Abstract
Context.—
Graft-versus-host disease of the gastrointestinal tract is a common complication of hematopoietic stem cell transplant associated with significant morbidity and mortality. Accurate diagnosis can be difficult and is a truly clinicopathologic endeavor.
Objectives.—
To assess the diagnostic sensitivity of gastrointestinal graft-versus-host disease using the 2015 National Institutes of Health (NIH) histology consensus guidelines and to analyze histologic findings that support the guidelines.
Design.—
Patients with allogeneic hematopoietic stem cell transplants were identified via a retrospective search of our electronic medical records from January 1, 2005, to January 1, 2011. Endoscopies with available histology were reviewed by 2 pathologists using the 2015 NIH guidelines. The clinical diagnosis was used as the gold standard. A nontransplant set of endoscopic biopsies was used as a control.
Results.—
Of the 250 total endoscopies, 217 (87%) had a clinical diagnosis of gastrointestinal graft-versus-host disease. Use of the NIH consensus guidelines showed a sensitivity of 86% and a specificity of 65%. Thirty-seven of 58 (64%) cases with an initial false-negative histopathologic diagnosis were diagnosed as graft-versus-host disease on our review.
Conclusions.—
Use of the NIH histology consensus guidelines results in a high sensitivity and specificity, thereby decreasing false-negatives. Additionally, use of the NIH guidelines aids in creating uniformity and diagnostic clarity. Correlation with clinical and laboratory findings is critical in evaluating the differential diagnosis and to avoid false-positives. As expected, increased apoptosis with decreased inflammation was associated with a pathologic diagnosis of graft-versus-host disease and supports the NIH guidelines.
Graft-versus-host disease (GVHD) of the gastrointestinal (GI) tract is a common complication of hematopoietic stem cell transplant (HSCT) and is associated with significant morbidity and mortality.1–6 Graft-versus-host disease develops when donor lymphocytes recognize recipient proteins as foreign and induce epithelial apoptosis, inflammation, and tissue injury.7–9 Most commonly, GVHD affects the skin, GI tract, liver, and lungs.1,5,6,10–12
Clinically, GI GVHD presents with nonspecific GI symptoms including nausea, vomiting, oropharyngeal mucositis, dysphagia, diarrhea, and GI bleeding.5,10,12,13 Common clinical and histologic mimickers of GI GVHD include side effects of chemotherapeutic and immunosuppressive agents, most notably mycophenolate mofetil (MMF), and infections, such as cytomegalovirus (CMV), adenovirus, and Clostridium difficile.13–23 A definitive diagnosis of GI GVHD can be challenging, given overlapping clinical presentations. An accurate diagnosis requires correlation with the patient’s history, clinical impression, endoscopic findings, laboratory studies, and histology.
In 2015, the US National Institutes of Health (NIH) published updated consensus guidelines to aid in the histologic diagnosis of GI GVHD and recommended using 3 diagnostic categories: (1) not, (2) possible, and (3) likely GVHD. The NIH consortium has previously suggested a threshold for minimal histologic features of GI GVHD to be ≥1 intraepithelial apoptosis (on average) per biopsy piece or crypts/glands with multiple apoptoses.14,15
The primary aim of this study was to assess the diagnostic accuracy of histology for GI GVHD within a large cohort of patients with HSCTs when using the 2015 NIH consensus guidelines. The secondary aim of this study was to analyze various histologic findings that could support the NIH consensus guidelines.
MATERIALS AND METHODS
A retrospective search of electronic medical records at Duke University Hospital in Durham, North Carolina, identified adult patients who had undergone HSCT for the treatment of malignant blood or bone marrow disease between January 1, 2005, and January 1, 2011. Of those patients, the ones who had undergone allogeneic HSCT and subsequent GI endoscopy for evaluation of GI-related symptoms were selected.
The clinical diagnosis at the time of endoscopy was used as the gold standard for the purpose of this study. The clinical diagnosis of each endoscopic event was determined by a thorough chart review performed by a clinical reviewer who assessed the impression of the treating transplant physician, whether empiric steroid treatment was initiated, the presence of a treatment response, and excluded other potential etiologies (eg, infection, medication-related injury, among others). The patient’s race was captured from the medical record as documented by the patient’s provider and was assessed to evaluate the diversity within the study population. Original pathology reports were reviewed by a pathologist, and the final diagnosis rendered by the pathologist of record was stratified into 1 of the 3 NIH categories described below, without reference to the recorded microscopic findings.
All available GI biopsies, excluding biopsies of the esophagus, were jointly reviewed by 2 pathologists who were blinded to the clinical, endoscopic, and laboratory findings and the original pathology diagnosis. In keeping with the NIH guidelines, a minimum of 8 serial sections were analyzed for each case. Immunohistochemistry (IHC) slides available at the time of the original diagnosis were reviewed (eg, CMV, adenovirus). Additionally, CMV IHC was retrospectively performed on all 24 biopsies in which the test was not performed or available. A consensus diagnosis was made for each biopsy site, independent of other concurrently biopsied sites, using the 2015 NIH diagnostic categories not GVHD, possible GVHD, and likely GVHD (Table 1). Not GHVD was used when there was no histologic evidence of GVHD, or another reason for the histologic findings was present. Possible GVHD was used when there was histologic evidence of GVHD, such as marked apoptosis, but there were also features of another potential etiology (eg, CMV inclusions), given that coexistent disease cannot be entirely excluded. Likely GVHD was used when there was histologic evidence of GVHD, ranging from minimal to marked epithelial injury, without another known or potential etiology (Figure 1, A and B). The likely GVHD category represents a fusion of 2 categories included in a previous version of the NIH guidelines published in 2006: consistent with GVHD and unequivocal GVHD. An overall diagnosis was subsequently assigned for each endoscopic event based on the highest histologic categorization of the biopsy set (likely > possible > not). If a diagnosis of likely GVHD was rendered at any biopsy site, a Lerner grade was also assessed (grade 0, normal mucosa; grade 1, epithelial apoptosis; grade 2, crypt/gland destruction; grade 3, focal erosion/ulcer; or grade 4, diffuse ulceration).24 Following initial statistical analysis, all the false-positive (FP) cases were studied, and if pertinent clinical information available at the time of diagnosis or findings from a concurrent biopsy sample indicated a probable alternative, the case was recategorized to possible GVHD, and statistical analyses were repeated.
Table 1.
National Institutes of Health Consensus Guidelines–Diagnostic Categories for Gastrointestinal Graft-Versus-Host Disease (GVHD)
| Category | Definition |
|---|---|
| Not GVHD | No histologic evidence of GVHD |
| Possible GVHD | Histologic evidence of GVHD, but other possible explanations also present |
For example:
|
|
| Likely GVHD | Clear histologic evidence of GVHD without a competing etiology; histologic evidence of GVHD, but also other mitigating factors or relevant clinical information is limited |
For example:
|
Abbreviations: CMV, cytomegalovirus; MMF, mycophenolate mofetil.
Figure 1.
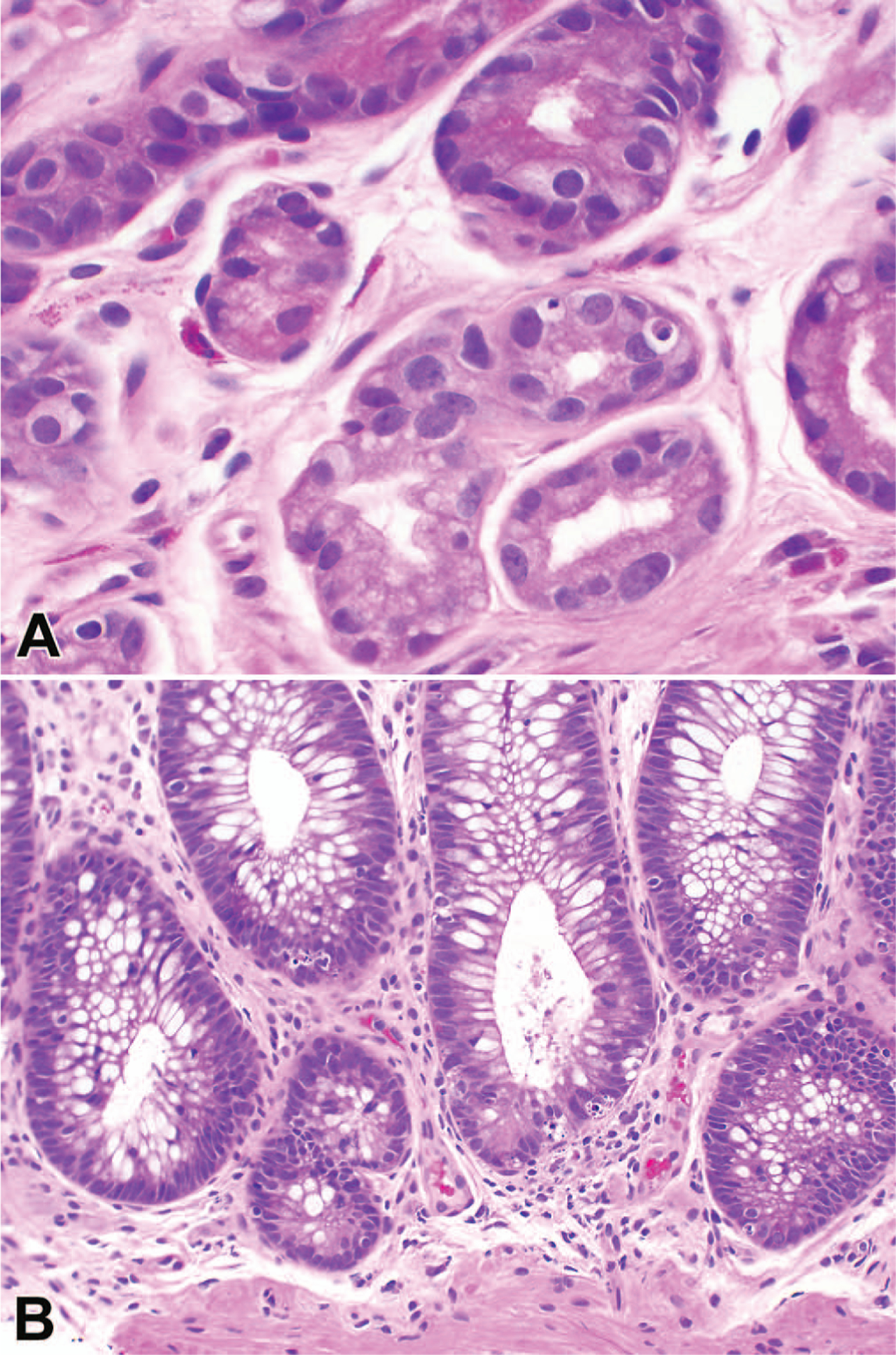
Photomicrographs of cases diagnosed on review as likely graft-versus-host disease A, Stomach. B, Colon (hematoxylin-eosin, original magnifications ×40 [A] and ×20 [B]).
Various histologic features were jointly evaluated and assessed by both pathologists. Using a qualitative scale of 0 to 4 (none, minimal, mild, moderate, and severe), the degree of apoptosis, crypt/gland destruction, crypt/gland dropout, lamina propria inflammation, and cryptitis was documented. For apoptosis assessment, less than 1 apoptosis per biopsy piece was scored as none (0), an average of 1 apoptosis per biopsy piece was scored as minimal (1), 2 or more apoptoses per biopsy piece (but distributed in separate glands) was diagnosed as mild (2), several apoptoses in a single crypt/gland was considered moderate (3), and confluent apoptosis was categorized as severe (4). The presence or absence of ulceration, basal lymphoplasmacytosis, crypt/gland distortion, foveolar cell metaplasia, pyloric cell metaplasia, granulomata, fibrosis, vasculitis, and CMV IHC positivity was recorded. The number of biopsy fragments was also noted.
A control group, composed of adult patients without transplants, was acquired via a retrospective search of the electronic medical records for 25 duodenal, 25 stomach, 25 right colon, and 25 left colon biopsies with a diagnosis of no pathologic diagnosis or no pathologic alteration. Those slides were reviewed for the same histologic features and with the same degree of scrutiny as the study slides. Clinical indication for the endoscopy was also recorded.
Statistical Methods
For statistical analysis, an overall histologic categorization of not GVHD was considered negative for pathologic GI GVHD and an overall histologic categorization of likely GVHD was considered positive for pathologic GI GVHD. Cases called possible GVHD were excluded from the initial statistical analysis. Measures of diagnostic accuracy assumed the gold standard to be the clinical diagnosis. Accuracy measures included sensitivity, defined as the proportion of all cases found to be positive clinically that had histologically positive results; specificity, defined as the proportion of all cases found to be negative clinically that had histologically negative results; positive predictive value, defined as the proportion histologically and clinically positive results from all cases found to be histologically positive; and negative predictive value, defined as the proportion histologically and clinically negative results from all cases found to be histologically negative. Fisher exact test was used to assess the significance of low-level apoptosis when comparing the patient cohort of interest to the control group.
The histologic features with sufficient spread to potentially discriminate between the presence or absence of GVHD or those with an overall incidence of more than 15% were analyzed. These ultimately were limited to apoptosis, inflammation, and crypt/gland destruction (each scored from 0 to 4) and the number of fragments (continuous). Exact binomial methods were used to calculate the 95% CIs.
RESULTS
During the 6-year study period, 397 adult patients underwent allogeneic HSCT. In-house histology from endoscopy was available for 169 (43%) of the 397 patients. The average time between HSCT and first endoscopy was 1.9 months. Of the 397 patients, 222 (56%) were male, 333 (84%) were white, and the median age was 49 years, with a range from 19 to 73 years. The most common indication for HSCT was acute myelogenous leukemia. The transplant sources were peripheral blood progenitor cells (330 of 397; 83%), cord blood (64 of 397; 16%), and bone marrow (3 of 397; 1%). The 169 patients included in our study underwent 250 upper and/or lower GI endoscopic events, resulting in 435 biopsy specimens. The most common symptoms cited as the indication for endoscopy included watery diarrhea (192 of 250; 77%), nausea (185 of 250; 74%), and anorexia (140 of 250; 56%). At the time of endoscopy, medication for GVHD prophylaxis was administered for 125 of the 250 events (50%), of which, 102 of the 125 endoscopies (82%) were for patients taking MMF specifically.
Of the 250 endoscopic events, GI GVHD was diagnosed clinically in 217 cases (87%). Of those 217 clinical GVHD cases, 148 (68%) had documented evidence of a potential coexistent disease complicating the entity or entities upon chart review. Culture, serologic, and/or molecular evidence of a coexisting infection or multiple infections was seen in 118 of those 148 cases (80%), including CMV (n = 82; 69%), polyomavirus (n = 17; 14%), parainfluenza virus (n = 12; 10%), herpes simplex virus (n = 7; 6%), human herpesvirus 6 (n = 3; 3%), varicella zoster virus (n = 2; 2%), adenovirus (n = 2; 2%), respiratory syncytial virus (n = 1; 1%), fungal infection (n = 4; 3%), parasitic infection (n = 2; 2%), and bacterial infection (n = 65; 55%); of which, 3 (5%) were C difficile. The above-mentioned 82 patients coinfected with CMV included those with CMV viremia without definitive evidence of CMV gut involvement as well as those with CMV disease of the gut. Additionally, 40 of the 169 patients (24%) in our study had other GI-related (n = 32) and non-GI-related (n = 15) medical conditions, including some patients with multiple medical comorbidities. The GI-related complications included peptic disease, celiac sprue, pancreatitis, cholecystitis, diverticulitis, esophagitis, irritable bowel syndrome, ischemic colitis, and dietary intolerances. Non–GI-related conditions included opioid withdrawal, diabetes mellitus, Guillain-Barré syndrome, mucositis, and thrombocytopenia.
Of the 250 endoscopic events, the 33 patients (13%) clinically diagnosed as not having GVHD were given diagnoses including 1 or more of the following: infectious etiologies (n = 15; 45%), medication related toxicity (n = 3; 9%), disease relapse (n = 1; 3%), other medical reasons (n = 13; 39%), and/or undetermined cause (n = 5; 15%).
Overall, the pathology diagnosis was likely GVHD for 117 of 250 endoscopic events (47%) on the original pathology report and for 188 of 250 endoscopic events (75%) on the pathology review for the study. Of the 217 GI GVHD cases found to be positive clinically, 116 (53%) and 173 (80%) were called likely GVHD on the original and study review pathology reports, respectively. In 58 of 250 endoscopic events (23%) initially diagnosed as not GVHD on the original pathology report, the patient was clinically diagnosed as having GI GVHD. Of those 58 endoscopic events, 37 originally false-negative (FN; 64%) cases were diagnosed as likely GVHD on the study review using the NIH consensus guidelines. In a subset of those cases there was a delay in treatment and repeat endoscopy was necessary; however, most patients did receive appropriate treatment given other key clinical and endoscopic findings. In the original pathology diagnoses, 49 of 250 (20%) were categorized as possible GVHD, in contrast to 18 (7%) following the study evaluation. Of the 49 cases originally called possible GVHD, 7 (14%) patients had clinically negative results for GVHD, whereas 42 (86%) were clinically positive for GVHD. In the 18 cases called possible GVHD during our study review, 3 (17%) were clinically negative for GVHD and 15 (83%) were clinically positive for GVHD.
In the study pathology review, of the 250 endoscopic events there were 15 FP cases (6%) and 29 FN cases (12%), when using histology alone and assessing biopsy sites independently. That led to a sensitivity of 86%, a specificity of 50%, a positive predictive value of 92%, and negative predictive value of 34%. The 15 FP cases were clinically diagnosed as one or more of the following: CMV infection (n = 5; 33%), gastroesophageal reflux (n = 3; 20%), MMF toxicity (n = 2; 13%), dietary intolerance (n = 2; 13%), pancreatitis/pancreatic cysts (n = 2; 13%), herpes simplex virus (HSV) esophagitis (n = 2; 13%), gastritis/gastroparesis (n = 2; 13%), C difficile colitis (n = 1; 7%), esophageal mass (n = 1; 7%), anxiety (n = 1; 7%), candidiasis (n = 1; 7%), and/or unknown (n = 1; 7%; Table 2). Of note, all 24 retrospectively performed CMV IHC results were negative. Understanding that certain key clinical findings are essential for accurate diagnosis at the time of histologic review, conservatively, 7 of the 15 FP cases (47%) were recategorized to possible GVHD, primarily because of results that were available at the time of initial diagnosis (eg, positive CMV polymerase chain reaction [PCR], positive C difficile toxin enzyme immunoassay, and/or positive CMV or HSV IHC results in a different biopsy site). Following that recategorization, the sensitivity remained at 86%, the specificity increased to 65%, the positive predictive value increased to 96%, the negative predictive value remained at 34%, and the number of FP cases decreased to 8 of 250 (3%).
Table 2.
False-Positive Cases Upon Initial National Institutes of Health (NIH) Graft-Versus-Host Disease (GVHD) Diagnostic Categorization
| Case No. | Clinical Diagnosis | NIH Diagnostic Category | Evidence of GVHD Elsewhere | CMV PCR | C difficile EIA | MMF Within 2 Wk of Endoscopy | Final NIH Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | MMF toxicity | Sto possible, duo likely | − | − | NP | + | Likely GVHD |
| 2 | Dietary problem | Sto likely, duo not, RS not | − | − | NP | + (trial of decreased dose ineffective) | Likely GVHD |
| 3 | HSV esophagitis, GERD, gastroparesis | (Eso + HSV IHC), sto likely, duo likely | Skin | − | − | + | Possible GVHD |
| 4 | Gastritis | Sto not, duo likely | − | − | NP | − | Likely GVHD |
| 5 | GERD, candidiasis | Sto possible, duo likely | Liver | − | − | − | Likely GVHD |
| 6 | Unknown, failure to thrive | Sto not, duo likely | − | − | − | − | Likely GVHD |
| 7 | CMV | Sto likely | − | + | NP | + | Possible GVHD |
| 8 | Pancreatic cysts | Sto likely | Skin | NP | NP | + | Likely GVHD |
| 9 | MMF toxicity, C difficile | RS likely | Lung | + (<100 copies/mL) | + | + | Possible GVHD |
| 10 | Esophageal inflammatory mass | Sto likely, duo likely | − | − | − (+1 mo prior) | − | Likely GVHD |
| 11 | CMV, HSV esophagitis | Sto possible, duo likely | GI (1 mo prior) | + | NP | − | Possible GVHD |
| 12 | CMV | Sto possible + CMV IHC, duo likely, RS possible + CMV IHC | − | + | − | + | Possible GVHD |
| 13 | CMV | Sto likely | − | + | − | + | Possible GVHD |
| 14 | GERD, anxiety | Sto likely, duo likely | Skin | − | − | − | Likely GVHD |
| 15 | CMV, pancreatitis, lactose intolerance | Duo likely, left colon likely | − | + | − | − | Possible GVHD |
Abbreviations: +, present; −, absent; C difficile, Clostridium difficile; CMV, cytomegalovirus; duo, duodenum; EIA, toxin enzyme immunoassay; Eso, esophagus; GERD, gastroesophageal reflux disease; GI, gastrointestinal; HSV, herpes simplex virus; IHC, immunohistochemistry; MMF, mycophenolate mofetil; NP, not performed; PCR, polymerase chain reaction; RS, rectosigmoid; sto, stomach.
Of the 250 endoscopic events, 82 (33%) had evidence of CMV disease (eg, positive CMV PCR and/or positive CMV IHC results). Of the 82, 10 cases (12%) were clinically positive for CMV disease of the gut and clinically negative for GI GVHD, of which 8 of 10 (80%) had a positive serum result from CMV PCR at the time of endoscopy and 3 of 10 (30%) had a positive CMV IHC result on at least 1 biopsy site. Both cases with PCR-negative results had a positive CMV PCR result the week prior and the week after endoscopy, and 1 patient had a positive CMV IHC result on their stomach biopsy. For the 10 cases clinically diagnosed as CMV gut disease and negative for GI GVHD, the pathology diagnoses were 4 not GVHD (40%), 1 possible GVHD (10%), and 5 likely GVHD (50%) before recategorization.
Three of the 250 endoscopic events (1%) had a clinical diagnosis of MMF toxicity. The pathology diagnosis in 2 cases was likely GVHD, and thus falsely positive.
There were 24 unique endoscopic events called not GVHD on pathology review with apoptosis scores of 0 of 4 (less than the minimal threshold) on all biopsy sites. Of those 24 cases, 18 (75%) were called positive for GVHD clinically, and 6 (25%) were called negative for GVHD clinically. Exploring the lower end of apoptosis further, there were 16 unique endoscopic events that were called likely GVHD and had an apoptosis score of 1 of 4 (minimal threshold for apoptosis) in at least one site and had apoptosis scores of 0 or 1 of 4 in all the biopsies. Of those 16 cases, 14 (88%) were called positive for GVHD clinically, and 2 (13%) were called negative for GVHD clinically.
Overall, there was a median of 4 biopsy fragments per biopsy site, with a range of 1–17. For the 29 FN cases identified in the study biopsy review, there was a median of 4 biopsy fragments per biopsy site, with a range of 1–14. For the 8 FP reread cases, there was a median of 3 biopsy fragments per biopsy site with a range of 1–5.
After controlling for other variables in the model, there was a significant effect of apoptosis (P < .001) and inflammation (P < .001) on the histologic diagnosis of GVHD. Greater apoptosis but lower inflammation was associated with a pathologic diagnosis of likely GVHD. Specifically, the odds of a GVHD diagnosis were 12 times higher with each 1-unit increase in apoptosis score (0–4; odds ratio [OR], 12.39). The median apoptosis score was 2. In contrast, each unit of increase in the inflammation score corresponded to an 86% decrease in the odds of a GVHD diagnosis (OR, 0.14). The remaining variables in the model did not statistically relate to the histologic diagnosis of GVHD (Table 3).
Table 3.
Observed Effects of Histologic Findings on the Pathologic Diagnosis
| Variable | Comparison | ORa | 95% CI | P Value |
|---|---|---|---|---|
| Apoptosis | 0–4 | 12.39 | 6.93–22.15 | <.001 |
| Inflammation | 0–4 | 0.14 | 0.08–0.25 | <.001 |
| Crypt destruction | 0–4 | 1.15 | 0.56–2.35 | .75 |
| Biopsy fragments, No. | Continuous | 1.06 | 0.90–1.25 | .49 |
Abbreviations: GVHD, graft-versus-host disease; OR, odds ratio.
Predicting to pathologic diagnosis of GVHD.
Of the 100 patients without transplants who provided control biopsies, 69 (69%) showed no evidence of significant apoptosis, 30 (30%) showed minimal apoptosis, and 1 (1%) showed mild apoptosis (Table 4). None of the cases (0%) showed moderate or severe apoptosis. Significant apoptosis was more commonly identified within the upper GI tract (20 of 31; 65% of cases with positive results) compared with the lower GI tract (11 of 31; 35% of cases with positive results), indicating a possibility of increased specificity in the lower GI tract (P = .08).
Table 4.
Control Biopsy Results
| Organ Biopsied, n = 25 | No Apoptosis, No. (%) | ≥ Minimal Apoptosis, ≥1, No. (%) |
|---|---|---|
| Stomach | 14 (56) | 11 (44) |
| Duodenum | 16 (64) | 9 (36) |
| Right colon | 19 (76) | 6 (24) |
| Left colon | 20 (80) | 5 (20) |
| Total, n = 100 | 69 (69) | 31 (31) |
DISCUSSION
The diagnosis of GI GVHD can be challenging both clinically and histopathologically. Biopsy specimens represent small samples of dynamic and complex pathologic processes. Patients undergoing histologic evaluation for GI GVHD have complicated clinical pictures involving immunosuppressive therapy and increased susceptibility to opportunistic infections. The subtlety of histologic changes and the frequent mimickers in this patient population make diagnosing GI GVHD difficult. The distinction between GVHD and other diagnoses is vital for proper therapy. Failure to treat GI GVHD can result in GI failure or death; therefore, prompt treatment can significantly aid in proper control of the disease. However, increasing immunosuppression of a patient without GI GVHD can result in increased susceptibility to opportunistic infections and would not provide treatment for the etiology of the patient’s symptoms (eg, infection, medication-related toxicity).2,3,25,26
Our study demonstrates that using the 2015 NIH consensus guidelines for GI GVHD allows for high sensitivity (86%) and specificity (65%). Most impressively, when compared with the initial pathology diagnoses, the number of FN diagnoses decreased. When using the NIH guidelines, 37 of 58 cases (64%) with an original FN result were correctly classified as likely GVHD. The high sensitivity and relatively low FN rate was likely due to the uniform application of the guidelines and an overall decreased threshold for the diagnosis of GI GHVD. Prior publications reported a wide range of sensitivities (33%–100%) for the diagnosis of GVHD by GI biopsy, of which many claim the biopsy site to be a contributing factor.27–30 There has been no consistent evidence supporting one biopsy site as definitively superior, and in a study published by our group, we did not find a significant difference between upper and lower GI endoscopies. In addition, the site of GI symptoms did not always correlate with the site of histologic changes seen on biopsy.31
Although our original, unaltered specificity was low (50%), that was in the context of our intentionally blinded study methods. Specifically, the pathologists were blind to all clinical information, and each biopsy site was evaluated independently and later given one overall score for the endoscopy, based on the most severe diagnosis, thus favoring a diagnosis of likely GVHD. There were 33 endoscopic events found to be negative clinically for GVHD. Of those, 15 (45%) were called likely GVHD on pathology review and were thus falsely positive. When the 15 FP cases were reviewed for clinical information, and the findings from the concurrent biopsies from other sites was unmasked, conservatively 7 of 15 cases (47%) were recategorized to possible GVHD given the definitive clinical evidence of a different or concurrent etiology. Thus, those 15 cases met minimal histologic criteria for GVHD, but in a realistic context, 7 of them would have been called possible GVHD because of coexisting etiologies. When recalculated, the specificity increased to 65%. As expected, these findings emphasize the importance of correlating the histologic, clinical, and laboratory findings when evaluating GI biopsies for GVHD to avoid false-positives. In day-to-day practice, interpretation of the histologic findings in the full clinical context of the patient should negate that limitation.
A second potential limitation of this study was the use of the clinical diagnosis as the gold standard for the diagnosis of GI GVHD. The original pathologic diagnosis, right or wrong, had a part in influencing the clinical impression; thus, there was a bias toward the original histology results. However, the benefit of hindsight has an effect. The clinical reviewers of our study used more of the clinical perspective, response to treatments, and outcome in determining the clinical diagnoses. Furthermore, the strength of the NIH guidelines employed in our review is illustrated by the fact that 64% (37 of 58) of cases assigned a clinical diagnosis of GVHD despite an initial negative histologic diagnosis were correctly assigned to the likely GVHD category upon review.
The most common clinical diagnosis with a FP pathology result was CMV infection, consisting of 5 of the 15 FP cases (33%). A CMV infection can cause histologic changes that mimic GI GVHD, namely epithelial apoptosis, and differentiation between the 2 diagnoses without the appropriate contextual information is difficult (Figure 2, A through D).14,15,17,21,23,32 Biopsies were evaluated for CMV with hematoxylin-eosin stain (eg, viral inclusions) and IHC, which was performed on all biopsy sites of all endoscopies. Clinical evidence of CMV infection (eg, positive CMV PCR results on peripheral blood and/or positive CMV IHC results on biopsy) was found in 82 of 250 cases (33%). Although all patients with significant CMV viremia were treated with antiviral therapy, not all patients with viremia were considered to have CMV disease of the gut. Additionally, patients with chronic low-level CMV viremia were not always treated. Of the 250 cases, 10 (4%) were found to be clinically positive for CMV disease of the gut and were felt to be clinically negative for GI GVHD. Thus, 72 (29%) of the 250 endoscopic events demonstrated GI GVHD with concurrent CMV viremia without definitive CMV gut disease. For those patients given antiviral therapy for CMV viremia and immunosuppressive therapy for GI GVHD simultaneously, it is unclear whether GI symptom resolution was attributable solely to the immunosuppressive therapy (and thus, GI GVHD with CMV viremia but without CMV gut disease) or was due to both therapies in combination (and thus, GI GVHD with CMV gut disease). Interestingly, 2 of the 5 FP reread cases (40%) clinically diagnosed as CMV infection were from patients on empiric steroid treatment for GVHD at the time of endoscopy, raising the possibility of GI GVHD also contributing to the patients’ GI symptoms. However, none of those patients were treated with additional immunosuppression, and their symptoms resolved after antiviral therapy. Clostridium difficile and HSV infection are additional examples in which clinical history, endoscopic findings, and laboratory results are invaluable to the diagnosis. Of the 15 FP reread pathology diagnoses, 1 (7%) was clinically diagnosed as C difficile colitis (Figure 2, E), and 2 of 15 (13%) were attributed to HSV infection. All 7 FP cases (100%) of CMV, C difficile, and HSV infection were recategorized as possible GVHD upon unblinding because the additional clinical information in those cases would have been warranted in a real-world setting.
Figure 2.
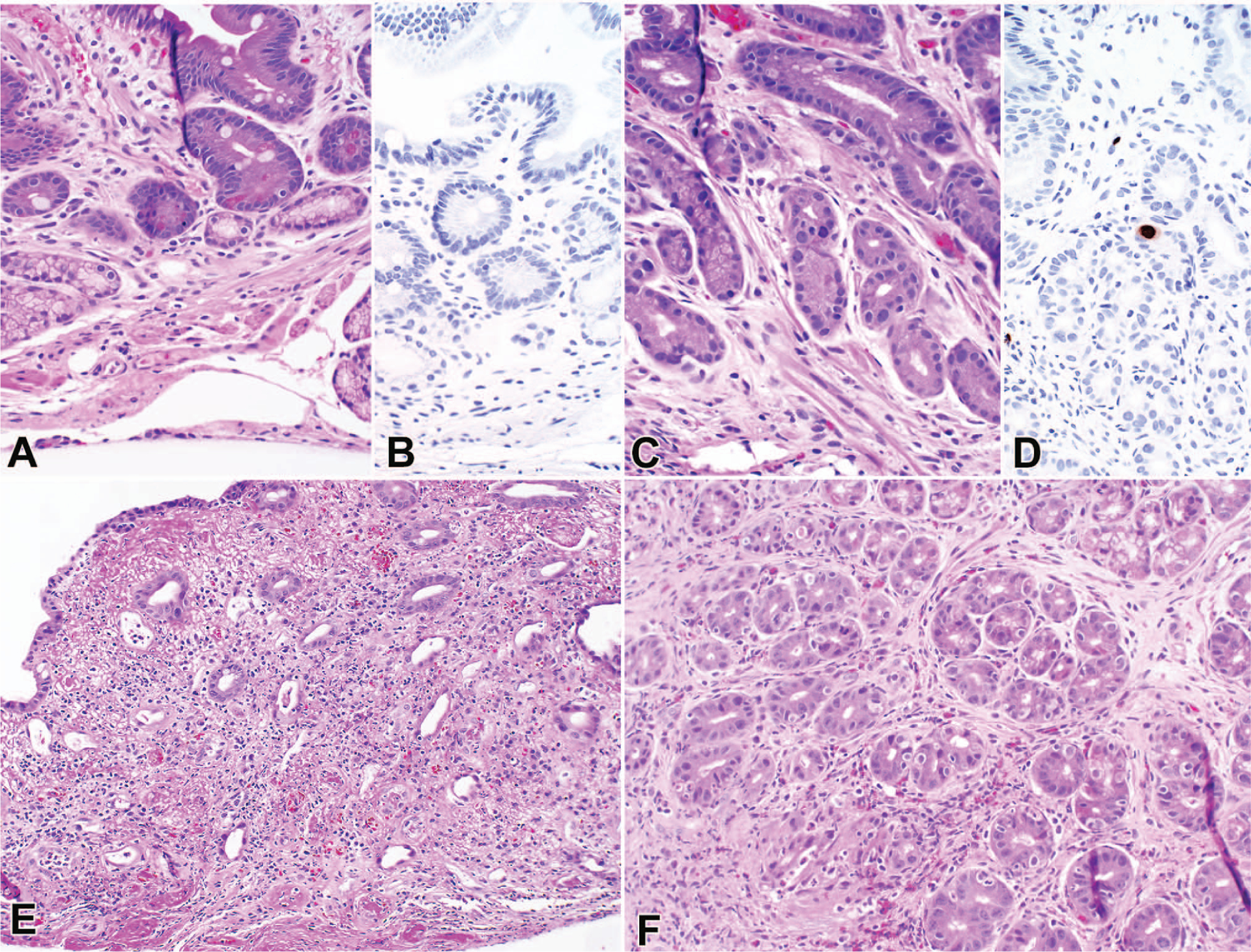
False-positive cases: clinical diagnosis of cytomegalovirus (CMV) and pathologic diagnosis of likely graft-versus-host disease (GVHD) on a duodenum biopsy (A), with negative CMV immunohistochemistry (B), and CMV gastritis and possible GVHD on a stomach biopsy (C), with CMV immunohistochemistry (D). Clostridium difficile infection and mycophenolate mofetil (MMF) toxicity from a rectosigmoid colon biopsy (E) and MMF toxicity from a stomach biopsy (F) (hematoxylin-eosin, original magnification ×20 [A, C, E, and F]; CMV immunohistochemistry, original magnification ×20 [B and D]).
Another clinical diagnosis with multiple FP results was MMF toxicity. Within our cohort of 102 endoscopic events in which the patient was administered MMF during the time of endoscopy, 3 patients (3%) were clinically diagnosed with MMF toxicity, 2 of which were called likely GVHD on pathology review and were thus falsely positive. Administration of MMF can cause histologic changes that mimic GI GVHD, namely epithelial apoptosis (Figure 2, E and F).18,21,22,33 Of the 2 FP cases, 1 (50%) showed a concurrent C difficile infection. That case was recategorized to possible GVHD after review because in day-to-day clinical practice it would be more appropriate to diagnose a patient with epithelial apoptosis and a positive C difficile enzyme immunoassay with possible GVHD than with likely GVHD. The diagnosis of MMF toxicity can be difficult to make without knowledge of the clinical history and medication profile. Ultimately, the diagnosis of MMF toxicity is primarily a clinical one based on improvement of the patient’s symptoms after discontinuation of MMF therapy. Studies have suggested that some histologic clues may support a diagnosis of MMF over GVHD-induced injury, such as increased lamina propria eosinophils; however, others have also demonstrated that eosinophils are activated in GI GVHD.34,35 Interestingly, for the 2 FP patients diagnosed clinically with MMF toxicity, the one case with concurrent C difficile showed zero eosinophils per 10 high-power fields, whereas the other case consisted of a stomach and duodenal biopsy with 106 and 197 eosinophils per 10 high-power fields, respectively.
Epithelial apoptosis correlates with GI GVHD severity and is the histologic hallmark of GVHD.36 Consistent with prior studies, increased apoptosis with decreased inflammation was associated with a pathologic diagnosis of GI GVHD in our cohort. The NIH consensus guidelines present a low threshold for intraepithelial apoptosis (≥1 intraepithelial apoptosis [on average] per biopsy piece or crypts/glands with multiple apoptosis). Shulman et al14,15 discuss in the consensus article how controversial the threshold for minimal histologic change is and they justify their decision as a balance between sensitivity and specificity.
Given the complexity of setting an absolute threshold, it is worth considering whether the ideal threshold might, in fact, be lower or higher than 1 apoptotic body on average per biopsy piece. In our study, three-quarters of our patients with less than the minimal threshold of apoptotic bodies in any of their biopsies by current NIH guidelines, nonetheless, received a clinical diagnosis of GI GVHD, suggesting that a lower threshold might be appropriate. A limited study by Nguyen et al37 suggested that even a single apoptotic body per total biopsy (all pieces in aggregate) might suffice for a diagnosis of GI GVHD in some circumstances. Recently, the use of a low threshold was further supported in a study by Myerson, et al,38 which validated the use of a modified Lerner grading system for GVHD that essentially subdivided the former grade 1 into 4 new grades and combined grades 2 to 4 in a new grade 5. Within their cohort, a diagnosis of their proposed grade 1 or grade 2 GVHD (≥0.07 to <0.25 apoptotic bodies and ≥0.25 to <4 apoptotic bodies per section, respectively) often still led to treatment for GVHD.
Taken to the extreme, one could consider a system in which all biopsies without evidence of a process other than GI GVHD, regardless of the presence or absence of apoptotic bodies, are interpreted as favoring GVHD. Such an approach would have a sensitivity of 100% (excepting patients with both GVHD and some other superimposed process). The issue with that system is, of course, its specificity and the potential for false-positives, resulting in overtreatment of patients with unnecessary immunosuppressive therapy. There would also likely be trepidation about making a diagnosis of this importance without objective support from histopathology results.
Balanced against those observations is the realization that a low level of apoptosis is present in (and indeed, physiologically important for) a healthy GI epithelium. When evaluating colorectal biopsies in healthy, nontransplanted patients, Lee et al39 reported that 75% of the time no apoptotic cells were present and approximately 0.2 apoptotic bodies per 20 crypts was the overall mean. Similarly, in our nontransplant control cases, 69% of the cases lacked significant epithelial apoptosis. Studies of GI pathology in solid organ transplant recipients treated with MMF have been shown to have considerable histologic overlap with the changes seen in GI GVHD and have employed apoptosis thresholds ranging from 2 to 5 apoptotic bodies per 100 glands.40,41 Translating those numbers into the typical biopsy piece that contains approximately 20 glands or crypts equates to 0.4 to 1 apoptotic body per biopsy piece, a range bordering the NIH guideline recommendation for GI GVHD.38
Perhaps in recognition of the low but significant baseline level of apoptosis, Lin et al42 have suggested the use of criteria for the histologic diagnosis of GI GVHD similar to those employed for acute cellular allograft rejection of small-bowel transplants, with 6 or more apoptotic bodies per 10 contiguous crypts required for a diagnosis of GVHD and biopsies falling short of that criterion being designated indeterminate. Although that approach would undoubtedly decrease the frequency of FP diagnoses, it would clearly lead to a decrease, possibly to an unacceptable level, in diagnostic sensitivity. Even in the Lin et al42 study cohort, 13 of 41 patients (32%) who were clinically diagnosed and treated for GI GVHD had fewer than 6 apoptosis per 10 contiguous crypts/glands. In our cohort, 48% (90 of 188) of likely GVHD cases showed minimal to mild apoptosis. Had those biopsies been considered indeterminate for GI GVHD, the diagnostic sensitivity would have decreased dramatically.
Ultimate resolution of the threshold dilemma may require refinement of our analytic and diagnostic methods. Within our control group, significant apoptosis was more commonly identified within the upper GI tract (65% [20 of 31] of cases with positive results) compared with the lower GI tract (35% [11 of 31] of cases with positive results), indicating a possibility of increased specificity in the lower GI tract (P = .08).
In summary, our study demonstrates that the use of the 2015 NIH guidelines increases diagnostic sensitivity and can decrease false-negative results for the histologic diagnosis of GI GVHD and can create uniformity and diagnostic clarity for pathologists and our treating colleagues. Other common diagnoses to consider in the HSCT population are infections (eg, CMV and C difficile) and medication-related toxicity (eg, MMF). This study confirms that in addition to the biopsy findings, access to relevant information, such as clinical presentation, symptoms and their severity, medications, laboratory tests, and endoscopic findings, is necessary to make an accurate diagnosis and to maintain a high diagnostic sensitivity and specificity, especially when considering whether minimal apoptosis is truly significant.
Acknowledgments
Dr Sung was supported by grants T32 HL007057-37 and KL2 TR001115-03 from the NIH and an American Society of Hematology research training award for fellows.
Footnotes
The authors have no relevant financial interest in the products or companies described in this article.
References
- 1.Jagasia M, Arora M, Flowers ME, et al. Risk factors for acute GVHD and survival after hematopoietic cell transplantation. Blood. 2012;119(1):296–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inagaki J, Moritake H, Nishikawa T, et al. Long-term morbidity and mortality in children with chronic graft-versus-host disease classified by national institutes of health consensus criteria after allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant. 2015;21(11):1973–1980. [DOI] [PubMed] [Google Scholar]
- 3.Carnevale-Schianca F, Leisenring W, Martin PJ, et al. Longitudinal assessment of morbidity and acute graft-versus-host disease after allogeneic hematopoietic cell transplantation: retrospective analysis of a multicenter phase III study. Biol Blood Marrow Transplant. 2009;15(6):749–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ayuk F, Veit R, Zabelina T, et al. Prognostic factors for survival of patients with newly diagnosed chronic GVHD according to NIH criteria. Ann Hematol. 2015;94(10):1727–1732. [DOI] [PubMed] [Google Scholar]
- 5.Filipovich AH, Weisdorf D, Pavletic S, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, I: diagnosis and staging working group report. Biol Blood Marrow Transplant. 2005;11(12):945–956. [DOI] [PubMed] [Google Scholar]
- 6.Jagasia MH, Greinix HT, Arora M, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, I: the 2014 Diagnosis and Staging Working Group report. Biol Blood Marrow Transplant. 2015;21(3):389–401.e381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sung AD, Chao NJ. Concise review: acute graft-versus-host disease: immunobiology, prevention, and treatment. Stem Cells Transl Med. 2013;2(1): 25–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrara JL, Deeg HJ. Graft-versus-host disease. N Engl J Med. 1991;324(10): 667–674. [DOI] [PubMed] [Google Scholar]
- 9.Socié G, Ritz J. Current issues in chronic graft-versus-host disease. Blood. 2014;124(3):374–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deeg HJ, Antin JH. The clinical spectrum of acute graft-versus-host disease. Semin Hematol. 2006;43(1):24–31. [DOI] [PubMed] [Google Scholar]
- 11.Kambham N, Higgins JP, Sundram U, Troxell ML. Hematopoietic stem cell transplantation: graft versus host disease and pathology of gastrointestinal tract, liver, and lung. Adv Anat Pathol. 2014;21(5):301–320. [DOI] [PubMed] [Google Scholar]
- 12.Hymes SR, Alousi AM, Cowen EW. Graft-versus-host disease: part I: pathogenesis and clinical manifestations of graft-versus-host disease. J Am Acad Dermatol. 2012;66(4):515.e1–518; quiz 533–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tuncer HH, Rana N, Milani C, Darko A, Al-Homsi SA. Gastrointestinal and hepatic complications of hematopoietic stem cell transplantation. World J Gastroenterol. 2012;18(16):1851–1860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shulman HM, Kleiner D, Lee SJ, et al. Histopathologic diagnosis of chronic graft-versus-host disease: National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease, II: pathology working group report. Biol Blood Marrow Transplant. 2006;12(1):31–47. [DOI] [PubMed] [Google Scholar]
- 15.Shulman HM, Cardona DM, Greenson JK, et al. NIH Consensus development project on criteria for clinical trials in chronic graft-versus-host disease, II: the 2014 pathology working group report. Biol Blood Marrow Transplant. 2015;21(4):589–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Castagnola E, Cappelli B, Erba D, Rabagliati A, Lanino E, Dini G. Cytomegalovirus infection after bone marrow transplantation in children. Hum Immunol. 2004;65(5):416–422. [DOI] [PubMed] [Google Scholar]
- 17.Cho BS, Yahng SA, Kim JH, et al. Impact of cytomegalovirus gastrointestinal disease on the clinical outcomes in patients with gastrointestinal graft-versus-host disease in the era of preemptive therapy. Ann Hematol. 2013;92(4): 497–504. [DOI] [PubMed] [Google Scholar]
- 18.Liapis G, Boletis J, Skalioti C, et al. Histological spectrum of mycophenolate mofetil-related colitis: association with apoptosis. Histopathology. 2013; 63(5):649–658. [DOI] [PubMed] [Google Scholar]
- 19.Soldini D, Gaspert A, Montani M, et al. Apoptotic enteropathy caused by antimetabolites and TNF-alpha antagonists. J Clin Pathol. 2014;67(7):582–586. [DOI] [PubMed] [Google Scholar]
- 20.Cox GJ, Matsui SM, Lo RS, et al. Etiology and outcome of diarrhea after marrow transplantation: a prospective study. Gastroenterology. 1994;107(5): 1398–1407. [DOI] [PubMed] [Google Scholar]
- 21.Washington K, Jagasia M. Pathology of graft-versus-host disease in the gastrointestinal tract. Hum Pathol. 2009;40(7):909–917. [DOI] [PubMed] [Google Scholar]
- 22.Papadimitriou JC, Drachenberg CB, Beskow CO, et al. Graft-versus-host disease-like features in mycophenolate mofetil-related colitis. Transplant Proc. 2001;33(3):2237–2238. [DOI] [PubMed] [Google Scholar]
- 23.Snover DC. Mucosal damage simulating acute graft-versus-host reaction in cytomegalovirus colitis. Transplantation. 1985;39(6):669–670. [PubMed] [Google Scholar]
- 24.Lerner KG, Kao GF, Storb R, Buckner CD, Clift RA, Thomas ED. Histopathology of graft-vs.-host reaction (GvHR) in human recipients of marrow from HL-A-matched sibling donors. Transplant Proc. 1974;6(4):367–371. [PubMed] [Google Scholar]
- 25.Weisdorf DJ, Snover DC, Haake R, et al. Acute upper gastrointestinal graft-versus-host disease: clinical significance and response to immunosuppressive therapy. Blood. 1990;76(3):624–629. [PubMed] [Google Scholar]
- 26.Baehr PH, Levine DS, Bouvier ME, et al. Oral beclomethasone dipropionate for treatment of human intestinal graft-versus-host disease. Transplantation. 1995;60(11):1231–1238. [PubMed] [Google Scholar]
- 27.Ip S, Marquez V, Schaeffer DF, Donnellan F. Sensitivities of biopsy sites in the endoscopic evaluation of graft-versus-host disease: retrospective review from a tertiary center. Dig Dis Sci. 2016;61(8):2351–2356. [DOI] [PubMed] [Google Scholar]
- 28.Sultan M, Ramprasad J, Jensen MK, Margolis D, Werlin S. Endoscopic diagnosis of pediatric acute gastrointestinal graft-versus-host disease. J Pediatr Gastroenterol Nutr. 2012;55(4):417–420. [DOI] [PubMed] [Google Scholar]
- 29.Ross WA, Ghosh S, Dekovich AA, et al. Endoscopic biopsy diagnosis of acute gastrointestinal graft-versus-host disease: rectosigmoid biopsies are more sensitive than upper gastrointestinal biopsies. Am J Gastroenterol. 2008;103(4): 982–989. [DOI] [PubMed] [Google Scholar]
- 30.Washington K, Bentley RC, Green A, Olson J, Treem WR, Krigman HR. Gastric graft-versus-host disease: a blinded histologic study. Am J Surg Pathol. 1997;21(9):1037–1046. [DOI] [PubMed] [Google Scholar]
- 31.Wild D, Sung AD, Cardona D, et al. The diagnostic yield of site and symptom-based biopsies for acute gastrointestinal graft-versus-host disease: a 5-year retrospective review. Dig Dis Sci. 2016;61(3):806–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.He JD, Liu YL, Wang ZF, Liu DH, Chen H, Chen YH. Colonoscopy in the diagnosis of intestinal graft versus host disease and cytomegalovirus enteritis following allogeneic haematopoietic stem cell transplantation. Chin Med J (Engl). 2008;121(14):1285–1289. [PubMed] [Google Scholar]
- 33.Selbst MK, Ahrens WA, Robert ME, Friedman A, Proctor DD, Jain D. Spectrum of histologic changes in colonic biopsies in patients treated with mycophenolate mofetil. Mod Pathol. 2009;22(6):737–743. [DOI] [PubMed] [Google Scholar]
- 34.Star KV, Ho VT, Wang HH, Odze RD. Histologic features in colon biopsies can discriminate mycophenolate from GVHD-induced colitis. Am J Surg Pathol. 2013;37(9):1319–1328. [DOI] [PubMed] [Google Scholar]
- 35.Daneshpouy M, Socie G, Lemann M, Rivet J, Gluckman E, Janin A. Activated eosinophils in upper gastrointestinal tract of patients with graft-versus-host disease. Blood. 2002;99(8):3033–3040. [DOI] [PubMed] [Google Scholar]
- 36.Kreft A, Mottok A, Mesteri I, et al. ; Gastrointestinal Pathology Group of the German-Austrian-Swiss GvHD Consortium. Consensus diagnostic histopathological criteria for acute gastrointestinal graft versus host disease improve interobserver reproducibility. Virchows Arch. 2015;467(3):255–263. [DOI] [PubMed] [Google Scholar]
- 37.Nguyen CV, Kastenberg DM, Choudhary C, Katz LC, DiMarino A, Palazzo JP. Is single-cell apoptosis sufficient for the diagnosis of graft-versus-host disease in the colon? Dig Dis Sci. 2008;53(3):747–756. [DOI] [PubMed] [Google Scholar]
- 38.Myerson D, Steinbach G, Gooley TA, Shulman HM. Graft-versus-host disease of the gut: a histologic activity grading system and validation. Biol Blood Marrow Transplant. 2017;23(9):1573–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee FD. Importance of apoptosis in the histopathology of drug related lesions in the large intestine. J Clin Pathol. 1993;46(2):118–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nguyen T, Park JY, Scudiere JR, Montgomery E. Mycophenolic acid (cellcept and myofortic) induced injury of the upper GI tract. Am J Surg Pathol. 2009;33(9):1355–1363. [DOI] [PubMed] [Google Scholar]
- 41.Parfitt JR, Jayakumar S, Driman DK. Mycophenolate mofetil-related gastrointestinal mucosal injury: variable injury patterns, including graft-versus-host disease-like changes. Am J Surg Pathol. 2008;32(9):1367–1372. [DOI] [PubMed] [Google Scholar]
- 42.Lin J, Fan R, Zhao Z, Cummings OW, Chen S. Is the presence of 6 or fewer crypt apoptotic bodies sufficient for diagnosis of graft versus host disease?: a decade of experience at a single institution. Am J Surg Pathol. 2013;37(4):539–547. [DOI] [PubMed] [Google Scholar]


