Abstract
We demonstrate the utility of risk stratification for postoperative delirium in geriatric patients and show that postoperative delirium can be prevented in high-risk patients when potentially inappropriate medications (PIMs) (medications that are best avoided in older adults) are avoided. In this case, a 65-year-old woman underwent two debridement procedures with similar presurgical risk for postoperative delirium. There was no risk stratification or preoperative cognitive assessment in the first procedure, she received PIMs and developed postoperative delirium. In the second procedure, PIMs were intentionally avoided and postoperative delirium did not occur. This case supports recent recommendations from the European Society of Anaesthesiology, the American Society of Anesthesiologists and the American Geriatrics Society that providers assess a patient’s cognitive function and delirium risk profile preoperatively to appropriately guide perioperative management.
Keywords: anaesthesia, geriatric medicine, unwanted effects / adverse reactions, delirium, surgery
Background
Delirium is defined as an acute change in mental status characterised by reduced awareness of the environment, attention disturbance and fluctuating course.1 It is a common postoperative complication, occurring in half of geriatric surgical patients.2 Postoperative delirium is associated with several short-term and long-term complications, including increased hospital length-of-stay, higher healthcare costs,3 dementia4 and increased mortality.5 Delirium occurs most commonly after cardiac surgery, but is common after many other procedures, including orthopaedic, general and spine surgeries.6
Postoperative delirium is the result of an interplay between predisposing (patient-related) and precipitating (perioperative) risk factors.7 8 Common predisposing factors include pre-existing cognitive impairment, advanced age and frailty.9 10 Medical comorbidities associated with increased risk include presence of infection, inadequately controlled pain, alcohol use, sleep deprivation or disturbance, renal failure and depression. Precipitating factors include type and duration of surgery, blood loss and specific perioperative medications administered.7 Because half of the delirium cases that occur in the hospital are thought to be preventable,11 experts recommend preoperative screening for delirium risk factors to identify patients at highest risk. For example, the American Geriatrics Society recommends, “Healthcare professionals caring for surgical patients should perform a preoperative assessment of delirium risk factors, including age greater than 65 years, chronic cognitive decline or dementia, poor vision or hearing, severe illness, and presence of infection”,12 and suggests that patients with two or more risk factors should be considered at high risk for postoperative delirium. In addition, the American Society of Anesthesiology Perioperative Neurotoxicity Working Group recommends, “Baseline cognition should be objectively evaluated with a brief screening tool during preoperative evaluation in all patients over the age of 65 and in any patient with risk factors for preexisting cognitive impairment.”13
While there is a paucity of evidence available to implicate specific anaesthetic agents or modalities in the development of postoperative delirium, current guidelines strongly recommend minimising the administration of deliriogenic medications in the perioperative period, particularly in high-risk geriatric patients.7 13 The American Geriatrics Society Beers Criteria is an explicit list of PIMs that should be avoided in geriatric patients whenever possible.14 The American Geriatrics Society Beers Criteria recommends against use of various medications that are commonly administered perioperatively, including benzodiazepines, anticholinergics, antipsychotics, H2-receptor antagonists and meperidine.
Here, we present a case of postoperative delirium following spine surgery in which the patient presented preoperatively with multiple risk factors and received several intraoperative potentially inappropriate medications (PIMs). In this self-controlled case report involving two operating room procedures within 4 days, we report improved postoperative neurocognitive recovery following PIM avoidance. This case illustrates that preoperative risk factors for postoperative delirium should be assessed formally and should guide perioperative care and medication administration in geriatric surgical patients. Because pre-existing cognitive impairment is one of the most important risk factors, formal assessment includes using a validated, brief cognitive screening tool such as the Mini-Cog.13 15 16
Case presentation
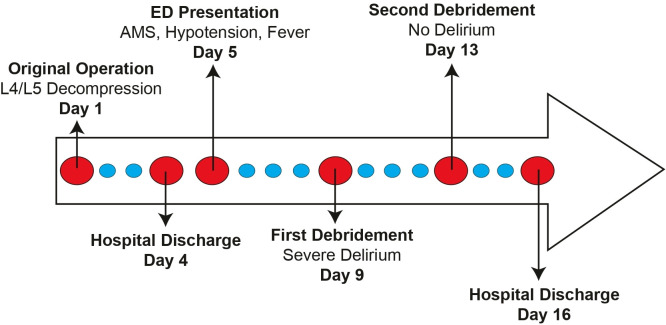
A 65-year-old woman presented to the emergency department on postoperative day 4 following an elective open decompression with posterior spinal fusion at the fourth and fifth vertebrae for lumbar stenosis with neurogenic claudication. The case timeline is depicted in figure 1. The patient presented with severe back pain and bloody drainage from her surgical site. Her medical history included congestive heart failure, stroke, chronic kidney disease, diabetes mellitus, hypertension, obesity (body mass index 34 kg/m2), peripheral neuropathy and insomnia. Her home medications included metformin, trazodone, labetalol, doxazosin, beclomethasone, albuterol, atorvastatin and gabapentin. The patient did not develop postoperative delirium after her initial spine surgery. She was taking oral hydromorphone for postoperative pain control. She reported no known history of prior anaesthesia complications.
Figure 1.
Case timeline. AMS, altered mental status; ED, emergency department; L, lumbar.
On presentation to the emergency department, she met three of four systemic inflammatory response syndrome (SIRS) criteria, as she was found to be febrile, tachycardic, hypotensive and had a leukocytosis of 18×109/L (up from 12×109/L the day prior). The patient was also anaemic with a haemoglobin of 100 g/L. The patient’s caregiver reported she was becoming more confused and forgetful. She was admitted for evaluation and management of SIRS.
The patient’s mental status improved by hospital day 2. She had mild intermittent confusion overnight, but was alert and oriented during the day. On hospital day 5, due to continued drainage from the surgical site and persistent leukocytosis, the decision was made to perform a surgical debridement. The incision was reopened, the surgical site was cleaned, and a wound vacuum was placed. The surgery team planned to return the patient to the operating room 2 days later for a second debridement, removal of the wound vacuum and closure of the surgical site. While the procedure was without complication, the patient received multiple perioperative PIMs, including midazolam, metoclopramide, glycopyrrolate and dexamethasone (table 1). When the patient arrived in the post-anaesthesia care unit, she developed severe hyperactive delirium, including altered mental status, impulsivity, agitation and paranoia. She continually attempted to get out of bed, pulled out her intravenous line and made irrational accusations. Multiple providers were required to safely transport the patient from the post-anaesthesia care unit to the ward.
Table 1.
Medications administered during surgical debridements
| Medications administered in debridement #1 | Medications administered in debridement #2 |
| Midazolam 2 mg* | Ketamine 50 mg |
| Fentanyl 250 µg | Fentanyl 100 µg |
| Lidocaine 2% 80 mg | Lidocaine 2% 60 mg |
| Propofol 170 mg | Propofol 100 mg |
| Rocuronium 40 mg | Rocuronium 50 mg |
| Dexamethasone 4 mg* | Sugammadex 400 mg |
| Metoclopramide 10 mg* | Dexmedetomidine 50 µg |
| Ondansetron 4 mg | Ondansetron 4 mg |
| Neostigmine 4 mg | Vasopressin 2 units |
| Cefazolin 2 g | Cefazolin 2 g |
| Phenylephrine 200 µg | Phenylephrine 594 µg |
| Glycopyrrolate 0.6 mg* |
*Metoclopramide is included in 2019 American Geriatrics Society Beers Criteria.13 Although midazolam, dexamethasone and glycopyrrolate are not specifically listed by name, benzodiazepines, steroids and anticholinergic medications are listed.
On the ward, the patient permitted an anaesthesiologist to place a new intravenous catheter. However, immediately after placement, she intentionally pulled it out and threw it across the room. The patient was then placed in four-point restraints, public safety was called, and intramuscular haloperidol was administered. The patient remained confused overnight, but returned to her cognitive baseline on postoperative day 1, becoming calm and cooperative with hospital staff. She did not have any focal neurological deficits, and her agitation subsided, so no brain imaging was performed.
Four days after the first wound debridement, the patient no longer met the criteria for SIRS and was afebrile, normotensive and normocardic. While her white cell count had decreased, it remained slightly elevated at 10.8×109/L. The patient also remained anaemic with a haemoglobin of 80 g/L and complained of severe back pain. At this point, the surgery team decided to proceed with the second debridement as planned. During this procedure, her anaesthesiology team intentionally avoided midazolam, metoclopramide and dexamethasone (table 1). To avoid cholinergic side effects, neuromuscular blockade was reversed with sugammadex, compared with standard reversal with neostigmine and glycopyrrolate. Her postoperative recovery was uneventful, and no postoperative delirium was observed.
Outcome and follow-up
No changes in mental status, fluctuations in awareness or episodes of inattention were recognised after her second surgical debridement and she was discharged home 3 days later (postoperative day 16 from her original spinal surgery). Three months postoperatively, the patient was recovering as expected. Her incision was well healed with no further concerns of drainage or infection. She reported no subjective cognitive deficits in the 3 months following hospital discharge. Unfortunately, the patient was found deceased in her home 6 months after hospital discharge. Her passing was attributed to complications from stage 4 chronic kidney disease and diabetes mellitus.
Discussion
Risk factors for postoperative delirium are well studied and multiple evidence-based prediction and prevention guidelines have been developed.13 17–20 However, many anaesthesia providers remain unaware of these recommendations.21
Multiple factors may have contributed to this patient’s postoperative delirium. The patient presented to the emergency department on postoperative day 4 from her original spine surgery with SIRS, a risk factor for postoperative central nervous system (CNS) dysfunction22 and delirium.23 SIRS induces prolonged altered mental status and sensitises the brain to future insults, even after other clinical signs have disappeared.24 In animal models, SIRS induces prolonged neuroinflammation, which persists months after systemic inflammation resolves.25
The American Geriatrics Society recommends preoperative screening for delirium risk factors: poor vision, severe illness, cognitive impairment and frailty.12 It is recommended that preoperative cognitive status is assessed via validated screening instruments such as the Mini-Cog.7 12 13 While various screening tools have been developed,26 a recent systematic review found that existing prediction models show inadequate predictive capabilities, and there is a need to develop robust models of delirium prediction.27 This patient had many predisposing risk factors for postoperative delirium. In addition to her age, her overall burden of comorbid conditions is a risk factor. She had chronic kidney disease, a sleep disorder and a previous stroke—all of which increase risk for postoperative delirium.28 Poorly controlled pain, as occurred in this case, can also precipitate postoperative delirium.29 In addition, while the patient was not overtly delirious on presentation, her caregiver did report increasing confusion. Various forms of preoperative cognitive impairment (mild cognitive impairment, dementia, delirium, etc) are strong risk factors for postoperative delirium.9 Thus, the patient’s altered mental status on presentation put her at higher risk for subsequent postoperative delirium.
PIMs such as midazolam and dexamethasone are among the 20 most commonly used medications by anaesthesiologists.30 While guidelines recommend avoiding these medications in geriatric patients,13 it was recently reported that anaesthesiologists are often unaware of these guidelines,21 suggesting that education is needed to change standard practice.
There is a lack of high-quality evidence to support particular anaesthetic techniques for the prevention of postoperative delirium. There is no consensus on whether there is a difference in the occurrence of delirium following inhalational versus intravenous anaesthetic agents, nor is there evidence to support general versus regional techniques.31 32 Similarly, there is conflicting data regarding the use of intraoperative processed electroencephalogram monitoring to reduce the risk of postoperative delirium.33–36 Despite limited prospective evidence of benefit from the avoidance of PIMs (as defined by the American Geriatrics Society Beers Criteria), most guidelines on the prevention of postoperative delirium strongly recommend avoiding them.7 13 17 This patient received multiple PIMs during her first debridement: metoclopramide, midazolam (a benzodiazepine) and dexamethasone (a steroid). The patient also received glycopyrrolate (an anticholinergic). Although glycopyrrolate has minimal CNS penetrance, it does cross the blood–brain barrier to a small extent,37 and the 2019 American Geriatrics Society Beers Criteria recommends avoiding anticholinergic medications. All of these medications have relatively short half-life elimination times (1–5 hours), so it is unclear if the resolution of the patient’s delirium (on postoperative day 1) coincided with medication clearance.
It is worth noting that several clinical variables changed between debridements, making it challenging to definitively ascribe the patient’s lack of postoperative delirium after the second surgery to the avoidance of PIMs. For example, the patient no longer met the SIRS criteria, which may have put her at a lower risk for delirium. However, the patient was anaemic and continued to complain of back pain, both of which are risk factors for postoperative delirium.38 39 Furthermore, by the second debridement, the patient had been hospitalised for 9 days. Length-of-hospital stay is associated with delirium.40 The challenge of assigning relative value to risk factors for postoperative delirium further emphasises the need for further research of the role of perioperative PIMs in postoperative delirium.
The anaesthetic team for the second debridement had the advantage of knowing the patient’s previous postoperative course. The patient was administered a one-time subanaesthetic dose of intraoperative ketamine. Ketamine attenuates the early postoperative inflammatory response,41 and is neuroprotective in vitro.42 In small clinical trials, subanaesthetic doses of ketamine have been associated with decreased postoperative delirium and less postoperative cognitive dysfunction.43 44 While a subsequent large randomised trial showed no difference in postoperative delirium after ketamine administration,45 there is still debate as to whether this medication provides a degree of neuroprotection or plays a role in preventing postoperative delirium.46 The patient was administered intraoperative dexmedetomidine, which has been shown to reduce the incidence of postoperative delirium in the intensive care unit, when compared with other sedatives.47–49
This case provides anecdotal evidence that avoiding PIMs may reduce postoperative delirium. A limitation of our case report is that the preoperative risk for postoperative delirium may not have been equal between the two surgeries, as the patient’s infection likely improved following the first debridement. Nevertheless, we agree with recent guidelines recommending preoperative cognitive screening for geriatric surgical patients, risk stratification and the avoidance of the American Geriatrics Society Beers Criteria PIMs in high-risk patients.7 13 17 32
Learning points.
Postoperative delirium is common in geriatric surgical patients.
Postoperative delirium is associated with increased hospital length-of-stay, higher cost, dementia and increased mortality.
Preoperative cognitive screening can identify patients who may benefit by avoidance of the American Geriatric Society Beers Criteria potentially inappropriate medications.
Acknowledgments
The authors would like to thank Marie Kane for her assistance with manuscript formatting and editing.
Footnotes
Contributors: The work was supervised by KS. The patient was under the care of KS. All authors shared in the analysis and interpretation of findings. The report was written by KGB, ATN, BMT and KS. All authors give final approval for the version submitted and agree to be accountable for all aspects of the work. KGB and ATN should be considered co-first authors as they shared equally in all aspects of this report.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.American Psychiatric Association . Diagnostic and statistical manual of mental disorders. 5th ed. Arlington, VA, 2013. [Google Scholar]
- 2.Dasgupta M, Dumbrell AC. Preoperative risk assessment for delirium after noncardiac surgery: a systematic review. J Am Geriatr Soc 2006;54:1578–89. 10.1111/j.1532-5415.2006.00893.x [DOI] [PubMed] [Google Scholar]
- 3.Leslie DL, Inouye SK. The importance of delirium: economic and societal costs. J Am Geriatr Soc 2011;59 Suppl 2:S241–3. 10.1111/j.1532-5415.2011.03671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lingehall HC, Smulter NS, Lindahl E, et al. Preoperative cognitive performance and postoperative delirium are independently associated with future dementia in older people who have undergone cardiac surgery: a longitudinal cohort study. Crit Care Med 2017;45:1295–303. 10.1097/CCM.0000000000002483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kat MG, Vreeswijk R, de Jonghe JFM, et al. Long-Term cognitive outcome of delirium in elderly hip surgery patients. A prospective matched controlled study over two and a half years. Dement Geriatr Cogn Disord 2008;26:1–8. 10.1159/000140611 [DOI] [PubMed] [Google Scholar]
- 6.Berian JR, Zhou L, Russell MM, et al. Postoperative delirium as a target for surgical quality improvement. Ann Surg 2018;268:93–9. 10.1097/SLA.0000000000002436 [DOI] [PubMed] [Google Scholar]
- 7.Hughes CG, Boncyk CS, Culley DJ, et al. American Society for enhanced recovery and perioperative quality initiative joint consensus statement on postoperative delirium prevention. Anesth Analg 2020;130:1572–90. 10.1213/ANE.0000000000004641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Inouye SK, Charpentier PA. Precipitating factors for delirium in hospitalized elderly persons. predictive model and interrelationship with baseline vulnerability. JAMA 1996;275:852–7. [PubMed] [Google Scholar]
- 9.Iamaroon A, Wongviriyawong T, Sura-Arunsumrit P, et al. Incidence of and risk factors for postoperative delirium in older adult patients undergoing noncardiac surgery: a prospective study. BMC Geriatr 2020;20:40. 10.1186/s12877-020-1449-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown CH, Max L, LaFlam A, et al. The association between preoperative frailty and postoperative delirium after cardiac surgery. Anesth Analg 2016;123:430–5. 10.1213/ANE.0000000000001271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hshieh TT, Yang T, Gartaganis SL, et al. Hospital elder life program: systematic review and meta-analysis of effectiveness. Am J Geriatr Psychiatry 2018;26:1015–33. 10.1016/j.jagp.2018.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.American Geriatrics Society Expert Panel on Postoperative Delirium in Older Adults . Postoperative delirium in older adults: best practice statement from the American geriatrics Society. J Am Coll Surg 2015;220:136–48. 10.1016/j.jamcollsurg.2014.10.019 [DOI] [PubMed] [Google Scholar]
- 13.Berger M, Schenning KJ, Brown CH, et al. Best practices for postoperative brain health: recommendations from the fifth International perioperative neurotoxicity Working group. Anesth Analg 2018;127:1406–13. 10.1213/ANE.0000000000003841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel . American geriatrics Society 2019 updated AGS beers Criteria® for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67:674–94. 10.1111/jgs.15767 [DOI] [PubMed] [Google Scholar]
- 15.Schenning KJ, Deiner SG. Postoperative delirium in the geriatric patient. Anesthesiol Clin 2015;33:505–16. 10.1016/j.anclin.2015.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tiwary N, Treggiari MM, Yanez ND. Agreement between the mini-cog in the preoperative clinic and on the day of surgery and association with postanesthesia care unit delirium: a cohort study of cognitive screening in older adults. Anesth Analg 2020;132. 10.1213/ANE.0000000000005197 [DOI] [PubMed] [Google Scholar]
- 17.Mohanty S, Rosenthal RA, Russell MM, et al. Optimal perioperative management of the geriatric patient: a best practices guideline from the American College of surgeons NSQIP and the American geriatrics Society. J Am Coll Surg 2016;222:930–47. 10.1016/j.jamcollsurg.2015.12.026 [DOI] [PubMed] [Google Scholar]
- 18.Inouye SK, Bogardus ST, Charpentier PA, et al. A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999;340:669–76. 10.1056/NEJM199903043400901 [DOI] [PubMed] [Google Scholar]
- 19.Inouye SK, Bogardus ST, Baker DI, et al. The hospital elder life program: a model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital elder life program. J Am Geriatr Soc 2000;48:1697–706. 10.1111/j.1532-5415.2000.tb03885.x [DOI] [PubMed] [Google Scholar]
- 20.Aldecoa C, Bettelli G, Bilotta F, et al. European Society of Anaesthesiology evidence-based and consensus-based guideline on postoperative delirium. Eur J Anaesthesiol 2017;34:192–214. 10.1097/EJA.0000000000000594 [DOI] [PubMed] [Google Scholar]
- 21.Deiner S, Fleisher LA, Leung JM, et al. Adherence to recommended practices for perioperative anesthesia care for older adults among US anesthesiologists: results from the ASA Committee on geriatric Anesthesia-Perioperative brain health Initiative ASA member survey. Perioper Med 2020;9:6. 10.1186/s13741-020-0136-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alam A, Hana Z, Jin Z, et al. Surgery, neuroinflammation and cognitive impairment. EBioMedicine 2018;37:547–56. 10.1016/j.ebiom.2018.10.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van den Boogaard M, Kox M, Quinn KL, et al. Biomarkers associated with delirium in critically ill patients and their relation with long-term subjective cognitive dysfunction; indications for different pathways governing delirium in inflamed and noninflamed patients. Crit Care 2011;15:R297. 10.1186/cc10598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Singer BH, Newstead MW, Zeng X, et al. Cecal ligation and puncture results in long-term central nervous system myeloid inflammation. PLoS One 2016;11:e0149136. 10.1371/journal.pone.0149136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin L, Wu X, Block ML, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia 2007;55:453–62. 10.1002/glia.20467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schenning KJ, Deiner SG. Postoperative delirium: a review of risk factors and tools of prediction. Curr Anesthesiol Rep 2015;5:48–56. 10.1007/s40140-014-0086-1 [DOI] [Google Scholar]
- 27.Lindroth H, Bratzke L, Purvis S, et al. Systematic review of prediction models for delirium in the older adult inpatient. BMJ Open 2018;8:e019223. 10.1136/bmjopen-2017-019223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Otomo S, Maekawa K, Goto T, et al. Pre-Existing cerebral infarcts as a risk factor for delirium after coronary artery bypass graft surgery. Interact Cardiovasc Thorac Surg 2013;17:799–804. 10.1093/icvts/ivt304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahanna-Gabrielli E, Schenning KJ, Eriksson LI, et al. State of the clinical science of perioperative brain health: report from the American Society of Anesthesiologists brain health Initiative Summit 2018. Br J Anaesth 2019;123:464–78. 10.1016/j.bja.2019.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodriquez LI, Smaka TJ, Mahla M, et al. Default drug doses in anesthesia information management systems. Anesth Analg 2017;125:255–60. 10.1213/ANE.0000000000001611 [DOI] [PubMed] [Google Scholar]
- 31.Miller D, Lewis SR, Pritchard MW, et al. Intravenous versus inhalational maintenance of anaesthesia for postoperative cognitive outcomes in elderly people undergoing non-cardiac surgery. Cochrane Database Syst Rev 2018;8:Cd012317. 10.1002/14651858.CD012317.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Velkers C, Berger M, Gill SS, et al. Association between exposure to general versus regional anesthesia and risk of dementia in older adults. J Am Geriatr Soc 2021;69:58–67. 10.1111/jgs.16834 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Radtke FM, Franck M, Lendner J, et al. Monitoring depth of anaesthesia in a randomized trial decreases the rate of postoperative delirium but not postoperative cognitive dysfunction. Br J Anaesth 2013;110 Suppl 1:i98–105. 10.1093/bja/aet055 [DOI] [PubMed] [Google Scholar]
- 34.Chan MTV, Cheng BCP, Lee TMC, et al. BIS-guided anesthesia decreases postoperative delirium and cognitive decline. J Neurosurg Anesthesiol 2013;25:33–42. 10.1097/ANA.0b013e3182712fba [DOI] [PubMed] [Google Scholar]
- 35.MacKenzie KK, Britt-Spells AM, Sands LP, et al. Processed electroencephalogram monitoring and postoperative delirium: a systematic review and meta-analysis. Anesthesiology 2018;129:417–27. 10.1097/ALN.0000000000002323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wildes TS, Mickle AM, Ben Abdallah A, et al. Effect of electroencephalography-guided anesthetic administration on postoperative delirium among older adults undergoing major surgery: the engages randomized clinical trial. JAMA 2019;321:473–83. 10.1001/jama.2018.22005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proakis AG, Harris GB. Comparative penetration of glycopyrrolate and atropine across the blood--brain and placental barriers in anesthetized dogs. Anesthesiology 1978;48:339–44. 10.1097/00000542-197805000-00007 [DOI] [PubMed] [Google Scholar]
- 38.Joosten E, Lemiengre J, Nelis T, et al. Is anaemia a risk factor for delirium in an acute geriatric population? Gerontology 2006;52:382–5. 10.1159/000095126 [DOI] [PubMed] [Google Scholar]
- 39.Nie H, Zhao B, Zhang Y-Q, et al. Pain and cognitive dysfunction are the risk factors of delirium in elderly hip fracture Chinese patients. Arch Gerontol Geriatr 2012;54:e172–4. 10.1016/j.archger.2011.09.012 [DOI] [PubMed] [Google Scholar]
- 40.Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age Ageing 2014;43:326–33. 10.1093/ageing/afu022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dale O, Somogyi AA, Li Y, et al. Does intraoperative ketamine attenuate inflammatory reactivity following surgery? A systematic review and meta-analysis. Anesth Analg 2012;115:934–43. 10.1213/ANE.0b013e3182662e30 [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Jing W, Hang YN. Glutamate-Induced c-Jun expression in neuronal PC12 cells: the effects of ketamine and propofol. J Neurosurg Anesthesiol 2008;20:124–30. 10.1097/ANA.0b013e3181667c27 [DOI] [PubMed] [Google Scholar]
- 43.Hudetz JA, Iqbal Z, Gandhi SD, et al. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiol Scand 2009;53:864–72. 10.1111/j.1399-6576.2009.01978.x [DOI] [PubMed] [Google Scholar]
- 44.Hudetz JA, Patterson KM, Iqbal Z, et al. Ketamine attenuates delirium after cardiac surgery with cardiopulmonary bypass. J Cardiothorac Vasc Anesth 2009;23:651–7. 10.1053/j.jvca.2008.12.021 [DOI] [PubMed] [Google Scholar]
- 45.Avidan MS, Maybrier HR, Abdallah AB, et al. Intraoperative ketamine for prevention of postoperative delirium or pain after major surgery in older adults: an international, multicentre, double-blind, randomised clinical trial. Lancet 2017;390:267–75. 10.1016/S0140-6736(17)31467-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hudetz JA, Pagel PS. Neuroprotection by ketamine: a review of the experimental and clinical evidence. J Cardiothorac Vasc Anesth 2010;24:131–42. 10.1053/j.jvca.2009.05.008 [DOI] [PubMed] [Google Scholar]
- 47.Deiner S, Luo X, Lin H-M, et al. Intraoperative infusion of dexmedetomidine for prevention of postoperative delirium and cognitive dysfunction in elderly patients undergoing major elective noncardiac surgery: a randomized clinical trial. JAMA Surg 2017;152:e171505. 10.1001/jamasurg.2017.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Su X, Meng Z-T, Wu X-H, et al. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiac surgery: a randomised, double-blind, placebo-controlled trial. Lancet 2016;388:1893–902. 10.1016/S0140-6736(16)30580-3 [DOI] [PubMed] [Google Scholar]
- 49.Duan X, Coburn M, Rossaint R, et al. Efficacy of perioperative dexmedetomidine on postoperative delirium: systematic review and meta-analysis with trial sequential analysis of randomised controlled trials. Br J Anaesth 2018;121:384–97. 10.1016/j.bja.2018.04.046 [DOI] [PubMed] [Google Scholar]