Abstract
NF-κB inhibitor ζ (NFKBIZ), a member of the IκB family that interacts with NF-κB, has been reported to be an important regulator of inflammation, cell proliferation and survival. However, the role of NFKBIZ in bladder cancer (BC) remains unknown. The present study aimed to investigate the functions of NFKBIZ in BC. First, the expression levels of NFKBIZ and the associations between NFKBIZ expression and the clinical survival of patients were determined using BC tissue samples, BC cell lines and datasets from different databases. Two BC cell lines (T24 and 5637) were selected to overexpress NFKBIZ, and the proliferative, migratory and invasive abilities of cells were determined; additionally, tumor growth following transplantation in in vivo mouse models was analyzed using T24 cells overexpressing NFKBIZ. Subsequently, the association between NFKBIZ and PTEN was determined using data from databases and immunohistochemistry analysis of clinical and nude mice tumor tissues. Finally, the interactions between NFKBIZ, PTEN and the downstream PI3K/AKT/mTOR signaling pathway were evaluated using western blotting. In conclusion, the present results indicated that NFKBIZ expression was low in BC, and NFKBIZ inhibited the proliferation of BC cells through the PTEN/PI3K/Akt signaling pathway, suggesting that NFKBIZ may represent a novel prognostic biomarker in BC and may provide a potential therapeutic tumor-associated antigen for BC.
Keywords: bladder cancer, NF-κB inhibitor ζ, PTEN, proliferation, migration, invasion
Introduction
According to global cancer data, bladder cancer (BC) is estimated to be the 9th most common type of cancer worldwide, with ~400,000 new cases diagnosed annually (1,2). The majority of BC cases are diagnosed as non-muscle invasive BC (NMIBC), for which the mortality rate is generally low due to its good prognosis; however, NMIBC often recurs and develops into MIBC (3). MIBC is characterized by rapid metastasis and progression, in addition to poor prognosis, and is the main cause of BC-associated mortality (4-6), with the 5-year overall survival rate being ~50% following surgery (7). Therefore, it remains urgent to identify novel molecular biomarkers and the underlying molecular mechanism of BC tumorigenesis.
NF-κB can be activated following the stimulation of various types of immune receptors, such as pattern recognition receptors and toll-like receptors, and its activation is controlled by the direct interaction with the IκB inhibitor family (8-10). The most well-established IκB members are the proteins IκBα, IκBβ and IκBε, which keep NF-κB in the cytoplasm of unstimulated cells (11). IκBζ is a newly identified IκB family member protein, which is encoded by the NF-κB inhibitor ζ (NFKBIZ) gene; it differs from IκBα, IκBβ and IκBε as it is atypically localized in the nucleus rather than in the cytoplasm (12). Notably, NFKBIZ has been reported to be an important regulator of inflammation, cell proliferation and survival (12-14). NFKBIZ is induced by toll-like receptor 12 signaling and can coactivate both the canonical and non-canonical NF-κB signaling pathways (15-17). Polymorphisms and mutations in NFKBIZ are associated with numerous types of disease, including psoriasis, spontaneous skin inflammation, invasive pneumococcal disease and Crohn's disease (18-21); the majority of these diseases are associated with inflammation. Furthermore, NFKBIZ has been associated with cancer. For example, NFKBIZ expression is upregulated in glioma and is associated with a poor prognosis (22). In addition, NFKBIZ expression is downregulated in mycosis fungoides, which is the most common type of primary cutaneous T-cell lymphoma (23). Additionally, it has been demonstrated that tumor formation in NFKBIZ-mutant mice is significantly decreased, and low NFKBIZ expression in human colorectal cancer cells affects cell proliferation (24). However, the mechanism underlying the inhibition of tumor growth and whether NFKBIZ can regulate other types of human carcinoma cells has not been elucidated.
Numerous signaling pathways are involved in the occurrence and development of BC. Among them, the PI3K/AKT/mTOR signaling pathway serves a crucial role in the pathogenesis of BC, where it is usually overactivated and plays a central role in stimulating cell proliferation, survival and metastasis (25-28). PTEN is an ubiquitous tumor suppressor that serves an essential role in tumor cell proliferation (25). Notably, PTEN inactivation has been associated with the pathogenesis and development of numerous types of solid tumors, such as colon, gastric and lung cancer (29-31). PTEN inhibits the PI3K/AKT signaling pathway, and a negative feedback association exists between PTEN and phosphorylated (p)-PI3K, indicating that a decrease in p-PI3K expression leads to an increase in PTEN expression (27). Conversely, the loss of PTEN function leads to the overactivation of the PI3K/AKT signaling pathway, which has been demonstrated to accelerate the growth, invasion and metastasis of BC (28). In addition, NF-κB and PTEN are closely associated in numerous types of cancer, such as colon, gastric and lung cancer (29-31), and NF-κB is regulated by NFKBIZ.
Therefore, the present study aimed to explore the association between NFKBIZ and PTEN, and whether NFKBIZ can affect the development of BC by regulating PTEN and its downstream PI3K/Akt signaling pathway. The expression levels of NFKBIZ in BC were analyzed, and bioinformatics analysis was used to determine the potential regulatory mechanism and clinical significance of NFKBIZ in BC. In addition, functional experiments were performed to investigate the function of NFKBIZ in BC. The current findings may provide novel insights into the role of NFKBIZ in BC tumorigenesis.
Materials and methods
Cell culture
The normal urinary tract epithelial cell line (SV-HUC-1) and the BC cell lines (J82, UMUC-3, T24 and 5637) were purchased from The Cell Bank of Type Culture Collection of The Chinese Academy of Sciences. The identification of the BC cell lines was conducted at the China Centre for Type Culture Collection. SV-HUC-1 cells were cultured in F-12K medium (HyClone; Cytiva) with 10% fetal calf serum (FCS; HyClone; Cytiva). J82 and UMUC-3 cells were cultured in Minimum Essential Media (HyClone; Cytiva) supplemented with 10% FCS. T24 and 5637 cells were cultured in RPMI-1640 medium (HyClone; Cytiva) supplemented with 10% FCS. Cells were maintained in a humidified atmosphere consisting of 5% CO2 at 37°C.
Bioinformatics analysis and NFKBIZ expression profile mining in The Cancer Genome Atlas (TCGA)
Relevant data or figures from TCGA database (http://cancergenome.nih.gov) were downloaded and analyzed using the Gene Expression Profiling Interacting Analysis (GEPIA; http://gepia.cancer-pku.cn), UCSC Xena (http://xena.ucsc.edu) and UALCAN (http://ualcan.path.uab.edu) databases. The data from GEPIA included NFKBIZ expression from 404 tumor and 28 adjacent normal control tissues. The data from UCSC Xena included NFKBIZ expression from 407 tumor and 29 adjacent normal control tissues. The data from UALCAN included NFKBIZ expression from 408 tumor and 19 adjacent normal control tissues. NFKBIZ mRNA expression was also explored at different stages, nodal metastasis status and histological subtypes using the UALCAN database. Finally, the 'TCGAbiolinks' package in R language (https://www.rdocumentation.org/packages/TCGAbiolinks/versions/1.2.5) was used to download the mRNA-reads count expression data from TCGA-BLCA, including 408 BC and 19 normal bladder tissue samples. An mRNA expression matrix was made with the raw counts of each RNA in each sample. The 'Deseq2' package in R (http://www.bioconductor.org/packages/release/bioc/html/DESeq2.html) was used to calculate the differential expression of mRNAs between the BC tissues samples and paracancerous normal specimens. A |fold-change| >2 and P<0.05 were used as the threshold values. Survival analysis was performed using these differentially expressed mRNAs using the 'ggsurv' package in R (https://www.rdocumentation.org/packages/GGally/versions/1.5.0/topics/ggsurv), with P<0.05 used as the screening threshold. The survival curves based on NFKBIZ expression were used to explore the effect of NFKBIZ expression on survival and prognosis of patients with BC. Kaplan-Meier analysis was used for survival analysis, and the log-rank test was used to calculate the P-values.
Chemicals and lentivirus infection
Matrigel was purchased from BD Biosciences and the PTEN inhibitor, VO-OHpic trihydrate (VO), was obtained from MedChemExpress. The treatment condition of VO was 35 nM/l at room temperature for 24 h. A lentiviral expression vector (pLV-NFKBIZ) [OBiO Technology (Shanghai) Corp., Ltd.] was used for NFKBIZ gene (NM_031419.4) delivery and stable overexpression. The MOI used to infect T24 and 5637 cells was 40 and 20, respectively. T24 and 5637 cells (1×105) were seeded in 6-well plates and grown to ~50% confluency. Subsequently, the culture medium was removed, and fresh culture medium containing lentiviral carrying NFKBIZ cDNA or a negative control was added according to the MOI. The cells were cultured in an incubator at 37°C with 5% CO2 for 18 h. Next, the culture medium was removed and replaced with fresh medium. After 48 h, culture medium containing 50 µg/ml puromycin (Sigma-Aldrich; Merck KGaA) was added to remove any non-transfected cells. The surviving cell clones were selected and expanded. The lentiviruses were designated as pcDNA-NFKBIZ (pSLenti-EF 1a-EGFP-P2A-Puro-CMV-NFKBIZ-3Flag). The empty vector was used as a negative control (pcDNA-vector; pSLenti-EF1a-E GFP-P2A-Puro-CMV-MCS-3Flag). Fluorescence microscopy (magnification, ×40; IX73; Olympus Corporation) and western blotting were used to evaluate infection efficiency.
Patient tissues
A total of 34 pairs of fresh BC and adjacent tissue specimens (>5 cm from the tumor) were obtained from patients (25 males and 9 females) with a median age of 65 years (range, 46-79 years) with BC who were diagnosed by the Pathology Department of The Renmin Hospital of Wuhan University (Wuhan, China) between July 2019 and December 2019. A small part of the resected tissue was removed within 30 min of surgical resection. The removed tissue was divided into two parts: A part of the tissue was washed with saline and then fixed with 4% paraformaldehyde (Beyotime Institute of Biotechnology) for 48 h at room temperature for paraffin embedding, and another part of the removed tissue was rapidly frozen in liquid nitrogen (-196°C) and stored at −80°C until required for RNA extraction. Most of the remaining resected tissues were sent to the pathology department for further diagnosis. BC was defined by two pathologists. The tumor stage and grade of all patients were diagnosed according to the 2009 TNM staging system and the 2004 World Health Organization grading system (32,33), respectively. All patients provided written informed consent and the research protocols were approved by the Research Ethics Committee of Renmin Hospital of Wuhan University (approval no. WDRY2019-K035).
Reverse transcription-quantitative PCR (RT-qPCR)
Total RNA was extracted from BC samples using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Inc.) and reverse transcribed into cDNA using a RevertAid First Strand cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. qPCR was subsequently performed using a SYBR Green mix (Takara Bio, Inc.), at a final volume of 20 µl, on an ABI 7500 Real-Time PCR Detection system (Applied Biosystems; Thermo Fisher Scientific, Inc.). The following primer sequences were used for qPCR: NFKBIZ forward, 5′-CCG ATT CGT TGT CTG ATG GAC C-3′ and reverse, 5′-GCA CTG CTC TCC TGT TTG GGT T-3′; and GAPDH forward, 5′-CCT TCA TTG ACC TCA ACT ACA-3′ and reverse, 5′-GCT CCT GGA AGA TGG TGA T-3′. The following thermocycling conditions were used for qPCR: Initial denaturation at 95°C for 5 min; followed by 40 cycles at 95°C for 10 sec and 60°C for 35 sec. The melting curve step occurred at 60-95°C, at increments of 0.5 sec for 5 sec. The relative expression levels of NFKBIZ were calculated using the 2−ΔΔCq method (34) and GAPDH served as the endogenous reference gene to normalize the expression levels of target mRNAs.
Western blotting
Western blotting was performed as previously described (35). Briefly, total protein was extracted from transfected T24 and 5637 cells using lysis buffer (cat. no. P0287; Beyotime Institute of Biotechnology) and the protein concentration was determined using BCA Protein assay reagent (Thermo Fisher Scientific, Inc.). Equal amounts of protein (10 µg protein/lane) were separated via 10% SDS-PAGE (Invitrogen; Thermo Fisher Scientific, Inc.) and transferred onto nitrocellulose membranes (EMD Millipore), then blocked in 5% skimmed milk for 2 h at room temperature. The membranes were incubated with the following primary antibodies (all Cell Signaling Technology, Inc.) at 4°C overnight: Anti-NFKBIZ (1:1,000; cat. no. 9244S), anti-β-actin (1:2,000; cat. no. 4970), anti-PTEN (1:1,000; cat. no. 9188), anti-PI3K (1:1,000; cat. no. 4249), anti-p-PI3K (1:1,000; cat. no. 17366), anti-AKT (1:1,000; cat. no. 4685), anti-p-AKT (1:1,000; cat. no. 4060), anti-mTOR (1:1,000; cat. no. 2983) and anti-p-mTOR (1:1,000; cat. no. 5536). Following the primary antibody incubation, the membranes were incubated with HRP-conjugated goat anti-mouse IgG (cat. no. ab205719) and goat anti-rabbit IgG (cat. no. ab205718) (both 1:1,000; Abcam) secondary antibodies for 2 h at room temperature. Protein bands were visualized using the Clarity Western ECL kit (Bio-Rad Laboratories, Inc.). β-actin was used as the internal loading control. Densitometric analysis was performed using ImageJ software (v1.8.0; National Institutes of Health).
Colony formation assay
Each group of transfected T24 and 5637 cells was digested with pancreatin, counted and plated into 6-well plates with complete medium at a density of 500 cells/well. During the 14-day incubation, the medium was changed once. Following incubation, the medium was discarded and cells were washed with PBS three times, fixed with 4% paraformaldehyde for 20 min at room temperature, then stained with 0.1% crystal violet solution for another 20 min at room temperature. Cells were subsequently washed with PBS and air-dried. The number of colonies was counted in four randomly selected fields of view using an Olympus digital camera (Olympus Corporation).
Wound healing assay
The transfected T24 and 5637 cells from each group were seeded into 6-well plates and cultured to 80-90% confluence. Scratches were subsequently made in the cell monolayer with a 200-µl sterile pipette tip. Each well was washed 2-3 times with PBS and the plates were then cultured in serum-free medium. The width of the wound was captured at 0 and 24 h using a light microscope (magnification, ×200; Nikon Corporation), and the area of cell migration (µm2) was measured using ImageJ software (v1.8.0).
Transwell invasion assay
A Matrigel chamber (BD Biosciences) was used for determining the invasive ability of the cells. Briefly, the transfected T24 and 5637 cells in each group were digested and counted, and 3×104 cells/well were plated in serum-free RPMI medium into the upper chamber of 24-well Transwell plates, which had been precoated with 40 µl Matrigel (BD biosciences) at 37°C for 1 h. Complete medium supplemented with 10% FCS was plated into the lower chambers. Following incubation for 24 h at 37°C, the cells remaining in the upper chamber were removed, while the invasive cells in the lower chamber were fixed with 4% paraformaldehyde at room temperature for 30 min and stained with 0.1% crystal violet solution at room temperature for 15 min (Sigma-Aldrich; Merck KGaA). The stained cells were counted in three randomly selected fields of view under a light microscope (magnification, ×200; CKX41; Olympus Corporation) for each chamber.
Tumor xenograft model
A total of 6 female BALB/c nude mice (age, 4 weeks; weight, 14±2 g) were obtained from the Animal Experiment Center of Wuhan University. All animals were randomly divided into two groups (n=3/group) and housed in a standard environment (25°C; 50% humidity; 12-h light/dark cycle) with free access to food and water. A volume of 200 µl PBS containing ~1×106 T24 cells transfected with pc-NC or pc-NFKBIZ were injected into the right scapular region of each mouse. The tumor volumes were measured every three days after injection and calculated using the following formula: (Length x width2)/2. After 1 month, the mice were sacrificed with CO2 (20% of the chamber volume was displaced per minute with CO2), and the tumor weight was measured immediately. The maximum tumor size observed in the present study was 1.5 cm3. The tumors were subsequently collected for further research. All animal protocols were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University, and all animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Immunohistochemistry (IHC) assay
The paraffin-embedded tissue samples were cut into 5-µm-thick sections and the immunohistochemical expression of Ki67, NFKBIZ and PTEN in tissue sections were determined using Strept Actividin-Biotin Complex IHC staining (Wuhan Boster Biological Technology, Ltd.). The tissue sections were deparaffinized in xylene for 15 min at room temperature, rehydrated with a graded series of alcohol and heated at 105°C for 10 min for antigen retrieval in citric acid buffer (0.01 M). The slides were subsequently washed with PBS for 10 min and then treated with 3% hydrogen peroxide solution to inhibit endogenous peroxidase activity, blocked with 10% normal goat serum (cat. no. AR0009; Wuhan Boster Biological Technology, Ltd.) for 30 min at 37°C, and subsequently incubated with the following primary antibodies: Anti-Ki67 (1:200; cat. no. 9449; Cell Signaling Technology, Inc.), anti-NFKBIZ (1:1,000; cat. no. 9244S; Cell Signaling Technology, Inc.) and anti-PTEN (1:125; cat. no. 9188; Cell Signaling Technology, Inc.) for 2 h at 37°C. The slides were washed three times with PBS and incubated with HRP-conjugated anti-mouse IgG secondary antibody (1:2,000; cat. no. sc-2005; Santa Cruz Biotechnology, Inc.) for 1 h at room temperature. The slides were subsequently stained using the diaminobenzidine substrate kit (cat. no. SK-4100; Vector Laboratories, Inc.). The nuclei were stained with hematoxylin for 4 min at room temperature, and the slides were observed under an inverted light microscope (Olympus Corporation; magnification, ×200). Brown staining was considered to be Ki67-, PTEN- or NFKBIZ-positive. A total of five fields of view were randomly selected from each slide, and the degree of positive staining and the proportion of positive cells were recorded and scored by blinded observation by two pathologists. The staining intensity was classified as follows: 0, negative; 1, weak; 2, moderate; and 3, strong. The scores 0 and 1 were defined as low expression, and the scores 2 and 3 were defined as high expression.
Statistical analysis
Statistical analysis was performed using GraphPad Prism 5 (GraphPad Software, Inc.) and SPSS 12.0 (SPSS, Inc.) softwares, and quantitative data are presented as the mean ± SD. The RT-qPCR data of tumor and non-tumor tissues from patients were compared using paired t-test. An independent-sample t-test was used to analyze the differences between two groups. One-way ANOVA with Tukey's post-hoc test was used to analyze the statistical differences among multiple groups. Categorical data were compared using Fisher's exact test. Correlation analysis was preformed using Spearman correlation coefficient algorithm. All experiments were independently repeated in triplicate. P<0.05 was considered to indicate a statistically significant difference.
Results
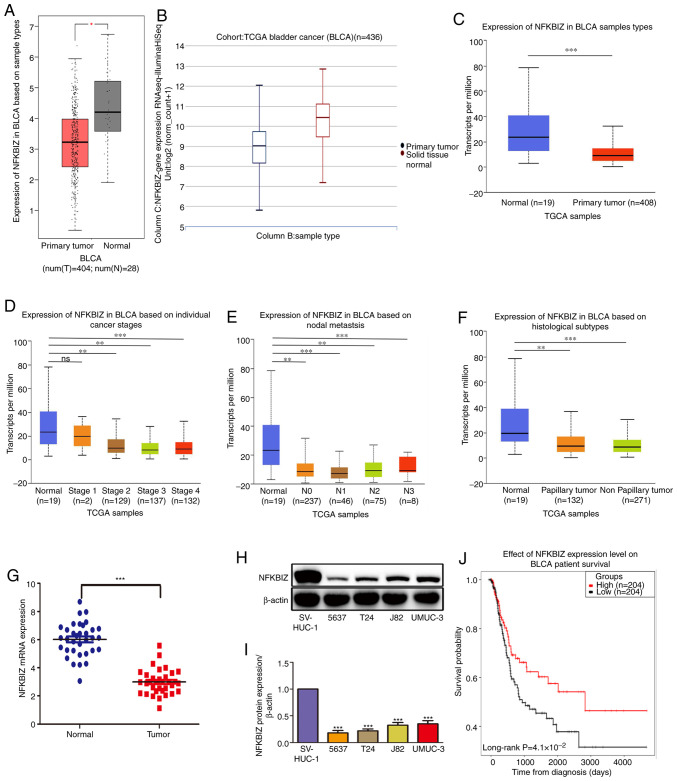
NFKBIZ expression is downregulated in human BC tissues and is associated with a poor prognosis
As shown in Table I, NFKBIZ expression in clinical patients with BC was associated with tumor stage (P=0.005) and tumor grade (P=0.018). However, no associations were observed between NFKBIZ expression and other clinical features, such as sex (P=0.306), age (P=0.154), tumor size (P=0.672) and tumor multiplicity (P>0.999). The bioinformatics analysis revealed that NFKBIZ mRNA expression was significantly downregulated in cancer tissues compared with in normal tissues (Fig. 1A-C), and at different stages, nodal metastasis status and histological subtypes compared with normal tissues, which suggests that the low NFKBIZ expression may be associated with a poor prognosis in patients with BC (Fig. 1D-F). To further validate the results obtained from databases, the expression levels of NFKBIZ in clinical BC tissues and cell lines were analyzed using RT-qPCR and western blotting, respectively. The results revealed that the expression levels of NFKBIZ in BC tissues and cell lines were significantly downregulated compared with in adjacent non-tumor tissues and normal bladder epithelial cells, respectively (Fig. 1G-I). Additionally, survival analysis indicated that patients with BC with high NFKBIZ expression had an increased overall survival rate compared with those with low NFKBIZ expression (Fig. 1J). The current results suggested that NFKBIZ may be act as a tumor suppressor gene and may serve an important role in BC progression.
Table I.
Association between NFKBIZ expression and clinical features of patients with bladder cancer (n=34).
| Variable | Total | Low NFKBIZ expression (n=28) | High NFKBIZ expression (n=6) | P-value |
|---|---|---|---|---|
| Sex | ||||
| Male | 25 | 22 | 3 | 0.306 |
| Female | 9 | 6 | 3 | |
| Age, years | ||||
| ≥60 | 22 | 20 | 2 | 0.154 |
| <60 | 12 | 8 | 4 | |
| Tumor size, cm | ||||
| ≥3 | 20 | 17 | 3 | 0.672 |
| <3 | 14 | 11 | 3 | |
| Multiplicity of tumor | ||||
| Single | 17 | 14 | 3 | >0.999 |
| Multiple | 17 | 14 | 3 | |
| Tumor grade | ||||
| PUNLMP, low grade | 8 | 4 | 4 | 0.018 |
| High grade | 26 | 24 | 2 | |
| Tumor stage | ||||
| Ta, T1 | 10 | 5 | 5 | 0.005 |
| T2-T4 | 24 | 23 | 1 | |
| Lymph node status | ||||
| Negative | 26 | 22 | 4 | 0.609 |
| Positive | 8 | 6 | 2 | |
| Distant metastasis | ||||
| Absent | 34 | 28 | 6 | |
| Present | 0 | 0 | 0 |
NFKBIZ, NF-κB inhibitor ζ; PUNLMP, papillary urothelial neoplasms of low malignant potential.
Figure 1.
NFKBIZ expression is significantly downregulated in BC, and its low expression predicts a poor prognosis. (A) NFKBIZ expression in BLCA based on sample types in the Gene Expression Profiling Interacting Analysis database. (B) Expression levels of NFKBIZ based on RNAseq data in BLCA based on sample types in the Xena database. NFKBIZ expression according to (C) sample types, (D) different BC stages, (E) nodal metastasis status and (F) histological subtypes in the UALCAN database. (G) Reverse transcription-quantitative PCR analysis of NFKBIZ expression in 34 pairs of BC tissues and corresponding adjacent normal bladder tissues. *P<0.05; **P<0.01; ***P<0.001. (H) Representative western blot images and (I) quantitative analyses of NFKBIZ and β-actin protein expression in different cell lines. ***P<0.001 vs. SV-HUC-1. (J) Survival analysis was performed using the mRNA-reads count expression data from TCGA-BLCA dataset in patients with BC with high and low NFKBIZ expression. ns, not significant; BLCA/BC, bladder cancer; TCGA, The Cancer Genome Atlas; RNAseq, RNA sequencing; NFKBIZ, NF-κB inhibitor ζ.
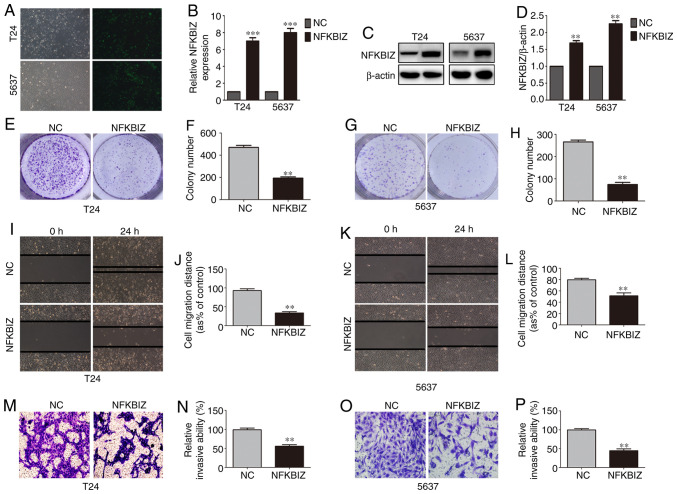
NFKBIZ suppresses cell proliferation, migration and invasion
To investigate the biological role of NFKBIZ in BC progression, T24 and 5637 cells were selected as in vitro cell models, since NFKBIZ expression was the lowest in these cell lines. The two cell lines were infected with lentivirus particles against puromycin to induce the stable overexpression of NFKBIZ, and the cell proliferative, migratory and invasive abilities were determined. Fluorescence microscopy, RT-qPCR and western blot analyses confirmed the transfection efficiency in the two cell lines (Fig. 2A-D). According to the results of the colony formation assay, the number of colonies formed from T24 (Fig. 2E and F) and 5637 (Fig. 2G and H) cells in the pc-NFKBIZ group was significantly decreased compared with in the pc-NC group. The results of the wound healing assay demonstrated that compared with in the pc-NC group, the migration rate of T24 (Fig. 2I and J) and 5637 (Fig. 2K and L) cells was significantly inhibited in the pc-NFKBIZ group. Moreover, the Transwell invasion assay results revealed that there were significantly fewer invasive T24 (Fig. 2M and N) and 5637 (Fig. 2O and P) cells in the pc-NFKBIZ group compared with in the pc-NC group. These results suggested that BC cell proliferation, migration and invasion were suppressed following the overexpression of NFKBIZ.
Figure 2.
Overexpression of NFKBIZ affects bladder cancer cell proliferation, migration and invasion in vitro. (A) Representative images of transfected T24 and 5637 cells were captured using a fluorescence microscope (magnification, ×200). NFKBIZ transfection efficiency was evaluated by (B) Reverse transcription-quantitative PCR and (C and D) western blotting in T24 and 5637 cell lines. Representative images and quantitative analyses of (E and F) T24 and (G and H) 5637 cell colonies in each group according to colony formation assays. Representative images and quantitative analyses of (I and J) T24 and (K and L) 5637 cell migration rates in each group according to wound healing assays (magnification, ×200). Representative images and quantitative analyses of invasive (M and N) T24 and (O and P) 5637 cells in each group according to Transwell invasion assays (magnification, ×200). **P<0.01 and ***P<001 vs. NC. NC, negative control; NFKBIZ, NF-κB inhibitor ζ.
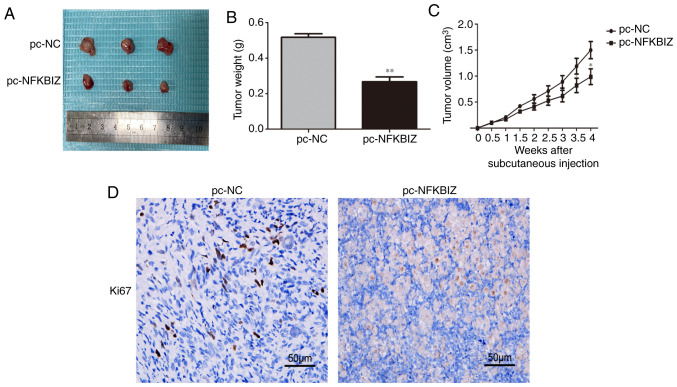
NFKBIZ expression affects BC growth in vivo
To determine the effects of NFKBIZ expression on BC growth in vivo, T24 cells were used to establish a xenograft model. The tumor weights and volumes were significantly decreased in the pc-NFKBIZ group compared with in the pc-NC group (Fig. 3A-C). In addition, the IHC results demonstrated that Ki-67 expression (a proliferation marker) was markedly decreased in the pc-NFKBIZ group compared with in the pc-NC group (Fig. 3D). These results indicated that BC growth was inhibited by the overexpression of NFKBIZ in vivo.
Figure 3.
Overexpression of NFKBIZ affects bladder cancer growth in vivo. (A) Representative images of the isolated tumors from mice (n=3/group), as well as the (B) weight and (C) volume of the tumors from T24 cells in the pc-NC and pc-NFKBIZ groups. (D) Representative immunohistochemistry images of Ki-67 expression in the mice tumors from T24 cells (magnification, ×200; scale bar, 50 µm). *P<0.05 and **P<0.01 vs. pc-NC. NC, negative control; NFKBIZ, NF-κB inhibitor ζ.
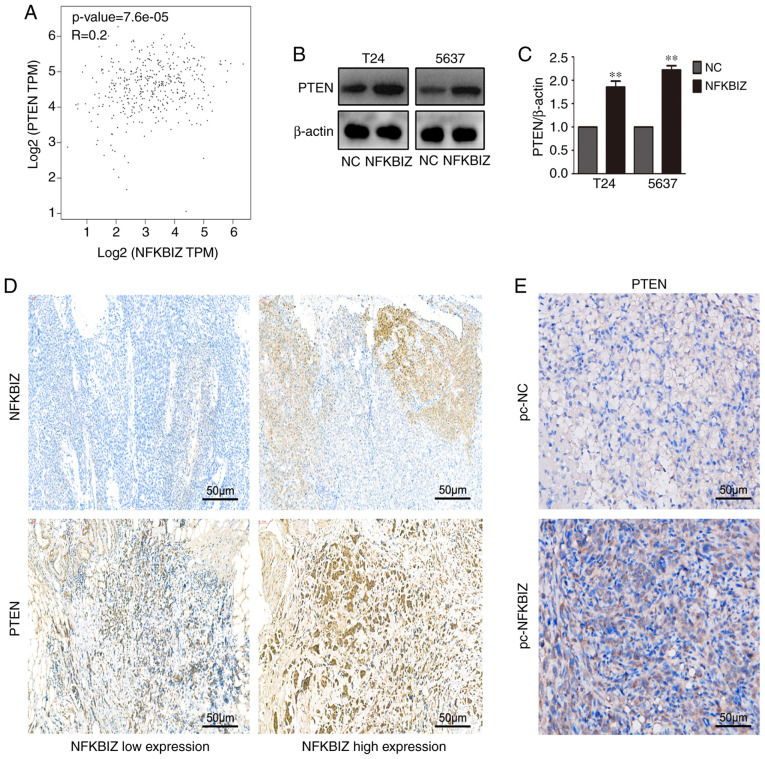
NFKBIZ and PTEN expression levels are positively correlated
To investigate the potential mechanism of the aforementioned biological functions of NFKBIZ in vivo and in vitro, correlation analysis was performed. The results identified a positive correlation between NFKBIZ and the tumor-inhibiting factor, PTEN (Fig. 4A). To determine whether the overexpression of NFKBIZ induced the expression levels of PTEN, its expression was analyzed in both T24 and 5637 cells using western blotting (Fig. 4B and C). Moreover, according to the RT-qPCR results of clinical BC tissues, samples in the NFKBIZ high expression and low expression groups were selected to conduct PTEN or NFKBIZ IHC, and to investigate whether PTEN expression was also high in the NFKBIZ high expression group or low in the NFKBIZ low expression group, to further explore whether NFKBIZ expression was positively correlated with PTEN expression. The IHC results from human BC and nude mice tumor tissues revealed that the overexpression of NFKBIZ upregulated PTEN expression (Fig. 4D and E). These results suggested the existence of a positive correlation between the expression levels of NFKBIZ and PTEN.
Figure 4.
Expression levels of NFKBIZ and PTEN are positively correlated. (A) NFKBIZ expression was significantly positively correlated with PTEN expression according to the Gene Expression Profiling Interacting Analysis database. (B and C) PTEN expression was increased in transfected T24 and 5637 cells overexpressing NFKBIZ. (D) Representative IHC images of NFKBIZ and PTEN expression in human bladder cancer tissues (magnification, ×200; scale bar, 50 µm). (E) Representative IHC images of PTEN expression in nude mice tumor tissues in the pc-NFKBIZ and pc-NC groups. **P<0.01 vs. NC. NC, negative control; NFKBIZ, NF-κB inhibitor ζ; IHC, immunohistochemistry; TPM, transcripts per million.
PTEN inhibition promotes BC cell proliferation, migration and invasion
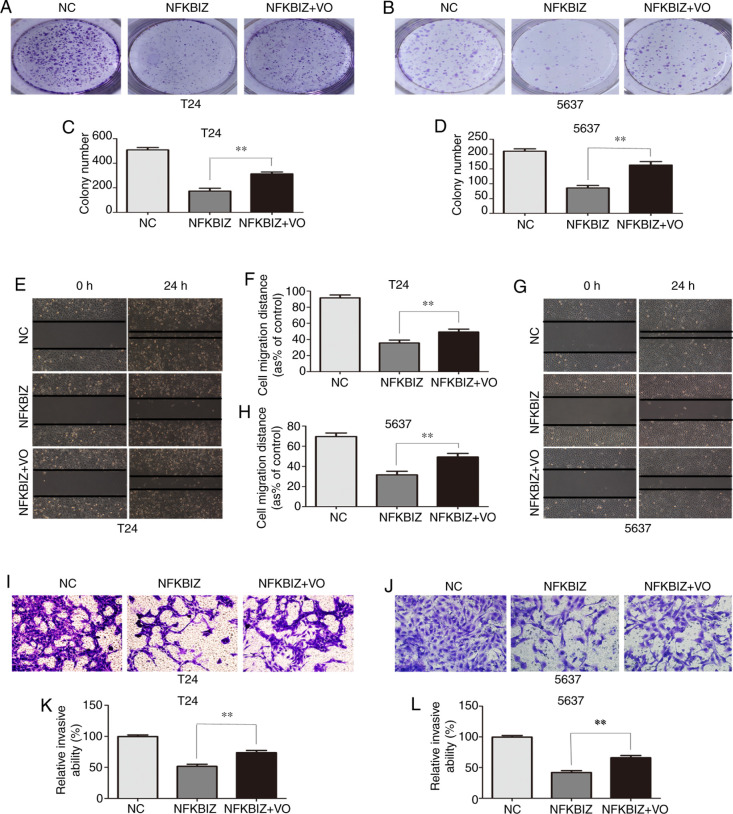
To further verify the positive correlation between NFKBIZ and PTEN, NFKBIZ-overexpressing T24 and 5637 cells were treated with the PTEN inhibitor VO (35 nM/l) at room temperature for 24 h, to observe the effect on cell proliferation, migration and invasion. According to the results of the colony formation assay, the number of colonies formed from T24 (Fig. 5A and C) and 5637 (Fig. 5B and D) cells in the pc-NFKBIZ + VO group was significantly increased compared with in the pc-NFKBIZ group. The wound healing assay results demonstrated that, compared with in the pc-NFKBIZ group, the migratory rate of T24 (Fig. 5E and F) and 5637 (Fig. 5G and H) cells was significantly increased in the pc-NFKBIZ + VO group. In addition, the results of the Transwell invasion assay revealed that the number of invasive T24 (Fig. 5I and K) and 5637 (Fig. 5J and L) cells was significantly increased in the pc-NFKBIZ + VO group compared with in the pc-NFKBIZ group.
Figure 5.
Treatment with the PTEN inhibitor VO enhanced the proliferation, migration and invasion in T24 and 5637 cells overexpressing NFKBIZ. (A and B) Representative images and (C and D) quantitative analyses of T24 and 5637 cell colonies in each group according to colony formation assays. Representative images and quantitative analyses of (E and F) T24 and (G and H) 5637 cell migration rates in each group according to wound healing assays (magnification, ×200). (I and J) Representative images and (K and L) quantitative analyses of invasive T24 and 5637 cells in each group according to Transwell invasion assays (magnification, ×200). **P<0.01. VO, VO-OHpic trihydrate; NC, negative control; NFKBIZ, NF-κB inhibitor ζ.
According to the aforementioned results, treatment with VO enhanced the proliferative, migratory and invasive abilities induced by NFKBIZ overexpression in T24 and 5637 cells. Thus, the findings suggested that NFKBIZ may function as an important tumor suppressor and may positively modulate the tumor-inhibiting factor, PTEN, in BC.
NFKBIZ suppresses BC by regulating the PTEN/PI3K/AKT signaling pathway in BC cells
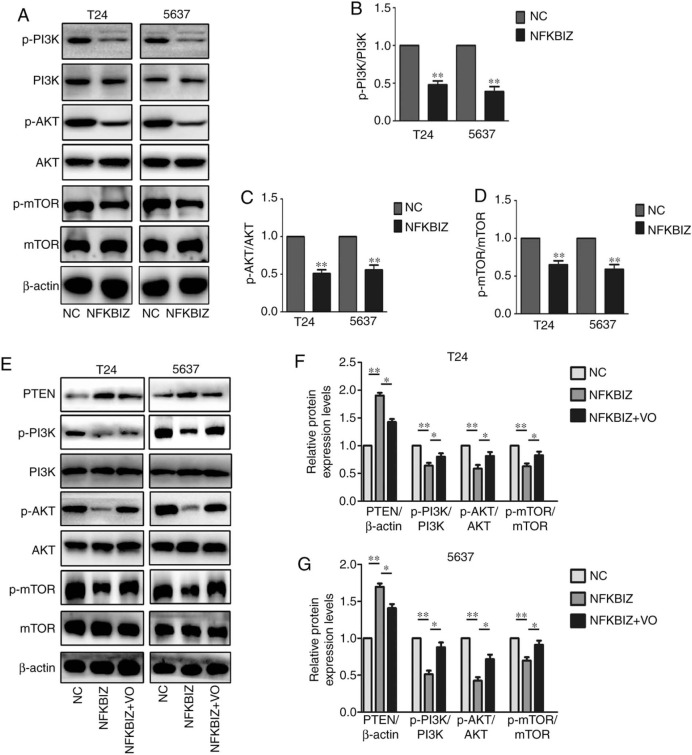
The PI3K/AKT/mTOR signaling pathway serves an important role in numerous types of cancer and regulates a wide range of cellular processes, including survival, proliferation, growth, metabolism, angiogenesis and metastasis (36). Thus, the present study determined the effect of NFKBIZ overexpression on the signaling activity of this pathway. The results revealed a significant downregulation in the levels of key molecules involved in the AKT signaling pathway, including p-PI3K, p-AKT and p-mTOR, in T24 and 5637 cells overexpressing NFKBIZ; however, the expression levels of the total proteins were unchanged (Fig. 6A-D). PTEN is directly upstream of the AKT signaling pathway (23). To further determine whether the activation of the PTEN/AKT/mTOR signaling pathway was important for the oncogenic activity of NFKBIZ in BC cells, NFKBIZ-overexpressing T24 and 5637 cells were treated with VO. The treatment of T24 and 5637 cells overexpressing NFKBIZ with VO rescued the inhibition of AKT signaling activity induced by the overexpression of NFKBIZ (Fig. 6E-G). These findings suggested that the overexpression of NFKBIZ may suppress BC cell proliferation, migration and invasion by inducing PTEN expression, and the mechanism may depend on PTEN/PI3K/AKT/mTOR signaling.
Figure 6.
Overexpression of NFKBIZ induces BC cell proliferation by regulating the PTEN/PI3K/Akt signaling pathway in BC cells. (A) Representative western blot images and quantitative analyses of related proteins, including (B) p-PI3K/PI3K, (C) p-AKT/AKT and (D) p-mTOR/mTOR in transfected T24 and 5637 cells overexpressing NFKBIZ. **P<0.01 vs. NC. (E) Representative western blot images and quantitative analyses of related proteins, including PTEN, p-PI3K/PI3K, p-AKT/AKT and p-mTOR/mTOR in transfected (F) T24 and (G) 5637 cells overexpressing NFKBIZ and after VO treatment. *P<0.05; **P<0.01. VO, VO-OHpic trihydrate; NC, negative control; NFKBIZ, NF-κB inhibitor ζ; p, phosphorylated; BC, bladder cancer.
Discussion
BC is one of the most common and invasive malignant tumors; however, the treatment of the disease is challenging. Although some progress has been made in the early diagnosis and comprehensive treatment of BC, the prognosis remains unsatisfactory (37). Therefore, there is an urgent requirement to elucidate the molecular mechanisms of the BC regulatory network.
NFKBIZ, an inhibitor of the IκB protein, is localized to the nucleus (12). Several previous studies have reported that NFKBIZ serves a potentially important role in modulating the progression of numerous inflammatory diseases, such as spontaneous skin inflammation, invasive pneumococcal disease and Crohn's disease (19,20,38,39). Notably, inflammation is also known to serve an important role in the development of cancer (40). However, the underlying pathological mechanisms of NFKBIZ in cancer remain elusive. Therefore, the present study aimed to determine the role of NFKBIZ in BC.
PTEN is a widely studied tumor suppressor gene involved in the majority of human malignant tumors (25). PTEN signaling has been found to inhibit the occurrence and development of cancer by inhibiting the proliferation, migration and invasion of cancer cells (25). Therefore, the activation of the PTEN signaling pathway has been considered to be a promising therapeutic strategy for the clinical treatment of cancer (41). In a previous study, the inactivation of the PTEN signaling pathway has been associated with the invasiveness of BC (42). PTEN, as an inhibitor of the PI3K/AKT signaling pathway, inhibits the growth and invasion of tumor cells by directly inhibiting the PI3K/AKT signaling pathway (43). In addition, the PI3K/AKT/mTOR signaling pathway is a classical signaling pathway that has a crucial role in regulating cell growth, metastasis and other cellular processes in various types of cancer, including BC (44-47). Previous studies have shown that the PI3K/AKT/mTOR signaling pathway is activated in BC, and patients with BC with simultaneous expression of PI3K/AKT/mTOR exhibit a worse prognosis (48,49). NF-κB correlates with PTEN (29-31) and is controlled by NFKBIZ (12). To determine the molecular mechanisms of NFKBIZ regulation over BC cell proliferation, invasion and migration, the present study analyzed the expression levels of proteins involved in the PTEN/PI3K/AKT/mTOR signaling pathway.
In the present study, the expression levels of NFKBIZ were significantly downregulated in BC samples and cell lines, and positively associated with the overall survival rate of patients. In addition, NFKBIZ expression at different stages, nodal metastasis status and histological subtypes was significantly decreased compared with in normal tissues, which suggested that low NFKBIZ expression may be associated with a poor prognosis in patients with BC. However, in 34 clinical samples, there was no association between lymph node metastasis and NFKBIZ expression, which was in contrast to the results obtained from the database analysis and may be due to the limited number of clinical specimens available for analysis. To verify whether NFKBIZ acted as a tumor suppressor, lentiviruses overexpressing NFKBIZ were transfected into BC cell lines to verify the results of the bioinformatics analysis. Mechanistically, NFKBIZ suppressed BC cell proliferation, migration and invasion. In addition, the data from the tumor xenograft model studies revealed that BC growth was inhibited by the overexpression of NFKBIZ, which provided further evidence for the suggested tumor-suppressive role of NFKBIZ in BC. Ki67 has been considered as a useful biomarker for BC, and its expression has been associated with a poor prognosis (50). In the current study, the upregulated expression levels of NFKBIZ were associated with the downregulated expression levels of Ki67, which is suggestive of an improved prognosis. Therefore, it may be inferred that the expression levels of NFKBIZ may be downregulated as the stage of the disease advances. Subsequently, the present study aimed to determine which pathway affected the progression of BC. The results of the current study revealed that PTEN expression was positively correlated with NFKBIZ expression, which was proven using the GEPIA database and IHC results obtained from clinical specimens. In addition, further results revealed that NFKBIZ affected BC cells by upregulating PTEN expression, which was followed by the inactivation of the PI3K/AKT/mTOR signaling pathway. These effects were reversed by the PTEN inhibitor VO. The current results suggested that the regulation of NFKBIZ expression may be a novel target in the treatment of BC.
In the present study, VO downregulated PTEN expression and indirectly activated the PI3K/Akt/mTOR signaling pathway, and then reversed the anticancer effects of NFKBIZ overexpression, which is consistent with previous studies (51-53). Additionally, another study has revealed that high concentration of VO can inhibit hepatocarcinoma cell proliferation and induce senescence (54). Whether high concentration of VO can directly inhibit BC cell proliferation should be further explored in future studies.
In conclusion, to the best of our knowledge, the findings of the present study revealed for the first time that NFKBIZ may serve as a tumor suppressor to inhibit tumor growth both in vitro and in vivo. In addition, NFKBIZ expression was positively correlated with PTEN expression, which affected BC tumorigenesis through the PTEN-mediated inhibition of the PI3K/AKT/mTOR signaling pathway. Furthermore, the results demonstrated that upregulated expression levels of NFKBIZ were an indicator of an improved prognosis in patients with BC. Therefore, the data of the present study revealed the molecular mechanism of the NFKBIZ-mediated regulation of BC, and the NFKBIZ/PTEN/PI3K/AKT axis may represent a novel clinical marker and therapeutic target for BC.
Acknowledgments
Not applicable.
Funding Statement
The present study was supported by grants from the National Natural Science Foundation of China (grant. nos. 81870471 and 81800617) and the Science and Technology Major Project of Hubei Province (grant. no. 2019AEA170).
Availability of data and materials
All data generated or analyzed during this study are included in this published article.
Authors' contributions
TX designed the study. TX, WMY, JZN and XY performed the experiments and drafted the manuscript. SMZ, KY and TB participated in data analysis. TX, TR and FC were involved in the discussion and interpretation of the results. TX and FC were responsible for confirming the authenticity of the data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
All patients provided written informed consent and the research protocols were approved by the Research Ethics Committee of Renmin Hospital of Wuhan University (approval no. WDRY2019-K035; Wuhan, China). All animal protocols were approved by the Animal Care and Use Committee of Renmin Hospital of Wuhan University, and all animal experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018;68:7–30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 2.Antoni S, Ferlay J, Soerjomataram I, Znaor A, Jemal A, Bray F. Bladder cancer incidence and mortality: A global overview and recent trends. Eur Urol. 2017;71:96–108. doi: 10.1016/j.eururo.2016.06.010. [DOI] [PubMed] [Google Scholar]
- 3.Song BN, Kim SK, Mun JY, Choi YD, Leem SH, Chu IS. Identification of an immunotherapy-responsive molecular subtype of bladder cancer. EBioMedicine. 2019;50:238–245. doi: 10.1016/j.ebiom.2019.10.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chou R, Selph SS, Buckley DI, Gustafson KS, Griffin JC, Grusing SE, Gore JL. Treatment of muscle-invasive bladder cancer: A systematic review. Cancer. 2016;122:842–851. doi: 10.1002/cncr.29843. [DOI] [PubMed] [Google Scholar]
- 5.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 6.Levi F, La Vecchia C, Randimbison L, Franceschi S. Incidence of infiltrating cancer following superficial bladder carcinoma. Int J Cancer. 1993;55:419–421. doi: 10.1002/ijc.2910550316. [DOI] [PubMed] [Google Scholar]
- 7.Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A, European Association of Urology EAU guidelines on muscle-invasive and metastatic bladder cancer: Summary of the 2013 guidelines. Eur Urol. 2014;65:778–792. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 8.Zhu G, Xu Y, Cen X, Nandakumar KS, Liu S, Cheng K. Targeting pattern-recognition receptors to discover new small molecule immune modulators. Eur J Med Chem. 2018;144:82–92. doi: 10.1016/j.ejmech.2017.12.026. [DOI] [PubMed] [Google Scholar]
- 9.Herrero-Beaumont G, Pérez-Baos S, Sánchez-Pernaute O, Roman-Blas JA, Lamuedra A, Largo R. Targeting chronic innate inflammatory pathways, the main road to prevention of osteoarthritis progression. Biochem Pharmacol. 2019;165:24–32. doi: 10.1016/j.bcp.2019.02.030. [DOI] [PubMed] [Google Scholar]
- 10.Napetschnig J, Wu H. Molecular basis of NF-κB signaling. Annu Rev Biophys. 2013;42:443–468. doi: 10.1146/annurev-biophys-083012-130338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baldwin AS., Jr The NF-kappa B and I kappa B proteins: New discoveries and insights. Annu Rev Immunol. 1996;14:649–683. doi: 10.1146/annurev.immunol.14.1.649. [DOI] [PubMed] [Google Scholar]
- 12.Willems M, Dubois N, Musumeci L, Bours V, Robe PA. IκBζ: An emerging player in cancer. Oncotarget. 2016;7:66310–66322. doi: 10.18632/oncotarget.11624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horber S, Hildebrand DG, Lieb WS, Lorscheid S, Hailfinger S, Schulze-Osthoff K, Essmann F. The Atypical Inhibitor of NF-κB, IκBζ, controls macrophage interleukin-10 expression. J Biol Chem. 2016;291:12851–12861. doi: 10.1074/jbc.M116.718825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu Z, Zhang X, Yang J, Wu G, Zhang Y, Yuan Y, Jin C, Chang Z, Wang J, Yang X, He F. Nuclear protein IkappaB-zeta inhibits the activity of STAT3. Biochem Biophys Res Commun. 2009;387:348–352. doi: 10.1016/j.bbrc.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 15.Nogai H, Wenzel SS, Hailfinger S, Grau M, Kaergel E, Seitz V, Wollert-Wulf B, Pfeifer M, Wolf A, Frick M, et al. IκB-ζ controls the constitutive NF-κB target gene network and survival of ABC DLBCL. Blood. 2013;122:2242–2250. doi: 10.1182/blood-2013-06-508028. [DOI] [PubMed] [Google Scholar]
- 16.Yamamoto M, Yamazaki S, Uematsu S, Sato S, Hemmi H, Hoshino K, Kaisho T, Kuwata H, Takeuchi O, Takeshige K, et al. Regulation of Toll/IL-1-receptor-mediated gene expression by the inducible nuclear protein IkappaBzeta. Nature. 2004;430:218–222. doi: 10.1038/nature02738. [DOI] [PubMed] [Google Scholar]
- 17.Krappmann D. Shaping oncogenic NF-κB activity in the nucleus. Blood. 2013;122:2146–2147. doi: 10.1182/blood-2013-08-516864. [DOI] [PubMed] [Google Scholar]
- 18.Coto-Segura P, Gonzalez-Lara L, Batalla A, Eiris N, Queiro R, Coto E. NFKBIZ and CW6 in adalimumab response among psoriasis patients: Genetic association and alternative transcript analysis. Mol Diagn Ther. 2019;23:627–633. doi: 10.1007/s40291-019-00409-x. [DOI] [PubMed] [Google Scholar]
- 19.Kim Y, Lee YS, Yang JY, Lee SH, Park YY, Kweon MN. The resident pathobiont Staphylococcus xylosus in Nfkbiz-deficient skin accelerates spontaneous skin inflammation. Sci Rep. 2017;7:6348. doi: 10.1038/s41598-017-05740-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sangil A, Arranz MJ, Guerri-Fernandez R, Perez M, Monzon H, Payeras A, Andres M, Torviso J, Ibanez L, Garau J, Calbo E. Genetic susceptibility to invasive pneumococcal disease. Infect Genet Evol. 2018;59:126–131. doi: 10.1016/j.meegid.2018.01.024. [DOI] [PubMed] [Google Scholar]
- 21.Kerami Z, Duijvis NW, Vogels EW, van Dooren FH, Moerland PD, Te Velde AA. Effect of interleukin-17 on gene expression profile of fibroblasts from Crohn's disease patients. J Crohns Colitis. 2014;8:1208–1216. doi: 10.1016/j.crohns.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 22.Brennenstuhl H, Armento A, Braczysnki AK, Mittelbronn M, Naumann U. IκBζ, an atypical member of the inhibitor of nuclear factor kappa B family, is induced by Y-irradiation in glioma cells, regulating cytokine secretion and associated with poor prognosis. Int J Oncol. 2015;47:1971–1980. doi: 10.3892/ijo.2015.3159. [DOI] [PubMed] [Google Scholar]
- 23.van Kester MS, Borg MK, Zoutman WH, Out-Luiting JJ, Jansen PM, Dreef EJ, Vermeer MH, van Doorn R, Willemze R, Tensen CP. A meta-analysis of gene expression data identifies a molecular signature characteristic for tumor-stage mycosis fungoides. J Invest Dermatol. 2012;132:2050–2059. doi: 10.1038/jid.2012.117. [DOI] [PubMed] [Google Scholar]
- 24.Kakiuchi N, Yoshida K, Uchino M, Kihara T, Akaki K, Inoue Y, Kawada K, Nagayama S, Yokoyama A, Yamamoto S, et al. Frequent mutations that converge on the NFKBIZ pathway in ulcerative colitis. Nature. 2020;577:260–265. doi: 10.1038/s41586-019-1856-1. [DOI] [PubMed] [Google Scholar]
- 25.Jiang BH, Liu LZ. PI3K/PTEN signaling in angiogenesis and tumorigenesis. Adv Cancer Res. 2009;102:19–65. doi: 10.1016/S0065-230X(09)02002-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang X, Huang H, Young KH. The PTEN tumor suppressor gene and its role in lymphoma pathogenesis. Aging (Albany NY) 2015;7:1032–1049. doi: 10.18632/aging.100855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Carracedo A, Pandolfi PP. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 28.Yang X, Cheng Y, Li P, Tao J, Deng X, Zhang X, Gu M, Lu Q, Yin C. A lentiviral sponge for miRNA-21 diminishes aerobic glycolysis in bladder cancer T24 cells via the PTEN/PI3K/AKT/mTOR axis. Tumour Biol. 2015;36:383–391. doi: 10.1007/s13277-014-2617-2. [DOI] [PubMed] [Google Scholar]
- 29.Zununi Vahed S, Barzegari A, Rahbar Saadat Y, Goreyshi A, Omidi Y. Leuconostoc mesenteroides-derived anticancer pharmaceuticals hinder inflammation and cell survival in colon cancer cells by modulating NF-κB/AKT/PTEN/MAPK pathways. Biomed Pharmacother. 2017;94:1094–1100. doi: 10.1016/j.biopha.2017.08.033. [DOI] [PubMed] [Google Scholar]
- 30.Zhou W, Fu XQ, Zhang LL, Zhang J, Huang X, Lu XH, Shen L, Liu BN, Liu J, Luo HS, et al. The AKT1/NF-kappaB/Notch1/PTEN axis has an important role in chemoresistance of gastric cancer cells. Cell Death Dis. 2013;4:e847. doi: 10.1038/cddis.2013.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Z, Fang S, Di Y, Ying W, Tan Y, Gu W. Modulation of NF-κB/miR-21/PTEN pathway sensitizes non-small cell lung cancer to cisplatin. PLoS One. 2015;10:e0121547. doi: 10.1371/journal.pone.0121547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sobin LH, Gospodarowicz MK, Wittekind C, editors. TNM Classification of Malignant tumors. 7th edition. Wiley-Blackwell; 2009. pp. 262–265. [Google Scholar]
- 33.Sauter G, Algaba F, Amin MB, Busch C, Cheville J, Gasser T, Grignon D, Hofstaedter F, Lopez-Beltran A, Epstein J. Tumours of the urinary system: Non-invasive urothelial neoplasias. In: Eble JN, Sauter G, Epstein JI, Sesterhenn I, editors. World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. IARC Press; Lyon: 2004. pp. 89–157. [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.Yang K, Xiao Y, Xu T, Yu W, Ruan Y, Luo P, Cheng F. Integrative analysis reveals CRHBP inhibits renal cell carcinoma progression by regulating inflammation and apoptosis. Cancer Gene Ther. 2020;27:607–618. doi: 10.1038/s41417-019-0138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ersahin T, Tuncbag N, Cetin-Atalay R. The PI3K/AKT/mTOR interactive pathway. Mol Biosyst. 2015;11:1946–1954. doi: 10.1039/C5MB00101C. [DOI] [PubMed] [Google Scholar]
- 37.Kluth LA, Black PC, Bochner BH, Catto J, Lerner SP, Stenzl A, Sylvester R, Vickers AJ, Xylinas E, Shariat SF. Prognostic and prediction tools in bladder cancer: A comprehensive review of the literature. Eur Urol. 2015;68:238–253. doi: 10.1016/j.eururo.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 38.Ishiguro-Oonuma T, Ochiai K, Hashizume K, Morimatsu M. The role of IFN-γ in regulating Nfkbiz expression in epidermal keratinocytes. Biomed Res. 2015;36:103–107. doi: 10.2220/biomedres.36.103. [DOI] [PubMed] [Google Scholar]
- 39.Muromoto R, Tawa K, Ohgakiuchi Y, Sato A, Saino Y, Hirashima K, Minoguchi H, Kitai Y, Kashiwakura JI, Shimoda K, et al. IκB-ζ expression requires both TYK2/STAT3 activity and IL-17-regulated mRNA stabilization. Immunohorizons. 2019;3:172–185. doi: 10.4049/immunohorizons.1900023. [DOI] [PubMed] [Google Scholar]
- 40.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keniry M, Parsons R. The role of PTEN signaling perturbations in cancer and in targeted therapy. Oncogene. 2008;27:5477–5485. doi: 10.1038/onc.2008.248. [DOI] [PubMed] [Google Scholar]
- 42.Puzio-Kuter AM, Castillo-Martin M, Kinkade CW, Wang X, Shen TH, Matos T, Shen MM, Cordon-Cardo C, Abate-Shen C. Inactivation of p53 and Pten promotes invasive bladder cancer. Genes Dev. 2009;23:675–680. doi: 10.1101/gad.1772909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tamura M, Gu J, Tran H, Yamada KM. PTEN gene and integrin signaling in cancer. J Natl Cancer Inst. 1999;91:1820–1828. doi: 10.1093/jnci/91.21.1820. [DOI] [PubMed] [Google Scholar]
- 44.Corti F, Nichetti F, Raimondi A, Niger M, Prinzi N, Torchio M, Tamborini E, Perrone F, Pruneri G, Di Bartolomeo M, et al. Targeting the PI3K/AKT/mTOR pathway in biliary tract cancers: A review of current evidences and future perspectives. Cancer Treat Rev. 2019;72:45–55. doi: 10.1016/j.ctrv.2018.11.001. [DOI] [PubMed] [Google Scholar]
- 45.Ediriweera MK, Tennekoon KH, Samarakoon SR. Role of the PI3K/AKT/mTOR signaling pathway in ovarian cancer: Biological and therapeutic significance. Semin Cancer Biol. 2019;59:147–160. doi: 10.1016/j.semcancer.2019.05.012. [DOI] [PubMed] [Google Scholar]
- 46.Xing X, Zhang L, Wen X, Wang X, Cheng X, Du H, Hu Y, Li L, Dong B, Li Z, Ji J. PP242 suppresses cell proliferation, metastasis, and angiogenesis of gastric cancer through inhibition of the PI3K/AKT/mTOR pathway. Anticancer Drugs. 2014;25:1129–1140. doi: 10.1097/CAD.0000000000000148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sathe A, Nawroth R. Targeting the PI3K/AKT/mTOR pathway in bladder cancer. Methods Mol Biol. 2018;1655:335–350. doi: 10.1007/978-1-4939-7234-0_23. [DOI] [PubMed] [Google Scholar]
- 48.Hou T, Zhou L, Wang L, Kazobinka G, Zhang X, Chen Z. CLCA4 inhibits bladder cancer cell proliferation, migration, and invasion by suppressing the PI3K/AKT pathway. Oncotarget. 2017;8:93001–93013. doi: 10.18632/oncotarget.21724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li Z, Hong S, Liu Z. lncRNA LINC00641 predicts prognosis and inhibits bladder cancer progression through miR-197-3p/KLF10/PTEN/PI3K/AKT cascade. Biochem Biophys Res Commun. 2018;503:1825–1829. doi: 10.1016/j.bbrc.2018.07.120. [DOI] [PubMed] [Google Scholar]
- 50.Zhang XG, Zhang T, Li CY, Zhang MH, Chen FM. CD164 promotes tumor progression and predicts the poor prognosis of bladder cancer. Cancer Med. 2018;7:3763–3772. doi: 10.1002/cam4.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li JY, Huang WX, Zhou X, Chen J, Li Z. Numb inhibits epithelial-mesenchymal transition via RBP-Jκ-dependent Notch1/PTEN/FAK signaling pathway in tongue cancer. BMC Cancer. 2019;19:391. doi: 10.1186/s12885-019-5605-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 52.Li X, Dai Y, Xu J. miR-21 promotes pterygium cell proliferation through the PTEN/AKT pathway. Mol Vis. 2018;24:485–494. [PMC free article] [PubMed] [Google Scholar]
- 53.Qin J, Fu M, Wang J, Huang F, Liu H, Huangfu M, Yu D, Liu H, Li X, Guan X, Chen X. PTEN/AKT/mTOR signaling mediates anticancer effects of epigallocatechin-3-gallate in ovarian cancer. Oncol Rep. 2020;43:1885–1896. doi: 10.3892/or.2020.7571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Augello G, Puleio R, Emma MR, Cusimano A, Loria GR, McCubrey JA, Montalto G, Cervello M. A PTEN inhibitor displays preclinical activity against hepatocarcinoma cells. Cell Cycle. 2016;15:573–583. doi: 10.1080/15384101.2016.1138183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.