Abstract
Meningeal solitary fibrous tumors (SFTs) and hemangiopericytomas (HPCs) are distinct entities in the World Health Organization (WHO) classification of central nervous system (CNS) tumors while they belong to the same spectrum of tumors in other locations. Well‐defined histological prognostic factors are also lacking for these tumors. In order to clarify the relationship between SFT and HPC and to find histological and immunohistochemical prognostic factors, we carried out a retrospective study in 89 patients. The following histological parameters were recorded: hypercellularity, collagenic areas, cytonuclear atypias, necrosis, mitotic count per 10 high‐power fields, vasculo‐nervous adherences defined by engulfment of vessel or nerve by the tumor, brain infiltration. We found overlapping histological and immunohistochemical features between SFT and HPC. The most relevant histological prognostic factors in the whole cohort for both progression‐free survival (PFS) and overall survival (OS) in univariate analysis were hypercellularity, high mitotic count (>5 per 10 high‐power fields) and necrosis. On the basis of these results, we propose a new grading scheme for these tumors which was of pronostic value for both PFS and OS in uni‐ and multivariate analysis. As extent of surgery was also a prognostic factor for both PFS and OS in univariate analysis, we propose that management of SFT/HPC might be based both on quality of removal and histological grade.
Keywords: grading, hemangiopericytoma, immunohistochemistry, meninges, prognostic factors, solitary fibrous tumor
INTRODUCTION
Hemangiopericytoma (HPC) is no longer recognized in the 2006 World Health Organization (WHO) classification of soft tissue tumors (17). “So‐called HPC” would be better classified as a “cellular” form of solitary fibrous tumor (SFT), an ubiquitous mesenchymal neoplasm of probable fibroblastic type. The heterogeneity of SFT has led to distinguishing a “fibrous” variant (the conventional SFT) which shows a patternless architecture characterized by alternating hypocellular and hypercellular areas separated from each other by thick bands of hyalinized collagen and branching HPC‐like vessels and a cellular variant characterized by a highly cellular monotonous appearance and thin‐walled branching vessels. In the conventional form of SFT CD34, expression by immunohistochemistry is diffuse while being more focal or absent in the cellular form (17). Actually, HPCs have many clinical and morphological features similar with SFT and do not show pericytic differentiation. True HPCs with myoid pericytic differentiation are found in the sinusonasal tract only. In soft tissue such tumors would be more appropriately called myopericytoma (18). Exceptional true myopericytomas have been reported in the central nervous system (CNS) (41). Even in bone, a recent study showed that HPC‐like features are a nonspecific growth pattern (53). However, in the last WHO classification of CNS tumors, HPC and SFT are listed as separate entities with different prognosis 19, 38. They both occur in adults while exceptional meningeal SFTs have been reported in children (15). HPCs are malignant neoplasms with a high rate of local recurrence, tendency to late leptomeningeal spread and distant delayed metastases. The probability of recurrence is 65% at 5 years and 90% at 12 years and of metastasis 80% at 12 years (8). On the other hand, most but not all SFTs have a benign course and are cured by gross total resection. In light of the difference of prognosis between HPC and SFT, care must be taken to achieve accurate diagnosis according to some authors (24). However, since its first description by Carneiro et al in 1996 (7), about 220 published cases have been reported (5). Some SFTs arising in atypical locations 1, 6, 29, 49 or with unusual presentations 26, 27, 28, 47 have been reported underscoring the need for reliable diagnostic criteria. Several cases of malignant or disseminated forms of SFT have also been described 32, 33, 35, 36, 40, 51. Some studies have also reported the difficulty of achieving an accurate diagnosis distinguishing between the two neoplasms which have many overlapping histological and immunohistochemical features 39, 46. Two other studies also showed that compared with soft tissue tumors, meningeal HPCs are indistinguishable from one another according to morphological and immunohistochemical criteria 2, 13. Furthermore, some authors have observed meningeal tumors initially diagnosed as conventional HPC that recurred as SFT‐like neoplasm, a finding in support of a common spectrum between these two entities 25, 51.
Pronostic factors are not clearly defined for meningeal SFT. In contrast, meningeal HPC are grade II or III tumors in the WHO classification of CNS tumors based on the criteria defined by Mena et al (31). Grade III HPCs exhibit necrosis or five or more than five mitotic figures per 10 high‐power fields (HPFs) as well as two or more of the following features: hemorrhage, moderate to high cellularity and moderate to marked nuclear pleiomorphism (38). However, these histological criteria do not always predict the clinical progression and no study had demonstrated its prognostic value among SFT.
In order to clarify the relationship between meningeal SFT and HPC and to define prognostic criteria, we performed a retrospective clinicopathological study in 89 patients with a SFT or HPC of the meninges.
MATERIALS AND METHODS
Patient population
The study was conducted under the auspices of the French Society of Neuropathology. Each participating center sent a representative paraffin block of a tumor diagnosed as meningeal SFT or HPC. One hundred five specimens were collected from the pathological files of each different center between 2005 and 2009. Sixteen specimens were recurrent tumors. Clinicoradiological and treatment data including age, symptoms, signs, Magnetic Resonance Imaging (MRI) aspect, extent of surgery and complementary treatment were available for all patients. Follow‐up data were available for 72 patients. The follow‐up ranged from 18 to 237 months with a median of 75 and a mean of 85 (±49).
Histological features
All tumors were reviewed by three of us (CB and AV or DFB). The following histopathological features were recorded in the two groups of tumors: areas of hypercellularity, collagenic areas, cytonuclear atypias, necrosis, mitotic count per 10 HPFs, vasculo‐nervous adherences defined by engulfment of a vessel or nerve by the tumor, brain or bone infiltration. Typical case of SFT was defined according to the criteria of the WHO classification of soft tissue tumors: “patternless architecture characterized by a combination of alternating hypocellular and hypercellular areas separated from each other by thick bands of hyalinized somewhat keloidal, collagen and branching haemangiopericytoma‐like vessels”(17). All tumors diagnosed as SFT showed diffuse immunoreactivity for CD34 except three cases which showed no consistent CD34 nor epithelial membrane antigen (EMA) immunoreactivity but had typical histological features. Foci of hypercellularity corresponded to areas devoid of collagen where the cells were arranged around staghorn vessels. Atypias, necrosis, vasculo‐nervous adherences, brain and bone infiltration were recorded as absent or present.
Immunohistochemical features
Immunohistochemical study was performed on Ventana Benchmark XT automate Device. The expression of the following antigens was searched for vimentin, EMA, smooth muscle actin (SMA), PS100, neural cell adhesion molecule (NCAM), estrogen and progesterone receptors (ER and PR), CD99 (Mic 2) and Ki67. The list of primary antibodies with their dilution is summarized in Table 1. Automated immunohistochemistry was performed on the sections with streptavidin‐biotin‐peroxidase complex on Ventana XT device (Ventana Medical Systems Inc, Tucson, AZ, USA) with Ventana kit including diaminobenzidine (DAB) reagent. For MIB1 immunohistochemistry, a semiquantitative score (MIB1 labeling index) was recorded by determining the percentage of positive neoplastic cells in comparison to the total cell number in the most highly stained areas. For the other markers, only positive (with a threshold of more than 5% of cells) or negative staining was recorded.
Table 1.
Antibodies used for immunohistochemistry.
| Clone | Source | Dilution | |
|---|---|---|---|
| Vimentin | V9 | Beckman | 1/200 |
| EMA (epithelial membrane antigen) | E29 | Dako | 1/30 |
| CD34 | QBEND10 | Dako | Prediluted |
| PS100 | Polyclonal | DBS | 1/100 |
| Alpha‐smooth actin | 1A4 | Microm | Prediluted |
| NCAM (neural cell adhesion molecule) | 1B6 | Menarini | 1/50 |
| ER (estrogen receptor) | SP1 | Ventana | Prediluted |
| PR (progesterone receptor) | 1E2 | Ventana | Prediluted |
| CD99 | 12E7 | Dako | 1/200 |
| Ki67 | MIB1 | Dako | 1/100 |
Statistical analysis
The chi‐square test (or Fisher's exact test when one subgroup was n < 5) was used to assess the histopathological and phenotypic distribution. Continuous variables were compared using the Mann–Whitney U‐test. All statistical tests were two sided, and the threshold for statistical significance was P = 0.05. As it was sometimes difficult to classify the tumor as SFT, we searched for a prognostic value for each clinical and/or histopathological parameters in the whole cohort. Survival curves were calculated according to the Kaplan–Meier method and compared using the log‐rank test. Variables with significant P‐value < 0.10 were used to build the multivariate Cox proportional hazard models. The results are reported as two‐sided P‐values with 95% confidence intervals [95% CI analyses were conducted with PASW for Windows version 17.0 (SPSS Inc, Chicago, IL, USA)].
RESULTS
Clinical data (Table 2)
Table 2.
Patients and treatment characteristics. Abbreviations: CT = chemotherapy; GTR = gross total resection; ICH = intracranial hypertension; KPS = Karnofsky performance status; SD = standard deviation.
| Variables | All patients Number of patients (%) (n = 72) | SFT Number of patients (%) (n = 29) | HPC Number of patients (%) (n = 43) | P‐value |
|---|---|---|---|---|
| Age | 0.789 | |||
| Median | 54.1 years | 54.1 years | 53.7 years | |
| Mean (±SD) | 53.1 ± 13.2 years | 54.0 ± 12.0 years | 52.4 ± 14.0 years | |
| Range | 22–81 years | 34–75 years | 22–81 years | |
| Sex | 0.162 | |||
| Male | 32 (44.4%) | 10 (34.5%) | 22 (51.2%) | |
| Female | 40 (55.6%) | 19 (65.5%) | 21 (48.8%) | |
| Preoperative KPS | 0.650 | |||
| ≥70 | 63 (87.5%) | 26 (89.7%) | 37 (86.0%) | |
| <70 | 9 (12.5%) | 3 (10.3%) | 6 (14.0%) | |
| Location | 0.439 | |||
| Intracranial | 56 (77.8%) | 20 (68.9%) | 36 (83.7%) | |
| Spinal | 16 (22.2%) | 9 (31.1%) | 7 (16.3%) | |
| Signs and symptoms | >0.05 | |||
| Intracranial tumors | ||||
| ICH | 42/56 (75.0%) | 16/20 (80.0%) | 26/36 (72.2%) | |
| Epilepsy | 19/56 (33.9%) | 8/20 (40.0%) | 11/36 (30.6%) | |
| Neurological deficit | 12/56 (50%) | 4/20 (20.0%) | 8/36 (22.2%) | |
| Spinal tumors | ||||
| Pain | 16/16 (100%) | 9/9 (100%) | 7/7 (100%) | |
| Neurological deficit | 7/16 (43.7%) | 2/9 (22.2%) | 5/7 (60.0%) | |
| Extent of surgery | 0.883 | |||
| GTR (−) | 38 (52.8%) | 15 (51.7%) | 23 (53.5%) | |
| GTR (+) | 34 (47.2%) | 14 (48.3%) | 20 (46.5%) | |
| Adjuvant treatment | 0.001 | |||
| RT (−) | 48 (66.7%) | 26 (89.7%) | 22 (51.2%) | |
| RT (+) | 24 (33.3%) | 3 (10.3%) | 21 (48.8%) |
The bold in P‐value underlines the statistically significant parameters.
No statistical significant difference relative to age, sex, Karnofsky performance status (KPS), location, signs and symptoms or extent of surgery was observed between SFT and HPC.
Among the classic SFT, we observed 10 local recurrences and four deaths that respectively occurred in a mean time of 53.7 months ± 29.4 [range: 21.8–95.0] and 104.5 months ± 7.9 [range: 98–115].
Histological features (Table 3)
Table 3.
Histological and immunohistochemical characteristics.
| Variables | All patients Number of patients (%) (n = 72) | SFT Number of patients (%) (n = 29) | HPC Number of patients (%) (n = 43) | P‐value | |
|---|---|---|---|---|---|
| Histological data | Collagenic areas | 55 (76.4%) | 29 (100%) | 26 (60.5%) | P = 0.0003 |
| High cellularity | 43 (59.7%) | 7 (24.2%) | 36 (83.7%) | P = 0.0001 | |
| Atypias | 33 (45.8%) | 3 (10.3%) | 30 (69.8%) | P < 0.0001 | |
| Mitoses > 5 | 21 (29.2%) | 1 (3.4%) | 20 (46.5%) | P = 0.002 | |
| Necrosis | 21 (29.2%) | 9 (31.0%) | 12 (27.9%) | P = 0.775 | |
| Vasculo‐nervous adherences | 11 (15.3%) | 9 (31.0%) | 2 (4.7%) | P = 0.005 | |
| Brain infiltration | 10 (13.9%) | 3 (10.3%) | 7 (16.3%) | P = 0.729 | |
| Immunohistochemical data | Vimentin | 72 (100%) | 29 (100%) | 43 (100%) | NS |
| EMA | 4 (5.6%) | 0 | 4 (9.3%) | P = 0.143 | |
| CD34 | 43 (59.7%) | 26 (89.7%) | 17 (39.5%) | P < 0.0001 | |
| PS100 | 0 | 0 | 0 | NS | |
| α smooth actin | 2 (2.8%) | 0 | 2 (4.7%) | P = 0.512 | |
| NCAM | 3 (4.2%) | 0 | 3 (7.0%) | P = 0.268 | |
| ER | 0 | 0 | 0 | NS | |
| PR | 25 (34.7%) | 11 (37.9%) | 14 (32.6%) | P = 0.828 | |
| MIC2 | 42 (47.2%) | 17 (58.6%) | 36 (83.7%) | P = 0.018 | |
| MIB1LI | Mean | Mean 5.35% | Mean 13.63% | ||
| Median | Median 5% | Median 10% | P = 0.001 | ||
The bold in P‐value underlines the statistically significant parameters.
We found 35 SFTs according to histological criteria of the WHO classification of soft tissue tumors: “SFT show a patternless architecture characterized by a combination of alternating hypocellular and hypercellular areas separated from each other by thick bands of hyalinized somewhat keloidal collagen and branching haemangiopericytoma‐like vessels”. Seventy HPCs (33 grade II and 37 grade III) (Figure 1A–C) were diagnosed according to the WHO classification of CNS tumors: “highly cellular and vascularized mesenchymal tumour exhibiting a characteristic monotonous low‐power appearance and a well‐developed, variably thick‐walled branching staghorn vasculature”. In 20% of cases we observed discordance between the diagnosis of the local pathologist which could be SFT or HPC and our diagnosis. The principal difficulty was the proportion of collagenic areas necessary to classify a tumor as SFT rather than HPC, as areas of hypercellularity with staghorn vessels were found in both tumors.
Figure 1.
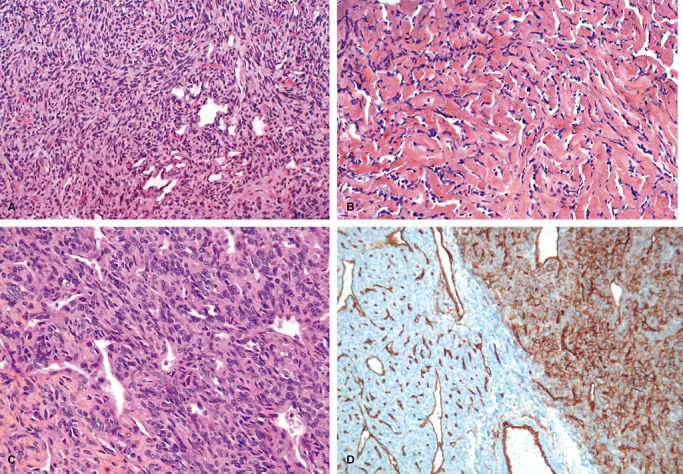
A,B. Microscopic features of a solitary fibrous tumor: characteristic biphasic pattern: cellular areas with staghorn vessels (A, HES X 25) and pseudokeloidal collagenous areas (B, HES X 25). C,D. Microscopic features of a hemangiopericytoma: highly cellular tumor made of oval cells arranged around vessels (C, HES X 40). Heterogenous expression of CD34 by immunohistochemistry (D, X 25).
Among SFT, three cases had been classified as fibroblastic meningioma before the immunohistochemical study. Three additional cases that showed a typical biphasic pattern at microscopic level with lack of EMA expression were diagnosed as SFT, although CD34 was negative. Among HPCs, one case was excluded after exhaustive sampling which showed areas of cartilaginous matrix. The tumor was reclassified as a mesenchymatous chondrosarcoma.
For statistical analysis, only patients with available clinical data were analyzed (72 cases). In the group of HPCs, high cellularity, atypias and high mitotic count (mitoses > 5) were more frequent than in SFT (Table 3) (Figure 2A,B). On the other hand, vasculo‐nervous adherences were more frequently observed in SFT. This was more often the result of engulfment of nerve or vessel by dense collagenic deposits rather than true infiltration by a cellular proliferation. Necrosis and brain invasion were not differentially recorded in SFT vs. HPC. Bone infiltration was present in only two recurrent HPCs (one grade II and one grade III). No hyperostotic reaction of bone as recorded in meningioma was seen.
Figure 2.
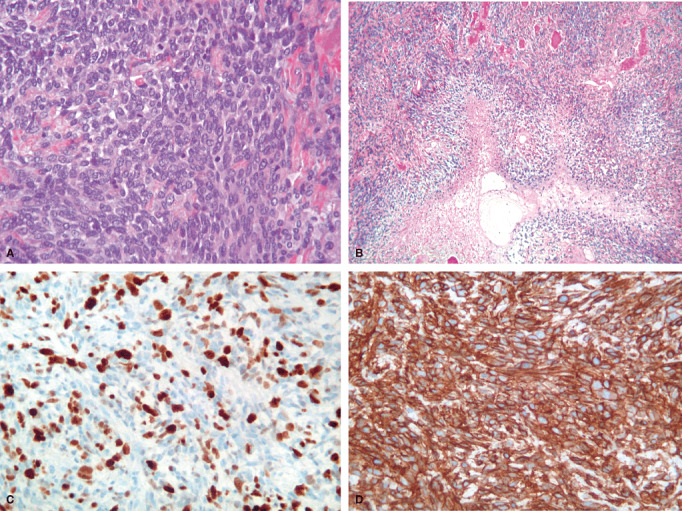
Microscopic features of malignancy in SFT/HPC. A. High cellularity and high mitotic count (HES X 25). B. Geographical areas of necrosis (HES X10). C. High MIB1LI (X 40). D. Retained positivity for CD34 in a grade III tumor of the SFT/HPC spectrum (X 40).
Among the 16 recurrences, one SFT and 10 HPCs had the same microscopic features as the first tumor. Four SFTs progressed to a more cellular form “HPC‐like” tumor. One HPC recurred with histological features of SFT.
Immunohistochemical data
All tumors were negative for PS100 and RE. CD34 expression was more frequent in SFT than in HPC (89.7% vs. 39.5%) (1, 2). HPC expressed more frequently Mic2 (83.7% vs. 58.6%) and their MIB1LI was higher than SFT (median 10% vs. 5%) (Table 3) (Figure 2C).
Prognostic factors
We searched for prognostic value on progression free survival (PFS) and overall survival (OS) for each clinical, histological and immunohistochemical parameter in the whole cohort whatever the pathological diagnosis, SFT or HPC. Hypercellularity, high mitotic count (>5 mitoses per 10 HPFs), histological subtype (SFT/HPC), high MIB1LI (>10%) and extent of surgery were significantly correlated with PFS. Hypercellularity, high mitotic count (>5 mitoses per 10 HPFs), histological subtype (SFT/HPC), necrosis and extent of surgery were significantly correlated with OS in univariate analysis (Table 4). As the cutoff for mitotic count of the WHO grading of HPC was equal or superior to five mitoses, we also tested its prognostic value. It was significant for both PFS and OS (P = 0.003, P = 0.0 43) but significance was higher if the cutoff was >5 (P = 0.001; P = 0.003).
Table 4.
PFS and OS in solitary fibrous tumors and hemangiopericytomas: univariate analysis. Abbreviations: 95 CI = 95% confidence interval; GTR = gross total removal; PFS = progression‐free survival; OS = overall survival; Grade I = hypercellularity (−), mitosis<5, necrosis (−); Grade IIa = mitosis < 5, variable cellularity; necrosis (−); Grade IIb = mitosis ≥ 5, variable cellularity, necrosis (−); Grade III = hypercellularity (+); mitosis ≥ 5; necrosis (+).
| Variables | Number of patients (%) | PFS months | OS months | ||||
|---|---|---|---|---|---|---|---|
| Median | 95% CI | Log rank | Median | 95% CI | Log rank | ||
| Extent of surgery | 0.016 | 0.043 | |||||
| GTR (+) | 38 (52.8%) | 128.7 | 74.5–182.9 | NR | — | ||
| GTR (−) | 34 (47.2%) | 60.0 | 20.9–99.1 | 170.4 | 55.1–285.4 | ||
| Histological subtype | 0.041 | 0.045 | |||||
| Solitary fibrous tumor | 29 (40.3%) | NR | — | NR | — | ||
| Hemangiopericytoma | 43 (59.7%) | 80.9 | 45.1–116.8 | 170.4 | — | ||
| Mitoses | 0.001 | 0.003 | |||||
| <5 per 10 high‐power fields | 51 (70.8%) | 128.7 | 59.1–198.2 | NR | — | ||
| >5 per 10 high‐power fields | 21 (29.2%) | 44.8 | 24.1–65.4 | 116.4 | 62.0–170.9 | ||
| Hypercellularity | 0.00 | 0.038 | |||||
| Absent | 29 (40.3%) | 128.7 | — | NR | — | ||
| Present | 43 (59.7%) | 60.0 | 17.3–102.7 | 170.4 | 86.5–254.3 | ||
| Necrosis | 0.065 | 0.016 | |||||
| Absent | 57 (79.2%) | 95.0 | 45.7–144.3 | NR | — | ||
| Present | 15 (20.8%) | 60.0 | 28.9–91.2 | 125.0 | 104.3–145.7 | ||
| MIB1 Index (55 patients) | 0.046 | 0.161 | |||||
| <10% | 27 (49.1%) | NR | — | NR | — | ||
| ≥10% | 28 (50.9%) | 47.3 | 37.1–57.4 | 170.4 | 66.3–274.5 | ||
| Marseille grading system | 0.003 | 0.001 | |||||
| Grade I | 25 (34.7%) | 128.7 | — | NR | — | ||
| Grade IIa | 26 (36.1%) | 84.5 | 73.8–95.2 | NR | — | ||
| Grade IIb | 14 (19.4%) | 44.8 | 34.1–55.4 | 90.6 | 41.3–140.0 | ||
| Grade III | 7 (9.7%) | 32.1 | 7.5–56.6 | 88.1 | 26.2–149.9 | ||
The bold in log rank underlines the statistically significant parameters.
In order to set up a grading scheme, we combined the presence and or absence of the following pathological features: hypercellularity, mitotic count (>5 mitoses/HPF), necrosis. Grade I was defined by lack of these criteria. Grade II tumors displayed no necrosis and low mitotic count (≤5, grade IIa) or high mitotic count (>5, grade IIb) whatever the cellularity. Grade III tumors all had pejorative criteria: hypercellularity, high mitotic count and necrosis. With this grading, we found 25 grade I tumors that encompassed 21 STFs and four HPCs, 26 grade IIa tumors (seven SFTs and 19 HPCs) and 14 grade IIb tumors (one SFT and 13 HPCs), and seven grade III tumors that corresponded to HPC. This grading scheme was of prognostic value for both PFS and OS (P = 0.003; P = 0.001) in univariate analysis. It was also a prognostic factor in multivariate analysis for both PFS and OS while extent of surgery or histological diagnosis was not (Table 5). When the parameters hypercellularity, mitoses, necrosis, extent of surgery and histological diagnosis were tested for prognostic significance in multivariate analysis, mitotic count was an independent prognostic factor for both PFS (P = 0.036) and OS (P = 0.035) and quality of removal for PFS (P = 0.036). Survival curves of significant prognostic factors for PFS and OS are provided in 3, 4
Table 5.
Predictors of PFS and OS in patients harboring solitary fibrous tumors and hemangiopericytomas: multivariate analysis. Abbreviations: CI = confidence interval; hemangiop. = hemangiopericytoma; PFS = progression‐free survival; OS = overall survival; SFT = solitary fibrous tumor.
| Variables | PFS | OS | ||||
|---|---|---|---|---|---|---|
| Hazard ratio | 95% CI | P‐value | Hazard ratio | 95% CI | P‐value | |
| Extent of surgery | 2.032 | 0.976–4.232 | 0.058 | 1.585 | 0.432–5.808 | 0.484 |
| Histology (hemangiop. vs SFT) | 1.240 | 0.502–3.061 | 0.641 | 1.858 | 0.75–10.97 | 0.458 |
| Marseille grading system | 1.522 | 1.038–2.232 | 0.031 | 2.452 | 1.238–4.854 | 0.010 |
The bold in P‐value underlines the statistically significant parameters.
Figure 3.
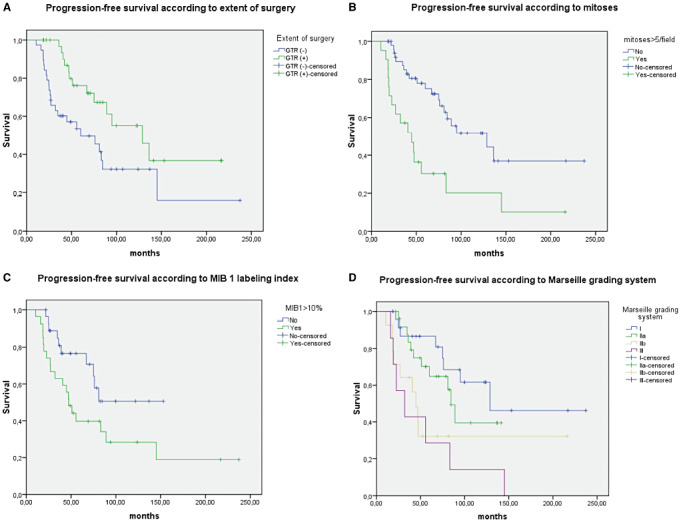
Survival curves for (A) progression‐free survival according to extent surgery, (B) mitoses, (C) MIB 1 labeling index, (D) Marseille grading system.
Figure 4.
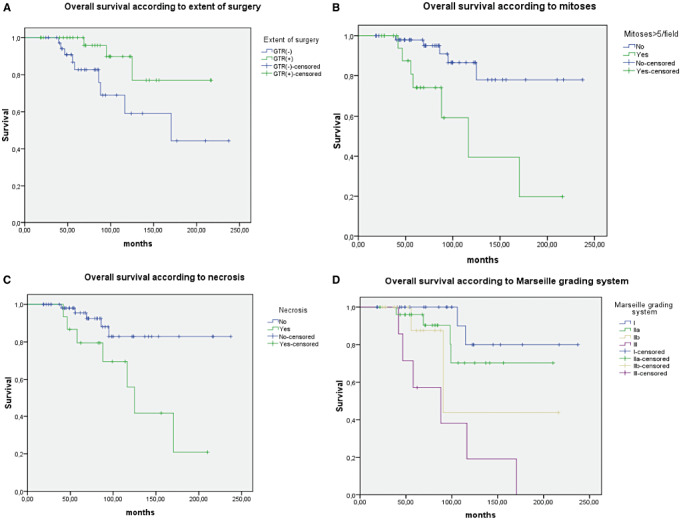
Survival curves for (A) overall survival according to extent of surgery, (B) mitoses, (C) necrosis, (D) Marseille grading system.
DISCUSSION
SFT and HPC belong to the same spectrum of tumors
SFT is rare spindle cell neoplasm first described in pleura and then reported to occur at almost any body site. Carneiro et al first reported cases arising in the meninges and provided histological and immunohistochemical criteria to distinguish them from meningiomas and HPCs (9). By the past, they might have been misdiagnosed as fibrous meningiomas or HPCs (50). SFT is a spindle cell tumor characterized by a combination of alternating hypocellular collagenic and hypercellular areas with branching HPC‐like vessels. They show diffuse intense immunopositivity for CD34 and lack EMA, PS100 and RE immunostaining compared to meningiomas 21, 30. CNS SFTs belong to the category of nonmeningothelial mesenchymal meningeal neoplasms in the WHO classification of CNS tumors (38). HPC is a separate entity from SFT in the CNS (19) although “indistinguishable” histologically from HPC occurring in somatic soft tissues. It is defined as a “highly cellular and richly vascularized tumor with a high tendency to recur and to metastasize outside the CNS”. In our study, no clinical or radiological features were found to accurately distinguish SFT from HPC. Symptoms depend on the location of the tumor only. On CT and MRI, both were well‐circumscribed, contrast‐enhancing and dura‐based tumors. They lack the broad‐based dural attachment (“dura tail sign”) of the meningiomas. On the basis of histological and immunohistochemical features alone, we had difficulty choosing between SFT and HPC in some cases. The main pitfall was to define the proportion of collagenic areas vs. cellular necessary to make a diagnosis of SFT rather than HPC, as in both tumors areas of hypercellularity with staghorn vessels could be found. We have classified tumors as SFT when collagenic areas represent more than 50% of the total tumoral volume but it seemed quite arbitrary. Immunohistochemistry was of little help in those cases. In particular, the distribution of CD34 immunoreactivity, even if statistically different between SFT and HPC, was not discriminant in some cases because of heterogeneity. CD34 expression is found in 90%–95% of fibrous cases of SFT in soft tissue (18) and is less frequently positive in cellular forms. In CNS HPC, CD34 expression varies from 33% to 100% of cases according to the series (39). In our study, 10.3% of SFT and 60.5% of HPC CD34 were negative by IHC which is in line with literature data. Several authors have already pointed out the difficulty of accurately diagnosing these tumors because of histological and immunohistochemical overlapping features (44). In the WHO classification of soft tissue tumors, HPC and SFT are listed as a single entity. The fibrous variant of SFT is a collagenic‐rich tumor highly positive for CD34 and corresponds to the classical description of SFT or conventional SFT. On the other hand, there is a “cellular form” with thin‐walled branching vessels and focal or absent CD34 reactivity, indistinguishable from HPC. Considering that these tumors belong to the same spectrum would also explain why some meningeal SFTs showed HPC features at recurrence and vice versa, as we and others observed (present study, 51). Changing the pathological diagnosis may be confusing for neurosurgeons and also for therapeutic purposes. Because of the relatively insensitive nature of both tumors to radiotherapy and chemotherapy in advanced disease, new therapies are needed for treatment. Novel‐targeted therapies are currently in development in soft tissue sarcomas and are starting to be assessed in the spectrum of SFT/HPC (37). They could also be assessed in SFT/HPC arising in the CNS. Finding common genetic alterations in both groups would be an additional argument for regrouping these two entities. Unfortunately, analyses of limited numbers of SFT and HPC to date have not found any consistent and recurrent cytogenetic abnormalities compared to synovialosarcomas (43). Cytogenetic aberrations are uncommon in small SFT but more frequent in larger ones, although variable in nature. However, recently a cDNA microarray study showed a homogeneous gene expression profile in SFT principally based on activation of the IGF2‐INSR pathway, independent of the anatomical location, suggesting that pleural and extra‐pleural SFTs are a single biological entity (22). The authors suggest that the artificial separation between SFT and HPC is merely a reflection of histological grading rather than indicating two distinct neoplasms.
SFTs/HPCs share common prognostic criteria
Whatever the location is prognosis of SFT/HPC remains difficult to predict 10, 23, 52. Most SFTs are thought to behave in a benign manner, but some recur (3) and some malignant forms have been described 7, 12, 34, 35, 40, and long‐term follow‐up is mandatory. There is no strict correlation between morphology and behavior. However, in soft tissues most but not all histologically benign SFTs prove to be nonrecurring and nonmetastasizing lesions, and most histologically “malignant” tumors defined by hypercellular lesions with moderate to marked cytological atypias, tumor necrosis and numerous mitoses (>4 mitoses per 10 HPFs), and/or infiltrative margins behave aggressively 17, 20. No pronostic criteria are validated for SFT occurring in the CNS although histological pronostic factors were well identified by Mena et al for HPC. These criteria are used in the WHO grading system of meningeal HPC. Grade III HPCs exhibit necrosis or five or more than five mitotic figures per 10 HPFs as well as two or more of the following features: hemorrhage, moderate to high cellularity, moderate to marked nuclear pleiomorphism. In our study, we searched for a prognostic value of common criteria defined by Mena et al.: hypercellularity, mitotic count (but with a different cutoff: >5 per 10 HPFs) and necrosis in the whole cohort of SFT/HPC. We have found that the most relevant histological prognostic features were hypercellularity, high mitotic count (>5 per 10 HPFs) and necrosis. These criteria in association are highly pejorative for both PFS and OS and suggest malignancy. We recommend using the terminology SFT/HPC and suggest a grading scheme with three grades. Grade I tumors are defined by the absence of hypercellularity, high mitotic count and necrosis. They correspond to the most “conventional” SFT. Grade II tumors display no necrosis but cellularity might be high or low. They correspond to SFT or HPC. They have an intermediate prognosis between grade I and grade III and could be stratified according to mitotic count less than or equal to five mitoses per 10 HPFs (grade IIa) or more than five mitoses per HPF (grade IIb). Grade III tumors have all pejorative criteria: hypercellularity, high mitotic count and necrosis. They correspond to grade III HPC. This grading was of prognostic value for both PFS and OS in univariate and multivariate analyses. MIB1LI was of prognostic value for PFS only in univariate analysis. The prognostic impact of MIB1LI has already been underlined by several authors 4, 33.
We have also found that extent of surgery was correlated to PFS and OS in univariate analysis but not in multivariate even if there was a trend to significance for PFS (P = 0.058) when opposed to the grading. In most studies, complete resection had a favorable impact on survival for HPC 11, 28, 42, 48, while in some it was not (16). This means that grading has a stronger prognostic impact than surgery. It also could be explained by the bias induced by retrospective studies spanned on many years with different surgical procedures and variability in assessing quality of removal with no systematic MRI control after resection. Extent of surgery was independent of histological diagnosis: SFT or HPC. Invasive nature for cerebral parenchyma, bone or vessels has already been reported as pejorative for prognosis with an increased risk of recurrence (51) in SFT of the CNS, probably because in those cases complete resection was not possible. SFTs located in the mediastin, abdomen, pelvis and/or retroperitoneum tend to behave more aggressively than in limbs probably because broad resection is difficult to achieve. Recently, a study comparing meningeal HPC and HPC/SFT of extracranial soft tissues showed a more aggressive behavior pattern in intracranial tumors, again because incomplete resection was more frequent for meningeal tumors (2). In pleura, sessile tumors that can not be completely resected have an unfavorable prognosis (8). The Perrot staging system was shown to be a reliable pronostic indicator 14, 45 for pleural SFT. It is based both on macroscopic features: sessile or pedunculated tumor and histological features. Pedunculated tumors have a better prognosis because complete resection is easier to achieve than in the sessile tumor. High mitotic count (>4 mitoses per 10 HPFs), mild to marked pleiomorphism, areas of high cellularity, necrotic or hemorrhagic zones, and stromal or vascular invasion are suggestive of malignancy (14). In parallel with this staging system, we proposed to take into account the grade and the extent of surgery to plan survey and complementary treatment for meningeal SFT/HPC. It seems that low‐grade tumors are cured by surgery alone while high grades need complementary treatment. The place of conventional radiotherapy, gamma knife radiosurgery, chemotherapy or targeted treatments has to be defined by prospective randomized clinical trials based on this new grading scheme.
ACKNOWLEDGMENTS
The authors thank the French Society of Neuropathology and the following pathologists who provided case materials: Pr D. Henin, Pr C. Godfrain, Pr MB. Delisle, Pr E. Uro‐Coste, Pr M Peoch'H, Dr A. Heitzmann, Dr G. Viennet, Pr JL Kemeny.
Financial support: AORC APHM 2007, Projet libre INCA 2007 GENOSTT, Cancéropole PACA, ARTCsud.
The authors declare to have no conflict of interest.
C. Bouvier and P. Métellus equally contributed to this work.
REFERENCES
- 1. Alston SR, Francel PC, Jane JA Jr (1997) Solitary fibrous tumor of the spinal cord. Am J Surg Pathol 21:477–483. [DOI] [PubMed] [Google Scholar]
- 2. Ambrosini‐Spaltro A, Eusebi V (2010) Meningeal hemangiopericytomas and hemangiopericytoma/solitary fibrous tumors of extracranial soft tissues: a comparison. Virchows Arch 456:343–354. [DOI] [PubMed] [Google Scholar]
- 3. Bikmaz K, Cosar M, Kurtkaya‐Yapicier O, Iplikcioglu AC, Gokduman CA (2005) Recurrent solitary fibrous tumour in the cerebellopontine angle. J Clin Neurosci 12:829–832. [DOI] [PubMed] [Google Scholar]
- 4. Bisceglia M, Dimitri L, Giannatempo G, Carotenuto V, Bianco M, Monte V et al (2011) Solitary fibrous tumor of the central nervous system: report of an additional 5 cases with comprehensive literature review. Int J Surg Pathol 19:476–486. [DOI] [PubMed] [Google Scholar]
- 5. Bisceglia M, Galliani C, Giannatempo G, Lauriola W, Bianco M, D'Angelo V et al (2011) Solitary fibrous tumor of the central nervous system: a 15‐year literature survey of 220 cases. Adv Anat Pathol 18:356–392. [DOI] [PubMed] [Google Scholar]
- 6. Bohinski RJ, Mendel E, Aldape KD, Rhines LD (2004) Intramedullary and extramedullary solitary fibrous tumor of the cervical spine. Case report and review of the literature. J Neurosurg 100(4 Suppl Spine): 358–363. [DOI] [PubMed] [Google Scholar]
- 7. Brunnemann RB, Ro JY, Ordonez NG, Mooney J, El‐Naggar AK, Ayala AG (1999) Extrapleural solitary fibrous tumor: a clinicopathologic study of 24 cases. Mod Pathol 12:1034–1042. [PubMed] [Google Scholar]
- 8. Burger PC, Scheithauer BW (2007) Tumors of the Central Nervous System in Atlas of Tumor Pathology. Series 4, Fascicle7. American Registry of Pathology and the Armed Forces Institute of Pathology: Washington, DC. [Google Scholar]
- 9. Carneiro SS, Scheithauer BW, Nascimento AG, Hirose T, Davis DH (1996) Solitary fibrous tumor of the meninges: a lesion distinct from fibrous meningioma. A clinicopathologic and immunohistochemical study. Am J Clin Pathol 106:217–224. [DOI] [PubMed] [Google Scholar]
- 10. Carretta A, Bandiera A, Melloni G, Ciriaco P, Arrigoni G, Rizzo N et al (2006) Solitary fibrous tumors of the pleura: immunohistochemical analysis and evaluation of prognostic factors after surgical treatment. J Surg Oncol 94:40–44. [DOI] [PubMed] [Google Scholar]
- 11. Chang IW, Lin JW, Wu YT (2011) The status of MGMT protein expression is a prognostic factor for meningeal hemangiopericytoma: a clinicopathologic and immunohistochemical study of 12 cases at a single institution. J Neurooncol 105:563–572. [DOI] [PubMed] [Google Scholar]
- 12. Choi CY, Han SR, Yee GT, Joo M (2011) An intracranial malignant solitary fibrous tumor. Neuropathology 31:177–182. [DOI] [PubMed] [Google Scholar]
- 13. D'Amore ES, Manivel JC, Sung JH (1990) Soft tissue and meningeal hemangiopericytomas: an immunohistochemical and ultrastructural study. Hum Pathol 21:414–423. [DOI] [PubMed] [Google Scholar]
- 14. De Perrot M, Fischer S, Brundler MA, Sekine Y, Keshavjee S (2002) Solitary fibrous tumors of the pleura. Ann Thorac Surg 74:285–293. [DOI] [PubMed] [Google Scholar]
- 15. De Ribaupierre S, Meagher‐Villemure K, Agazzi S, Rilliet B (2006) Meningeal solitary fibrous tumour in a child. Childs Nerv Syst 22:619–622. [DOI] [PubMed] [Google Scholar]
- 16. Ecker RD, Marsh WR, Pollock BE, Kurtkaya‐Yapicier O, McClelland R, Scheithauer BW, Buckner JC (2003) Hemangiopericytoma in the central nervous system: treatment, pathological features, and long‐term follow up in 38 patients. J Neurosurg 98:1182–1187. [DOI] [PubMed] [Google Scholar]
- 17. Fletcher CDM, Unni KK, Mertens F (2002) WHO Classification of Tumours. Pathology and Genetics of Tumours of Soft Tissue and Bone. IARC Press: Lyon. [Google Scholar]
- 18. Gengler C, Guillou L (2006) Solitary fibrous tumour and haemangiopericytoma: evolution of a concept. Histopathology 48:63–74. [DOI] [PubMed] [Google Scholar]
- 19. Giannini C, Rushing EJ, Hainfelier JA (2007) Hemangiopericytoma. In: WHO Classification of Tumours of the Central Nervous System, 4th edn. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds), pp. 178–180. IARC: Lyon. [Google Scholar]
- 20. Gold JS, Antonescu CR, Hajdu C, Ferrone CR, Hussain M, Lewis JJ et al (2002) Clinicopathologic correlates of solitary fibrous tumors. Cancer 94:1057–1068. [PubMed] [Google Scholar]
- 21. Guevara P, Escobar‐Arriaga E, Saavedra‐Perez D, Martinez‐Rumayor A, Flores‐Estrada D, Rembao D et al (2010) Angiogenesis and expression of estrogen and progesterone receptors as predictive factors for recurrence of meningioma. J Neurooncol 98:379–384. [DOI] [PubMed] [Google Scholar]
- 22. Hajdu M, Singer S, Maki RG, Schwartz GK, Keohan ML, Antonescu CR (2010) IGF2 over‐expression in solitary fibrous tumours is independent of anatomical location and is related to loss of imprinting. J Pathol 221:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hanau CA, Miettinen M (1995) Solitary fibrous tumor: histological and immunohistochemical spectrum of benign and malignant variants presenting at different sites. Hum Pathol 26:440–449. [DOI] [PubMed] [Google Scholar]
- 24. Hayashi Y, Uchiyama N, Hayashi Y, Nakada M, Iwato M, Kita D et al (2009) A reevaluation of the primary diagnosis of hemangiopericytoma and the clinical importance of differential diagnosis from solitary fibrous tumor of the central nervous system. Clin Neurol Neurosurg 111:34–38. [DOI] [PubMed] [Google Scholar]
- 25. Hori E, Kurimoto M, Fukuda O, Takahashi C, Nagai S, Oya T, Endo S (2007) Recurrent intracranial solitary fibrous tumor initially diagnosed as haemangiopericytoma. Brain Tumor Pathol 24:31–34. [DOI] [PubMed] [Google Scholar]
- 26. Hu SW, Tsai KB, Yang SF, Lee KS, Chai CY (2005) Unusual solitary fibrous tumors in the central nervous system: a report of two cases. Kaohsiung J Med Sci 21:179–184. [DOI] [PubMed] [Google Scholar]
- 27. Kataoka H, Akiyama Y, Kubo S, Itoh H, Hamasuna R, Tajima N, Koono M (1999) Solitary fibrous tumor of the spinal nerve rootlet: case report and literature survey. Pathol Int 49:826–830. [DOI] [PubMed] [Google Scholar]
- 28. Kim KA, Gonzalez I, McComb JG, Giannotta SL (2004) Unusual presentations of cerebral solitary fibrous tumors: report of four cases. Neurosurgery 54:1004–1009, discussion 1009. [DOI] [PubMed] [Google Scholar]
- 29. Kocak A, Cayli SR, Sarac K, Aydin NE (2004) Intraventricular solitary fibrous tumor: an unusual tumor with radiological, ultrastructural, and immunohistochemical evaluation: case report. Neurosurgery 54:213–216, discussion 216–217. [DOI] [PubMed] [Google Scholar]
- 30. Leães CG, Meurer RT, Coutinho LB, Ferreira NP, Pereira‐Lima JF, Da Costa Oliveira M (2010) Immunohistochemical expression of aromatase and estrogen, androgen and progesterone receptors in normal and neoplastic human meningeal cells. Neuropathology 30:44–49. [DOI] [PubMed] [Google Scholar]
- 31. Mena H, Ribas JL, Pezeshkpour GH, Cowan DN, Parisi JE (1991) Hemangiopericytoma of the central nervous system: a review of 94 cases. Hum Pathol 22:4–91. [DOI] [PubMed] [Google Scholar]
- 32. Metellus P, Bouvier C, Guyotat J, Fuentes S, Jouvet A, Vasiljevic A et al (2007) Solitary fibrous tumors of the central nervous system: clinicopathological and therapeutic considerations of 18 cases. Neurosurgery 60:715–722. [DOI] [PubMed] [Google Scholar]
- 33. Miyashita K, Hayashi Y, Fujisawa H, Hasegawa M, Yamashita J (2004) Recurrent intracranial solitary fibrous tumor with cerebrospinal fluid dissemination. Case report. J Neurosurg 101:1045–1048. [DOI] [PubMed] [Google Scholar]
- 34. Ng HK, Choi PC, Wong CW, To KF, Poon WS (2000) Metastatic solitary fibrous tumor of the meninges. Case report. J Neurosurg 93:490–493. [DOI] [PubMed] [Google Scholar]
- 35. Ogawa K, Tada T, Takahashi S, Sugiyama N, Inaguma S, Takahashi SS, Shirai T (2004) Malignant solitary fibrous tumor of the meninges. Virchows Arch 444:459–464. [DOI] [PubMed] [Google Scholar]
- 36. Pakasa NM, Pasquier B, Chambonniere ML, Morrison AL, Khaddage A, Perret AG et al (2005) Atypical presentations of solitary fibrous tumors of the central nervous system: an analysis of unusual clinicopathological and outcome patterns in three new cases with a review of the literature. Virchows Arch 447:81–86. [DOI] [PubMed] [Google Scholar]
- 37. Park MS, Araujo DM (2009) New insights into the hemangiopericytoma/solitary fibrous tumor spectrum of tumors. Curr Opin Oncol 21:327–331. [DOI] [PubMed] [Google Scholar]
- 38. Paulus W, Scheithauer BW, Perry A (2007) Mesenchymal non‐meningothelial tumors. In: WHO Classification of Tumours of the Central Nervous System, 4th edn. Louis DN, Ohgaki H, Wiestler OD, Cavenee WK (eds), pp. 173–177. IARC: Lyon. [Google Scholar]
- 39. Perry A, Scheithauer BW, Nascimento AG (1997) The immunophenotypic spectrum of meningeal hemangiopericytoma: a comparison with fibrous meningioma and solitary fibrous tumor of meninges. Am J Surg Pathol 21:1354–1360. [DOI] [PubMed] [Google Scholar]
- 40. Pizem J, Matos B, Popovic M (2004) Malignant intracranial solitary fibrous tumour with four recurrences over a 30‐year period. Neuropathol Appl Neurobiol 30:696–701. [DOI] [PubMed] [Google Scholar]
- 41. Rousseau A, Kujas M, van Effenterre R, Boch AL, Carpentier A, Leroy JP, Poirier J (2005) Primary intracranial myopericytoma: report of three cases and review of the literature. Neuropathol Appl Neurobiol 31:641–648. [DOI] [PubMed] [Google Scholar]
- 42. Rutkowski MJ, Jian BJ, Bloch O, Chen C, Sughrue ME, Tihan T et al (2011) Intracranial hemangiopericytoma: clinical experience and treatment considerations in a modern series of 40 adult patients. Cancer [Epub ahead of print, doi: 10.1002/cncr.26411]. [DOI] [PubMed] [Google Scholar]
- 43. Sandberg AA, Bridge JA (2002) Updates on the cytogenetics and molecular genetics of bone and soft tissue tumors. Synovial sarcoma. Cancer Genet Cytogenet 133:1–23. [DOI] [PubMed] [Google Scholar]
- 44. Scheithauer BW, Fuller GN, Wandenberg SR (2008) The 2007 WHO classification of tumors of the nervous system: controversies in surgical neuropathology. Brain Pathol 18:307–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Schirosi L, Lantuejoul S, Cavazza A, Murer B, Yves Brichon P, Migaldi M et al (2008) Pleuro‐pulmonary solitary fibrous tumors: a clinicopathologic, immunohistochemical, and molecular study of 88 cases confirming the prognostic value of de Perrot staging system and p53 expression, and evaluating the role of c‐kit, BRAF, PDGFRs (alpha/beta), c‐met, and EGFR. Am J Surg Pathol 32:1627–1642. [DOI] [PubMed] [Google Scholar]
- 46. Shidoh S, Yoshida K, Takahashi S, Mikami S, Mukai M, Kawase T (2010) Parasagittal solitary fibrous tumor resembling hemangiopericytoma. Brain Tumor Pathol 27:35–38. [DOI] [PubMed] [Google Scholar]
- 47. Slavik T, Bentley RC, Gray L, Fuchs HE, McLendon RE (1998) Solitary fibrous tumor of the meninges occurring after irradiation of a mixed germ cell tumor of the pineal gland. Clin Neuropathol 17:55–60. [PubMed] [Google Scholar]
- 48. Soyuer S, Chang EL, Selek U, Shi W, Maor MH, DeMonte F (2004) Radiotherapy after surgery for benign cerebral meningioma. Radiother Oncol 71:85–90. [DOI] [PubMed] [Google Scholar]
- 49. Surendrababu NR, Chacko G, Daniel RT, Chacko AG (2006) Solitary fibrous tumor of the lateral ventricle: CT appearances and pathologic correlation with follow‐up. AJNR Am J Neuroradiol 27:2135–2136. [PMC free article] [PubMed] [Google Scholar]
- 50. Suzuki SO, Fukui M, Nishio S, Iwaki T (2000) Clinicopathological features of solitary fibrous tumor of the meninges: an immunohistochemical reappraisal of cases previously diagnosed to be fibrous meningioma or hemangiopericytoma. Pathol Int 50:808–817. [DOI] [PubMed] [Google Scholar]
- 51. Tihan T, Viglione M, Rosenblum MK, Olivi A, Burger PC (2003) Solitary fibrous tumors in the central nervous system. A clinicopathologic review of 18 cases and comparison to meningeal hemangiopericytomas. Arch Pathol Lab Med 127:432–439. [DOI] [PubMed] [Google Scholar]
- 52. Vallat‐Decouvelaere AV, Dry SM, Fletcher CD (1998) Atypical and malignant solitary fibrous tumors in extrathoracic locations: evidence of their comparability to intra‐thoracic tumors. Am J Surg Pathol 22:1501–1511. [DOI] [PubMed] [Google Scholar]
- 53. Verbeke SL, Fletcher CD, Alberghini M, Daugaard S, Flanagan AM, Parratt T et al (2010) A reappraisal of hemangiopericytoma of bone; analysis of cases reclassified as synovial sarcoma and solitary fibrous tumor of bone. Am J Surg Pathol 34:777–783. [DOI] [PubMed] [Google Scholar]


