A20) gene and C-X-C Motif Chemokine Receptor 6 (CXCR6) gene have a relatively high rank in our CRISPR screen (Fig. 1c). Next, we analyzed if the COVID-19 ANA_C2_V2 GWAS lead variant 3:45867022:C:G affects expression of any of these genes. Using data from the eQTL Catalogue [9] and GTEx v8 [7], we analyzed all eQTL associations for this variant, and performed colocalization analysis [10, 11] to assess if the eQTL and GWAS signal share a genetic cause. CXCR6 and SLC6A20 stood out, with the eQTL data indicating that the COVID-19-associating variant affects the expression of these two genes (Fig. 1c, Additional file 1: Figure S2, Additional file 2: Table S1). The eQTL affecting CXCR6 is active in memory T follicular helper (Tfh) CD4+ T cells [12] and has a suggestive colocalization with the GWAS (Fig. 1d–e). The colocalization signal was very strong for the cis-eQTL for SLC6A20 in four tissues from GTEx - esophagus muscularis, breast mammary tissue, skeletal muscle, and tibial nerve (Additional file 1: Figure S3). Other genes in the region, such as LZTFL1, FYCO1, and XCR1, also have regulatory variants affecting their expression, but two distinct variants likely drive the GWAS and eQTL signals (Fig 1d–e). Given that both the CRISPR screen and eQTL data support the causal role of SLC6A20 and CXCR6, it is possible that the COVID-19 GWAS association in the 3p21.31 locus is driven by pleiotropic effects of the same variant on multiple genes in different cell types.
In addition to human genetic evidence and support from experimental data, both SLC6A20 and CXCR6 have a plausible biological function that could affect COVID-19. Notably, SLC6A20 functionally interacts with ACE2 [13], the receptor of the SARS-CoV-2 Spike protein that is the key host gene for viral entry [14]. In contrast, CXCR6 regulates the localization of resident memory T (TRM) cells in the lung and maintains a pool of airway TRM cells, critical for cellular immunity against respiratory pathogens [15]. It is also an alternate coreceptor for HIV [16, 17], raising a hypothesis of a similar function in lung cells for SARS-CoV-2. The fact that we observed eQTLs for SLC6A20 and CXCR6 in different tissues and cell types other than lung highlights the complexity and possibility of extrapulmonary spread of SARS-CoV-2 [18], as well as potential pleiotropic effects of these genes in multiple cell types and physiological processes leading to COVID-19. Importantly, GWAS can only show associations for common variants in the human population, and thus, the functional role of these genes in lung cells, captured by the screen, may not be captured by GWAS and eQTL data. Nevertheless, corroborating evidence from entirely independent approaches pinpointing SLC6A20 and CXCR6 warrants further studies exploring the virological or mechanistic mode of action of these two genes.
Conclusion
In this work, we demonstrated that genes required for SARS-CoV-2 infection in vitro also contribute to COVID-19 susceptibility in humans. Our integrative analysis of the 3p21.31 GWAS locus indicated SLC6A20 and CXCR6 as potential protein-coding genes through which noncoding variants associated with COVID-19 risk in human patients may function. This integrative approach should prove useful for other human diseases and pathogens to bridge the divide between correlational and causal studies of human biology.
Methods
Genome-wide CRISPR screen and guide RNA analysis
Details regarding the SARS-2 CRISPR screen in A549ACE2 human lung epithelial cells that overexpress ACE2 (A549ACE2) have been described before [8]. Briefly, human GeCKOv2 A and B libraries (Addgene, 1000000048) were used for the genome-wide CRISPR-Cas9 screen [19]. After transduction and selection for the GeCKOv2 library (maintaining ~1000-fold library representation), we infected 125 million A549ACE2 cells with SARS-CoV-2 virus (NIAID BEI isolate USA-WA1/2020) at two multiplicity of infections (MOIs): 0.01 (low MOI) and 0.3 (high MOI), which differ by the amount of virus used to infect the cells. Surviving cells were collected on day 6 post-infection, when genomic DNA was isolated, guide RNAs were recovered by PCR, and guide representation was determined by next generation sequencing (Illumina).
We aligned trimmed sequencing reads to the GeCKOv2 reference using bowtie v1.1.2 [-a --best --strata -v 1 –norc] allowing 1 nucleotide mismatch to determine the read counts per guide. Alignment rates were ~80% for all samples. We normalized read counts between biological samples and computed a fold-change by comparison of SARS-CoV-2-infected samples to the uninfected control. Genes were then ranked based on the second-best guide as described before [20–22]. Briefly, each gene in the human GeCKOv2 A+B library is targeted by 6 different guides. Genes are ranked based on the guide RNA with second highest fold-change (sbScore).
Inflation of COVID-19 GWAS signal for variants that are cis-eQTLs
To estimate the importance of CRISPR screen hit genes in susceptibility to COVID-19 in humans, we tested if we observe inflation of COVID-19 GWAS signal for variants that are eQTLs in GTEx v8 lung [7] for top-ranked genes enriched in at least one CRISPR screen (n = 588 genes). We used summary statistics of the six COVID-19 GWAS generated by the COVID-19 Human Genetics Initiative (HGI) [4] (round 3 meta-analyses, released July 2, 2020): 1) ANA_A2_V2 - very severe respiratory confirmed covid vs. population, 2) ANA_B1_V2 - hospitalized covid vs. not hospitalized covid, 3) ANA_B2_V2 - hospitalized covid vs. population, 4) ANA_C1_V2 - covid vs. lab/self-reported negative, 5) ANA_C2_V2 - covid vs. population, 6) ANA_D1_V2 - predicted covid from self-reported symptoms vs. predicted or self-reported non-covid. We measured inflation using the lambda inflation statistic relative to the chi-square quantile function of 0.1, λ0.1, i.e., estimating the inflation among 10% of the most significant tests. We calculated λ0.1 for two sets of eQTLs: 1) all lead eQTLs in lung (14,113 genes with significant eQTLs in lung at FDR 5%, serving as a background set), 2) all lead eQTLs in lung for CRISPR hit genes (274 out 588 top-ranked genes have eQTLs in lung at FDR < 0.05).
To test the significance of λ0.1, we used a permutation-based test. We selected n number of lead eQTLs from the background set k = 10,000 times, where n is the number of lead eQTLs for CRISPR hit genes tested in a given COVID-19 GWAS, and calculated λ0.1 on the permuted data. To calculate two-sided permutation P-values, we applied log-transformation on the permuted λ0.1 values to get a symmetric null distribution. We then calculated permutation P-value as the proportion of permuted log(λ0.1) as extreme as or more extreme than the observed log(λ0.1).
Prioritization of genes in the 3p21.31 locus based on CRISPR screen and eQTL data
We focused on eight genes, LIMD1, SACM1L, SLC6A20, LZTFL1, CCR9, FYCO1, CXCR6, and XCR1, in the 3p21.31 locus that has been shown to robustly associate with COVID-19 susceptibility [3–5]. Firstly, we ranked the genes based on the sbScore in the CRISPR screen. Secondly, we gathered summary statistics for 110 eQTL data sets from the eQTL Catalogue [9], (61 data sets, mostly immune cell types with and without treatment) and GTEx v8 [7] (49 tissues). We prioritized the genes based on the P-value in eQTL studies for the lead GWAS variant 3:45867022:C:G from the covid vs population (ANA_C2_V2, largest COVID-19 GWAS to date with 6,696 total cases and 1,073,072 total controls) GWAS generated by the COVID-19 HGI. Since observing low eQTL P-value for the lead GWAS variant does not necessarily translate into shared genetic causality, we further performed colocalization analysis.
Colocalization analysis of COVID-19 GWAS and cis-eQTLs in the 3p21.31 locus
To assess evidence for shared causal variant of a cis-eQTL and a COVID-19 GWAS, we used the Bayesian statistical test for colocalization, coloc [10], assuming one causal variant per trait. We only included cis-eQTLs for genes for colocalization test, if there was a cis-eQTL with a nominal P-value < 10−4 within 100kb of the lead ANA_C2_V2 COVID-19 GWAS variant. Coloc was run on a 1Mb region centered on the lead GWAS variant (+/− 500 kb from the variant) with prior probabilities set to p1 = 10−4, p2 = 10−4, p3 = 5×10−6. We defined suggestive support for colocalization between the ANA_C2_V2 COVID-19 GWAS and cis-eQTL signal if posterior probability for one shared causal variant (PP4) > 0.25.
Allelic heterogeneity of gene expression in cis is widespread [7], and it violates the assumption of one causal variant per trait. Thus, we also used the development version of coloc [11] (https://github.com/chr1swallace/coloc/tree/condmask) in a wider 2Mb region centered at the lead ANA_C2_V2 COVID-19 GWAS variant. The enhanced version of coloc allows conditioning and masking to overcome one single causal variant assumption. For eQTL data sets from the eQTL Catalogue, we used method = mask with LD data from the 1000 Genomes Project [23] CEU population that matches the genetic ancestry of the population in majority of the studies in the eQTL Catalogue. For eQTL data sets from the GTEx Project, we used method = cond with LD calculated from the individuals that had gene expression data in the given tissue in GTEx. We used the mode = iterative and allowed for a maximum of three variants to condition/mask. We set the r2 threshold to 0.01 to call two signals independent when masking, and we set the P-value threshold to 10−4 to call the secondary signal significant. We used method = single for the ANA_C2_V2 COVID-19 GWAS.
As a result, the maximum PP4 (posterior probability for shared causal variants) estimates with conditioning/masking were similar to PP4 estimates from the standard coloc run (Additional file 2: Table S1), suggesting no additional colocalization events with secondary independent cis-eQTLs.
Regional association plots for the 3p21.31 locus
Data points in the locuszoom-like regional association plots are colored by the LD between the lead ANA_C2_V2 COVID-19 GWAS variant 3:45867022:C:G and other variants in the region. For plotting data for COVID-19 GWAS and eQTLs from the eQTL Catalogue, we used genotype data from the 1000 Genomes Project, and calculated weighted average r2 value based on the counts of global ancestral populations in the analysis (if multiple global ancestry populations were studied). Note that for European populations, we only included non-Finnish populations in the 1000 Genomes Project. For plotting data for eQTLs from the GTEx project, we used genotype data available via dbGaP, accession phs000424.v8, and calculated r2 using the set of individuals that had expression data from the given tissue.
Supplementary Material
Acknowledgements
We thank the COVID-19 Human Genetics Initiative for making the results from meta-analyses public.
Funding
S.K. and T.L. are supported by NIH/NHLBI (R01HL142028). Z.D. is supported by an American Heart Association postdoctoral fellowship (grant no. 20POST35220040). Postdoctoral fellowship support for T.X.J. is provided by the NIH (grant no. R01AI123155). B.R.t. is supported by the Marc Haas Foundation, the National Institutes of Health, and DARPA’s PREPARE Program (HR0011-20-2-0040). N.E.S. is supported by NYU and NYGC startup funds, NIH/NHGRI (DP2HG010099), NIH/NCI (R01CA218668), DARPA (D18AP00053), the Sidney Kimmel Foundation, and the Brain and Behavior Foundation. T.L. is supported by NIH/NIMH (R01MH106842) and NIH/NHGRI (UM1HG008901).
Footnotes
Ethics approval and consent to participate
Not applicable
Consent for publication
Not applicable
Availability of data and materials
The datasets analyzed during the current study are available from the following repositories: CRISPR screen data can be accessed from GEO repository under an accession number GSE158298 (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE158298), summary statistics of the COVID-19 GWAS (release 3) by the COVID-19 Human Genetics Initiative are available from https://www.covid19hg.org/results/r3/, eQTL summary statistics can be downloaded from the eQTL Catalogue (https://www.ebi.ac.uk/eqtl/) and the GTEx Portal (https://gtexportal.org/).
Competing interests
N.E.S. is an advisor to Vertex. T.L. advises and has equity in Variant Bio, and is a member of the scientific advisory board of Goldfinch Bio. The remaining authors declare that they have no competing interests.
References
- 1.Guan W-J, Ni Z-Y, Hu Y, Liang W-H, Ou C-Q, He J-X, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N Engl J Med. 2020;382:1708–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Goyal P, Choi JJ, Pinheiro LC, Schenck EJ, Chen R, Jabri A, et al. Clinical Characteristics of Covid-19 in New York City. N Engl J Med. 2020;382:2372–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ellinghaus D, Degenhardt F, Bujanda L, Buti M, Albillos A, Invernizzi P, et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N Engl J Med. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.COVID-19 Host Genetics Initiative. The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur J Hum Genet EJHG. 2020;28:715–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shelton JF, Shastri AJ, Ye C, Weldon CH, Filshtein-Somnez T, Coker D, et al. Trans-ethnic analysis reveals genetic and non-genetic associations with COVID-19 susceptibility and severity. Preprint at medRxiv 10.1101/2020.09.04.20188318. 2020. [DOI] [PubMed] [Google Scholar]
- 6.Gamazon ER, Segrè AV, van de Bunt M, Wen X, Xi HS, Hormozdiari F, et al. Using an atlas of gene regulation across 44 human tissues to inform complex disease- and trait-associated variation. Nat Genet. 2018;50:956–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Consortium GTEx. The GTEx Consortium atlas of genetic regulatory effects across human tissues. Science. 2020;369:1318–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Daniloski Z, Jordan TX, Wessels H-H, Hoagland DA, Kasela S, Legut M, et al. Identification of required host factors for SARS-CoV-2 infection in cells. Cell. 2021;184(1):92–105.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerimov N, Hayhurst JD, Manning JR, Walter P, Kolberg L, Peikova K, et al. eQTL Catalogue: a compendium of uniformly processed human gene expression and splicing QTLs. Preprint at bioRxiv 10.1101/2020.01.29.924266. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giambartolomei C, Vukcevic D, Schadt EE, Franke L, Hingorani AD, Wallace C, et al. Bayesian test for colocalisation between pairs of genetic association studies using summary statistics. PLoS Genet. 2014;10:e1004383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace C. Eliciting priors and relaxing the single causal variant assumption in colocalisation analyses. PLoS Genet. 2020;16:e1008720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schmiedel BJ, Singh D, Madrigal A, Valdovino-Gonzalez AG, White BM, Zapardiel-Gonzalo J, et al. Impact of Genetic Polymorphisms on Human Immune Cell Gene Expression. Cell. 2018;175:1701–1715.e16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vuille-dit-Bille RN, Camargo SM, Emmenegger L, Sasse T, Kummer E, Jando J, et al. Human intestine luminal ACE2 and amino acid transporter expression increased by ACE-inhibitors. Amino Acids. 2015;47:693–705. [DOI] [PubMed] [Google Scholar]
- 14.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181:271–280.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wein AN, McMaster SR, Takamura S, Dunbar PR, Cartwright EK, Hayward SL, et al. CXCR6 regulates localization of tissue-resident memory CD8 T cells to the airways. J Exp Med. 2019;216:2748–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liao F, Alkhatib G, Peden KW, Sharma G, Berger EA, Farber JM. STRL33, A novel chemokine receptor-like protein, functions as a fusion cofactor for both macrophage-tropic and T cell line-tropic HIV-1. J Exp Med. 1997;185:2015–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Blaak H, Boers PHM, Gruters RA, Schuitemaker H, van der Ende ME, Osterhaus ADME. CCR5, GPR15, and CXCR6 Are Major Coreceptors of Human Immunodeficiency Virus Type 2 Variants Isolated from Individuals with and without Plasma Viremia. J Virol. 2005;79:1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gupta A, Madhavan MV, Sehgal K, Nair N, Mahajan S, Sehrawat TS, et al. Extrapulmonary manifestations of COVID-19. Nat Med. 2020;26:1017–32. [DOI] [PubMed] [Google Scholar]
- 19.Sanjana NE, Shalem O, Zhang F. Improved vectors and genome-wide libraries for CRISPR screening. Nat Methods. 2014;11:783–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen S, Sanjana NE, Zheng K, Shalem O, Lee K, Shi X, et al. Genome-wide CRISPR screen in a mouse model of tumor growth and metastasis. Cell. 2015;160:1246–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shalem O, Sanjana NE, Hartenian E, Shi X, Scott DA, Mikkelson T, et al. Genome-scale CRISPR-Cas9 knockout screening in human cells. Science. 2014;343:84–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel SJ, Sanjana NE, Kishton RJ, Eidizadeh A, Vodnala SK, Cam M, et al. Identification of essential genes for cancer immunotherapy. Nature. 2017;548:537–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.1000 Genomes Project Consortium, Auton A, Brooks LD, Durbin RM, Garrison EP, Kang HM, et al. A global reference for human genetic variation. Nature. 2015;526:68–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.