INTRODUCTION
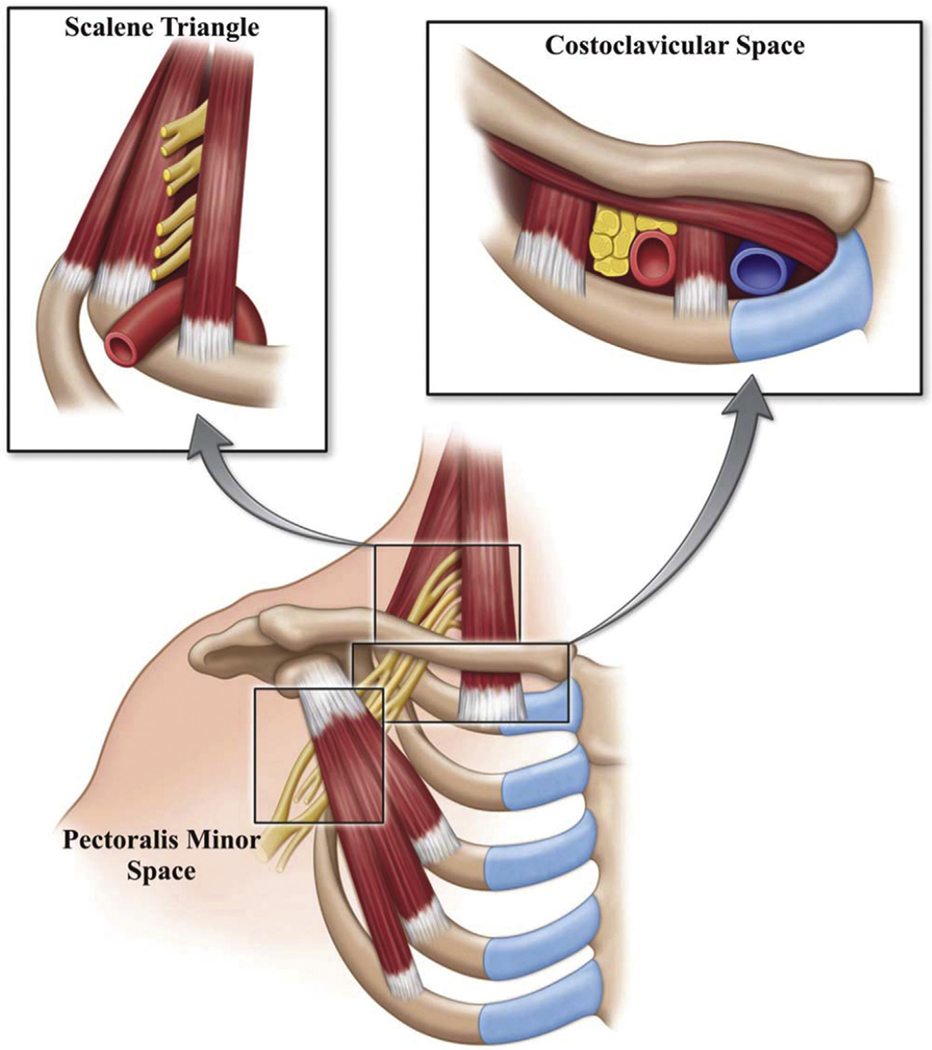
The thoracic outlet is defined as the space in the lower neck between the thorax and axilla through which the subclavian vein, subclavian artery, and brachial plexus travel from their central origins to their peripheral termini. It is bounded by the clavicle anteriorly, the first thoracic rib posteriorly, the insertion of the pectoralis minor muscle onto the coracoid process of the humerus laterally, and the sternum medially. It is subdivided into three areas: the scalene triangle above the clavicle, the costoclavicular space or cervicoaxillary canal between the clavicle and first rib, and the subcoracoid or pectoralis minor space below the clavicle1,2 (Fig. 1).
Fig. 1.
Three spaces of the thoracic outlet. (From Klaassen Z, Sorenson E, Tubbs RS, et al. Thoracic outlet syndrome: a neurological and vascular disorder. Clin Anat. 2014;27:724–32; with permission.)
The subclavian vasculature and brachial plexus—together termed the neurovascular bundle—pass from the scalene triangle into the costoclavicular space before exiting through the subcoracoid space. The thoracic outlet is both a confined and a dynamic space: compression of the neurovascular bundle resulting in the clinical syndrome generally termed thoracic outlet syndrome (TOS) may occur constantly or intermittently with movement of the neck, thorax, and arm (Fig. 2).
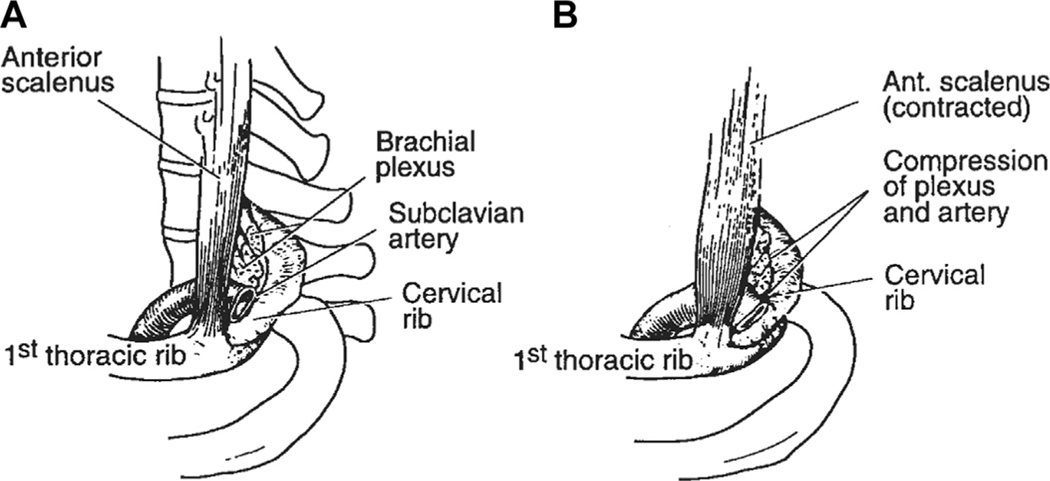
Fig. 2.
Compression with movement of the scalene muscle. As the anterior scalene muscle goes from rest (A) to contracted (B), it pulls the first thoracic rib cranially and compresses the brachial plexus and subclavian within the costoclavicular space. Presence of a supernumerary cervical rib, as demonstrated in this figure, may constrict this space further. (From Adson AW. Surgical treatment for symptoms produced by cervical ribs and the scalenus anticus muscle. Surg Gynecol Obstet. 1947;85:687. Reprinted with permission from the Journal of the American College of Surgeons, formerly Surgery Gynecology & Obstetrics.)
Aberrant anatomy is common in the thoracic outlet. Such anatomy predisposes patients to compression of the neurovascular bundle and development of clinical TOS. Much of this aberrancy is explained by the embryologic origins of the structures that comprise the thoracic outlet. A thorough understanding of this anatomy and embryology is therefore critical to the understanding of TOS.
NEUROVASCULAR ANATOMY AND EMBRYOLOGY
Subclavian Artery
The subclavian arteries are the primary blood supply to the upper extremities. The left subclavian artery branches directly from the aorta, whereas the right subclavian artery arises from the brachiocephalic artery. Each subclavian artery ascends superiorly into the neck before arching laterally and traveling posterior to the anterior scalene muscle through the scalene triangle and exiting the thoracic outlet via the costoclavicular space (above the first rib, below the clavicle) to become the axillary artery. Because of the close proximity of the clavicle, first rib, and anterior and middle scalene muscles, the costoclavicular space is the most frequent site of arterial compression.
The left subclavian artery develops from the left seventh intersegmental artery, whereas the right subclavian artery arises from the fourth aortic arch, right dorsal aorta, and right seventh intersegmental artery.3 Anomalies of the distal subclavian artery are rare, although anomalies of the aortic arch frequently encompass the origin of the either artery. In the thoracic outlet, the subclavian artery occasionally penetrates the anterior scalene muscle and rarely may pass entirely anterior to it.
Subclavian Vein
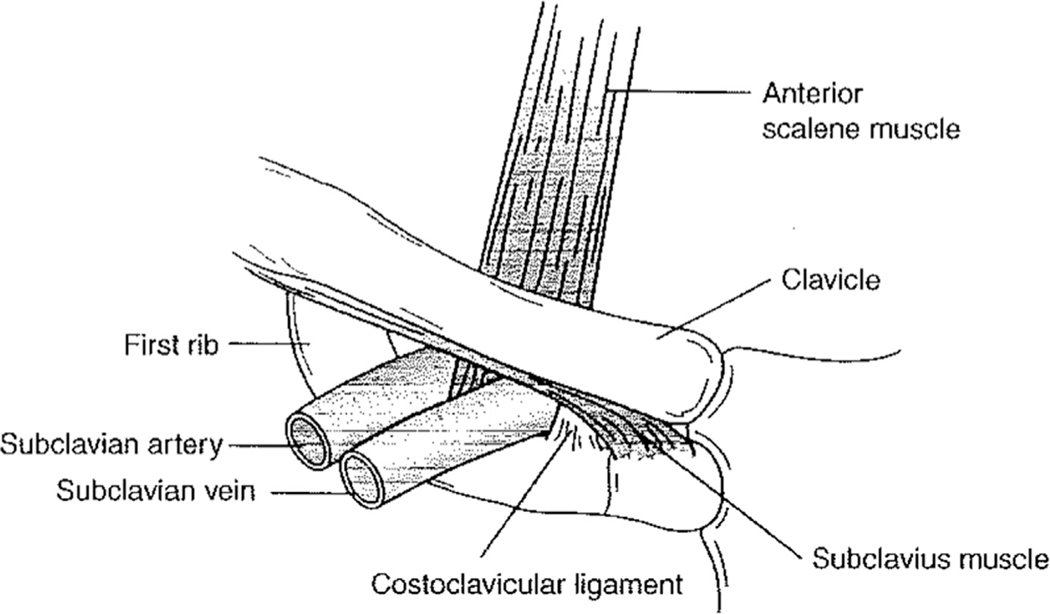
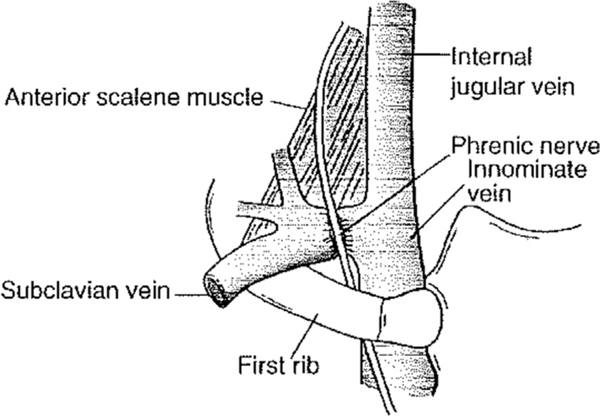
The subclavian veins provide the venous drainage of the upper extremities. The left subclavian vein also receives chyle from the thoracic duct drainage. On either side, the vein ascends superiorly with the subclavian artery into the neck. It then travels anterior to the anterior scalene muscle (outside of the technical scalene triangle) and continues in parallel to the artery with the anterior scalene muscle separating the two structures. It then exits through the costoclavicular space as the axillary vein. The subclavian vein is bound by the anterior scalene muscle laterally, the costoclavicular ligament medially, the subclavius tendon cranially, and first rib caudally1 (Fig. 3). This course explains the compression of the subclavian vein against the subclavius tendon found in patients with an abnormally anterior insertion of the anterior scalene muscle.1,2
Fig. 3.
The subclavian vein. (From Sanders RJ, Haug CE. Thoracic outlet syndrome: a common sequela of neck injuries. Philadelphia: JB Lippincott; 1991. p. 236; with permission.)
The subclavian vein develops in the fourth gestational week and is formed by the fusion of venous tributaries from the upper limb bud.3 The vein can be found in an anomalous location posterior to the anterior scalene muscle, immediately adjacent to the subclavian artery. More rarely, the subclavian vein splits to form a “clavicular loop” or travels between the clavicle and the subclavius muscle.
Brachial Plexus
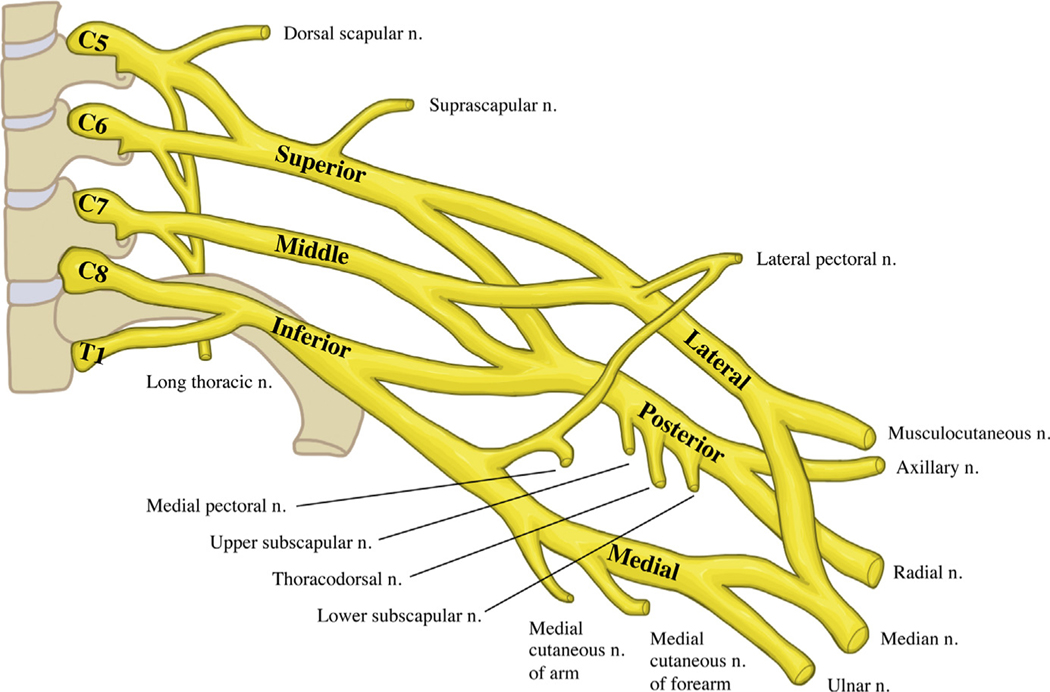
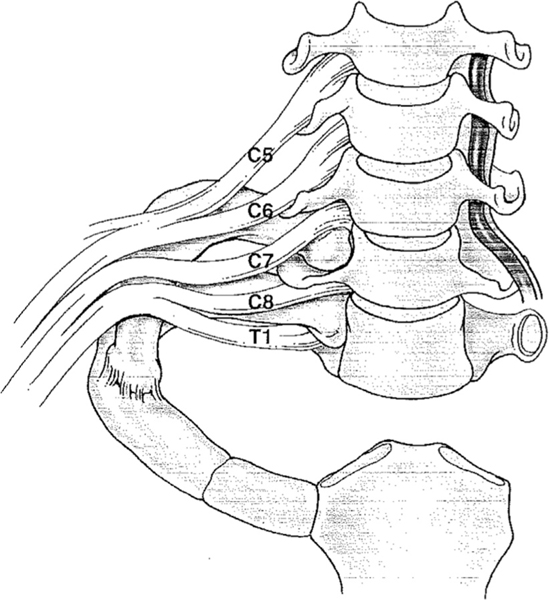
The brachial plexus is composed of nerves from the C5 to T1 roots that innervate most of the shoulder and arm. As the nerve roots exit the spinal cord and form the brachial plexus trunks, they travel through the scalene triangle, posterior to the subclavian artery and anterior to the middle scalene muscle, split into anterior and posterior divisions, and exit through the costoclavicular space alongside the subclavian artery. In the axilla, they transition from trunks to divisions and then cords (Fig. 4).
Fig. 4.
The brachial plexus.
The brachial plexus develops before bone maturation and influences bony development. At the end of the first gestational month, the ventral rami begin budding from the neural tube and begin to form the brachial plexus as they grow toward their sclerotomes and myotomes.3 The developing brachial plexus splits the scalene muscles into anterior and middle components. This gives rise to considerable variability in the relationship between the components of the plexus and the anterior scalene muscle. Common variations include a C5 root anterior to the anterior scalene muscle, C5 and C6 anterior to the anterior scalene muscle, C5 and C6 through the anterior scalene muscle, C5 and C6 through a double anterior scalene muscle, and C5 anterior with C6 through the anterior scalene muscle.2,4,5
The contribution of nerve roots to the brachial plexus may also vary. A prefixed plexus occurs when a branch of C4 contributes to the brachial plexus with or without contribution from T1. This has a tendency to pull the plexus in a cephalad direction. Alternatively, a postfixed plexus is drawn closer to the thorax by contribution from T2 with little or no contribution from C5. It is postulated that the prefixed and postfixed plexi may be associated with anomalies of the cervical or first rib.6
Phrenic Nerve
The phrenic nerve innervates the diaphragm and forms from branches of C3–5, of which C3 and C4 normally combine cephalad to the thoracic outlet.1 The combined branches and the C5 branch descend along the anterior surface of the anterior scalene muscle posterior to the subclavian vein. The C5 branch typically joins as the combined branches cross the anterior scalene muscle from lateral to medial. If the C5 branch continues along a separate course, it is termed an “accessory phrenic nerve.”1 Rarely, the phrenic nerve may travel anterior to the subclavian vein and may even obstruct it. This is termed a “prevenous” nerve1,7 (Fig. 5).
Fig. 5.
Prevenous phrenic nerve. (From Sanders RJ, Haug CE. Thoracic outlet syndrome: a common sequela of neck injuries. Philadelphia: JB Lippincott; 1991. p. 236; with permission.)
Long Thoracic Nerve
The long thoracic nerve innervates the serratus anterior muscle and forms from branches of C5–7. The C5 and C6 branches travel through the belly of the middle scalene muscle where they combine and exit the muscle as a single nerve which crosses the lateral edge of the first rib.1 The location of the C7 contribution to the nerve is highly variable and occasionally occurs within the belly of the middle scalene muscle. It can be easily injured during dissection of the middle scalene muscle.
Dorsal Scapular Nerve
The dorsal scapular nerve innervates the rhomboid muscles and part of the levator scapulae muscle. It arises from C5, travels briefly with the C5 branch of the long thoracic nerve, and then separates at the top of the middle scalene muscle to descend through its lateral edge.1
Cervical Sympathetic Nerve Chain
The cervical sympathetic nerve chain is part of the autonomic nervous system and travels along the anterior surface of the cervical transverse processes. Although not technically located in the thoracic outlet, operations to decompress the thoracic outlet place the sympathetic chain at risk for injury, particularly when the middle scalene muscle is released from the proximal rib in the costotransverse space.1 Injury to the sympathetic chain results in Horner’s Syndrome, although this is typically self-limited.
BONY ANATOMY AND EMBRYOLOGY
First Rib
The first rib develops from the T1 costal process and joins the manubrium. Together with the T1 vertebral body and manubrium, the first rib forms the opening of the superior thoracic cage, also known as the thoracic inlet or superior thoracic aperture. Normal ribs have a costotransverse ligament posteriorly and are joined to the manubrium anteriorly. The anterior portion of the rib may form a symphysis with the sternal head of the clavicle. Abnormal first ribs have a tendency to fuse to with the second rib and are often thinner and more cephalad in location.1 They often look like cervical ribs on the radiograph but may be distinguished by their T1 origin. Abnormal first ribs result in similar pathology as do cervical ribs. They are primarily associated with arterial or neurogenic TOS. It should be noted, however, that most patients with bony abnormalities are asymptomatic.
Cervical Rib
A cervical rib arises anomalously from a cervical vertebral body, mostly commonly C7. It was first described in dissections by Galen and Vesalius8 (Fig. 6). A complete cervical rib attaches to the normal first rib by fusion or with a true joint. The incidence is reported in 0.1% to 6% of the adult population and is more frequent in women.2,9–12
Fig. 6.
Anomalous cervical rib.
Costal elements for cervical ribs 5 to 7 form during embryologic development and then regress to become transverse processes as a result of the rapid development of brachial plexus nerve roots during the formation of the upper extremity limb bud. The formation of a cervical rib is considered an error in HOX gene expression, which is responsible for segmental development of the vertebral column.3,13–15 Ossification of the C7 costal element becomes a precursor to development of a supernumerary cervical rib.3
The neurovascular structures are considered to be the main limiting factor in the development of a cervical rib. The contribution of the nerve root to the brachial plexus has been linked to the presence or size of the rib, as the embryonic nerve trunk is proportionally larger than the cervical rib.3,16 This is demonstrated by the association of persistent C7 rib in the prefixed plexus in which the C4 nerve root may be included, and the T1 nerve root is often small, as opposed to the postfixed plexus, which has a larger T2 nerve root and is associated with a rudimentary first thoracic rib.2,16–18
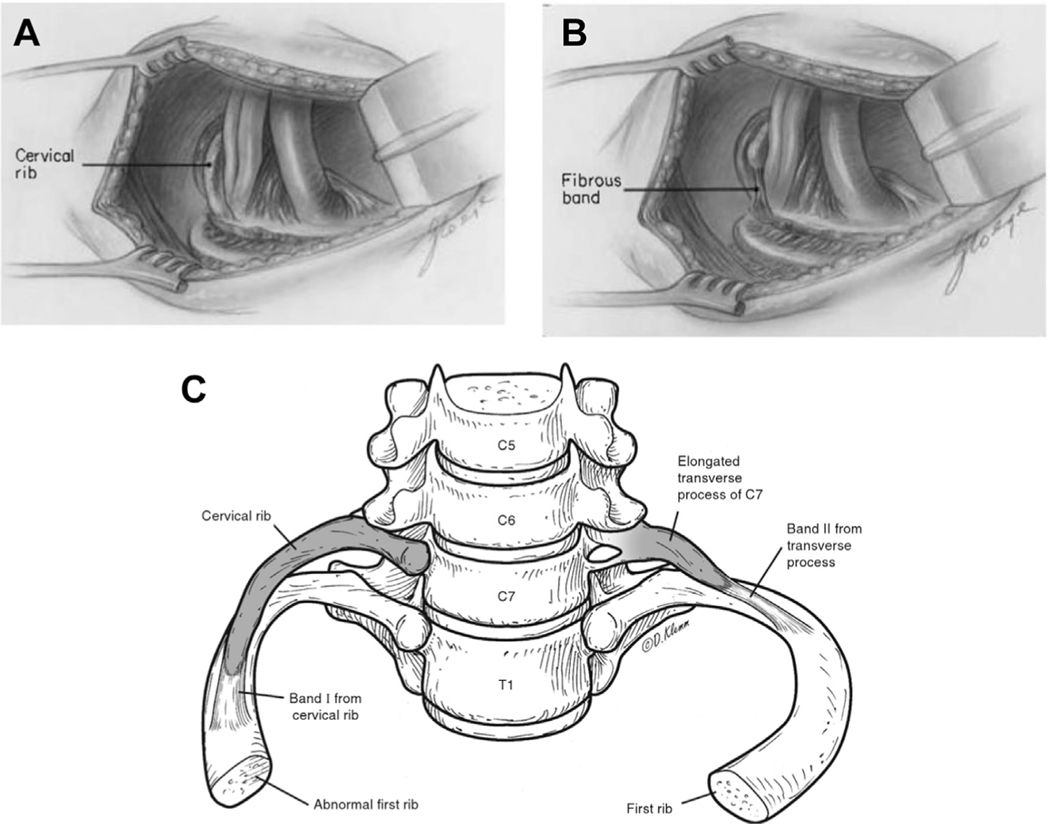
Rather than a complete cervical rib, some people may develop an enlarged C7 transverse process or a rudimentary incomplete C7 rib 0.5 to 3 cm in length with a thick fibrocartilaginous band attaching it to the first rib1,16,19 (Fig. 7). This band is not visible on the radiograph but can cause compression similar to a complete C7 rib. When the band extends from a rudimentary cervical rib, it is termed a Type I band. When it extends from an enlarged C7 transverse process, it is termed a Type II band. These were first described by Roos, who identified and numbered many fibromuscular anomalies3,20 (Table 1). The enlarged C7 transverse process has been reported in familial forms of TOS and is thought to be inherited in an autosomal dominant pattern.21–23
Fig. 7.
The cervical rib may be complete, in which it is frequently fused to the first thoracic rib (A), or it may be incomplete with a fibrous band to the first thoracic rib (B). The elongated transverse process of C7 may function similarly to an incomplete cervical rib, but is anatomically distinct from a rib in its continuity with the C7 vertebra (C). ([A] From Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet. J Vasc Surg. 1992;16:538; with permission; and [B] From Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet. J Vasc Surg. 1992;16:538; with permission; and [C] Courtesy of Dave Klemm, Georgetown University School of Medicine.)
Table 1.
Congenital bands and ligaments described by Roos
| Type 1 | A band from the anterior tip of an incomplete cervical rib to the middle of the first thoracic rib inserts into the upper rib surface posterior to the scalene tubercle. |
| Type 2 | A band arising from an elongated C7 transverse process attaches to the first rib just behind the scalene tubercle in the same place as a type 1 band. |
| Type 3 | A band both originating from and inserting into the first rib arises posteriorly, near the neck of the rib, and inserts more anteriorly, just behind the scalene tubercle. |
| Type 4 | A band originating along with the middle scalene muscle from a transverse process runs along the anterior edge of the middle scalene muscle and inserts with it into the first rib. The lower nerves of the plexus may lie against it. |
| Type 5 | The scalene minimus muscle is the 5th type of band. It arises with the lower fibers of the anterior scalene muscle and runs parallel to it but passes deep into it, behind the subclavian artery, in front of the plexus, to insert into the first rib. Normally, the entire anterior scalene muscle passes anterior to the artery. Any fibers that pass anterior to the plexus but posterior to the artery belong to the scalene minimus muscle. |
| Type 6 | When the scalene minimus muscle inserts into Sibson’s fascia over the cupola of the pleura and lung instead of into the first rib, it is labeled separately to distinguish its point of insertion. |
| Type 7 | A fibrous cord running along the anterior surface of the anterior scalene muscle down to the first rib attaches to the costochondral junction or sternum. In this position, the band lies immediately behind the subclavian vein and can be the cause of partial venous obstruction. |
| Type 8 | A band arising from the middle scalene muscle runs under the subclavian artery and vein to attach to the costochondral junction. |
| Type 9 | A web of muscle and fascia filling the inside posterior curve of the first rib forms the ninth type of band. |
| Type 10 | Some of the anterior scalene muscle fibers form a band that connects to the perineurium of the brachial bundle. |
| Type 11 | A band formed by fibers existing between the anterior and middle scalene muscles passes between nerve roots. |
| Type 12 | The upper part of an anomalous anterior scalene muscle passes behind the C5 and C6 roots. |
| Type 13 | Fused scalene muscles form a band, and the brachial nerve roots pass through the muscle like arrows. |
| Type 14 | Fibrous bands passing vertically in front of the nerve roots behind the anterior scalene muscle form the 14th type of band. |
From Tokat AO, Atınkaya C, Fırat A, et al. Cadaver analysis of thoracic outlet anomalies. Turkish J Thorac Cardiovasc Surg. 2011;19(1):72–6; with permission.
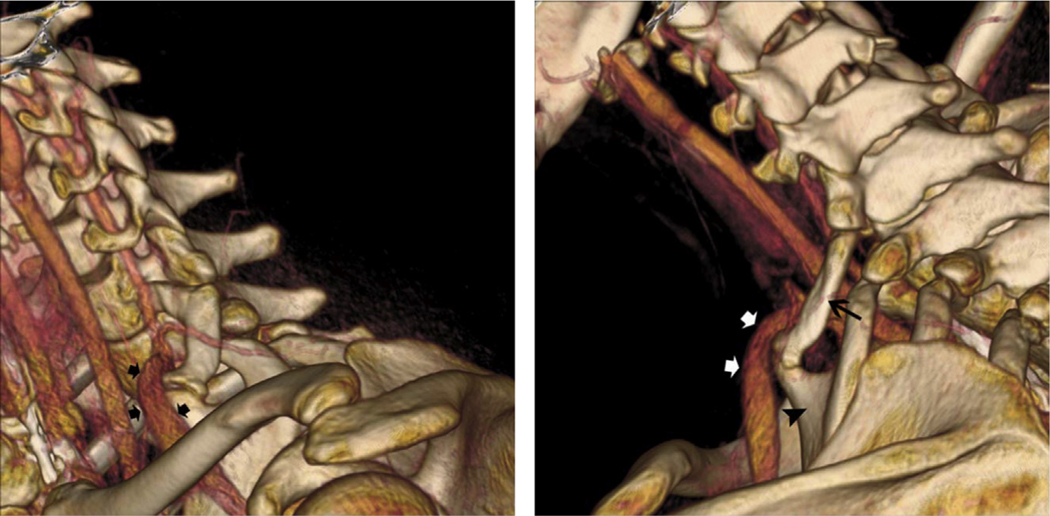
The presence of a cervical rib tightens the scalene triangle and elevates the thoracic outlet such that when the subclavian artery exits the costoclavicular space to become the axillary artery, it must ascend over the cervical rib.1,2 Not surprisingly, cervical ribs are associated with arterial TOS2 (Fig. 8). Neurogenic TOS may also occur as the brachial plexus follows the same course artery over the supernumerary rib.
Fig. 8.
Relationship of the subclavian artery to the cervical rib. In the lateral view of this 3D reconstruction of a CT angiogram (A), compression of the subclavian artery is visible (black arrows) as it passes over a cervical rib. In the posterolateral view (B), the compression is again seen (white arrows) as the subclavian vein passes over the cervical rib (black arrow) at its joint with the tubercle from the first thoracic rib (black arrowhead). (From Klaassen Z, Sorenson E, Tubbs RS, et al. Thoracic outlet syndrome: a neurological and vascular disorder. Clin Anat. 2014;27:724–32; with permission.)
FIBROMUSCULAR
Scalene Muscles
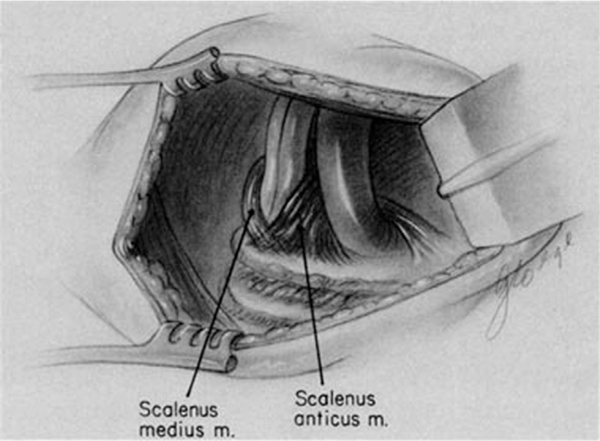
The scalene muscles are accessory muscles of respiration in that they function to elevate the upper thoracic cage. They consist of the anterior, middle, and posterior scalene muscles and extend from the cervical vertebrae to the first and second ribs. The anterior scalene muscle separates the subclavian vein (anterior) from the subclavian artery (posterior). The middle scalene is posterior to the brachial plexus, which is posterior and lateral to the subclavian artery. The posterior scalene is posterior to the middle scalene, inserts on the second rib, and does not play a role in the thoracic outlet.
The development of the scalene muscles is similar to that of intercostal muscles.3 Like cervical ribs, the embryologic development of the scalene muscle is influenced by the neurovascular structures.16,24 They originate from the hypaxial mesoderm of somital myotomes, beginning as a common scalene muscle in the eighth gestational week. This common scalene muscle is then separated into two distinct components (anterior and middle) by the brachial plexus.
Anomalies occur as a result of the location of the traversing neurovascular bundle and are more common than osseous anomalies. These include supernumerary muscles and muscles with irregular shapes or fibrotic components. In cadaver dissections, muscle fibers connecting the anterior and middle scalene muscles were found interdigitating through the brachial plexus in 75% of dissections of patients with TOS.25 This can create a sling that traps the brachial plexus and subclavian artery. This is known as a Type 4 band of Roos.20 (Fig. 9).
Fig. 9.
Sling-like entrapment of neurovascular structures from incomplete scalene separation. (From Makhoul RG, Machleder HI. Developmental anomalies at the thoracic outlet. J Vasc Surg. 1992;16:539; with permission.)
The division of the common scalene muscle may lead to the development of supernumerary muscles, most commonly the “scalenus minimus.” This small muscle passes between the subclavian artery and brachial plexus and may irritate the lower trunk of the brachial plexus. It may result from segmentation defects of the common scalene muscle or failure of regression of the mesodermal mass.3 The scalenus minimus may be less of a muscle and more of a residual ligament, defined as a Type 5 band if it inserts on the rib (“costovertebral”). The scalenus pleuralis similarly may be defined as a Type 6 band if it inserts on the apical suprapleural fascia of Sibson (“pleurospinal”).3,16,20 In a study of human fetus thoracic outlet anatomy, fibromuscular bands were identified in 15% of 80 dissections, with Roos’ Types 5 and 11 found most frequently26 (see Table 1).
Symptomatic TOS is more commonly seen in patients with anomalies that occur posterior to the brachial plexus.3,27 The most common anomaly, termed an “outlet band,” is a ligamentous band extending from the neck of the first rib to its inner surface posterior to the scalene tubercle where the anterior scalene muscle inserts.3
TOS may occur after hyperextension injuries to the neck. The hyperextension tears some of the anterior and middle scalene muscle fibers, which form scar tissue in the process of healing. This scarred muscle is less pliable than normal muscle, which can compress the brachial plexus and cause neurogenic TOS.1
Subclavius Muscle
The subclavius muscle stabilizes the clavicle with shoulder motion. It originates at the junction of the first rib and its cartilage, travels along the inferior surface of the clavicle, and inserts at the subclavian groove of the clavicle. The subclavius muscle tendon compresses the subclavian vein against the first rib when the shoulder is abducted or retracted.16 Hypertrophy of the subclavius tendon has been noted in Paget-Schroetter deformity.28–30
Pectoralis Minor Muscle
The pectoralis minor moves the scapula anterior and inferior. It also functions as an accessory muscle of respiration by elevating the upper ribs. It originates on the anterior surfaces of ribs 3 to 5 and inserts on the coracoid process of the scapula. The pectoral nerve to the pectoralis major muscle travels within the pectoralis minor muscle. The tendon of the pectoralis minor plays a role in compression of the neurovascular bundles as it transits the subcoracoid tunnel.31 Excising the tendon from the coracoid process and removing 2 to 3 cm of tissue results in decompression of this space and is a useful adjunct to the first rib resection and scalenectomy for TOS.1
THORACIC DUCT
The thoracic duct is a lymphatic vessel that transports chyle and lymphatic drainage from the left upper extremity, left thorax, and lower body to the venous system. In the neck, it is located in the left scalene fat pad, posterior and inferior to the clavicle as it travels to the junction of the left internal jugular and left subclavian veins. It has an associated network of fine lymphatic channels along the internal jugular vein. Lymphatic leaks in the left neck are avoided by careful attention to all small lymphatic tributaries during dissection and by avoiding separating the scalene fat pad from the internal jugular vein.1
SUMMARY
The anatomy of the thoracic outlet is highly variable. Understanding this variation and its roots in embryology is crucial to safe and effective surgical management of the myriad conditions that comprise TOS.
KEY POINTS.
The subclavian artery and brachial plexus travel together through the costoclavicular space posterior to the anterior scalene muscle, whereas the subclavian vein travels anterior to the anterior scalene muscle.
The brachial plexus is composed of C5-T1 nerve roots, trunks, divisions, cords, and branches, and variability in development may influence anomalies of the surrounding structures.
The first rib normally develops from the T1 costal process to the manubrium. Anomalous first ribs originate from T1 and may fuse to the second rib, whereas cervical ribs arise from cervical vertebral bodies (usually C7).
The scalene muscles may have significant variability, including several anomalies associated with thoracic outlet syndrome.
Clinics care points.
The thoracic outlet is both a confined and a dynamic space: compression of the neurovascular bundle at varying sites may result in symptoms of thoracic outlet syndrome (TOS).
Abnormally anterior insertion of the anterior scalene muscle may compress the subclavian vein against the subclavius tendon.
Prefixed and postfixed brachial plexi may be associated with cervical or first rib anomalies.
Abnormal first ribs and cervical ribs are typically asymptomatic, but when symptoms are present, they are usually related to arterial or neurogenic TOS.
The presence of a cervical rib tightens the scalene triangle and elevates the thoracic outlet as the subclavian artery and brachial plexus must ascend over the supernumerary rib.
The scalene muscles originate from one common muscle that is later separated by the developing brachial plexus, and therefore, anomalies commonly include supernumerary or irregularly shaped muscles that may compress aspects of the brachial plexus.
Understanding anatomic anomalies and their roots in embryology is crucial to safe and effective surgical management of TOS
Footnotes
DISCLOSURE
The authors have nothing to disclose.
REFERENCES
- 1.Sanders RJ. Anatomy of the thoracic outlet andrelated structures. In: Illig KA, Thompson RW, Freischlag JA, et al. , editors. Thoracic outlet syndrome. Springer, London; 2013. p. 17–24. 10.1007/978-1-4471-4366-6_3. [DOI] [Google Scholar]
- 2.Klaassen Z, Sorenson E, Tubbs RS, et al. Thoracic outlet syndrome: a neurological and vascular disorder. Clin Anat 2014;27:724–32. [DOI] [PubMed] [Google Scholar]
- 3.Tubbs RS, Shoja MM. Embryology of the thoracic outlet. In: Illig KA, Thompson RW, Freischlag JA, et al. , editors. Thoracic outlet syndrome. London: Springer, London; 2013. p. 11–6. [Google Scholar]
- 4.Roos DB. The place for scalenectomy and first-rib resection in thoracic outlet syndrome. Surgery 1982;92(6):1077–85. [PubMed] [Google Scholar]
- 5.Natsis K, Totlis T, Tsikaras P, et al. Variations of the course of the upper trunk of the brachial plexus and their clinical significance for the thoracic outlet syndrome: A study on 93 cadavers. Am Surg 2006;72(2):188–92. [PubMed] [Google Scholar]
- 6.Pellerin M, Kimball Z, Shane Tubbs R, et al. The prefixed and postfixed brachial plexus: a review with surgical implications. Surg Radiol Anat 2010. 10.1007/s00276-009-0619-3. [DOI] [PubMed] [Google Scholar]
- 7.Jackson NJ, Nanson EM. Intermittent subclavian vein obstruction. Br J Surg 1961;49(215):303–6. [DOI] [PubMed] [Google Scholar]
- 8.Adson AW, Coffey JR. Cervival rib: A method of anterior approach for relief of symptoms by division of the scalenus anticus. Ann Surg 1927;85(6): 839–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Etter L. Osseous abnormalities of the thoracic cage seen in forty thousand consecutive chest photo roentgenograms. Am J Roentgenol 1944;51: 359–63. [Google Scholar]
- 10.Adson AW. Surgical treatment for symptoms produced by cervical ribs and the scalenus anticus muscle. Surg Gynecol Obstet 1947;85(6): 687–700. [PubMed] [Google Scholar]
- 11.Adson AW. Cervical ribs: symptoms, differential diagnosis, and indication for section of the insertion of scalenous anticus muscle. J Int Coll Surg 1951; 16:546–59. [PubMed] [Google Scholar]
- 12.Firstov G. Cervical ribs and their distinction from under-developed first ribs. Arkh Anat Gistol Embriol 1974;67:101–3. [PubMed] [Google Scholar]
- 13.Galis F. Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. J Exp Zool 1999;285(1): 19–26. [PubMed] [Google Scholar]
- 14.Burke AC, Nelson CE, Morgan BA, et al. Hox genes and the evolution of vertebrate axial morphology. Development 1995;121(2):333–46. [DOI] [PubMed] [Google Scholar]
- 15.Horan GSB, Kovacs EN, Behringer RR, et al. Mutations in paralogous hox genes result in overlapping homeotic transformations of the axial skeleton: evidence for unique and redundant function. Dev Biol 1995;169(1):359–72. [DOI] [PubMed] [Google Scholar]
- 16.Machleder HI. Thoracic outlet syndrome. In: Hollier LH, White RA, editors. Vascular surgery: basic science and clinical correlations. Malden: Blackwell Publishing Inc.; 2005. p. 146–61. Available at: https://www.google.com/books/edition/Vascular_Surgery/PluZG–0VEcC?hl5en&gbpv51. [Google Scholar]
- 17.Guday E, Bekele A, Muche A. Anatomical study of prefixed versus postfixed brachial plexuses in adult human cadaver. ANZ J Surg 2017;87(5):399–403. [DOI] [PubMed] [Google Scholar]
- 18.White J, Poppel M, Adams R. Congenital malformations of the first thoracic rib; a cause of brachial neuralgia which simulates the cervical rib syndrome. Surg Gynecol Obstet 1945;81:643–59. [PubMed] [Google Scholar]
- 19.Todd TW. The relations of the thoracic operculum considered in reference to the anatomy of cervical ribs of surgical importance. J Anat 1911;45: 293–304. [PMC free article] [PubMed] [Google Scholar]
- 20.Roos DB. Congenital anomalies associated with thoracic outlet syndrome. Am J Surg 1976;132(6): 771–8. [DOI] [PubMed] [Google Scholar]
- 21.Weston WJ. Genetically determined cervical ribs - a family study. Br J Radiol 1956;29(344):455–6. [DOI] [PubMed] [Google Scholar]
- 22.Boles J, Missoum A, Mocquard Y, et al. A familial case of thoracic outlet syndrome. Clinical, radiological study with treatment [French]. Sem Hop 1981; 57(25–28):1172–6. [PubMed] [Google Scholar]
- 23.Schapera J. Autosomal dominant inheritance of cervical ribs. Clin Genet 1987;31(6):386–8. [DOI] [PubMed] [Google Scholar]
- 24.Milliez P. Contribution a l’Etude de l’Ontogenese DesMuscles Scalenes (Reconstruction d’un Embryon de 2.5 Cm). Paris; 1991. [Google Scholar]
- 25.Sanders RJ, Roos DB. The surgical anatomy of the scalene triangle. Contemp Surg 1989;35:11–6. [Google Scholar]
- 26.Fodor M, Fodor L, Ciuce C, et al. Anomalies ofThoracic Outlet in Human Fetuses: Anatomical Study. Ann Vasc Surg 2011. 10.1016/j.avsg.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Redenbach DM, Nelems B. A comparative study of structures comprising the thoracic outlet in 250 human cadavers and 72 surgical cases of thoracic outlet syndrome. Eur J Cardiothorac Surg 1998;13(4):353–60. [DOI] [PubMed] [Google Scholar]
- 28.Sampson J. Medico-Surgical Tribute to Harold Brunn. Berkeley: University of California Press; 1942. p. 453. [Google Scholar]
- 29.Aziz S, Straehley CJ, Whelan TJ. Effort-related axillosubclavian vein thrombosis. A new theory of pathogenesis and a plea for direct surgical intervention. Am J Surg 1986;152(1):57–61. [DOI] [PubMed] [Google Scholar]
- 30.Kunkel JM, Machleder HI. Treatment of pagetschroetter syndrome: a staged, multidisciplinary approach. Arch Surg 1989;124(10):1153–8. [DOI] [PubMed] [Google Scholar]
- 31.Charon JPM, Milne W, Sheppard DG, et al. Evaluation of MR angiographic technique in the assessment of thoracic outlet syndrome. Clin Radiol 2004; 59(7):588–95. [DOI] [PubMed] [Google Scholar]