Abstract
The last three decades have seen the biotherapeutic drug market evolve from promising concept to market dominance in a range of clinical indications. This growth has been spurred by the success of established drug classes like monoclonal antibodies, but also by the introduction of biosimilars, and more recently, multiple novel cell and gene therapies. Biotherapeutic drug development presents many unique challenges, but unintended immune responses are among the most common reasons for program attrition. Anti-drug antibodies can impact the safety and efficacy of drug products, and related immune responses, like the cytokine release syndrome that occurred in the infamous TGN-1412 clinical trial, can be challenging to predict with nonclinical models. For this reason, it is important that development programs proceed with a scientifically grounded and measured approach to these responses. This process begins at the discovery stage with the application of “quality by design,” continues into the clinic with the development of quality assays and management strategies, and culminates in the effective presentation of this information in regulatory documents. This review provides an overview of some of the key strategic and regulatory considerations for biotherapeutics as they pertain to immunogenicity and related responses.
Keywords: Regulatory science, immunogenicity, anti-drug antibodies, biotherapeutics, drug development
Introduction
Although many advances have been made in their design, biotherapeutics still generally carry a larger risk of unanticipated immune responses than traditional small molecule drugs [1]. This risk has become increasingly relevant over the last three decades in concert with the global growth in approvals for these products. Indeed, more than 20% of the 79 monoclonal antibodies that are currently approved by the US Food and Drug Administration (FDA) were approved in the last 2 years, and many established products have now begun to see heavy competition from biosimilars as their initial patents and/or exclusivity periods have ended. Along with monoclonal antibodies and other well-established drug classes (hormones, cytokines, therapeutic replacement enzymes, and blood factors) [2], the last few years have also seen the introduction of multiple novel cell and gene therapies (e.g., sipuleucel-T, alipogene tiparvovec, tisagenlecleucel, and remestemcel-L). While only a handful of these products are currently available [3,4], this number is estimated to increase dramatically, with FDA anticipating the approval of 10–20 such products per year by 2025 [5].
In addition to radically expanding the treatment options for many indications, this continued growth has also produced substantial financial returns. In 2018, 8 of the top 10 bestselling drugs worldwide were biologics, and the monoclonal antibody market alone is projected to reach $300 billion by 2025 [6]. Although sponsors entering the biotherapeutic space are confronted by a number of unique development challenges, unintended immune responses are among the most important contributors to failure for these products [7]. Not only do anti-drug antibodies (ADAs) have the potential to impact the efficacy of investigational therapies, they can also cause serious adverse responses in subjects, and even lead to death in rare cases [8,9]. In addition, the development of other aberrant immune responses, like cytokine release syndrome (CRS), can be more problematic than ADAs for some therapies, such as chimeric antigen receptor (CAR) T-cells [10].
In some cases, these realities have led drug developers to avoid a full assessment of a product’s immunomodulatory potential until late in clinical development when such analysis is required for marketing approval, but this approach can be problematic since it is generally more difficult to introduce control measures at this time. Instead, regulatory bodies including the European Medicines Agency (EMA) and FDA recommend that a product’s risk be given appropriate consideration at all phases of a development program. This begins at the design stage with an understanding of how each of the finished product’s properties (i.e., size, method of production, structural modifications, and propensity for aggregation) may influence its interactions with components of the immune system and continues throughout the development of the product [11,12]. In this review, we provide an overview of immunogenicity and related responses as they pertain to biotherapeutic program planning and execution with an emphasis on how a systematic, proactive, and scientifically grounded approach can contribute to overall program success.
The Impact of Immunogenicity and Related Responses
In its 2014 guidance “Immunogenicity Assessment for Therapeutic Protein Products,” the FDA defines immunogenicity as the “propensity of the therapeutic protein product to generate immune responses to itself and to related proteins or to induce immunologically related adverse clinical events” [13]. Although the word immunogenicity refers to all adaptive responses (both cell and antibody-mediated), the terms ADA and immunogenicity are often used interchangeably in pharmaceutical development [14]. This practice stems from the use of ADA monitoring as the chief criterion for predicting the risk of adverse immune responses. Nevertheless, regulatory authorities generally include discussion of a broad range of immune-related responses in the guidance documents they issue (both antibody-dependent and independent) since these responses tend to overlap with regard to clinical presentation and management [12,13,15]. Furthermore, while the above definition specifically refers to therapeutic protein products, immunogenicity assessments are also routinely performed for several non-biologic drug classes where the potential for immunogenicity has been established, including peptides, oligonucleotides, and some combination products.
What follows is a discussion of the impact of these responses as they pertain to biopharmaceuticals. A more detailed discussion on management and mitigation strategies is also provided in the section “clinical management and treatment of immune responses.” While a detailed discussion of the mechanistic basis of antibody and cell-based responses is beyond the scope of this article, there are many excellent resources that provide considerable detail on this topic [16,17,18].
Impact of Immunogenicity
Although immunogenic responses have considerable potential to disrupt development, it is important to note that most biotherapeutics are immunogenic to some extent. In fact, some approved products have ADA incidence levels that reach over 90% with little to no overt impact on clinical safety or efficacy [19]. While rare, safety effects can be generally grouped into acute or non-acute categories based on whether they develop within minutes to hours, or hours to days after the therapy is administered. Among the acute responses are type I hypersensitivity reactions like anaphylaxis, systemic inflammatory responses like CRS, and inflammatory responses that are more localized to the site of administration. Non-acute responses include type III and IV hypersensitivity reactions (antigen-antibody, complex-mediated, and cell-mediated reactions), such as serum sickness and contact dermatitis. Importantly, both acute and non-acute responses can vary in severity from mild to life-threatening [19,20].
Impacts to efficacy are more commonly encountered but can also pose substantial challenges to development since they are capable of modifying the established pharmacologic profile of a drug. For example, depending upon where the ADA binds, it may be able to interfere with the drug’s ability to interact with its target. Known as neutralization, this interaction can partially or completely prevent a drug from exerting its therapeutic effect. While this may seem innocuous, neutralizing antibodies (NAbs) are often not readily apparent since they may not be accompanied by other symptoms, which is why early ADA assessments and frequent monitoring of well-established biomarkers (i.e., those with a clearly established correlation between clinical response and relevance) of activity are recommended for high-risk products [13]. Both neutralizing and non-neutralizing ADAs can also change the rate at which a drug is cleared, either by sustaining it in circulation, or by hastening its metabolism/elimination [13]. The former can be dangerous since increased exposure can increase the likelihood of adverse events, but hastened metabolism/elimination can also be problematic for the same reasons outlined above.
It is important to note that while novel modalities are often more likely to be recognized as foreign by the immune system, patients can and do develop ADAs against wildtype human proteins as well. This point is especially salient for replacement therapies where treatment involves administering a common human factor to patients in which the factor is either deficient or entirely absent, or where substantial differences between the endogenous and therapeutic forms (e.g., due to naturally occurring polymorphisms) may promote the development of an undesirable response against the treatment [13]. For example, severe congenital factor (F) XI, FIX-, and FVIII-deficient patients commonly develop NAbs following treatment with these proteins [19,21].
Finally, ADAs that develop may also cross-react with other marketed drug(s) or endogenous protein(s) that are similar or identical to the biotherapeutic. For example, recombinant interferon (IFN)-β has been widely used as a treatment for newly diagnosed patients with relapsing-onset multiple sclerosis. However, NAbs that develop during treatment have been found to cross-react with the endogenous IFN-β protein, which may compromise immune function in these patients [22,23]. Likewise, administration of recombinant thrombopoietin to healthy volunteers has been shown to result in the development of NAbs that cross-react with endogenous thrombopoietin, resulting in severe thrombocytopenia [24]. In its 2014 guidance, the FDA suggested several special evaluations for products that are the counterpart of normally endogenous proteins. Among others, these include quantitating the level of the endogenous protein in serum at steady state, and investigating the mechanisms that normally lead to its production. These and additional measures are especially justified in instances where the endogenous protein in question serves a non-redundant physiological role in the body [13].
Impact of Related Immune Responses
Although circulating antibody is considered to be the chief criterion for defining an immune response to biologics, regulators also recognize the importance of antibody-independent processes in assessing safety [12,13]. For example, although anaphylaxis is generally caused by IgE antibody-mediated degranulation of basophils and mast cells, antibody-independent anaphylactoid responses can also occur [25]. Likewise, CRS development is known to be orchestrated through a number of different mechanisms, including FC receptor and complement cascade-mediated activation [26,27]. Therefore, testing for these and other responses may also be needed on a case-by-case basis when justified.
The most notorious example of CRS occurred with a product known as TGN-1412 (also theralizumab). TGN-1412 was a CD28 superagonist that was designed to be able to activate T-cells without the requirement for overt T-cell receptor engagement [28]. It was intended to treat B-cell chronic lymphocytic leukemia and rheumatoid arthritis but was withdrawn from clinical testing after inducing severe CRS in a first-in-human (FIH) study in London in 2006. Six patients were administered TGN-1412 in relatively quick succession, and all six were ultimately hospitalized, with several suffering from multiple organ dysfunction [29,30]. Notably, the dose that was administered, 0.1 mg/kg, was about 500 times lower than the dose that was found to be safe in animal studies using a no observable adverse effect level (NOAEL) approach [31].
Among other actions, this event prompted the EMA (at that time known as EMEA) to release a guidance on identifying and mitigating risks in FIH trials in September 2007. In this guidance, they introduced the concept of using a minimally anticipated biological effect level (MABEL) in place of an NOAEL for calculating FIH dose selection [32,33]. Although the FDA’s initial 2005 guidance on FIH dose selection prioritized use of the NOAEL [34], the MABEL is currently recommended for both bispecific antibodies, and biologic products directly or indirectly intended to stimulate immune responses in separate 2019 and 2020 draft guidance documents [35,36].
A further finding of subsequent investigations was that the preclinical models used were not appropriate for predicting risk to patients. CD4+ effector memory T-lymphocytes, which were responsible for the cytokine release, were found to be negative for the target (i.e., CD28) in cynomolgus macaques (the non-human primate species used for preclinical in vivo testing). Furthermore, while in vitro studies with human cells were included in the preclinical program, these assays also failed to predict the hazard as they did not adequately model in vivo presentation of TGN-1412. Subsequent analysis showed that TGN-1412 would have produced a substantial cytokine response in these experiments if it had been directly immobilized onto a cell culture plate, or presented utilizing coculture with an endothelial cell monolayer [29,37,38].
Despite considerable progress in the advancement of in vitro and ex vivo assays to predict toxicity [39,40], FIH trials with biotherapeutics still carry a risk of unanticipated immune responses. Nevertheless, it is worth noting that TGN-1412 was ultimately purchased and rebranded as TAB08. The new company was then able to successfully re-enter clinical trials with TAB08 in 2011 using a more appropriate starting dose of 0.1 µg/kg (1000-fold below the dose used in 2006) (NCT01885624) [28]. This story not only highlights the value of a reasoned and scientific approach in addressing risk, but it demonstrates that the correct interpretation of the data can mean the difference between tragedy and success for these programs.
Quality-by-Design in Program Development and Planning
While current estimates place the mean development expenditure for a new drug at $1.3 billion, it can be much higher for certain therapeutic areas. For example, the estimated cost for new antineoplastic and immunomodulatory agents is nearly $4.5 billion. Low clinical success rates are an important driver of these costs as 9 out of 10 drugs are expected to fail during clinical development [41]. Although lack of efficacy is the most common overall cause of trial failure, early-phase failures are often due to safety issues [42]. For biologics, immunogenicity is one of the principal reasons for this attrition; hence, it is important for sponsors to design their therapeutics in a way that maximizes their chances of success in this area [43].
Maximizing the success of biologic programs can be accomplished by holistic application of the concept of quality-by-design (QbD) in early development. The ICH Q8(R2) guidance defines QbD as “a systematic approach to development that begins with predefined objectives and emphasizes product and process understanding and process control, based on sound science and quality risk management” [44]. While this concept has traditionally been used to ensure quality within manufacturing processes, it can be expanded to include therapeutic design as well. In this model, sponsors develop an ideal quality target product profile for their finished pharmaceutical product that includes considerations such as intended route of administration, strength, and stability. They then identify critical quality attributes (CQA) that are essential to produce that profile, and these serve as a guideline for subsequent development and control activities. As an example, protein aggregation is generally considered to be a CQA for biotherapeutics since it is known to contribute to immunogenic responses in patients. Aggregates can form at many different stages of manufacturing due to a host of extrinsic factors (pH, temperature, osmolality, and mechanical stress), but as the propensity of an antibody to aggregate can also be partially predicted from its primary amino acid sequence and structure using various in silico algorithms, the most prudent approach is to start by selecting a drug candidate with a low propensity for aggregation [45]. Likewise, residual host cell proteins (HCPs) in the finished drug product are also considered to be a key CQA for biotherapeutics given their potential to induce ADA, function as adjuvants, and directly impact the quality of the finished product [46]. For these reasons, HCPs are routinely reduced to acceptable levels that ensure product quality and safety during downstream purification activities, which can result in substantial cost [47]. Nevertheless, since the HCP profile is also dependent upon many upstream processes including the expression system, cell viability, and culture duration, it makes sense to reduce the need for such controls wherever possible by optimizing the initial production process [48]. This strategy, often described as “starting with the end in sight,” is perhaps the easiest way to reduce the cost of additional control measures later in development [43].
Factors That Contribute to Immune Responses
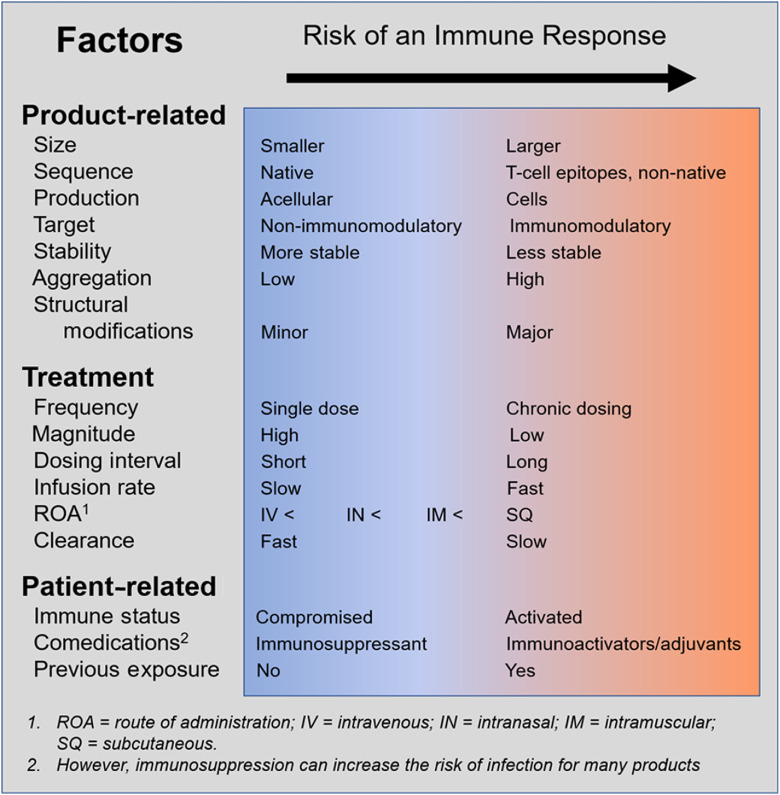
While the idea of controlling immunogenicity at the design stage may sound simple, it is much more difficult in practice. The list of known factors that shape these responses is extensive and has been the subject of several excellent reviews [49,50–52]. For this reason, it can be challenging to predict what elements will be important for new classes of drugs, such as cell and gene therapies. Moreover, for many products, like T-cell engaging antibodies, it may not be possible to prevent toxicity without sacrificing efficacy to some extent [53]. Nevertheless, Fig. 1 outlines some of the key factors to consider when developing a drug candidate.
Fig. 1.
Factors that influence immune responses.
Assessment, Interpretation, and Presentation of Immunogenicity Data
When Is Immunogenicity Information Required?
Immunogenicity testing is required by regulatory authorities for therapeutic proteins, and other relevant classes of drugs as justified by mechanism, history, or experience. Fully validated, multi-tier assessments should be performed for these products during pivotal clinical trials, and regulators recommend that fit for purpose assays also be included during early clinical trials and repeat-dose nonclinical studies [12,13]. Per ICH S6(R1), nonclinical immunogenicity data are not considered to be predictive of clinical safety. Although humans can and do develop immunogenic responses against human(ized) proteins (e.g., the first approved human monoclonal antibody, adalimumab, has an ADA occurrence rate of 20–30% in some analyses [54,55]), it has been well established that species differences generally make such responses more likely to occur in animal studies than in patients [12,13,56]. Even so, assessing ADA in repeat-dose toxicology studies can be incredibly valuable for the interpretation of nonclinical data [12,13,57]. If, for example, deaths were observed in the mid-dose group of an animal study, but not the high-dose group due to the presence of neutralizing ADAs in the latter group, this information would be important for understanding the relationship between exposure and toxicity in that species.
In addition to pivotal clinical studies, comparative clinical immunogenicity assessments are required for most biosimilars and may be needed for certain manufacturing changes in innovator products when there is a high potential to impact immunogenicity [12,13,58,59]. Biosimilar insulin products are an interesting exception to these requirements. In 2015, the EMA updated its position on recombinant human insulin and insulin analogs to waive prelicensing immunogenicity assessments in instances where sufficient biosimilarity to a low-risk reference product has been demonstrated, and where the impurity profile/excipients do not give cause for concern [60]. The FDA followed suit in a recent 2019 draft guidance, and justified the change by pointing to numerous factors, including “extensive experience and literature survey that confirm minimal or no clinical relevance of immunogenicity with insulin product use” [61].
As manufacturing changes are typically more minor in nature than those that are needed to develop a new biosimilar, they can usually be justified without additional nonclinical and/or clinical analysis. However, additional studies may be needed in some special cases where existing data are insufficient to ensure comparable safety and efficacy with the pre-change product [62,63].
Finally, immunogenicity concerns can be important considerations for post-approval pharmacovigilance plans. Risk-management plans are required in the EU [64], and risk evaluation and mitigation strategies may be required in the USA [65] – both of which may contain specific measures related to immunogenicity (including additional clinical studies) where justified by known safety concerns, or identified areas of missing information.
Designing a Sampling Approach
The sampling scheme for ADA assessments should be tailored to each specific product being tested and should aim to fully characterize the nature, impact, and significance of the immune response. In general, pre-study sampling is recommended in order to identify pre-existing antibodies and to allow calculation of treatment-boosted titers. Post-dose sampling should be performed frequently following administration, especially when drug concentrations are anticipated to be at their lowest to avoid interference by free drug in ADA assays (see discussion of drug tolerance below). Regulatory authorities typically expect at least one year of clinical immunogenicity data to support marketing authorization for chronically administered products unless a shorter time can be justified [13,66]. However, a longer evaluation may be needed where ADAs persist or clearance is slow. One possible approach would be to collect patient samples at baseline, and at 0.5, 1, 2, 3, 6, 9, 12, 18, and 24 months, and yearly thereafter, but this may not be appropriate for all programs and the sampling strategy should be carefully considered based upon all available information [19]. For example, a recent investigation in rheumatoid arthritis patients found that the median time to ADA onset for infliximab and adalimumab with methotrexate cotreatment was 4.5 and 13 months, respectively [55]. Accordingly, increased sampling should be considered whenever ADAs are detected or suspected, and when adverse events are observed (where possible) in order to aid in their interpretation.
ADA Laboratory Assays
In-depth guidance on how to design an appropriate, multi-tiered suite of ADA assays is available from the EMA and FDA [11,12]. In general, these documents establish consistent regulatory expectations that apply to all investigational products with potential to produce immunogenic effects. Accordingly, a successful analysis program should aim to provide as much of the following information as possible with regard to any ADA responses.
Nature: the incidence, magnitude (i.e., titer), relevant isotype distribution, the duration of the response, whether ADA is transient or persistent, and any other pertinent information.
Impact: neutralizing ability, cross-reactivity with endogenous molecules or other pharmaceuticals, and any impact on pharmacokinetics (PK; either drug-clearing or sustaining), or pharmacodynamics (PD).
Significance: any association with clinical sequelae and observed relevant thresholds for adverse events.
ADA laboratory assays fall into three tiers: (1) screening assays, (2) confirmatory assays, and (3) assays to characterize the nature and impact of the response (neutralization, antibody isotyping, and titering). Validation involves assessing various parameters of each assay, including sensitivity, specificity, selectivity, drug tolerance, precision, reproducibility, and robustness where appropriate to assess if they are fit for their intended use on study. During study analysis, samples that are identified as presumptively positive during the screening assay are confirmed in a separate confirmatory assay. These samples are then identified as positive, negative, unevaluable, or inconclusive. Best practice for high-risk products is to then follow those ADA positive samples until they are ADA negative (e.g., via a patient registry) to determine off-treatment persistence of ADA [67].
ADA assays are performed using a variety of methods, including enzyme-linked immunosorbent assay-based methods, surface plasmon resonance, and radioimmunoprecipitation. However, the current industry standard is to use meso scale discovery electrochemiluminescence immunoassays (ECLIA). This shift has been driven in part by the improved sensitivity that ECLIA affords over more traditional methods [68,69], which is important since these assays are typically only semi-quantitative. Regulators tend to prefer that ADA magnitude be expressed as a titer (i.e., the inverse of the greatest dilution that still yields a positive result) rather than absolute concentration since the latter requires interpolation of data from standard curves made using positive controls that likely do not reflect the structure, avidity, and specificity of antibodies found in patient samples [11,12]. In the absence of standard curves, assays are dependent upon the establishment of an assay cut-point to make a determination of ADA status (e.g., positive or negative), which is in turn highly dependent upon the sensitivity of the assay [70]. If the assay is not sensitive enough (current industry guidelines have reduced the recommended sensitivity value for an assay from 250 to 500 ng/mL, to ≤ 100 ng/mL), the assay may under-report the incidence of clinically impactful ADA [11]. It is also worth noting that sensitivity can vary considerably between assays (especially between older assays and newer ones). As such, regulators do not tend to recommend drawing conclusions from ADA incidence alone (i.e., in the absence of PK/PD and safety outcomes) or even comparing the results of two analyses that have utilized different analytical methods [19].
In addition to sensitivity, the ECLIA and other newer approaches are also preferred due to their improved drug tolerance [11,19]. The drug tolerance of an assay is the highest concentration at which free drug in the patient sample (from administration to the patient) does not interfere with the ability of the assay to detect ADAs. Such interference is problematic since it can result in an underestimation of the amount of ADA present, and hence, an underestimation of risk [70]. In the past, low drug tolerance values have been common and have often led to a considerable number of study samples being labeled as inconclusive. In fact, Wang et al. reviewed 28 biologics that were approved by the FDA between 2005 and 2011 and found that greater than 50% had drug tolerance levels that were lower than their steady-state drug concentrations [71]. These results greatly limit the utility of such data in drawing meaningful conclusions about ADA.
While many different options are available for improving both the sensitivity and drug tolerance of assays, including advanced platforms and novel mitigation strategies [72–74], the value of early discussions with regulators in determining the suitability of an individual assay or an overall approach cannot be over-emphasized. Ultimately, the most important thing is that any data that were derived from study samples be interpreted utilizing an in-depth understanding of the detection method, drug tolerance range, and overall risk posed by the product.
Presenting and Interpreting Data
Effective presentation of immunogenicity information in regulatory documents is exceedingly important. Not only does it facilitate efficient review by regulatory assessors, but an understanding of immunogenic potential can help inform clinical monitoring, as well as overall regulatory strategy. The common technical document (CTD) format, as outlined in the ICH M4 guidance, was introduced across the founding International Council for Harmonisation regions (Japan, EU, and USA) in July 2003 as a way to harmonize the presentation of technical information in regulatory submissions [75,76]. However, many non-member countries have also adopted the CTD format, including Mexico, Australia, Canada, and Switzerland [77,78].
The components of an immunogenicity program have historically been spread across many different parts of the regulatory dossier: clinical study reports, including reports of bioanalytical and analytical methods, are found in CTD module 5.3; an overview of clinical pharmacology is presented in CTD Module 2.5.3; and immunogenicity studies that correlate with PK, PD, safety, and/or efficacy data are summarized in Special Studies (CTD Module 2.7.2.4) [79,80]. In some cases, it may also be necessary to reference nonclinical and quality information (CTD Modules 2 and 3, respectively). Unfortunately, this wide disbursement of information can make it challenging to reconstruct the overall risk assessment strategy and provide an integrated view of the potential impact to the development program.
While the concept of an integrated assessment of immunogenicity risk has been around for several years [81], final guidance on the structure and organization of this assessment has only recently been made available by regulatory agencies. As such, the EMA (in 2017) and FDA (in 2019) issued guidance requiring inclusion of an Integrated Summary of Immunogenicity (ISI) in marketing applications for biological products (and other immunogenic products as needed) [11,12]. Although technically only required with applications for marketing authorization (the full document should be included in CTD Module 5.3.5.3 and briefly outlined in Module 2.7.2.4), the ISI should be initiated early in development to maximize value to the development program. Real-time population of this “living” document ensures that relevant immunogenicity data are given appropriate consideration in study planning and enables alignment with regulators at pivotal points through the lifecycle of the product (e.g., investigational new drug application/clinical trial application submission and end-of-phase II program meetings). The EMA and FDA diverge with regard to overall format, but the basic information to provide is consistent and includes: 1) an analysis of a product’s unique risk factors, 2) an outline of the assay strategy and clinical sampling approach used or proposed, 3) immunogenicity results from studies to date, and 4) conclusions and any risk-mitigation strategies proposed based on these results [79,82]. While the ISI might begin as just a general description of possible product risk factors, the addition of study information over time will eventually make it a strategic tool for understanding the likely impact of immunogenicity as it relates to the overall clinical program.
Clinical Management and Treatment of Immune Responses
Despite their best efforts, it is often necessary for sponsors to implement strategies to mitigate unintended immune responses once drugs have progressed into the clinic. In some cases, this need may be anticipated given historical experience with the therapeutic class, or other knowledge/experience gathered in preclinical development through a growing number of in silico and in vitro methods [39,83,84] (although generally not animal toxicology studies for the reasons described previously). However, in many cases, the full nature of these responses cannot be understood until after the candidate enters clinical trials, and even then, limited subject numbers and the potential for substantial inter-subject variability from both genetic and non-genetic factors can make it difficult to get a full understanding of impact prior the initiation of pivotal studies [12,21,85].
In theory, developing a strategy to prevent or manage undesirable immune responses would start by considering the same factors that were pertinent during the design stage (i.e., treatment, patient, and product-related factors). However, since the indication, target, population and drug product itself are established by this point, efforts typically focus on changes to the dosing regimen, or the use of pharmacological solutions that treat, mitigate or prevent these outcomes.
Dosing Regimen
Where ADA development is the chief concern, two of the more common approaches for modifying the dosing regimen are to either decrease the interval between doses, or to increase the dose itself, with the ultimate goal of inducing immune tolerance to the drug. These approaches, collectively referred to as dose intensification, have been particularly effective in restoring responses to tumor necrosis factor (TNF)-α blockers (e.g., adalimumab and infliximab) in conditions like Crohn’s disease, where secondary loss of therapeutic response due to ADA can be up to 61% in TNF-α-treatment naïve patients [86]. However, it should be noted that high-dose/high-frequency regimens do not always overcome ADA responses, and since these strategies can increase the risk of any toxic effects that are already known to be associated with the product, any such strategy should be performed using a well-defined safety monitoring plan and stopping rules [13,87].
The development of acute infusion reactions can also be managed by changing the dosing approach. This may include slowing or suspending the infusion, or using a lower, “priming dose,” when a high “first dose effect” is anticipated. The latter has been used extensively for T-cell engaging bispecific antibodies, which are particularly susceptible to this effect [88].
Pharmacological Treatment of Symptoms
Pharmacological management of symptoms is also common, and several groups including the National Comprehensive Cancer Network, American Society of Clinical Oncology, Society for Immunotherapy of Cancer, and European Society for Medicinal Oncology have issued detailed clinical guidelines for treating immune-related adverse events resulting from immunotherapy treatment [89–92]. These guidelines are broken down by organ system-based toxicity (e.g., dermatologic, gastroenterological, endocrine, and respiratory), frequency of presentation in the clinic, and severity according to the current Common Terminology Criteria for Adverse Events Grading System (v5.0 published in November 2017) from the US National Cancer Institute. In general, corticosteroids (e.g., prednisone) are recommended as the mainstay for low-severity outcomes (Grades 1–2). Other immunosuppressants are recommended for higher grades, or where corticosteroid treatment is not effective. While the general goal is to continue therapy, the drug may be withheld for Grades 2–4 until the event has resolved, or the decision to discontinue has been made [93].
Such guidelines are extremely useful, but it should be emphasized that the clinical approach for a new product should ultimately be determined on a case-by-case basis. For example, corticosteroids may not be the preferred treatment for all complications associated with CAR T-cell therapies since high doses have been reported to impair T-cell function, which is necessary for clinical effectiveness. Instead, interleukin-6 blockade via tocilizumab has often been used to manage CRS mediated by CAR T-cells, and in 2017, the FDA extended the approval of tocilizumab to include this indication [94]. Likewise, combination treatment with multiple biologic disease-modifying antirheumatic drugs (DMARDs) like tocilizumab or abatacept can cause severe immunosuppression and substantially increase the risk of life-threatening infections as a result; therefore, a conventional DMARD (e.g., hydroxychloroquine and methotrexate) may be a better choice than a second biologic when considering combination therapy with these drugs [95]. Thus, the decision to use a particular pharmacological intervention should be made following careful consideration of the mechanism of action of the biologic, the mechanism of the immune response, and the overall risk–benefit profile of the therapy.
Use of Co-Medications and Pre-Medications
Many products also take a more proactive approach to managing unintended immune responses by permitting or requiring the use of pre- and co-medications. For example, premedication with antihistamines (anti-H1 ± anti-H2) is allowed at physician’s discretion with infliximab due to the risk for severe IgE-mediated anaphylaxis [96,97], and both infliximab and golimumab require co-medication with methotrexate in treating rheumatoid arthritis [98]. These approaches are especially common among high-risk products, and those where the development of ADAs is known to impact the overall success of the treatment.
Manufacturing Changes
It may be that some change to the drug product itself is needed to gain product approval, to ensure long-term competitiveness in the marketplace, or to address changes in the safety profile of the product. Among these modifications, some are more feasible than others. For example, reformulation of the active pharmaceutical ingredient (e.g., to deimmunize or humanize the drug) can lead to considerable rework. Other examples include interventions to reduce the formation of aggregates, or to address issues with the integrity of a container closure system.
The latter became an issue in the late 1990s/early 2000s when an increase in the incidence of a rare disorder known as pure red cell aphasia (PRCA) was noted among patients receiving a synthetic form of erythropoietin, known as epoetin alfa. These cases were determined to be due to the cross-reaction of NAbs with the endogenous protein, but the underlying reason for the sudden increase was not clear until it was noticed that most of the cases were occurring outside the USA. This fact eventually implicated a manufacturing change in 1998 that saw the original stabilizer, human serum albumin, swapped out for polysorbate 80 in most countries (except the USA). Polysorbate 80 was later found to contribute to immunogenicity by reacting with leachates from the uncoated rubber stoppers used in the product (a prefilled syringe) [99]. In April 2003, this issue was resolved when the uncoated stoppers were replaced with stoppers containing a fluororesin coating that minimized interactions with the drug, and substantially reduced the incidence of PRCA [100,101].
Conclusion
The impact of immunogenicity and related immune responses on clinical safety and efficacy can be substantial. While responses are not generally adverse, they do have the potential to result in adverse events, including death, cause ambiguity in study interpretation, and halt a single study or an entire program. The potential for immune-mediated outcomes should be given adequate consideration beginning at the design stage; however, clinical management of these responses is also very common, and there are many options available to drug developers and clinicians. Designing, implementing, and presenting a successful risk assessment and integrated management approach for biotherapeutics requires careful analysis, utilizing all available information regarding the nature, impact, and significance of individual responses, as well as the overall risk–benefit profile of the program. However, the inherent value in these products has been demonstrated time and time again for those that are willing to navigate these challenges, and their potential for treating complex and rare diseases will likely only increase in the future.
Acknowledgments
The authors would like to thank Ambrose Sanjeewa Goonasekera, Gary Freeman, Beth Ann Klotz, Matthew Barthel, and Julie Hopkins at Medpace for their critical review during the development of this manuscript.
Disclosures
All authors are full-time employees of Medpace, Inc.
References
- 1. Wan H. An overall comparison of small molecules and large biologics in ADME testing. ADMET & DMPK 2016; 4(1): 1–22. [Google Scholar]
- 2. Dimitrov DS. Therapeutic proteins. Methods in Molecular Biology 2012; 899: 1–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. The Alliance for Regenerative Medicine. Available Products [Internet]. (https://alliancerm.org/available-products/).
- 4. Office of Tissues and Advanced Therapies. Approved cellular and gene therapy products [Internet], 2019. (https://www.fda.gov/vaccines-blood-biologics/cellular-gene-therapy-products/approved-cellular-and-gene-therapy-products).
- 5. US Food and Drug Administration (FDA). FDA Statement: Statement from FDA Commissioner Scott Gottlieb, M.D. and Peter Marks, M.D., Ph.D., Director of the Center for Biologics Evaluation and Research on new policies to advance development of safe and effective cell and gene therapies [Internet], 2019. (https://www.fda.gov/news-events/press-announcements/statement-fda-commissioner-scott-gottlieb-md-and-peter-marks-md-phd-director-center-biologics).
- 6. Lu R, et al. Development of therapeutic antibodies for the treatment of diseases. J Biomed Sci 2020; 27: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Strand V, et al. Immunogenicity of biologics in chronic inflammatory diseases: a systematic review. BioDrugs 2017; 31(4): 299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Doevendans E, Schellekens H. Immunogenicity of innovative and biosimilar monoclonal antibodies. Antibodies (Basel) 2019; 8(1): 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tourdot S, et al. 10Th European immunogenicity platform open symposium on immunogenicity of biopharmaceuticals. MAbs 2020; 12(1): 1725369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Novartis Pharmaceuticals. Kymriah™ (tisagenlecleucel): highlights of prescribing information [Internet], 2018. (https://www.fda.gov/files/vaccines%2C%20blood%20%26%20biologics/published/Package-Insert---KYMRIAH.pdf).
- 11. US Food and Drug Administration (FDA). Guidance for industry: immunogenicity testing of therapeutic protein products – developing and validating assays for anti-drug antibody detection. [Internet], 2019. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-testing-therapeutic-protein-products-developing-and-validating-assays-anti-drug).
- 12. European Medicines Agency (EMA). Guideline on immunogenicity assessment of therapeutic proteins. [Internet], 2017. (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-immunogenicity-assessment-therapeutic-proteins-revision-1_en.pdf).
- 13. US Food and Drug Administration (FDA). Guidance for industry: immunogenicity assessment for therapeutic protein products. [Internet], 2014. (https://www.fda.gov/regulatory-information/search-fda-guidance-documents/immunogenicity-assessment-therapeutic-protein-products).
- 14. Mahanty S, Prigent A, Garraud O. Immunogenicity of infectious pathogens and vaccine antigens. BMC Immunology 2015; 16: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. European Medicines Agency (EMA). Guideline on immunogenicity assessment of monoclonal antibodies intended for in vivo clinical use. [Internet], 2012. (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-immunogenicity-assessment-monoclonal-antibodies-intended-vivo-clinical-use_en.pdf).
- 16. Warrington R, et al. An introduction to immunology and immunopathology. Allergy, Asthma & Clinical Immunology 2011; 7(Suppl): S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Janeway, C. Immunobiology: The Immune System in Health and Disease. 6th ed. New York: Garland Science. [Google Scholar]
- 18. Chaplin DD. Overview of the immune response. The Journal of Allergy and Clinical Immunology 2010; 125(2 Suppl 2): S3–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shankar G, et al. Assessment and reporting of the clinical immunogenicity of therapeutic proteins and peptides-harmonized terminology and tactical recommendations. The American Association of Pharmaceutical Scientists Journal 2014; 16(4): 658–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Baldo BA. Adverse events to monoclonal antibodies used for cancer therapy: focus on hypersensitivity responses. Oncoimmunology 2013; 2(10): e26333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kuriakose A, Chirmule N, Nair P. Immunogenicity of biotherapeutics: causes and association with posttranslational modifications. Journal of Immunology Research 2016; 2016: 1298473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sominanda A, Lundkvist M, Fogdell-Hahn A. Inhibition of endogenous interferon beta by neutralizing antibodies against recombinant interferon beta. Archives of Neurology 2010; 67(9): 1095–1101. [DOI] [PubMed] [Google Scholar]
- 23. Hurtado-Guerrero I, et al. Cross-reactivity of antibodies against interferon beta in multiple sclerosis patients and interference of the JAK-STAT signaling pathway. Scientific Reports 2017; 7: 16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li J, et al. Thrombocytopenia causes by the development of antibodies to thrombopoietin. Blood 2001; 98(12): 3241–3248. [DOI] [PubMed] [Google Scholar]
- 25. Doessegger L, Banholzer ML. Clinical development methodology for infusion-related reactions with monoclonal antibodies. Clinical & Translational Immunology 2015; 4(7): e39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Van der Kolk LE, et al. Complement activation plays a key role in the side-effects of rituximab treatment. British Journal of Haematology 2001; 115(4): 807–811. [DOI] [PubMed] [Google Scholar]
- 27. Wing MG, Lachmann PJ, Compston A. Mechanism of first-dose cytokine-release syndrome by CAMPATH 1-H: involvement of CD16 (FCgammaRIII) and CD11a/CD18 (LFA-1) on NK cells. Journal of Clinical Investigation 1996; 98(12): 2819–2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tyrsin D, et al. From TGN1412 to TAB08: the return of CD28 superagonist therapy to clinical development for the treatment of rheumatoid arthritis. Clinical and Experimental Rheumatology 2016; 34(98): S45–S48. [PubMed] [Google Scholar]
- 29. Eastwood D, et al. Severity of the TGN1412 trial disaster cytokine storm correlated with IL-2 release. British Journal of Clinical Pharmacology 2013; 76(2): 299–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Suntharalingam G, et al. Cytokine storm in a phase I trial of the anti-CD28 monoclonal antibody TGN1412. The New England Journal of Medicine 2006; 355: 1018–1028. [DOI] [PubMed] [Google Scholar]
- 31. Attarwala H. TGN1412: from discovery to disaster. Journal of Young Pharmacists 2010; 2(3): 332–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. European Medicines Agency. Guideline on strategies to identify and mitigate risk for first-in-human clinical trials with investigational medicinal products. [Internet], 2007. (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-strategies-identify-mitigate-risks-first-human-clinical-trials-investigational-medicinal_en.pdf).
- 33. European Medicines Agency. ‘First-in-man’ clinical trial guideline release for public consultation. [Internet], 2007. (https://www.ema.europa.eu/en/news/first-man-clinical-trials-guideline-released-public-consultation).
- 34. US Food Drug Administration. Guidance for industry: Estimating the Maximum Safe Starting Dose in Initial Clinical Trials for Therapeutics in Adult Healthy Volunteers. [Internet], 2005. (http://www.fda.gov/downloads/drugs/guidances/ucm078932.pdf).
- 35. US Food and Drug Administration (FDA). Draft guidance: bispecific antibody development programs. [Internet], 2019. (https://www.fda.gov/media/123313/download).
- 36. US Food and Drug Administration (FDA). Draft guidance: nonclinical safety of the immunotoxic potential of drugs and biologics. [Internet], 2020. (https://www.fda.gov/media/135312/download).
- 37. Eastwood D, et al. Monoclonal antibody TGN1412 trial failure explained by species differences in CD28 expression on CD4+ effector memory T-cells. British Journal of Pharmacology 2010; 161(3): 512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stebbings R, et al. “Cytokine storm” in the phase I trial of monoclonal antibody TGN1412: better understanding the causes to improve preclinical testing of immunotherapeutics. Journal of Immunology 2007; 179(5): 3325–3331. [DOI] [PubMed] [Google Scholar]
- 39. Finco D, et al. Cytokine release assays: current practices and future directions. Cytokine 2014; 66(2): 143–155. [DOI] [PubMed] [Google Scholar]
- 40. Ferbas J, et al. A novel assay to measure B cell responses to keyhole limpet haemocyanin vaccination in health volunteers and subjects with systemic lupus erythematosus. British Journal of Clinical Pharmacology 2013; 76(2): 188–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Wouters OJ, McKee M, Luyten J. Estimated research and development investment needed to bring a new medicine to market, 2009-2018. JAMA 2020; 323(9): 844–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Fogel DB. Factors associated with clinical trials that fail and opportunities for improving the likelihood of success: a review. Contemporary Clinical Trials Communications 2018. 11: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Zurdo J, et al. Early implementation of QbD in biopharmaceutical development: a practical example. BioMed Research International 2015; 2015: 605427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. International Council for Harmonisation (ICH). Pharmaceutical Development Q8(R2). Current Step 4 version [Internet], 2009. (https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf).
- 45. Yi Y, Zang L. Factors influencing biotherapeutic monoclonal antibody aggregation. American Pharmaceutical Review 2018; March. [Google Scholar]
- 46. US Pharmacopeia National Formulary. USP 39-NF 34 residual host cell protein measurement in biopharmaceuticals [Internet]. Rockville, MD: United States Pharmacopeial Convention; 2016. (https://www.usp.org/sites/default/files/usp/document/our-work/biologics/USPNF810G-GC-1132-2017-01.pdf).
- 47. International Council for Harmonisation (ICH). Specifications: test procedures and acceptance criteria for biotechnological/biological products Q6B. Current Step 4 version [Internet], 1999. (https://database.ich.org/sites/default/files/Q6B_Guideline.pdf).
- 48. Goey CH, Alhuthali S, Kontoravdi C. Host cell protein removal from biopharmaceutical preparations: towards the implementation of quality by design. Biotechnology Advances 2018; 36(4): 1223–1237. [DOI] [PubMed] [Google Scholar]
- 49. Krishna M, Nadler SG. Immunogenicity to biotherapeutics – the role of anti-drug immune complexes. Frontiers in Immunology 2016; 7: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mukovozov L, et al. Factors that contribute to the immunogenicity of therapeutic recombinant human proteins. Thrombosis and Haemostasis 2008; 99: 874–882. [DOI] [PubMed] [Google Scholar]
- 51. Singh SK. Impact of product-related factors on immunogenicity of biotherapeutics. Journal of Pharmaceutical Science 2010; 100(2): 354–387. [DOI] [PubMed] [Google Scholar]
- 52. Krishna M. Product-related factors and immunogenicity of biotherapeutics. Journal of Pharmaceutical Innovation 2019. 10.1007/s12247-019-09423-2 [DOI] [Google Scholar]
- 53. Maude SL, et al. Managing cytokine release syndrome associated with novel T cell-engaging therapies. Cancer Journal 2014; 20(2): 119–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bartelds GM, et al. Development of Antidrug Antibodies Against Adalimumab and Association With Disease Activity and Treatment Failure During Long-term Follow-up. JAMA 2011; 305(14): 1460–1480. [DOI] [PubMed] [Google Scholar]
- 55. Quistrebert J, et al. Incidence and risk factors for adalimumab and infliximab anti-drug antibodies in rheumatoid arthritis: a European retrospective multicohort analysis. Seminars in Arthritis and Rheumatism 2018;48(6): 967–975. [DOI] [PubMed] [Google Scholar]
- 56. International Council for Harmonisation (ICH). Preclinical safety evaluation of biotechnology-derived pharmaceuticals S6(R1). Current Step 4 version [Internet], Parent guideline data 16 July 1997 “addendum data 12 June 2011 incorporated at the end of June 2011). (https://database.ich.org/sites/default/files/S6_R1_Guideline_0.pdf).
- 57. Swanson SJ, Bussiere J. Immunogenicity assessment in non-clinical studies. Current Opinion in Microbiology 2012; 15(3): 337–347. [DOI] [PubMed] [Google Scholar]
- 58. US Food and Drug Administration (FDA). Guidance for industry: scientific considerations in demonstrating biosimilarity to a reference product [Internet], 2015. (https://www.fda.gov/media/82647/download).
- 59. European Medicines Agency (EMA). Guideline on similar biological medicinal products containing biotechnology-derived proteins as active substance: non-clinical and clinical issues [Internet], 2014. (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-similar-biological-medicinal-products-containing-biotechnology-derived-proteins-active_en-2.pdf).
- 60. European Medicines Agency (EMA). Guideline on non-clinical and clinical development of similar biological medicinal products containing recombinant human insulin and insulin analogues [Internet], 2015. (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-non-clinical-clinicaldevelopment-similar-biological-medicinal-products-containing_en-0.pdf).
- 61. US Food and Drug Administration (FDA). Draft guidance for industry: clinical immunogenicity consideration for biosimilar and interchangeable insulin products [Internet], 2019. (https://www.fda.gov/media/133014/download).
- 62. European Medicines Agency (EMA). Guideline on comparability of biotechnology-derived medicinal products after a change in the manufacturing process: non-clinical and clinical issue [Internet], 2007. (https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-comparability-biotechnology-derived-medicinal-products-after-change-manufacturing-process_en.pdf).
- 63. US Food and Drug Administration. Guidance for industry: Scientific consideration in demonstrating biosimilarity to a reference product [Internet], 2015. (https://www.fda.gov/media/82647/download).
- 64. European Medicines Agency. Risk-management plans [Internet]. (https://www.ema.europa.eu/en/human-regulatory/marketing-authorisation/pharmacovigilance/risk-management/risk-management-plans#integrated-pdf-version-section).
- 65. US Food and Drug Administration (FDA). Risk evaluation and mitigation strategies I REMS [Internet], 2019. (https://www.fda.gov/drugs/drug-safety-and-availability/risk-evaluation-and-mitigation-strategies-rems).
- 66. International Council for Harmonisation (ICH). The extent of population exposure to assess clinical safety for drugs intended for long-term treatment of non-life-threatening conditions E1. Current Step 4 version [Internet], 1994. (https://database.ich.org/sites/default/files/E1_Guideline.pdf).
- 67. Pineda C, et al. Assessing the immunogenicity of biopharmaceuticals. BioDrugs 2016; 30: 195–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Song S, et al. Understanding the supersensitive anti-drug antibody assay: unexpected high anti-drug antibody incidence and its clinical relevance. Journal of Immunology Research 2016; 2016: 3072586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Nath N, et al. Development of NanoLuc bridging immunoassays for detection of anti-drug antibodies. Journal of Immunological Methods 2017. 450: 17–26. [DOI] [PubMed] [Google Scholar]
- 70. Gorovits B. Antidrug antibody assay validation: industry survey results. The American Association of Pharmaceutical Scientists Journal 2009; 11(1): 133–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang YM, et al. A survey of applications of biological products for drug interference of immunogenicity assays. Pharmaceutical Research 2012; 29(12): 3384–3392. [DOI] [PubMed] [Google Scholar]
- 72. Collet-Brose J, et al. Evaluation of multiple immunoassay technology platforms to select the anti-drug antibody assay exhibiting the most appropriate drug and target tolerance. Journal of Immunology Research 2016; 2016: 5069678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Zhong ZD, et al. Drug target interference in immunogenicity assays: recommendations and mitigation strategies. The American Association of Pharmaceutical Scientists Journal 2017; 19(6): 1564–1575. [DOI] [PubMed] [Google Scholar]
- 74. Partridge MA, et al. Emerging technologies and generic assays for the detection of anti-drug antibodies. Journal of Immunology Research 2016; 2016: 6262383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. International Council for Harmonisation. Organisation of the common technical document for the registration of pharmaceuticals for human use: M4 [Internet]. (https://www.ich.org/page/ctd).
- 76. Jordan D. An overview of the common technical document (CTD) regulatory dossier. Medical Writing 2014; 23(2): 101– 105. [Google Scholar]
- 77. Carter M, Tabaniag J. Chapter 3: Premarket requirements/dossier requirements. In: Fundamentals of international pharmaceutical and biologics regulations. Regulatory Affairs Professionals Society (MD); 2018.
- 78. López-Morales CA, et al. Regulatory Pathway for Licensing Biotherapeutics in Mexico. Front Med (Lausanne) 2018; 5: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Chamberlain P. Effective presentation of immunogenicity risk assessments and related data in regulatory dossiers. Bioanalysis 2019; 11(17): 1581–1592. [DOI] [PubMed] [Google Scholar]
- 80. International Council for Harmonisation (ICH). Revision of M4E guideline on enhancing the format and structure of benefit-risk information in ICH efficacy—M4E(R2). Current Step 4 version [Internet] 2016. (https://database.ich.org/sites/default/files/M4E_R2__Guideline.pdf).
- 81. Chamberlain P. Addressing immunogenicity-related risks in an integrated manner. Regulatory Affairs Pharma 2011; January: 10–15. [Google Scholar]
- 82. Pedras-Vasconcelos J. Integrated summaries of immunogenicity: an FDA reviewer’s wish list. Presented at: EIP symposium. Nov 2017, Lison [Internet]. (https://slideplayer.com/slide/14552262/).
- 83. Joubert MK, et al. Use of in vitro assays to assess immunogenicity risk of antibody-based biotherapeutics. PLoS One 2016; 11(8): e0159328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Groell F, Jordan O, Borchard G. In vitro models for immunogenicity prediction of therapeutic proteins. European Journal of Pharmaceutics and Biopharmaceutics 2018; 130: 128–142. [DOI] [PubMed] [Google Scholar]
- 85. Van Schouwenburg PA, Rispens T, Wolbink GJ. Immunogenicity of anti-TNF biologic therapies for rheumatoid arthritis. Nature Reviews Rheumatology 2013; 9(3): 164–172. [DOI] [PubMed] [Google Scholar]
- 86. Srinivasan A, et al. Anti-TNF re-induction is as effective, simpler, and cheaper compared with dose interval shortening for secondary loss of response in Crohn’s disease. Journal of Crohn’s and Colitis 2018; 12(3): 280–288. [DOI] [PubMed] [Google Scholar]
- 87. Van Brummelen EMJ, et al. Antidrug antibody formation in oncology: clinical relevance and challenges. Oncologist 2016; 21(10): 1260–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Chen X, et al. A modeling framework to characterize cytokine release upon T-cell-engaging bispecific antibody treatment: methodology and opportunities. Clinical and Translational Science 2019; 12: 600–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Thompson JA, et al. Management of immunotherapy-related toxicities, version 1.2019. Journal of the National Comprehensive Cancer Network 2019; 17(3): 255–289. [DOI] [PubMed] [Google Scholar]
- 90. Brahmer JR, et al. Management of immune-related adverse events in patients treated with immune checkpoint inhibitor therapy: American Society of Clinical Oncology Clinical Practice Guideline. Journal of Clinical Oncology 2018; 36(17): 1714–1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Puzanov I, et al. Managing toxicities associated with immune checkpoint inhibitors: consensus recommendations from the Society for Immunotherapy of Cancer (SITC) toxicity management working group. Journal for ImmunoTherapy of Cancer 2017; 5(1): 95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Haanen JBAG, et al. Management of toxicities from immunotherapy: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2017; 28(suppl_4):iv119–142. [DOI] [PubMed] [Google Scholar]
- 93. Trinh S, et al. Management of immune-related adverse events associated with immune checkpoint inhibitor therapy: a minireview of current clinical guidelines. Asia-Pacific Journal of Oncology Nursing 2019; 6(2): 154–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Le RQ, et al. FDA approval summary: tocilizumab for treatment of chimeric antigen receptor T cell-induced severe or life-threatening cytokine release syndrome. Oncologist 2018; 23(8): 943–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Benjamin O, et al. Disease Modifying Anti-Rheumatic Drugs (DMARD). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020 Jan. (https://www.ncbi.nlm.nih.gov/books/NBK507863/).
- 96. Janssen Biotech, Inc. Remicade (infliximab): highlights of prescribing information [Internet], 2013. (https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/103772s5359lbl.pdf).
- 97. Vultaggio A, et al. Anti-infliximab IgE and non-IgE antibodies and induction of infusion-related severe anaphylactic reactions. Allergy 2010; 65(5): 657–661. [DOI] [PubMed] [Google Scholar]
- 98. Janssen Biotech, Inc. Simponi (golimumab): highlights of prescribing information. [Internet], 2019. (http://www.janssenlabels.com/package-insert/product-monograph/prescribing-information/SIMPONI-pi.pdf).
- 99. McKoy JM, et al. Epoetin-associated pure red cell aplasia: past, present, and future considerations. Transfusion 2008; 48(8): 1754–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bennett CL, et al. Pure red-cell aplasia and epoetin therapy. The New England Journal of Medicine 2004; 351: 1403–1408. [DOI] [PubMed] [Google Scholar]
- 101. Boven K, et al. The increased incidence of pure red cell aplasia with an Eprex formulation in uncoated rubber stopper syringes. Kidney International 2005; 67(6): 2346–2353. [DOI] [PubMed] [Google Scholar]