Supplemental Digital Content is available in the text
Keywords: COVID-19, Efficacy, Immunogenicity, Safety, SARS-CoV-2, Vaccine clinical trial
Abstract
The coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to scale up around the world, costing severe health and economic losses. The development of an effective COVID-19 vaccine is of utmost importance. Most vaccine designs can be classified into three camps: protein based (inactivated vaccines, protein subunit, VLP and T-cell based vaccines), gene based (DNA or RNA vaccines, replicating or non-replicating viral/bacterial vectored vaccines), and a combination of both protein-based and gene-based (live-attenuated virus vaccines). Up to now, 237 candidate vaccines against SARS-CoV-2 are in development worldwide, of which 63 have been approved for clinical trials and 27 are evaluated in phase 3 clinical trials. Six candidate vaccines have been authorized for emergency use or conditional licensed, based on their efficacy data in phase 3 trials. This review summarizes the strengths and weaknesses of the candidate COVID-19 vaccines from various platforms, compares, and discusses their protective efficacy, safety, and immunogenicity according to the published clinical trials results.
Introduction
Coronavirus disease 2019 (COVID-19) pandemic caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) continues to scale up around the world. Over 105 million COVID-19 cases and 2.3 million deaths have been reported globally till 6 February, 2021, according to WHO.[1] Approximately 40% to 45% of those infected with SARS-CoV-2 will remain asymptomatic,[2,3] and most people (about 80%) can recover from the disease without treatment.[4] Thus, the actual number of COVID-19 infected cases is supposed to be much higher than what have been reported.[5,6] Nevertheless, the level of antibody seropositivity in the general population was still low,[7,8] indicating that most of the population in the world remain susceptible.
Most patients displayed an antibody response after infection with SARS-CoV-2,[7] and reinfection incidents with valid evidence were few.[9] Convalescent plasma transfusion has been indicated as an effective therapy against COVID-19.[10] These evidences together highlighted the necessity and feasibility of COVID-19 vaccine development.
The SARS-CoV-2 genome contains four main structural proteins: the spike (S), membrane (M), envelope (E) and nucleocapsid (N) protein. The main target for antigen epitopes of COVID-19 vaccine is S protein[11]: the S1 domain, which contains the receptor-binding domain (RBD) for the host cell receptor angiotensin-converting enzyme-2 (ACE2),[12] the N-terminal domain (NTD), which has been proven as another site with potent neutralizing activity[13–15] and the S2 domain containing the fusion peptide.[16,17] Currently, 237 candidate vaccines against SARS-CoV-2 are in development worldwide according to the survey of WHO.[18] Among them, 63 vaccines have been approved for clinical trials and 27 are evaluated in phase 3 clinical trials. Up to now, six COVID-19 vaccines, including two mRNA vaccines, two inactivated vaccines, and two viral-vectored vaccines have been authorized for emergency use or conditional licensed in some countries or regions, based on their efficacy data in phase 3 trials.[18]
Platforms for COVID-19 candidate vaccines
Currently, COVID-19 candidate vaccines can be classified into three camps: protein based (inactivated vaccines, protein subunit, VLP and T-cell based vaccines), gene based (DNA or RNA vaccines, replicating or non-replicating viral/bacterial vectored vaccines), and a combination of both protein-based and gene-based (live-attenuated virus vaccines).[19] Most of the COVID-19 candidate vaccines underdevelopment belong to the first two camps.
Protein based vaccines
Many of the vaccines in clinical use today fall into this category. This approach utilizes the entire or a part of the pathogen as antigen to elicit protective immune responses. This type of vaccine is generally well tolerated, safe and can be used to most people, even the elderly or people with immunodeficiency.[20,21] Besides, the immunogenicity of vaccines from protein-based platform were stable, fluctuates very little due to the stability of the protein content. At the time of writing, the number of protein subunit vaccines is the most among the candidate vaccines being clinically studied (n = 21). There are ten inactivated vaccines evaluated in clinical trials, and six of which have been evaluated at phase 3 study.
In the production of inactivated vaccines, conserving the viral antigen of high quality is the key to induce protective immunity.[21] In general, inactivated vaccines are highly immunogenic. However, in the case of COVID-19, the inactivated vaccine's immunogenicity could be jeopardized by the immune evasion capacity of SARS-CoV-2, such as “Glycan shield” and the lying-down of RBD.[22–25] In addition, non-neutralizing epitopes contained in the inactivated whole virus might be able to induce high level of non-neutralizing antibodies, which have the potential to cause antibody dependent enhancement (ADE).
Protein subunit or VLP designed vaccines may address the concern of ADE, by removing as much non-neutralizing epitopes as possible, however, this would be accompanied by a long production time.[26] Furthermore, protein subunit vaccine can hardly induce cellular immune response with the traditional adjuvant of alum, and thus an appropriate adjuvant may in need.[27]
Gene based vaccines
Gene based vaccines have the potential to elicit broad immune responses and are easier to achieve mass production compared with protein-based vaccines.[28] To date, there are 11 non-replicating viral vectored vaccines (4 at phase 3), five replicating viral vectored vaccines, seven RNA-based vaccines (3 at phase 3), and eight DNA-based vaccines (3 at phase 3) are evaluated in clinical trials.
The RNA or DNA-based vaccine could be swiftly advanced towards the targeted antigen and be manufactured massively. Therefore, even it's a relative new platform for vaccine development, and never been approved for marketing before this pandemic, this platform is expected to contribute to accelerating COVID-19 vaccine development. However, the efficacy of an RNA or DNA-based vaccine in human strongly depends on its formulation and the delivery system for introducing the target genes into cells, which may vary a lot in different individuals.
Viral vectored vaccines deliver the target gene into the cells for compilation and expression through an infectious attenuated vector virus. For replication-incompetent vectored vaccines, the pre-existing immunity to the vectors could affect the efficiency of deliver significantly, with reduced the vaccine-induced immune responses in those with pre-exposure to vector at baseline. While, using replication-competent adenovirus as a vector would raise a safety concern that the vector virus may recombine or revert to a parental or wild-type phenotype at a low frequency, or cause clinical infections in some immunocompromised populations.[29]
Though most of the viral vectored vaccines underdevelopment are administrate intramuscularly, there is a potential advantage for this type vaccine to be is given by inhalation or intranasal administration. Intranasal vaccination is supposed to provide a better protection compared to subcutaneous inoculation in terms of respiratory pathogens, due to the ability of inducing high level of specific IgA antibodies.[30] Some vaccines that induce mucosal immunity against SARS-CoV-1 and MERS-CoV have succeeded in producing IgA in the respiratory tract, preventing corresponding viral dissemination to the lung.[31] As ACE2 expression is abundant in nasal epithelial cells of human upper respiratory tract, and nasal goblet cells and ciliated cells might be the initial infection sites of SARS-CoV-2.[32,33] Four intranasal vaccines were developed and evaluated in clinical trials.[18]
The results of clinical trials on COVID-19 vaccines
Up to now, a total of ten vaccine candidates have reported the results of human clinical trials, including four non-replicating viral vectored vaccines, four inactivated vaccines, two mRNA vaccines and two protein subunit vaccines [Table 1].
Table 1.
The profile of published clinical studies
| Vaccine platform | Vaccine | Phase | Age | Study design | Location | Sample size | Number of doses | Schedule | Administration | Doses |
|---|---|---|---|---|---|---|---|---|---|---|
| Non-replicating viral vectored | ||||||||||
| Ad5-vectored COVID-19 | 2 | ≥18 | Randomised, double-blind, placebo-controlled | Wuhan, China | 508 | 1 | – | IM | 0.5 or 1 × 1011 VP | |
| ChAdOx1 nCoV-19 | 1/2 | 18–55 | Randomised, single-blind, placebo-controlled | UK | 1077 | 0 or 1 | 0, 28 days | IM | 0.5 × 1011 VP | |
| rAd26-S+rAd5-S COVID-19 | 1/2 | 18–60 | Open, non-randomised | Moscow, Russia | 76 | 2 | 0, 21 days | IM | 1 × 1011 VP | |
| Ad26.COV2.S | 1/2 | 18–55 | Randomised, multi-center, placebo-controlled | US | 1045 | 0 or 1 | 0, 56 days | IM | 0.5 or 1 × 1011 VP | |
| Inactivated | ||||||||||
| Inactivated whole-virus COVID-19 vaccine | 1, 2 | 18–59 | Randomised, single-blind, placebo-controlled | Henan, China | 224 | 2 | 0, 14 or 0, 21 days | IM | 2.5, 5,10 μg | |
| BBIBP-CorV | 1, 2 | ≥3 | Randomised, single-blind, placebo-controlled | Henan, China | 448 | 2 | 0, 14 or 0, 21 or 0, 28 days | IM | 2, 4, 8 μg | |
| CoronaVac | 2 | 18–59 | Randomised, double-blind, placebo-controlled | Jiangsu, China | 600 | 2 | 0, 14 days or 0, 28 days | IM | 3, 6 μg | |
| BBV152 | 1, 2 | 12–65 | Double-blind, randomised, controlled | India | 755 | 2 | 0, 14 days | IM | 3, 6 μg | |
| mRNA | ||||||||||
| mRNA-1273 | 1 | 18–55 | Dose-escalation, open-label | Seattle and Atlanta, US | 45 | 2 | 0, 28 days | IM | 25, 100, 250 μg | |
| mRNA-1273 | 1 | ≥56 | open-label, dose-ranging | US | 120 | 2 | 0, 28 days | IM | 25, 100 μg | |
| BNT162b1 | 1/2 | 18–55 | Randomised, dose-escalation, single-blind, placebo-controlled | Germany | 45 | 2 | 0, 28 days | IM | 10, 30,100 μg | |
| DNA | ||||||||||
| INO-4800 | 1 | 18–55 | open-label, multi-center | US | 20 | 2 | 0, 4 weeks | ID | 1.0, 2.0 mg | |
| Protein subunit | ||||||||||
| NVX-CoV2373 | 1/2 | 18–59 | Open, non-randomised | Australia | 76 | 2 | 0, 21 days | IM | 5, 25 μg | |
| SCB-2019 | 1 | 18–54, 55–75 | Randomised, double-blind, placebo-controlled | Australia | 150 | 2 | 0, 21 days | IM | 3, 9, 30 μg | |
ID: Intradermal; IM: Intramuscular; NA: No data; VP: Virus particle.
Safety
The accumulated safety data from clinical trials shows that candidate COVID-19 vaccines from different platforms are generally safe and tolerable, but with distinct safety profiles. Viral vectored vaccines and mRNA vaccines were associated with increased adverse reactions including fever, fatigue, headache, myalgia compared with inactivated vaccines. Significantly, people aged 18 to 55 reported more adverse reactions than that in elderly.
Around 22% of the recipients of chimpanzee adenovirus-vectored vaccine (ChAdOx1 nCoV-19) reported severe adverse reactions in phase 1 clinical trial, in which participants received only single dose, in phase 2/3 trial however, all people administrated two doses, none of whom reported adverse reactions. Besides, all the vaccines of the recombinant adenovirus type 26 vector vaccine and recombinant adenovirus type 5 vector vaccine (rAd26-S+rAd5-S nCoV-19) had fever.[34–37]
Of note, mRNA-1273 caused pain in all vaccine recipients aged 18 to 55 and severe adverse reaction was reported by 6.7% participants between the ages of 18 to 55 years, and 5.3% participants over 55 years old.[38,39] For another mRNA vaccine BNT162b2, 8.0% participants between the ages of 18 to 55 years reported severe adverse reaction.[40]
Protein subunit (Matrix-M1 adjuvanted NVX-CoV2373 and SCB-2019) also induced some adverse reactions, especially fatigue and pain, and 11.4% of the recipients of NVX-CoV2373 developed severe adverse reactions.[41,42]
Comparing to above mentioned vaccines, alum-adjuvanted inactivated vaccines and INO-4800 generated the least adverse reactions with only a small proportion of participants having mild to moderate adverse reactions, probably because of the limited capacity of producing cellular immunity.[43–44] However, it is worth noting that some individuals had abnormal increase of blood glucose, or facial neuritis after vaccination. The relationship between these abnormal changes and inactivated vaccines is uncertain yet, which needs to be further investigated [Figure 1; Supplementary Table 1].
Figure 1.
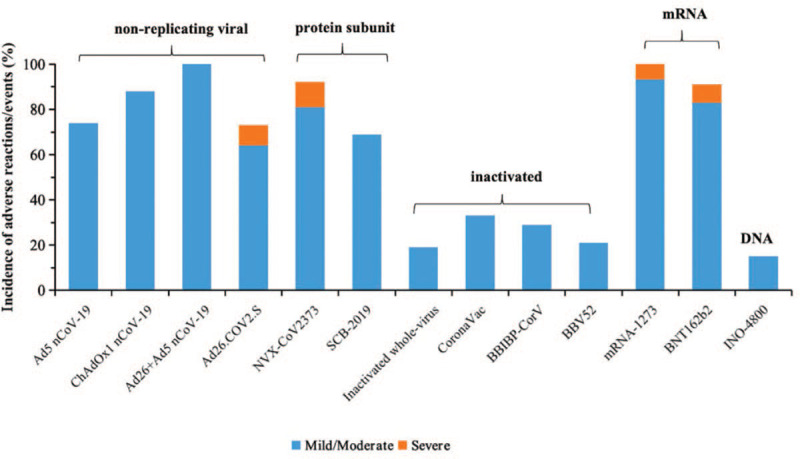
Incidence of adverse reactions/events of COVID-19 vaccines. Incidence of adverse reactions/events was reported by participants receiving target dose or immunization procedure in phase 3 studies.
Immunogenicity
Humoral immune responses in terms of S- or RBD-binding antibodies measured by glycoprotein-specific enzyme-linked immunosorbent assay (ELISA), and neutralizing antibodies (Nab) to live SARS-CoV-2 virus or pseudo virus were measured in clinical trials.
Protein subunit (NVX-CoV2373 and SCB-2019) induced the strongest antibodies levels with the GMT of 3906 and 1810, respectively, by microneutralization assay (MNA).[41,42] mRNA vaccines also generated good specific antibodies. GMTs of Nab were 654.3, 361 and 102.3 elicited by mRNA-1273 (by PRNT80), BNT162b2 (by MNA50).[38,39] The humoral immunity elicited by non-replicating viral vectored vaccines, inactivated vaccines and DNA vaccine was comparable, and was minor than that of other types of vaccines. The detected GMTs of Nab ranged from 27.6 (GMTs by MNA50) to 300 (GMTs by PRNT50) for inactivated vaccines and DNA vaccine, and ranged from 18.3 (GMTs by MNA50) to 827 (median by MNA50) for non-replicating viral vectored vaccines.[35–37,43–47] The Nab titers of 827 was induced by Ad26.COV2.S, evidently higher than other non-replicating viral vectored vaccines.[48]
Since there are no standardized methods for these serological tests, these published data of the antibody tests in clinical trials are impossible to compare across the different studies. Nevertheless, a panel of convalescent serum from COVID-19 patients was provided as active competitors in most studies, and we can get some comparative information from these data.
The mRNA vaccine BNT162b2 showed better humoral responses, 1.7 to 4.6 times higher for Nab results compared with convalescent patients after vaccination, and Ad26.COV2.S also generated ELISA antibodies and Nab titers of 1.9 and 1.0 times higher than human convalescent serum. The rAd26-S+rAd5-S, mRNA-1273 and INO-4800 vaccination group presented a higher ELISA titres or Nab results than detected in convalescent patients. The NVX-CoV2373 and SCB-2019 vaccine induced approximately four times greater than that in outpatients for Nab, and also resulted in similar GMT levels of ELISA antibodies and Nab compared with hospitalized patients. The Nab titres after vaccination of inactivated vaccine were lower in participants than that was detected in convalescent serum from patients who has previously had COVID-19.
To sum up, the protein subunit vaccine performed notably best, followed by the mRNA vaccines and the non-replicating Chimpanzee adenovirus and Ad26 vectored vaccines, which induced relatively high humoral immune response than inactivated vaccines, DNA vaccine and Ad5-vectored vaccines. The differences in disease severity, age, and sampling time points post-infection could affect the level of antibody titer of the panel of convalescent serum, so the comparisons still have some uncertainty.
According to the reported data in terms of the Nab, two doses administration were preferred for most of the candidate COVID-19 vaccines, in order to induce more satisfying antibody responses than one shot did. Nevertheless, the two viral vectored vaccines (Ad26 and Ad5) were evaluated in phase 3 trials with one shot regimen for efficacy estimation, expecting to generate acceptable protection for COVID-19 after one dose.
As for cellular immunity, the protein subunit vaccine (NVX-CoV2373), one of the mRNA vaccines (mRNA-1273), Ad26.COV2.S and SCB-2019 induced significant CD4+ T-cell responses, especially Th1, and three non-replicating viral vectored vaccines (ChAdOx1 nCoV-19, Ad5-vectored and rAd26-S+rAd5-S COVID-19 vaccine) and DNA vaccine induced significant interferon-γ response. Whereas, T-cell response induced by inactivated vaccines was relatively weak [Figure 2; Supplementary Table 2].
Figure 2.
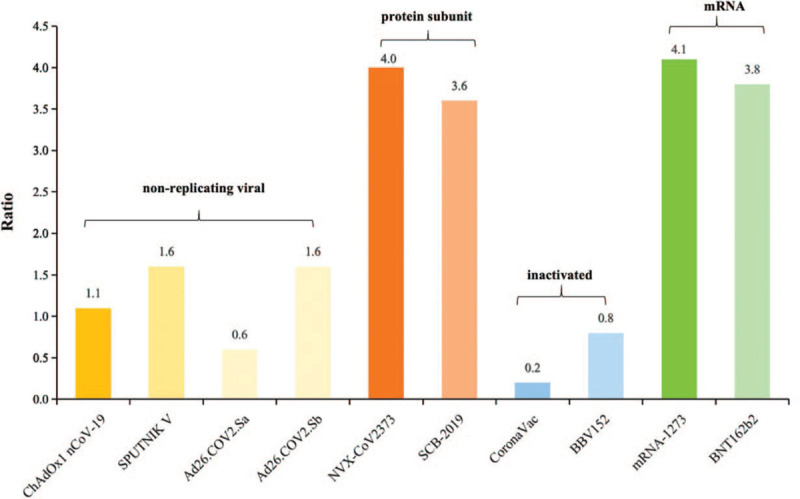
The ratio of neutralizing antibody induced by candidate COVID-19 vaccines to a panel of convalescent serum from COVID-19 patients. The neutralizing antibody referred above is induced in health adults receiving target dose or immunization procedure in main phase 3 clinical studies. The method for neutralizing antibodies induced by the vaccine is similar to those of convalescent patients who previously had COVID-19. Ad26.COV2.Sa: Single dose immunization procedure; Ad26.COV2.Sb: Two doses immunization procedure.
Protective efficacy
Either laboratory-confirmed COVID-19 or laboratory-confirmed SARS-CoV-2 infection is an acceptable primary endpoint for a COVID-19 vaccine efficacy trial, and the estimate of the primary efficacy endpoint should be at least 50% for a placebo-controlled efficacy trial.[49,50] In addition, Food and Drug Administration requests that a total of five or more severe COVID-19 cases should be observed in the placebo group, so as to assess the efficacy and VED sufficiently.[51] Currently, the interim analysis results of the efficacy data from the phase 3 clinical trials of eight COVID-19 vaccines have been reported and shown efficacies ranged from 50.4% to 95% against COVID-19. However, there are many factors influencing the efficacy estimation, such as primary endpoints, case monitoring system, and the definition of confirmed cases, all of which are different used in each vaccine. BNT162b2 was 95% effective in preventing confirmed symptomatic COVID-19 with onset at least 7 days after the second dose in participants, and similar efficacy (generally 90 to 100%) was observed across subgroups defined by age, sex, race, ethnicity, baseline body-mass index, and the presence of coexisting conditions. In this efficacy trials, a total of 170 cases were occurred, only eight in vaccine group, and ten severe cases occurred after the first dose, nine in placebo group and one in BNT162b2 group.[52] The efficacy of mRNA-1273 was 94.1% in preventing a first occurrence of symptomatic COVID-19 with onset at least 14 days after the second injection in per-protocol population, with 196 cases being observed (11 in vaccine group), of which ten were severe COVID-19 cases in the placebo group. In terms of secondary analyses, including analyses in participants who had evidence of SARS-CoV-2 infection at baseline, and analyses in participants aged over 65 years old, the efficacy was similar.[53] As for SPUTNIK V, PCR confirmed COVID-19 from day 21 after receiving the first dose occurred in 62 placebo recipients including 10 moderate or severe cases and in 16 vaccine recipients, so the efficacy was shown 91.6%.[54] An interesting phenomenon happened to the ChAdOx1 nCoV-19 in phase 3 study, in which the primary outcome was virologically confirmed, symptomatic COVID-19 after last vaccination.[55] In participants who received two standard doses, vaccine efficacy was 62.1% and in participants who received a low dose followed by a standard dose, efficacy was 90.0%. All the cases hospitalized for COVID-19 or severe cases were occurred in the control arm. Also, Janssen announced the efficacy results of 66% in preventing confirmed moderate to severe COVID-19 14 days after single dose.[56] The most immunogenic vaccine, Novavax, demonstrated 89.3% efficacy, based on 62 cases, of which 56 cases (including one severe case) were observed in the placebo group.[57] Two inactivated vaccines were shown the efficacy of 79.3% and 91.3% (in Turkey), 50.4% (in Brazil), respectively. More details are shown in Table 2.
Table 2.
The efficacy results from published clinical studies
| Vaccine | Number of vaccines | Targeted dose | Immunization procedure | Time for observation | Number of COVID-19 cases | Estimate of efficacy | Severe COVID-19 cases |
|---|---|---|---|---|---|---|---|
| BNT162b2 | 43,661 | 30 μg | 0-21 days apart | 7 days after two doses | 170 (8 in vaccine group) | 95% | 10 (1 in vaccine group) |
| mRNA-1273 | 30,000 | 100 μg | 0-28 days apart | 14 days after two doses | 196 (11 in vaccine group) | 94.1% | 30 |
| ChAdOx1 nCoV-19 | 22,690 | 2.55 × 1010/ 5 × 1010 vp | 0-28 days apart | 14 days after two doses | 131 (30 in vaccine group) | Low dose+standard dose: 90%; Two standard dose: 62%; Overall: 70% | 10 cases hospitalised (2 severe cases) all in placebo group |
| Ad26.COV2.S | 34,000 | 5 × 1010 vp | 0-21 days apart | 14 days after two doses | NA | 66% | NA |
| SPUTNIK V | 22,714 | 1 × 1011 vp | 0-21 days apart | 14 days after first dose | 78 (16 in vaccine group) | 91.6% | 20 |
| Inactivated whole-virus nCov--19 vaccine | 60,000 | 5 μg | 0-21 days apart | 14 days after two doses | NA | 79.3% | NA |
| CoronaVac | 7371 | 3 μg | 0-14 days apart | 14 days after two doses | NA | 91.25% (in Turkey); 50.38% (in Brazil) | NA |
| Novavax | 15,000 | 5 μg | 0-21 days apart | 7 days after two doses | 62 (56 in vaccine group) | 89.3% | 1 in placebo group |
NA: Not available.
Outlook
The most efficient approach to halt the pandemic is to achieve herd immunity with a valid COVID-19 vaccine. The history of vaccine development tells us that not all the vaccine candidates would succeed, particularly for a novel emerging respiratory virus. Thus, the more candidates we test, the bigger chance we gain to have safe and efficacious vaccines against COVID-19.[58] Up to now, at least nine candidate COVID-19 vaccines from different platforms evaluated in clinical trials, appeared to be safe, and able to elicit significant immune responses. Of them, eight COVID-19 vaccines had their preliminary efficacy data being released, meeting the minimum requirement of 50% efficacy, and were authorized for emergency use or conditional licensed in some countries or regions. Since a massive immunization campaign of the effective vaccine is implementing, we are expecting a slowing down of the COVID-19 epidemic later this year. However, there are still a lot scientific questions remain to be answered, including durability of vaccine-induced immune responses, safety and rare severe adverse reactions, vaccine effectiveness and its clinical evaluation and correlates of protection.
The duration of the antibodies following natural infection with SARS-CoV-2 has not been fully understood, although some researches demonstrated that the specific antibody level could be stable for at least 3 to 4 months.[59,60] Some experts believe that a vaccine could provide stronger and more durable immune response than a natural infection. One reason is that the vaccine could be designed to contain highly-concentrated antigens, which is capable of triggering high level of Nab titers. A report of immunogenicity of mRNA-1273 three months after the second vaccination showed that despite a slight expected decline in titers of binding and neutralizing antibodies, a durable humoral immunity was observed.[61] Although the evidence on the immunity durability is limited now, most of the ongoing trials are designed to perform the follow-up for at least 6 months or longer, and could give an answer to the duration of vaccine-induced immunity.[62]
Safety profile of vaccine candidates must be solid, which requests a well-established monitoring system for adverse events in clinical trials and post-market surveillance. Phase 3 studies of ChAdOx1 nCoV-19 and Ad26COVS1 vaccines have been requested to suspend because a transverse myelitis case and an unexplainable case occurred. A few BNT162b2 recipients were attacked by acute hypersensitive reaction and Bell facial paralysis after vaccination. Although the relationships of these adverse reactions and vaccines were not determined yet, continuing the surveillance for these risk signals would be extremely important.
Another highly controversial issue about the safety of the COVID-19 vaccine is ADE. ADE is commonly identified in vivo or animal models, which mainly occurs in flavivirus, coronavirus, respiratory virus and arthropod-borne viruses,[63] but the fact in humans can be exemplified only in dengue viruses with clinical, epidemiological, biological, or pathological evidence.[64–66] To address this issue, systematic evaluation endpoints have been put forward to measure ADE, including Nab versus binding antibodies (low Nab titer, low ratio of Nab to total binding antibody, low affinity of IgG antibody binding to RBD receptor), cellular immunity (low CD4+ but high CD8+ proliferative responses, CD4 T-cell responses biased toward expression of Th2 cytokines), inflammatory reactions (IL-1, IL-6, IL-8, TNF, IFN-I increased) and immunopathology (eosinophilic, Th2 cytokines IL4, IL5, IL10, IL13 increased).[60]
The lack of a standardized serum antibody and available evidence on immunoassays being correlated to functional/neutralization assays or to clinical protection is another hurdle in clinical trials to evaluate the immunogenicity of vaccine candidates. In recent published clinical studies, researchers tend to compare Nab levels between vaccines and convalescent COVID-19 patients. However, antibody response varies by time and between convalescent patients, with severe or older patients having higher antibody titers.[67,68] Hence, it is important to clearly state the sampling time and clinical severity of convalescent patients in clinical studies.
In addition to humoral immunity, cellular immunity and local mucosal immunity play important roles in protection against SARS-CoV-2 infection as well.[69,70] Mucosal immunity is critical in the prevention of respiratory infection, such as influenza, respiratory syncytial virus and pneumococcal.[71,72] A single mucosal inoculation of Ad5-nCoV could induce better protection than intramuscular vaccination for the upper and lower respiratory tracts against SARS-CoV-2 challenge in mice and ferrets.[73] These findings suggest that a COVID-19 vaccine that can not only induce humoral immunity but also a protective T cell response and mucosal immunity may maximize the protection against SARS-CoV-2, which should be investigated in future studies.
Mutations in spike protein, especially the RBD predicting conformational changes in the S1 domain, may compromise the efficacy of vaccines.[74–76] Unfortunately, there has been the evidence that the neutralization of antibody generated by two RNA vaccines (mRNA-1273 and BNT162b2) might be reduced caused by some variants, but whether the vaccine protective efficacy being influenced should be further investigated,[77] and a close monitor on the viral evolution should be continued and amplified.
The granting of emergency use designation to candidate vaccines and licensed vaccines being available raise some issues. In some countries and regions with intensive COVID-19 vaccination campaign, a randomized, double-blind placebo-controlled phase 3 efficacy clinical trial is tough to develop and maintain. Investigators might be requested to unmask trial subjects to guarantee that those who received placebo are offered or actively seek approved candidate vaccines. Also, risk-benefit profile of normal placebo-controlled trial will be unacceptable, and the compliance of the trial can be impacted by drop-outs or “contamination”, alternative strategies to evaluate those vaccines are needed. Head-to-head comparative design, stepped-wedge design and cross-over design are suggested as alternative study design to avoid ethical issue as all people in the trial are offered protective vaccine,[78,79] but at a considerable cost to efficiency and benefit. If possible, a serological correlate of protection and an immunological surrogate endpoint are expected to be justified by scientific evidence.[62]
At the present, vaccine efficacy results are just the relatively short-term data, and the durability of protection needs to be observed. Also, the number of severe cases observed in trials were still limited, in order to obtain a solid vaccine efficacy for severe cases, more severe COVID-19 cases need to be captured in the continuing surveillance of phase 3 trials. Furthermore, the protective efficacy data of the inactivated vaccines is mainly in 18 to 59 adults, and more data of other populations should be collected to support the vaccine efficacy. Since the emergency use authorization and conditional licensure are not full licensures, WHO suggested it is ethically applicable to continue blinded follow-up of placebo recipients in existing studies and to continue perform placebo-controlled trials in order to yield unbiased evidence for the next vaccine candidates.[80]
Although the existing efficacy results of vaccine against SARS-CoV-2 show the full expectations to reduce the disease and economic burden resulted from COVID-19 pandemic, we are still devoting to developing various kinds of vaccines in order to satisfy the demand of the whole world.
Author Contributions
Feng-Cai Zhu, Xiang Huo and Jing-Xin Li contributed to critical review and revision of the manuscript. Hu-Dachuan Jiang drafted of the manuscript. Peng Zhang contributed to the literature search.
Conflicts of Interest
None.
Supplementary Material
References
- [1].World Health Organization. Coronavirus disease (COVID-19)-situation report; 2021. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports. Accessed February 6, 2021. [Google Scholar]
- [2].Jeyanathan M, Afkhami S, Fiona S, et al. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol 2020;20(10):615–632. doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Oran DP, Topol EJ. Prevalence of asymptomatic SARS-CoV-2 infection: a narrative review. Ann Intern Med 2020;173(5):362–367. doi: 10.7326/M20-3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].World Health Organization. What happens to people who get seriously ill? Available from: https://www.who.int/news-room/q-a-detail/q-a-coronaviruses. Accessed October 15, 2020. [Google Scholar]
- [5].Cheryl LG, Marie JM, Dietrich P, et al. Measuring underreporting and under-ascertainment in infectious disease datasets: a comparison of methods. BMC Public Health 2014;14:147. doi: 10.1186/1471-2458-14-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Russell TW, Golding N, Hellewell J, et al. Reconstructing the early global dynamics of under-ascertained COVID-19 cases and infections. BMC Med 2020;18(1):332. doi: 10.1186/s12916-020-01790-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].European Centre for Disease Prevention and Control. Immune responses and immunity to SARS-CoV-2. Available from: https://www.ecdc.europa.eu/en/covid-19/latest-evidence/immune-responses. Accessed October 15, 2020. [Google Scholar]
- [8].Anand S, Montez-Rath M, Han J, et al. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet 2020;396(10259):1335–1344. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Iwasaki A. What reinfections mean for COVID-19. Lancet Infect Dis 2021;21(1):3–5. doi: 10.1016/S1473-3099(20)30783-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Liu STH, Lin HM, Baine I, et al. Convalescent plasma treatment of severe COVID-19: a propensity score-matched control study. Nat Med 2020;26(11):1708–1713. doi: 10.1038/s41591-020-1088-9. [DOI] [PubMed] [Google Scholar]
- [11].Watanabe Y, Allen JD, Wrapp D, et al. Site-specific glycan analysis of the SARS-CoV-2 spike. Science 2020;369(6501):330–333. doi: 10.1126/science.abb9983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Zhang L, Cao L, Gao XS, et al. A proof of concept for neutralizing antibody-guided vaccine design against SARS-CoV-2. bioRxiv doi: 10.1101/2020.09.23.309294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Chi X, Yan R, Zhang J, et al. A neutralizing human antibody binds to the N-terminal domain of the Spike protein of SARS-CoV-2. Science 2020;369(6504):650–655. doi: 10.1126/science.abc6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Liu L, Wang P, Nair MS, et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature 2020;584(7821):450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- [16].Wrapp D, Wang NS, Corbett KS, et al. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 2020;367(6483):1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Walls AC, Park YJ, Tortorici MA, et al. Structure, function, and antigenicity of the SARS-CoV-2 spike glycoprotein. Cell 2020;181(2):281–292.e6. doi: 10.1016/j.cell.2020.02.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].World Health Organization. Draft landscape of COVID-19 candidate vaccines. Available from: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines. Accessed January 29, 2021. [Google Scholar]
- [19].Abbasi J. COVID-19 and mRNA vaccines-first large test for a new approach. JAMA 2020;324(12):1125–1127. doi: 10.1001/jama.2020.16866. [DOI] [PubMed] [Google Scholar]
- [20].Vartak A, Sucheck SJ. Recent advances in subunit vaccine carriers. Vaccines (Basel) 2016;4(2):12. doi: 10.3390/vaccines4020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Delrue I, Verzele D, Madder A, et al. Inactivated virus vaccines from chemistry to prophylaxis: merits, risks and challenges. Expert Rev Vaccines 2012;11(6):695–719. doi: 10.1586/erv.12.38. [DOI] [PubMed] [Google Scholar]
- [22].Watanabe Y, Bowden TA, Wilson IA, et al. Exploitation of glycosylation in enveloped virus pathobiology. Biochim Biophys Acta Gen Subj 2019;1863(10):1480–1497. doi: 10.1016/j.bbagen.2019.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Vankadari N, Wilce JA. Emerging COVID-19 coronavirus: glycan shield and structure prediction of spike glycoprotein and its interaction with human CD26. Emerg Microbes Infect 2020;9(1):601–604. doi: 10.1080/22221751.2020.1739565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Shang J, Wan YS, Luo C. Cell entry mechanisms of SARS-CoV-2. Proc Natl Acad Sci U S A 2020;117(21):11727–11734. doi: 10.1073/pnas.2003138117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Sternberg A, Cord Naujokat C. Structural features of coronavirus SARS-CoV-2 spike protein: targets for vaccination. Life Sci 2020;257:118056. doi: 10.1016/j.lfs.2020.118056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Amanat F, Krammer F. SARS-CoV-2 vaccines: status report. Immunity 2020;52(4):583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Karch CP, Burkhard P. Vaccine technologies: from whole organisms to rationally designed protein assemblies. Biochem Pharmacol 2016;120:1–14. doi: 10.1016/j.bcp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rauch S, Jasny E, Schmidt KE, et al. New vaccine technologies to combat outbreak situations. Front Immunol 2018;9:1963. doi: 10.3389/fimmu.2018.01963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].U.S. Department of Health and Human Services Food and Drug Administration. Chemistry, manufacturing, and control (CMC) information for human gene therapy Investigational New Drug applications (INDs), guidance for industry. Available from: https://www.fda.gov/vaccines-blood-biologics/guidance-compliance-regulatory-information-biologics/biologics-guidances. Accessed September 20, 2020. [Google Scholar]
- [30].Halifa SA, Gauthier L, Arpin D, et al. Nanoparticle-based vaccines against respiratory viruses. Front Immunol 2019;10:22. doi: 10.3389/fimmu.2019.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fierros LM, Silva IG, Mendoza SR. Development of SARS-CoV-2 vaccines: should we focus on mucosal immunity? Expert Opin Biol Ther 2020;20(8):831–836. doi: 10.1080/14712598.2020.1767062. [DOI] [PubMed] [Google Scholar]
- [32].Sungnak W, Huang N, Bécavin C, et al. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med 2020;26(5):681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ziegler CGK, Allon SJ, Nyquist SK, et al. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell 2020;181(5):1016–1035. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Ramasamy MN, Minassian AM, Ewer KJ, et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): a single-blind, randomised, controlled, phase 2/3 trial. Lancet 2021;396(10267):1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Folegatti PM, Ewer KJ, Aley PK, et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020;396(10249):467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: two open, non-randomised phase 1/2 studies from Russia. Lancet 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Zhu FC, Guan XY, Li YH, et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020;396(10249):479–488. doi: 10.1016/S0140-6736(20)31605-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jackson LA, Anderson EJ, Rouphael NG, et al. An mRNA vaccine against SARS-CoV-2-preliminary report. N Engl J Med 2020;383(20):1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Walsh EE, Frenck RW, Falsey AR, et al. Safety and immunogenicity of two RNA-based Covid-19 vaccine candidates. N Engl J Med 2020;383(25):2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Anderson EJ, Rouphael NG, Widge AT, et al. Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 2020;383(25):2427–2438. doi: 10.1056/NEJMoa2028436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Keech C, Albert G, Cho I, et al. Phase 1-2 Trial of a SARS-CoV-2 recombinant spike protein nanoparticle vaccine. N Engl J Med 2020;383(24):2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Peter R, Lara H, Min D, et al. Safety and immunogenicity of S-Trimer (SCB-2019), a protein subunit vaccine candidate for COVID-19 in healthy adults: a phase 1, randomised, double-blind, placebo-controlled trial. Lancet 2021;397(10275):682–694. doi: 10.1016/S0140-6736(21)00241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Xia SL, Duan K, Zhang YT, et al. Effect of an inactivated vaccine against SARS-CoV-2 on safety and immunogenicity outcomes: interim analysis of 2 randomized clinical trials. JAMA 2020;324(10):1–10. doi: 10.1001/jama.2020.15543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Xia SL, Zhang YT, Wang YX, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: a randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet Infect Dis 2021;21(1):39–51. doi: S1473-3099(20)30831-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Zhang YJ, Zeng GZ, Pan HX, et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect Dis 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Ella R, Vadrevu KM, Jogdand H, et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBV152: a double-blind, randomised, phase 1 trial. Lancet Infect Dis 2021;21. doi: 10.1016/S1473-3099(20)30942-7. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tebas P, Yang SP, Boyer JD, et al. Safety and immunogenicity of INO-4800 DNA vaccine against SARS-CoV-2: a preliminary report of an open-label, Phase 1 clinical trial. EClinicalMedicine 2021;31:100689. doi: 10.1016/j.eclinm.2020.100689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Sadoff J, Gars ML, Shukarev G, et al. Interim results of a Phase 1-2a trial of Ad26.COV2 S Covid-19 vaccine. N Engl J Med 2021;384. doi: 10.1056/NEJMoa2034201. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].U.S. Department of Health and Human Services Food and Drug Administration. Development and licensure of vaccines to prevent COVID-19 guidance for industry. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/development-and-licensure-vaccines-prevent-covid-19. Accessed October 2, 2020. [Google Scholar]
- [50].World Health Organization. WHO target product profiles for COVID-19 vaccines. Available from: https://www.who.int/who-documents-detail/who-target-product-profiles-for-covid-19-vaccines. Accessed October 2, 2020. [Google Scholar]
- [51].Food and Drug Administration. Emergency use authorization for vaccines to prevent COVID-19 guidance for industry. Available from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/emergency-use-authorization-vaccines-prevent-covid-19. Accessed October 2, 2020. [Google Scholar]
- [52].Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Baden LR, Sahly HME, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Logunov DY, Dolzhikova IV, Zubkova OV, et al. Safety and efficacy of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine: an interim analysis of a randomised controlled phase 3 trial in Russia. Lancet 2020;396(10255):887–897. doi: 10.1016/S0140-6736(20)31866-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Voysey M, Clemens SAC, Madhi SA, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet 2021;397(10269):99–111. doi: 10.1016/S0140-6736(20)32661-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Janssen. Johnson & Johnson announces single-shot Janssen COVID-19 vaccine candidate met primary endpoints in interim analysis of its phase 3 ENSEMBLE trial. Available from: https://www.janssen.com/johnson-johnson-announces-single-shot-janssen-covid-19-vaccine-candidate-met-primary-endpoints. Accessed January 31, 2021. [Google Scholar]
- [57].Novavax. Novavax COVID-19 vaccine demonstrates 89.3% efficacy in UK phase 3 trial. Available from: https://www.usatoday.com/story/news/health/2021/01/28/novavaxs-covid-19-vaccine-shown-nearly-90-effective-uk-clinical-trial-also-provided-immunity-against/4294584001/. Accessed January 31, 2021. [Google Scholar]
- [58].World Health Organization. WHO Director-General's opening remarks at the media briefing on COVID-19-21 September 2020. Available from: https://www.who.int/dg/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---21-september-2020. Accessed September 17, 2020. [Google Scholar]
- [59].Wajnberg W, Amanat F, Firpo A, et al. SARS-CoV-2 infection induces robust, neutralizing antibody responses that are stable for at least three months. medRxiv 2020;[2020-07-14]. doi: 10.1101/2020.07.14.20151126. [DOI] [Google Scholar]
- [60].Gudbjartsson DF, Norddahl GL, Melsted P, et al. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med 2020;383(18):1724–1734. doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Widge AT, Rouphael NG, Jackson LA, et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N Engl J Med 2021;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].World Health Organization. Considerations for evaluation of COVID-19 vaccines points to consider for manufacturers of COVID19 vaccines Version 24 September 2020. Available from: https://www.who.int/medicines/regulation/prequalification/prequal-vaccines/WHO_Evaluation_Covid_Vaccine.pdf?ua=1. Accessed Oct 2, 2020. [Google Scholar]
- [63].Taylor A, Foo SS, Bruzzone R, et al. Fc receptors in antibody-dependent enhancement of viral infections. Immunol Rev 2015;268(1):340–364. doi: 10.1111/imr.12367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Halstead SB, Chow JS, Marchette NJ. Immunological enhancement of dengue virus replication. Nature 1973;243:24–26. [PubMed] [Google Scholar]
- [65].Sridhar S, Luedtke A, Langevin E, et al. Effect of dengue serostatus on dengue vaccine safety and efficacy. N Engl J Med 2018;379(4):327–340. doi: 10.1056/NEJMoa1800820. [DOI] [PubMed] [Google Scholar]
- [66].Wilder-Smith A, Ooi EE, Horstick O, et al. Dengue. Lancet 2019;393:350–363. doi: 10.1016/S0140-6736(18)32560-1. [DOI] [PubMed] [Google Scholar]
- [67].Moore JP, Klasse PJ. COVID-19 vaccines: “warp speed” needs mind melds, not warped minds. J Virol 2020;94(17):e01083–e1120. doi: 10.1128/JVI.01083-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Huang AT, Garcia-Carreras B, Hitchings MDT, et al. A systematic review of antibody mediated immunity to coronaviruses: antibody kinetics, correlates of protection, and association of antibody responses with severity of disease. medRxiv 2020;[2020-04-14]. doi: 10.1101/2020.04.14.20065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Chandrashekar A, Liu J, Martinot AJ, et al. SARS-CoV-2 infection protects against rechallenge in rhesus macaques. Science 2020;369(6505):812–817. doi: 10.1126/science.abc4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yu J, Tostanoski LH, Peter L, et al. DNA vaccine protection against SARS-CoV-2 in rhesus macaques. Science 2020;369(6505):806–811. doi: 10.1126/science.abc6284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Holmgren J, Czerkinsky C. Mucosal immunity and vaccines. Nat Med 2005;11(4 Suppl):S45–S53. doi: 10.1038/nm1213. [DOI] [PubMed] [Google Scholar]
- [72].Mazur NI, Higgins D, Nunes MC, et al. The respiratory syncytial virus vaccine landscape: lessons from the graveyard and promising candidates. Lancet Infect Dis 2018;18(10):e295–e311. doi: 10.1016/S1473-3099(18)30292-5. [DOI] [PubMed] [Google Scholar]
- [73].Wu S, Zhong G, Zhang J, et al. A single dose of an adenovirus-vectored vaccine provides protection against SARS-CoV-2 challenge. Nat Commun 2020;11(1):4081. doi: 10.1038/s41467-020-17972-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Singh PK, Kulsum U, Rufai SB, et al. Mutations in SARS-CoV-2 leading to antigenic variations in spike protein: a challenge in vaccine development. J Lab Physicians 2020;12(2):154–160. doi: 10.1055/s-0040-1715790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Becerra-Flores M, Cardozo T. SARS-CoV-2 viral spike G614 mutation exhibits higher case fatality rate. Int J Clin Pract 2020;74(8):e13525. doi: 10.1111/ijcp.13525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Korber B, Fischer WM, Gnanakaran S, et al. Spike mutation pipeline reveals the emergence of a more transmissible form of SARS-CoV-2. bioRxiv 2020, [2020-05-05]. doi: 10.1101/2020.04.29.069054. [DOI] [Google Scholar]
- [77].Wang ZJ, Schmidt F, Weisblum Y, et al. mRNA vaccine-elicited antibodies to SARS-CoV-2 and circulating variants. bioRxiv, 2021 [2021-01-30]. doi: 10.1101/2021.01.15.426911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].World Health Organization Solidarity Vaccines. Trial Expert Group COVID-19 vaccine trials should seek worthwhile efficacy. Lancet 2020;396(10253):741–743. doi: 10.1016/S0140-6736(20)31821-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Cohen J, Kupferschmidt K. Infectious diseases. Ebola vaccine trials raise ethical issues. Science 2014;346(6207):289–290. doi: 10.1126/science.346.6207.289. [DOI] [PubMed] [Google Scholar]
- [80].Krause PR, Fleming TR, et al. WHO Ad Hoc Expert Group on the Next Steps for Covid-19 Vaccine Evaluation. Placebo-controlled trials of Covid-19 vaccines – why we still need them. N Engl J Med 2020;384(2):e2. doi: 10.1056/NEJMp2033538. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


