Abstract
Although caseating granulomas are classically associated with infectious processes, a subgroup of intracranial caseating granulomas without identifiable infectious pathology (ICGN) is described. We aimed to identify clinical, laboratory, radiological and histological markers with potential to distinguish patients with ICGN from those with intracranial caseating granulomas with infectious etiology (ICGI) on tissue microbiological examinations. In a referral hospital setting, we identified 11 patients with ICGNs and 6 patients with ICGI over an 11‐year period. The two groups had similar demographics (other than higher infection risk factors in ICGIs), clinical presentation, serology, location of lesions and cellular composition of the inflammatory infiltrate. Significant differences were the homogenous vs. ring pattern of enhancement on neuroimaging and small (<1 mm) vs. large (>1 mm) area of necrosis on histological examination, in ICGNs and ICGIs, respectively. The dichotomy was best reflected in the response of ICGNs to immunomodulatory and not antimicrobial treatment and the reverse pattern in ICGIs. Based on these findings, we suggest a scheme for the diagnosis of ICGN: (i) caseating granulomas with areas of necrosis predominantly <1 mm in diameter; (ii) absence of an identifiable infectious agent in extensive tissue examinations; and (iii) no clinical and radiological response within 2 months of appropriate antimicrobial treatment.
Keywords: caseating granuloma, CNS autoimmune diseases, tuberculosis
INTRODUCTION
A subset of the granulomatous diseases of the central nervous system (CNS) remains unclassified (11). Among these unclassified granulomas are caseating granulomas in the context of noninfectious pathologies 3, 5, 12. We recently described intracranial caseating granulomas without identifiable infectious pathology (ICGNs) and reported that immunomodulation therapy is often effective in their treatment (4), suggesting that an immune‐mediated process may underlie these pathologies. A delay in starting appropriate treatment in these patients may result in progression and irreversible neurological impairment (4). Given the considerable difference in treatment of ICGNs vs. intracranial caseating granulomas with infectious etiology (ICGIs), an early distinction between the two pathologies is an essential component of the management of these patients.
The differentiation of ICGNs from ICGIs is often delayed, because both histological and time‐consuming microbiological studies are required before identifying a caseating granuloma as noninfectious (4). Even then, it is unclear if negative microbiological examinations reflect a true absence of infection or represent a false‐negative laboratory finding. Clinical, laboratory and radiological markers with potential to distinguish ICGNs from their infectious counterparts are therefore necessary to expedite the recognition and improve the management of these patients. We aimed to address this issue by comparing the clinical, laboratory, radiological and histological markers between patients with ICGN and ICGI using the eventual response to treatment as the gold standard for diagnosis.
METHODS
We searched the pathology database of St. Michael's Hospital, Toronto for “granuloma” and “brain.” The search was limited to biopsies, because the intention of the study was to correlate the pathological findings with the subsequent clinical course and response to treatment. The database search retrieved 59 records during an 11‐year period between January 2000 and December 2010. We reviewed and selected intracranial granulomas with caseating necrosis defined as structureless coagulative necrosis on hematoxylin‐phloxine‐saffron (HPS) staining (17 patients). Retrieved records that showed noncaseating granulomas consisted of foreign body granuloma (19 records), sarcoidosis, granuloma related to an abscess and granuloma related to vasculitis (3 records each), granuloma seen within histiocytosis, xanthogranuloma, lymphomatoid granulomatosis, and granuloma seen within meningioma (2 records each), granuloma surrounding cysticercosis, healing granulation, inflammatory myofibroblastic pseudotumor, osteomyelitis, rheumatoid arthritis related bone lesion and Rathke's cyst (1 record each). We reviewed the histopathological features of the 17 identified caseating granulomas including the cellular composition and the diameter of the area of necrosis. Reticulin staining was not performed.
We next reviewed the presence or absence of an infectious agent in tissue samples. All tissues were examined with an rRNA Amplified Mycobacterium tuberculosis Direct Test (AMTD), Ziehl Neelsen and Auramine Rhodamine stains, one or more staining for fungi (Silver Methenamine, Periodic Acid‐Schiff), Gram stains, other stains for bacteria (Fite, Steiner), and ordinary bacterial, mycobacterial and fungal cultures. Tissue AMTD and mycobacterial cultures were performed in the local public health laboratory on fresh tissue at the time of biopsy.
We defined ICGNs when the said tissue microbiological examinations were negative. Otherwise, patients were included in the ICGI group. One patient had positive fungal culture for Aureobasidium pullulans, a fungus widely found in humid environment with no clinical relevance in immune‐competent individuals 6, 7. This finding was considered contamination and patient was included in the ICGN group. We retrieved and compared the clinical, pathological and radiological data on these two groups of patients. HIV‐positive status, malnutrition, extreme of age, recent measles, alcoholism, malignancy, immunosuppressed state and endemic tuberculosis (TB) exposure were considered as infection risk factors.
Student's t‐test was used for statistical comparisons involving continuous variables. Chi‐square and Fisher's exact tests were used to compare categorical variables. The research ethics board of St. Michael's Hospital approved the study.
RESULTS
We identified 11 patients (6 women and 5 men) with ICGN, as compared with 6 patients (2 men and 4 women) with ICGI. Table 1 compares the demographics, clinical and laboratory data of these patients. There was no significant difference in gender distribution or age on presentation. In both groups, the most common presentation was seizures followed by confusion and headache. While constitutional symptoms of malaise and/or weight loss were seen in two patients with ICGN and one patient with ICGI, none had fever, chills, headache or neck‐stiffness. All patients with ICGI had risk factors for infection (two patients had HIV, one patient had malignancy, one patient was on immunosuppressive treatment and two patients had endemic TB exposure). In contrast, two patients with ICGN had infection risk factor (endemic TB exposure). The difference in infectious risk factors between the two groups was statistically significant (Table 1).
Table 1.
Clinical and laboratory comparison of patients with ICGN vs. ICGI. Abbreviations: ICGI = intracranial caseating granulomas with infectious etiology; ICGN = intracranial caseating granulomas without identifiable infectious pathology; ACE = angiotensin‐converting enzyme; ESR = erythrocyte sedimentation rate; ANA = anti‐nuclear antibody; ANCA = anti‐neutrophil cytoplasmic antibodies; CSF = cerebrospinal fluid; WBC = white blood cells.
| ICGN (%) | ICGI (%) | P‐value | |
|---|---|---|---|
| Age (years) | 0.96 | ||
| Range | 21–69 | 37–66 | |
| Mean | 48 ± 4.9 | 48 ± 4.3 | |
| Male : Female | 5:6 | 2:4 | 0.62 |
| Constitutional symptoms | 2/11 (18) | 1/6 (17) | 0.93 |
| Risk factors | |||
| Total | 2/11 (18) | 6/6 (100) | 0.001 |
| Endemic TB exposure | 2/11 (18) | 2/6 (33) | 0.48 |
| Immunosuppression | 0/11 (0) | 4/6 (67) | 0.001 |
| Laboratory findings | 0.99 | ||
| Elevated ACE | 1/9 (11) | 0/1 | |
| Serum‐corrected Ca2+ (mmol/L) | 2.34 ± 0.03 | 2.34 ± 0.04 | |
| Elevated serum Ca2+ | 0/11 (0) | 0/6 (0) | |
| ESR (mm/h) | 48 ± 9 | 8 | |
| Elevated ESR | 5/6 (83) | 0/1 (0) | |
| Positive ANA | 0/7 (0) | 0/1 (0) | |
| Positive ANCA | 0/6 (0) | Not tested | |
| CSF analysis | |||
| WBC/mm3 | 104 ± 48 | Not tested | |
| Elevated WBC | 5/6 (83) | Not tested | |
| Protein (g/L) | 1.7 ± 0.4 | Not tested | |
| Glucose (mmol/L) | 2.4 ± 0.3 | Not tested | |
| Radiological findings | |||
| Pattern of enhancement | |||
| Homogenous | 7/11 (64) | 0/6 (0) | 0.01 |
| Ring | 3/11 (27) | 5/6 (83) | 0.02 |
| Anatomical location of lesion | |||
| Leptomeningeal | 7/11 (64) | 1/6 (17) | 0.06 |
| Dural | 2/11 (18) | 1/6 (17) | 0.93 |
| Supratentorial intraparenchymal | 10/11 (91) | 5/6 (83) | 0.64 |
| Infratentorial intraparenchymal | 4/11 (36) | 2/6 (33) | 0.90 |
| Spinal cord | 5/11 (45) | 1/6 (17) | 0.23 |
| Hilar lymphadenopathy | 3/11 (27) | 4/6 (67) | 0.28 |
Laboratory data did not distinguish between the two groups (Table 1). Elevated angiotensin‐converting enzyme level was seen in one patient with ICGN and none of the patients with ICGI. All patients had normal corrected calcium levels. Erythrocyte sedimentation rate (ESR) was elevated in five (out of six tested) patients with ICGN. The only patient with ICGI tested for ESR had a normal result. Anti‐nuclear antibody was negative in seven tested patients with ICGN. Anti‐neutrophil cytoplasmic antibodies was negative in six tested patients with ICGN and one tested patient with ICGI. Cerebrospinal fluid (CSF) showed elevated protein concentrations, lymphocytic pleocytosis and normal to mildly decreased glucose concentrations in six tested patients with ICGN. None of patients with ICGI had a CSF analysis. Infectious agents identified in tissue were M. tuberculosis in four patients, Mycobacterium avium complex in one patient and fungal pathogen (histoplasma) in one patient.
Radiological findings were somewhat distinctive (Table 1). In patients with ICGN, contrast‐enhanced magnetic resonance imaging (MRI) or computed tomography (CT) of brain typically showed lesions with homogeneous gadolinium enhancement (7/11, 64%). In three (27%) patients, there was ring pattern of enhancement. In comparison, most patients with ICGI had ring enhancement on MRI or CT of brain (5/6, 83%; Figure 1). This difference in pattern of enhancement was statistically significant. Anatomical location of the lesion was not distinctive. In both group, supratentorial intraparenchymal lesions were most prevalent. While ICGNs showed a tendency to involve leptomeninges as compared with ICGIs, the difference was not statistically significant. Dural, infratentorial intraparenchymal and concurrent spinal cord lesions occurred in a minority of patients in both groups. Hilar lymph nodes were detected in three patients with ICGN and four patients with ICGI. One patient with an infectious etiology also had lung parenchymal infiltration and one also had a lung nodule.
Figure 1.
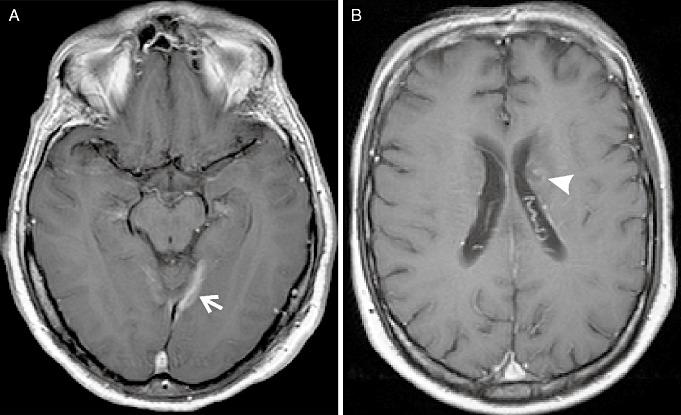
Comparison of radiological findings. Magnetic resonance imagong (MRI) of brain with contrast in a patient with ICGN (A) shows homogeneous enhancement of an intraparenchymal lesion (arrow) as well as the overlaying meninges. Ring enhancement (arrowhead) is seen on MRI of brain with contrast in a patient with ICGI (B). ICGI = intracranial caseating granulomas with infectious etiology; ICGN = intracranial caseating granulomas without identifiable infectious pathology.
We compared the histopathological material in the two groups. Biopsy specimens in patients with ICGN consisted of dural tissue (four specimens) and brain tissue (six specimens, temporal lobe in three, frontal lobe in two and site unspecified in two). In patients with ICGI, the specimens consisted of brain tissue (four patients, site unspecified in two, frontal lobe in one and occipital lobe in one), dura and the adjacent bone in one and dura, the adjacent bone and brain tissue in one. The biopsy volume was 2.8 ± 1.6 for ICGNs and 2.4 ± 1.2 for ICGIs (P = 0.8). Histological examination of tissue showed no difference in the cellular composition of the infiltrate, which included multinucleated giant cells, epithelioid histiocytes, lymphocytes and plasma cells in both conditions. A distinctive difference was that lesions in ICGN patients were solid, with small areas of necrosis that were always less than 1 mm in diameter and restricted to the center of the granulomata. In contrast, lesions in ICGI patients showed extensive areas of necrosis larger than 1 mm in diameter in addition to the above pattern (Figure 2).
Figure 2.

Comparison of histological findings. The lesions in ICGN patients (A,C) were always solid, with small areas of necrosis (stars) less than 1 mm in diameter restricted to the center of granulomata, as seen in C. In contrast, areas of necrosis (stars) larger than 1 mm in diameter were seen only in ICGI (B,D). Multinucleated giant cells (arrows) are present in the inflammatory cell infiltrate in both conditions. ICGI = intracranial caseating granulomas with infectious etiology; ICGN = intracranial caseating granulomas without identifiable infectious pathology.
Clinical and radiological responses to treatment were distinctive in the two groups (Table 2). None of the patients were treated prior to the biopsy. In patients with ICGN, six out of six patients who received a trial of anti‐mycobacterial treatment (isoniazid, rifampin, pyrazinamide and vitamin B6 for at least 9 months) showed no improvement or worsening. Clinical improvement with either radiological resolution or improvement was observed with immunomodulation in seven out of eight ICGN patients who received this treatment. Three of these patients had previously showed no response or worsening on antimycobacterial treatment. Immunomodulation regimen used consisted of prednisone with or without azathioprine. In 4 patients, long‐term prednisone treatment along with azathioprine was required. One patient was stable on immunomodulation, after having worsened on anti‐mycobacterial and antifungal (fluconazole) treatments. Aureobasidium pullulans was isolated from tissue in this patient. He was included in ICGN group as explained in the Methods section. Immunomodulation was not tried in three patients with ICGN. One of these patients returned to his home country and as a result was lost to follow‐up. On the other hand, an excellent response to the appropriate antimicrobial agent, typically within weeks of treatment and always within 2 months, was observed in all patients with an identifiable infectious agent. Antimicrobial regimen consisted of isoniazid, rifampin, pyrazinamide and vitamin B6 for ≥9 months in all cases. None of these patients required a trial of immunomodulation.
Table 2.
Response of patients to treatment. Abbreviations: ICGI = intracranial caseating granulomas with infectious etiology; ICGN = intracranial caseating granulomas without identifiable infectious pathology.
| ICGN | ICGI | P‐value | |
|---|---|---|---|
| Antimicrobial therapy | 0.002 | ||
| Improved | 0/6 (0%) | 6/6 (100%) | |
| Unchanged | 3/6 (50%) | 0/6 (0%) | |
| Worsened | 3/6 (50%) | 0/6 (0%) | |
| Not tried | 5 | 0 | |
| Immunomodulation therapy | |||
| Improved | 7/8 (87.5%) | 0/0 (NA) | |
| Unchanged | 1/8 (12.5%) | 0/0 (NA) | |
| Worsened | 0/8 (0%) | 0/0 (NA) | |
| Not tried | 3 | 6 | |
| Overall response | 0.0006 | ||
| Antimicrobial therapy | 0 | 6 | |
| Immunomodulation therapy | 7 | 0 | |
| Follow‐up length (months) | 37.6 | 27.4 |
The average follow‐up was 32.75 months (37.6 months in patients with ICGN and 27.4 months in patients with an infectious etiology).
DISCUSSION
This study provides relevant clinical information on intracranial caseating granulomas. First, the two types of caseating granulomas, infectious and noninfectious, occur with similar low frequency, and the noninfectious is the predominant type at our institution. Most importantly, the response to treatment can be reliably predicted from the histopathological/microbiological tissue examination. Risk factors for infection, in particular immunosuppressed state, and the pattern of enhancement on MRI and the area of necrosis on histological examination are good predictors in this small series. However, the two entities were not easily distinguishable based on other clinical and laboratory data. Surprisingly, constitutional symptoms and signs were scarce.
ICGN is likely an immune‐mediated process (4). In at least 7 out of 11 patients, the response to immunomodulation treatment in addition to the lack of response to antimicrobial agents supports this inference. In three patients, the noninfectious mechanism can only be inferred from the latter, as the immunomodulation was not tried in them. One patient was clinically stable on immunomodulation after worsening clinical and radiological conditions on antimicrobial agents. An immune‐mediated process may have been the underlying nature of the granulomas in this latter patient. Thomas et al have previously described a group of biopsy‐proven pathogen‐free granulomatous disease of the CNS (11). In their series, 5 out of 11 patients were not definitively classifiable as sarcoidosis. In only one of the patients, the granulomata were caseating. There was a high mortality rate (8 out of 11) in these patients, mainly secondary to the complications of steroids. The patient with caseating granulomata was one of the few survivors. This is in sharp contrast with the absence of deaths and generally good response of our ICGN patients to immunomodulation. We cannot go beyond speculation on whether this represents a difference in patient population between the studies or a difference in the nature of caseating vs. noncaseating granulomata. Nevertheless, the association between histopathological/microbiological characteristics of granulomata and response to immunomodulation appears to be robust in ICGN. Hence, the inference that ICGNs likely result from an immune dysregulation rather than an active infection is well supported. Whether this immune dysregulation is triggered by an infectious agent such as M. tuberculosis remains a plausible explanation that needs to be addressed in a separate study.
The specificity and sensitivity of negative extensive tissue microbiological examination [defined as including a polymerase chain reaction (PCR)‐based test (AMTD), staining and culture for mycobacterium, gram stain and culture for other bacteria, and staining and culture for fungal agents] were calculated using response to immunomodulation treatment as the gold standard for diagnosis of ICGN. Under these conditions, and considering the patient with A. pullulans isolated from tissue as false results and excluding patients in whom immunomodulation was not tried, tissue examination conferred a sensitivity of 87.5% and a specificity of 100% for ICGN. Conversely, tissue examination conferred a sensitivity of 100% and a specificity of 87.5% for detecting ICGI. The small number of the patients in our series does not allow for an evaluation of the individual sensitivity and specificity of the said three mycobacterial tests. Current literature on the sensitivity and specificity of these tests mainly describes non‐CNS tissue examinations. The reported sensitivity of Zeil Neilsen staining and culture for detection of mycobacterial in tissue are 20%–90% and 55%, respectively 8, 9. Tissue PCR‐based assays such as AMTD are often more sensitive at 70%–80% 2, 9. Given the variability and suboptimal sensitivity of the individual mycobacterial tests, it seems prudent to use a combination of the said three tests before commiting a patient with caseating granuloma to immunosuppressive therapy.
There are some limitations to the clinical utility of extensive tissue microbiological examination alone, despite its excellent ability to distinguish ICGNs from infectious granulomas. Most notably is the long turnover time for some of these examinations such as tissue mycobacterial culture. PCR based tissue examinations such as AMTD are only available in public health reference laboratories. Delay in treatment of CNS TB is associated with poor outcome (8). Thus, clinical situation does not always permit for a delay in antimycobacterial treatment. Current guidelines on treatment of TB suggest stopping treatment and considering an alternative diagnosis when there is no clinical and radiological improvement after two months of appropriate antimycobacterial treatment in patients with negative acid fast bacilli smear and culture (1). A 2‐month wait in assessing the response to antimycobacterial treatment is underscored by the fact that there may be a worsening of CNS tuberculosis in the first two weeks of treatment (10). All of the patients with an infectious etiology in our series responded to anti‐microbial agents within 2 months of treatment. Unfortunately, a ≥9‐month course of antimycobacterial treatment was administered in the six patients with ICGN who received this treatment in our institution. One of these patients received two full courses of antimycobacterial treatment before an alternative diagnosis was entertained. In three patients, this delay resulted in significant neurological morbidity. Hence, antimicrobial treatment beyond 2 months in patients with negative tissue microbiological examination and no clinical and radiological improvement is likely more harmful than beneficial.
Based on these findings, we suggest the following scheme for the diagnosis of ICGN:
-
(i)
presence of caseating granulomas with areas of necrosis, predominantly less than 1 mm in diameter, in tissue;
-
(ii)
absence of an identifiable infectious agent in extensive tissue examination that includes an amplified M. tuberculosis direct test, staining and culture for mycobacterium, gram stain and culture for other bacteria, and staining and culture for fungi; and
-
(iii)
Lack of clinical and radiological response within 2 months of appropriate antimycobacterial treatment.
This scheme is supported by our findings that extensive tissue examination and response to treatment are the major features with the ability to distinguish ICGNs from infectious intracranial granulomas. It also fulfills the minimum 2‐month treatment period in laboratory negative suspected tuberculosis recommended by the CDC (1) and supported by our observations.
The major limitations of this study are the small number of the subjects, because of the rarity of this entity, and the retrospective nature of the investigations. A larger prospective study is required to validate our findings and establish the optimal criteria for the diagnosis of ICGNs. In the mean time, the above‐proposed scheme provides a basis for future clinical research in this area. It will also potentially reduce the risk of further neurological morbidity because of delayed recognition of ICGN, to which patients are currently exposed.
REFERENCES
- 1. American Thoracic Society, Center for Disease Control and Infectious Diseases Society of America (2003) Treatment of tuberculosis. MMWR Recomm Rep 52:1–77. [Google Scholar]
- 2. Chawla K, Gupta S, Mukhopadhyay C, Rao PS, Bhat SS (2009) PCR for M. tuberculosis in tissue samples. J Infect Dev Ctries 3:83–87. [DOI] [PubMed] [Google Scholar]
- 3. Fleming MG, Gewurz AT, Pearson RW (1991) Caseating cutaneous granulomas in a patient with X‐linked infantile hypogammaglobulinemia. J Am Acad Dermatol 24:629–633. [DOI] [PubMed] [Google Scholar]
- 4. Ghavanini AA, Munoz DG (2011) Intracranial caseating granulomas with no infectious organism detected. Can J Neurol Sci 38:82–87. [DOI] [PubMed] [Google Scholar]
- 5. Johnson LN, Iseri O, Knodell RG (1990) Caseating hepatic granulomas in Hodgkin's lymphoma. Gastroenterology 99:1837–1840. [DOI] [PubMed] [Google Scholar]
- 6. Joshi A, Singh R, Shah MS, Umesh S, Khattry N (2010) Subcutaneous mycosis and fungemia by Aureobasidium pullulans: a rare pathogenic fungus in a post allogeneic BM transplant patient. Bone Marrow Transplant 45:203–204. [DOI] [PubMed] [Google Scholar]
- 7. Mershon‐Shier KL, Deville JG, Delair S, Fothergill AW, Wickes B, de Hoog GS et al (2011) Aureobasidium pullulans var. melanigenum fungemia in a pediatric patient. Med Mycol 49:80–83. [DOI] [PubMed] [Google Scholar]
- 8. Rock RB, Olin M, Baker CA, Molitor TW, Peterson PK (2008) Central nervous system tuberculosis: pathogenesis and clinical aspects. Clin Microbiol Rev 21:243–261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Salian NV, Rish JA, Eisenach KD, Cave MD, Bates JH (1998) Polymerase chain reaction to detect Mycobacterium tuberculosis in histologic specimens. Am J Respir Crit Care Med 158:1150–1155. [DOI] [PubMed] [Google Scholar]
- 10. Sütlaş PN, Unal A, Forta H, Senol S, Kirbaş D (2003) Tuberculous meningitis in adults: review of 61 cases. Infection 31:387–391. [DOI] [PubMed] [Google Scholar]
- 11. Thomas G, Murphy S, Staunton H, O'Neill S, Farrell MA, Brett FM (1998) Pathogen‐free granulomatous diseases of the central nervous system. Hum Pathol 29:110–115. [DOI] [PubMed] [Google Scholar]
- 12. Torrelo A, Mediero IG, Zambrano A (1995) Caseating cutaneous granulomas in a child with common variable immunodeficiency. Pediatr Dermatol 12:170–173. [DOI] [PubMed] [Google Scholar]


