Abstract
Background
The present study was designed to reveal the trajectory of self-reported somatic symptom burden and sleep quality over time in patients with COVID-19 and to identify prognostic factors for greater somatic symptom burden and sleep disturbance.
Material/Methods
Seventy-four patients with COVID-19 were prospectively followed for longitudinal assessment of somatic symptom burden and sleep quality. We used the 8-item Somatic Symptom Scale (SSS-8) and the modified Medical Research Council (mMRC) scale for somatic symptom burden and the Pittsburgh Sleep Quality Index for sleep quality investigation. Univariate and multivariate analyses were performed to identify independent factors associated with somatic symptom burden and sleep quality.
Results
Although the degree of physical discomfort and sleep quality issues tended to decline during self-quarantine, patients still experienced these problems to a certain degree. Univariate and multivariate analyses showed that SSS-8 scores at admission (relative risk [RR] 1.234, 95% CI 1.075–1.417, P=0.003) and mMRC scores at discharge (RR 2.420, 95% CI 1.251–4.682, P=0.009) were 2 independent prognostic indicators of somatic symptom burden. In addition, muscle pain as a chief complaint (RR 4.682, 95% CI 1.247–17.580, P<0.022) and history of use of hypnotic drugs (RR 0.148, 95% CI 0.029–0.749, P<0.019) were 2 independent indicators of patient sleep quality during hospitalization.
Conclusions
To the best of our knowledge, the present study was the first dynamic assessment of the somatic symptom burden and sleep quality in patients with COVID-19 during hospitalization and quarantine after discharge. Patients with high somatic symptom burden at admission, especially muscle pain as the chief complaint, are prone to having a higher physical burden and more sleep disturbance at discharge.
Keywords: COVID-19, Signs and Symptoms, Sleep Disorders
Background
Numerous studies have revealed the initiation and development of different clinical manifestations of coronavirus disease 2019 (COVID-19) [1–12]. Huang et al described the clinical characteristics of clustered COVID-19 cases in early January 2020 [1] in one of the earliest studies of symptoms in patients with COVID-19. Subsequently, articles on various aspects of COVID-19 features, such as distinct regions [7,11], patient groups [2,6,12], symptoms in specific organ systems [4,5], and mortality rates [8–10], have been published. These studies have provided a better understanding of the evolution of clinical symptoms of COVID-19 and revealed different aspects of the physiological burden of common somatic symptoms (somatic symptom burden) in patients over time. How somatic symptom burden develops over time, however, is still unclear. Furthermore, there is a paucity of studies on the physiological status of patients with COVID-19 after they are discharged from the hospital [13–15].
Apart from the need to trace the development of somatic symptom burden, researchers should also pay attention to psychological stress in patients with COVID-19. Among the psychological factors that have been investigated [16,17], sleep quality has seldom been addressed [18,19]. No study has compared sleep disturbances during hospitalization versus the self-quarantine period.
Given the increase in the number of patients with COVID-19 who have been discharged from the hospital, it is important to study the trajectory of their rehabilitation during the self-quarantine period. There is a need to understand chronological changes in somatic symptom burden and sleep quality in patients with COVID-19 to improve post-discharge medical care and more easily identify patients in need of close follow-up.
The present prospective study was designed to assess the sequential development of somatic symptom burden and sleep disturbance in patients with COVID-19 and to identify clinicopathological factors associated with these issues.
Material and Methods
Study Design
A convenience sample of 74 patients with COVID-19 from Huoshenshan Hospital was enrolled in this prospective cohort study from February 4, 2020 to May 5, 2020. Well-designed and validated questionnaires were used to collect data from these patients on their physiological burden and sleep quality. In a nationwide, population-based study, the 8-item Somatic Symptom Scale (SSS-8), modified from the well-known 15-item Patient Health Questionnaire (PHQ-15) [20], was shown to have excellent item characteristics and reliability, with a Cronbach α value of 0.81 [21]. With advantages such as briefness, excellent practicality, and verified cutoff values, the SSS-8 has enabled researchers to identify individuals with mild, low, medium, high, and very high somatic symptom burdens [21]. Moreover, because respiratory symptoms are frequently reported in patients with COVID-19 [1,22,23], we assessed the severity of dyspnea using the modified Medical Research Council (mMRC) scale, which has been widely used in patients with chronic respiratory diseases [24]. The Pittsburgh Sleep Quality Index (PSQI) is effective in distinguishing individuals with distinct sleep quality [25–27] and was used in the present study to evaluate the sleep quality of patients with COVID-19.
All of the patients were required to complete the questionnaires at admission (T1), on discharge (T2), and 2 weeks (T3) and 1 month after discharge (T4). The SSS-8 and mMRC were completed at 4 time points. Because the PSQI was designed to evaluate sleep quality and mental health status over a period of time, it was only completed twice: during hospitalization (T2) and subsequent self-quarantine at home (T4). All of the questionnaires were distributed online. Patients who had 2 consecutive negative results on a nucleic acid test for severe acute respiratory syndrome coronavirus 2 virus were discharged from the hospital and underwent self-quarantine at home.
Institutional Review Board Approval
The present study was approved by the Ethics Committees of 3 hospitals: Huoshenshan Hospital (Wuhan, China), Guangdong Provincial People’s Hospital (Guangzhou, China), and General Hospital of Southern Theater Command (Guangzhou, China). Informed consent was obtained from all patients when the online questionnaire was distributed. The authors are accountable for all aspects of the work and for ensuring that questions related to the accuracy or integrity of any part of the work were appropriately investigated and resolved. The study conformed to the provisions of the Declaration of Helsinki (as revised in 2013).
Patient Diagnosis
All of the patients were diagnosed and treated according to the 6th edition of the Diagnosis and Treatment Guideline for COVID-19 issued by the National Health Commission of the People’s Republic of China [28]. Patients with fever, respiratory symptoms, and evidence of pneumonia on imaging at admission were classified as having moderately severe cases, while severe cases were those that met any of the following criteria: dyspnea and respiratory rate ≥30/min; oxygen saturation ≤93% at resting state; or PaO2/FiO2 ≤300 mmHg (1 mmHg=0.133 kPa).
Patient Inclusion and Exclusion Criteria
The patients included in the study had a confirmed diagnosis of moderately severe or severe COVID-19, were aged ≥18 years, and were willing to sign the informed consent. Patients were excluded if they were suspected of having but not confirmed to have COVID-19, had incomplete medical records that could affect statistical analyses, or died during the follow-up period (Figure 1).
Figure 1.
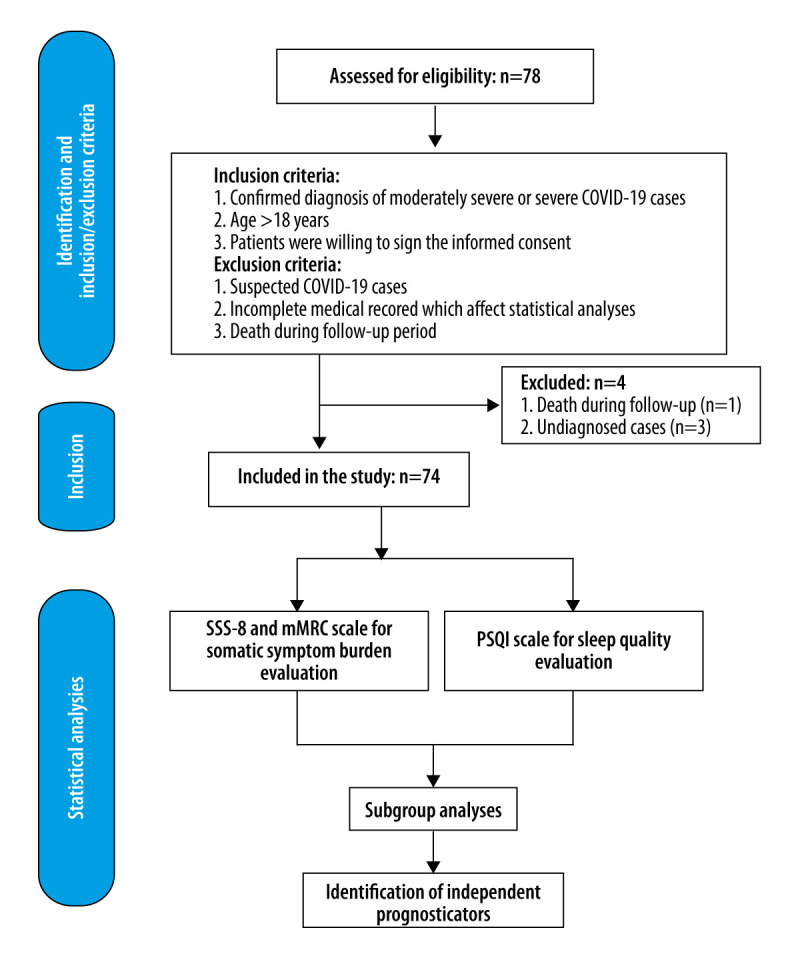
Study design and data analyses.
Statistical Analysis
For statistical purposes, the SSS-8 scores were divided into 2 groups: high and low somatic symptom burden. Patients with minimal (0–3) and low (4–7) physical discomfort were defined as the low somatic symptom burden group, while patients with SSS-8 scores from 7 to 32 were defined as the high somatic symptom burden group. Patients also were divided into groups for better (PSQI ≤5) or worse (PSQI >5) sleep quality [29]. The SSS-8 scale, mMRC scale, and PSQI were validated by calculating the Kaiser-Meyer-Olkin Measure of Sampling Adequacy and with Bartlett’s test of sphericity, whereas the reliability of the questionnaires was presented as Cronbach’s alpha. Descriptive statistics (frequency, mean, standard deviation, and median) were obtained for demographic, epidemiological, and clinical variables. The SSS-8 scores were stratified using previously validated cutoff values (0–3: no to minimal severity, 4–7: low severity; 8–11: medium severity; 12–15: high severity; 16–32: very high severity) [21]. Intergroup comparisons of continuous variables were conducted using the t test and one-way analysis of variance. Non-parametric tests were used for the variables that failed to meet the assumptions of normal distribution or equal variance. Categorical variables were compared by using a chi-square test when appropriate (expected frequency >5). Otherwise, the Fisher exact test was performed. Univariate and multivariate binary logistic regression were performed using the stepwise forward procedure to identify independent factors associated with stratified severity of physiological and psychological burden in patients with COVID-19. All statistical analyses were two-sided and P<0.05 was considered statistically significant. The analyses were carried out with IBM Statistical Package for Social Science (SPSS), version 26 for Windows (SPSS, Inc., Chicago, Illinois, United States). GraphPad Prism 8.0 (GraphPad Software, La Jolla, California, United States) was used to generate all of the figures.
Results
Clinicopathological Characteristics of Patients with COVID-19
We included 74 patients with COVID-19 (44 men [59.46%] and 30 women [40.54%]; median age, 56 years). To shorten the time it took to distribute the questionnaires, we combined them at different time points. At T1 and T3, we combined the SSS-8 with the mMRC, while at T2 and T4, we combined the SSS-8, mMRC, and the PSQI. The overall response rates for the questionnaires at the T1, T2, T3, and T4 time points were 100%, 100%, 98.6%, and 94.5%, respectively. Of the 74 enrolled patients, 60 were diagnosed with moderately severe COVID-19. Common chief complaints included shortness of breath (36.5%), muscle pain (20.3%), fever (73.0%), fatigue (29.7%), productive cough (43.2%), and dry cough (24.3%) (Table 1). The median length of hospital stay was 21 days. Eighty-three percent of the patients (n=61) had a previous history of COVID-19-related admission. Further detailed clinicopathological information is provided in Table 1.
Table 1.
Clinicopathological characteristics of COVID-19 patients.
| Clinicopathological characteristics | n (%) |
|---|---|
| Sex | |
| Male | 44 (59.46) |
| Female | 30 (40.54) |
| Age (mean±SD) | 52.3±13.3 |
| Chief complaint | |
| Shortness of breath | 27 (36.5) |
| Muscle pain | 15 (20.3) |
| Fever | 54 (73.0) |
| Chills | 6 (8.1) |
| Fatigue | 22 (29.7) |
| Headache | 3 (4.1) |
| Dry mouth | 1 (1.35) |
| Sore throat | 1 (1.35) |
| Dyspnea | 5 (6.8) |
| Cough (sputum production) | 32 (43.2) |
| Dry cough | 18 (24.3) |
| Palpitation | 3 (4.1) |
| Chest tightness | 10 (13.5) |
| Chest pain | 3 (4.1) |
| Nausea | 2 (2.7) |
| Diarrhea | 5 (6.8) |
| Duration of chief complain (mean±SD) | 23.7±13.7 |
| Diagnosis | |
| Moderate cases | 60 (81.08) |
| Severe cases | 14 (18.92) |
| Comorbidities | |
| Hypertension | 21 (28.38) |
| Aneurysm | 1 (1.35) |
| Hyperlipidemia | 2 (2.70) |
| Coronary atherosclerotic heart disease | 3 (4.05) |
| Cardiac insufficiency | 1 (1.35) |
| Bradycardia | 1 (1.35) |
| DVT | 1 (1.35) |
| Hypothyroidism | 2 (2.70) |
| Diabetes mellitus type II | 11 (14.86) |
| Bulla | 3 (4.05) |
| COPD | 2 (2.70) |
| Lung cancer | 1 (1.35) |
| Hyperuricemia | 1 (1.35) |
| Gouty arthritis | 1 (1.35) |
| Chronic gastritis | 1 (1.35) |
| Fatty liver | 2 (2.70) |
| Hepatitis B | 3 (4.05) |
| Gallstone | 1 (1.35) |
| Kidney cyst | 1 (1.35) |
| Chronic Kidney disease | 1 (1.35) |
| Kidney cancer | 1 (1.35) |
| Breast cancer | 1 (1.35) |
| Length of hospital stay/median (IQR) | 21 (12.0–34.3) |
| Multi-hospital admission history | |
| No | 13 (17.57) |
| Yes | 61 (82.43) |
| Drinking history | |
| No | 71 (95.95) |
| Yes | 3 (4.05) |
| Smoking history | |
| No | 68 (91.89) |
| Yes | 6 (8.11) |
| Antibiotics use during hospitalization | |
| No | 32 (43.24) |
| Yes | 42 (56.76) |
| Antiviral use during hospitalization | |
| No | 43 (58.11) |
| Yes | 31 (41.89) |
| Glucocorticoid use during hospitalization | |
| No | 61 (82.43) |
| Yes | 13 (17.57) |
| Hypnotic use during hospitalization | |
| No | 46 (62.2) |
| Yes | 28 (37.8) |
| Key lab results on admission (mean±SD) | |
| WBC (×109/L) | 6.14±1.94 |
| RBC (×1012/L) | 4.14±0.59 |
| Hemoglobin (g/L) | 128.36±16.92 |
| Platelet (×109/L) | 244.14±78.69 |
| Neutrophil (×109/L) | 3.98±1.80 |
| Lymphocyte (×109/L) | 1.55±0.57 |
| Monocyte (×109/L) | 0.46±0.17 |
| Eosinophil (×109/L) | 0.12±0.11 |
| Basophil (×109/L) | 0.02±0.01 |
| C-reactive protein (mg/L) | 14.65±33.03 |
| hs-C-reactive protein (mg/L) | 3.00±2.49 |
| PCT (ng/ml) | 0.07±0.09 |
| Additional key lab results during hospitalization (mean±SD) | |
| SARS-CoV-IgM antibody | 76.46±97.14 |
| SARS-CoV-IgG-antibody | 143.97±46.11 |
Reliability and Factorial Validity of the SSS-8 and mMRC Scales and PSQI
The reliability ratings for the SSS-8 and mMRC scales and PSQI in patients with COVID-19 patients, as assessed by Cronbach α, were 0.926, 0.837, and 0.877, respectively. In addition, the 3 scales showed high factorial validity (SSS-8: Kaiser-Meyer-Olkin [KMO]=0.737, X2=1881.762, P<0.001; mMRC: KMO=0.772, X2=142.033, P<0.001; PSQI: KMO=0.776, X2=522.820, P<0.001).
Change in Somatic Symptom Burden and Sleep Quality Over Time
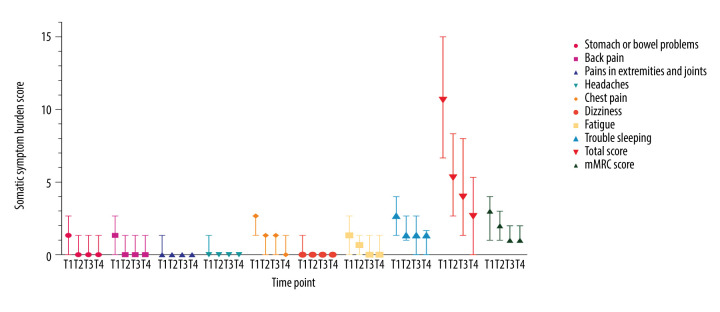
The overall somatic symptom burden decreased significantly over time. Results of all intergroup comparisons of the SSS-8 and mMRC scores at different time points (T1, T2, T3, and T4) were statistically significant (P<0.001). The rates of remission for the investigated symptoms were much more pronounced during hospitalization than during the self-quarantine period (Figure 2).
Figure 2.
Changes in somatic symptom burden over time in patients with COVID-19. Median scores of for somatic symptom burdens were evaluated using the Somatic Symptom Score (SSS-8) and modified Medical Research Council (mMRC) scales at 4 time points (T1, T2, T3, and T4). The baseline score for each symptom recorded at admission was the highest. A declining trend in the scores was observed throughout hospitalization and quarantine.
Moreover, the residual somatic symptom burden was observed at the time of discharge and during the self-quarantine period. At discharge, nearly one-third of the patients were still experiencing a high somatic symptom burden (SSS-8 score >7).
The median total SSS-8 scores at 2 and 4 weeks after discharge were 4 and 3, respectively. Chest pain and trouble sleeping were the 2 most pronounced complaints among those assessed.
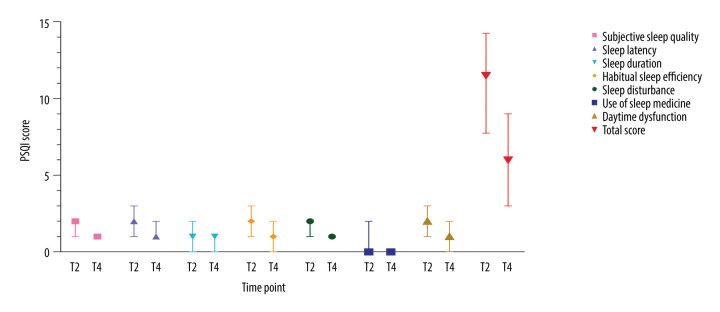
A Wilcoxon signed rank test revealed a significantly decreased PSQI score during the self-quarantine period compared to that during hospitalization (P<0.001) (Figure 3). However, the median score of 6 was relatively high (interquartile range [IQR]=3.0 to 9.0), indicating that more than 50% of the patients were disturbed by poor sleep quality. Subjective sleep quality and sleep latency were the 2 main components that accounted for the high PSQI score.
Figure 3.
Alterations in sleep quality over time in patients with COVID-19. Sleep quality during hospitalization and self-quarantine was assessed via median Pittsburgh Sleep Quality Index (PSQI) scores. Overall, sleep disturbances were more serious during hospitalization than during self-quarantine. Pronounced sleep quality issues (PSQI >5) were observed during self-quarantine.
Assessment of Prognostic Indicators of Somatic Symptom Burden at Discharge
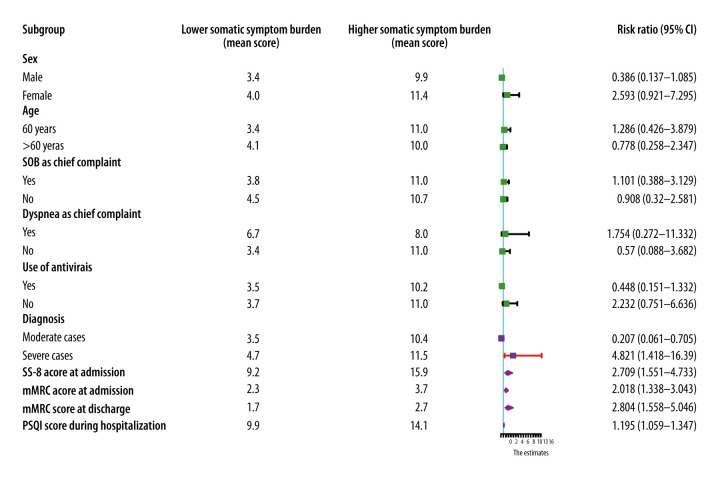
The median SSS-8 and mMRC scores at discharge were 5 (IQR: 3–8) and 2 (IQR: 1–3), respectively. The SSS-8 total scores at discharge were classified into low and high somatic symptom burden groups. Univariate analyses revealed that the severity of disease (severe vs moderate: RR 4.821, 95% CI 1.418–16.390, P<0.012), SSS-8 score at admission (RR 2.709, 95% CI 1.551–4.733, P<0.001), mMRC scores at admission (RR 2.018, 95% CI 1.338–3.043, P=0.001), mMRC scores at discharge (2.804, 95% CI 1.558–5.046, P=0.001), and PSQI scores during hospitalization (RR 1.195, 95% CI 1.059–1.347, P=0.004) were significantly associated with higher somatic symptom burden (Figure 4). Furthermore, multivariate analyses suggested that the SSS-8 scores at admission (RR 1.234, 95% CI 1.075–1.417, P=0.003) and the mMRC scores at discharge (RR 2.420, 95% CI 1.251–4.682, P=0.009) were 2 independent prognostic indicators of somatic symptom burden.
Figure 4.
Forest plot of prognostic indicators for somatic symptom burden at discharge. The severity of the disease, Somatic Symptom Score-8 and modified Medical Research Council scores at admission and Pittsburgh Sleep Quality Index score during hospitalization were significantly associated with patient self-reported physical outcomes. (Purple squares and diamonds indicate relative risk.)
Assessment of Prognostic Indicators of Sleep Quality During Hospitalization
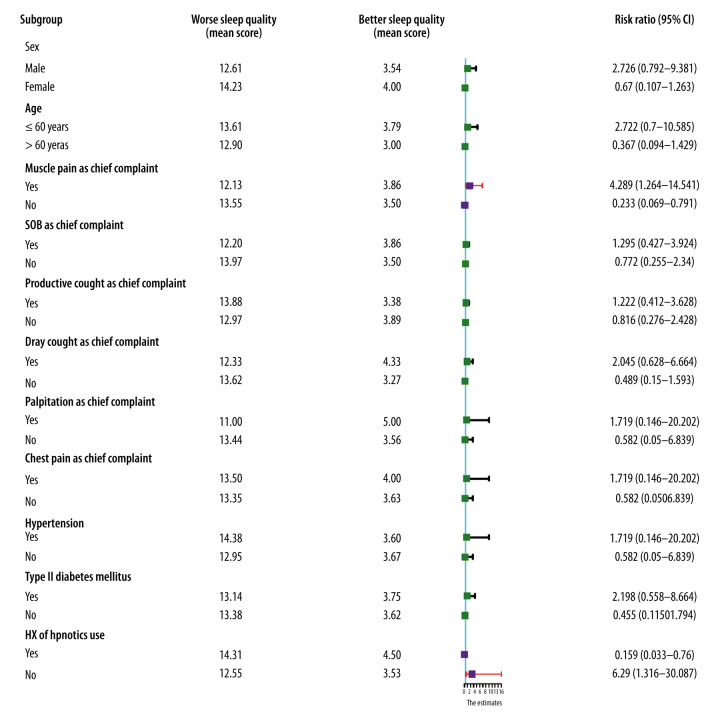
The median PSQI scores were 11.5 (IQR 7.75–14.25) and 6 (IQR 3.00–9.00) during hospitalization and 1-month self-quarantine, respectively. Univariate analyses indicated that muscle pain as the chief complaint (RR 4.287, 95% CI 1.264–14.541, P=0.019) was significantly associated with worse sleep quality than in patients who did not have that complaint. Use of hypnotic drugs during hospitalization (RR 0.159, 95% CI 0.033–0.760, P=0.021) was identified as a protective factor (Figure 5). A multivariate regression model suggested that muscle pain as the chief complaint (RR 4.682, 95% CI 1.247–17.580, P=0.022) and use of hypnotic drugs (RR 0.148, 95% CI 0.029–0.749, P=0.019) were 2 independent prognostic indicators of sleep quality.
Figure 5.
Forest plot of prognostic indicators for sleep quality during hospitalization. Muscle pain as the chief complaint was identified as a risk factor for sleep quality. A history of use of hypnotic drugs during hospitalization was a protective factor for ensuring better sleep quality. (Purple squares and diamonds indicate relative risk.)
Discussion
Despite the substantial number of studies of COVID-19 that have been ongoing in the last 7 months, little is known about the overall evolution of somatic symptom burden and sleep quality in patients with the virus. To address this issue, we conducted a 3-month prospective follow-up study of 74 patients with COVID-19. By tracing patient self-reported physiological and psychological burdens at 4 consecutive time points, we were able to record the development of somatic symptom burden and sleep quality in these patients. Furthermore, independent prognostic indicators associated with somatic symptom burden and sleep disturbance were identified.
More than half of the patients recruited for this study were older than age 50 years. Fever and productive cough were the 2 most common chief complaints, which is in keeping with previous findings [1,6,22]. We observed an overall declining trend in both somatic symptom burden and sleep quality in patients with COVID-19. It was worth noting that the residual somatic symptom burden and sleep disturbance persisted throughout the 1-month self-quarantine period. These results suggest that early close follow-up should be initiated and treatment of symptoms should be recommended to lessen the physiological and psychological burden for patients. Lack of regular follow-up could lead to worse clinical outcomes. Wang et al reported that although readmission is uncommon, the risk is higher in patients who have been discharged and have a latent or recurring physiological burden [30]. In a 4-week follow-up of elderly patients with COVID-19, individuals with dyspnea, cardiovascular disease, and chronic obstructive pulmonary disease had poor clinical outcomes after discharge [31]. To date, few studies have investigated the post-discharge status of patients with COVID-19. However, evidence from the present study underscores the value that physicians and other caregivers should place on post-discharge surveillance. To date, more than 83 million cases of COVID-19 have been confirmed and the moderate and severe cases account for a considerably large proportion [32]. Therefore, if post-discharge surveillance were initiated among patients with COVID-19 who are self-quarantining, a significant number of individuals would stand to benefit from the lessened somatic symptom burden and improved sleep quality.
The somatic symptom burden in patients with COVID-19 has been investigated in several studies [2,6,11,12]. Unfortunately, these descriptive studies did not stratify the severity of the overall somatic symptom burden; therefore, the information was insufficient on which to base the development of personalized intervention and prevention for individuals who are currently hospitalized. Also, few articles have specifically targeted mental health and sleep quality in patients with COVID-19 who have no preexisting psychiatric problems. Bo et al showed that posttraumatic stress syndrome occurs in more than 95% of patients who are hospitalized [33]. Nearly 30% of patients who recover from COVID-19 develop depressive episodes [34]. However, neither of these studies stratified the severity of the psychological problem nor did they identify patients at higher risk of suffering from prolonged psychological problems, such as depressive episodes and sleep disturbances. In the present study, we performed binary logistic analyses to identify patients with COVID-19 who had a potentially high somatic symptom burden and sleep disturbance. A univariate and multivariate binary logistic regression model revealed that SSS-8 score at admission and mMRC score at discharge were 2 independent predictors of somatic symptom burden at discharge. Our data suggest that the patients with a higher somatic symptom burden at admission were prone to poor recovery. The severity of dyspnea significantly contributed to the degree of somatic symptom burden. Hence, treatment of dyspnea symptoms during the self-quarantine period could be beneficial.
Our results further indicate that muscle pain as the chief complaint and use of hypnotic drugs during hospitalization were independent prognostic indicators for sleep quality. Previous reports of the close association between muscle pain and sleep disturbance [35] were echoed by our findings in patients with COVID-19. Moreover, patients with severe COVID-19 and comorbidities reportedly are likely to have muscle pain [1,22,35–37]. In this regard, we suggest close monitoring of patients with COVID-19 who have muscle pain on admission. So far, data on the protective effects of hypnotic drugs on sleep quality in patients with COVID-19 have not been captured in the documented reports, likely because few studies have investigated sleep disturbance in patients with COVID-19. Our findings on the beneficial effects of hypnotic drugs could provide evidence for early initiation of these agents in patients who have sleep disturbance during hospitalization and suggest a need to further investigate the specific timing and duration of use of the drugs.
The present prospective study was the first to establish the overall trend in both somatic symptom burden and sleep quality in patients with COVID-19, and to identify independent prognostic indicators for them. Identification of factors that significantly increase and decrease risk would facilitate timely psychological intervention during hospitalization and post-discharge follow-up, at an early stage.
Although our findings about patient-reported outcomes over time were significant, the present study had some limitations. The assessment had a small sample size of only 74 patients. Furthermore, because it was the first study to explore somatic symptom burden and sleep quality in patients with COVID-19, we are cautious about drawing any conclusions based on the findings. Therefore, future prospective studies, based on large sample sizes, are required to confirm the results of the present study.
Conclusions
To the best of our knowledge, the present study is the first dynamic assessment of somatic symptom burden and sleep quality in patients with COVID-19 during hospitalization and the quarantine period after discharge. Persistent somatic symptoms and sleep disturbance were detected in a considerable number of patients despite their discharge from the hospital. Patients with high somatic symptom burden at admission, especially muscle pain as the chief complaint, were prone to have sleep disturbance. This suggests to us that symptom-specific treatment, such as a painkiller for muscle pain, should be considered to manage sleep disturbance before hypnotic drugs are prescribed.
Supplementary Data
Supplementary Table 1.
The Somatic Symptom Scale-8 (SSS8).
| Not at all | A little bit | Somewhat | Quite a bit | Very much | |
|---|---|---|---|---|---|
| 1. Stomach or bowel problems | 0 | 1 | 2 | 3 | 4 |
| 2. Back pain | 0 | 1 | 2 | 3 | 4 |
| 3. Pain in your arms, legs, or joints | 0 | 1 | 2 | 3 | 4 |
| 4. Headaches | 0 | 1 | 2 | 3 | 4 |
| 6. Dizziness | 0 | 1 | 2 | 3 | 4 |
| 5. Chest pain or shortness of breath | 0 | 1 | 2 | 3 | 4 |
| 7. Feeling tired or having low energy | 0 | 1 | 2 | 3 | 4 |
| 8. Trouble sleeping | 0 | 1 | 2 | 3 | 4 |
Supplementary Table 2.
The modified Medical Research Council scale (mMRC).
| Severity | Grade | |
|---|---|---|
| Dyspnea only with strenuous exercise | No dyspnea | 0 |
| Dyspnea when hurrying or walking up a slight hill | Slight dyspnea | 1 |
| Walks slower than people of the same age because of dyspnea or has to stop for breath when walking at own pace | Moderate dyspnea | 2 |
| Stops for breath after walking 100 yards (91m) or after a few minutes | Severe dyspnea | 3 |
| Too dyspneic to leave house or breathless when dressing | Very severe dyspnea | 4 |
Supplementary Table 3.
Pittsburgh Sleep Quality Index (PSQI).
|
Instructions: The following questions were designed to investigated your sleep quality during your hospitalization and self-quarantine period. Please answer all questions. 1. During the past month, what time have you usually gone to bed at night? ________________________________________ 2. During the past month, how long (in minutes) has it usually taken you to fall asleep each night? ______________________ 3. During the past month, what time have you usually gotten up in the morning? ____________________________________ 4. During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spent in bed.) __________________________________________________________________________________________ | ||||
| None | Less than once a week | Once or twice a week | Three or more times a week | |
| 5. During hospitalization/self-quarantine period, how often have you had trouble sleeping because you… | ||||
| a. Cannot get to sleep within 30 minutes | ||||
| b. Wake up in the middle of the night or early morning | ||||
| c. Have to get up to use the bathroom | ||||
| d. Cannot breathe comfortably | ||||
| e. Cough or snore loudly | ||||
| f. Feel too cold | ||||
| g. Feel too hot | ||||
| h. Have bad dreams | ||||
| i. Have pain | ||||
| j. Other reason(s), please describe: | ||||
| 6. During hospitalization/self-quarantine period, how often have you taken medicine to help you sleep (prescribed or “over the counter”)? | ||||
| 7. During hospitalization/self-quarantine period, how often have you had trouble staying awake while eating meals? | ||||
| No problem at all | Only a very slight problem | Somewhat of a problem | A very big problem | |
| 8. During hospitalization/self-quarantine period, how much of a problem has it been for you to keep up enough enthusiasm to get things done? | ||||
| Very good | Fairly good | Fairly bad | Very bad | |
| 9. During hospitalization/self-quarantine period, how would you rate your sleep quality overall? | ||||
| No bed partner or room mate | Partner/room mate in other room | Partner in same room but not same bed | Partner in same bed | |
| 10. Do you have a bed partner or roommate? | ||||
| None | Less than once a week | Once or twice a week | Three or more times a week | |
| If you have a roommate or bed partner, ask him/her how often in hospitalization/self-quarantine period, you have had: | ||||
| a. Loud snoring | ||||
| b. Long pauses between breaths while asleep | ||||
| c. Legs twitching or jerking while you sleep | ||||
| d. Episodes of disorientation or confusion during sleep | ||||
| e. Other restlessness while you sleep, please describe: | ||||
Acknowledgments
We are grateful to the Freescience Editing Service for English language editing.
Footnotes
Availability of Data and Material
All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Tables 1–3.
Conflict of Interest
None.
Source of support: This work was supported by a grant from the 2020–2021 Popularization of Science and Technology Innovation Special Project of Guangdong Province of China (No. 2020A1414070007)
References
- 1.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen T, Dai Z, Mo P, et al. Clinical characteristics and outcomes of older patients with coronavirus disease 2019 (COVID-19) in Wuhan, China (2019): A single-centered, retrospective study. J Gerontol A Biol Sci Med Sci. 2020;75(9):1788–95. doi: 10.1093/gerona/glaa089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zu ZY, Jiang MD, Xu PP, et al. Coronavirus disease 2019 (COVID-19): A perspective from China. Radiology. 2020;296(2):E15–25. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han C, Duan C, Zhang S, et al. Digestive symptoms in COVID-19 patients with mild disease severity: Clinical presentation, stool viral RNA testing, and outcomes. Am J Gastroenterol. 2020;115(6):916–23. doi: 10.14309/ajg.0000000000000664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin X, Lian JS, Hu JH, et al. Epidemiological, clinical and virological characteristics of 74 cases of coronavirus-infected disease 2019 (COVID-19) with gastrointestinal symptoms. Gut. 2020;69(6):1002–9. doi: 10.1136/gutjnl-2020-320926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang Y, Lu X, Li Y, et al. Clinical course and outcomes of 344 intensive care patients with COVID-19. Am J Respir Crit Care Med. 2020;201(11):1430–34. doi: 10.1164/rccm.202003-0736LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Young BE, Ong SWX, Kalimuddin S, et al. Epidemiologic features and clinical course of patients infected with SARS-CoV-2 in Singapore. JAMA. 2020;323(15):1488–94. doi: 10.1001/jama.2020.3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weiss P, Murdoch DR. Clinical course and mortality risk of severe COVID-19. Lancet. 2020;395(10229):1014–15. doi: 10.1016/S0140-6736(20)30633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–62. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–81. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cummings MJ, Baldwin MR, Abrams D, et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: A prospective cohort study. Lancet. 2020;395(10241):1919–26. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee LYW, Cazier JB, Starkey T, et al. COVID-19 mortality in patients with cancer on chemotherapy or other anticancer treatments: A prospective cohort study. Lancet. 2020;395(10241):1919–26. doi: 10.1016/S0140-6736(20)31173-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu HQ, Yuan B, An YW, et al. Clinical characteristics and follow-up analysis of 324 discharged COVID-19 patients in Shenzhen during the recovery period. Int J Med Sci. 2021;18(2):347–55. doi: 10.7150/ijms.50873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao YM, Shang YM, Song WB, et al. Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine. 2020;25:100463. doi: 10.1016/j.eclinm.2020.100463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George PM, Barratt SL, Condliffe R, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. 2020;75(11):1009–16. doi: 10.1136/thoraxjnl-2020-215314. [DOI] [PubMed] [Google Scholar]
- 16.Guo Q, Zheng Y, Shi J, et al. Immediate psychological distress in quarantined patients with COVID-19 and its association with peripheral inflammation: A mixed-method study. Brain Behav Immun. 2020;88:17–27. doi: 10.1016/j.bbi.2020.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vindegaard N, Eriksen Benros M. COVID-19 pandemic and mental health consequences: Systematic review of the current evidence. Brain Behav Immun. 2020;89:531–42. doi: 10.1016/j.bbi.2020.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu K, Chen Y, Wu D, et al. Effects of progressive muscle relaxation on anxiety and sleep quality in patients with COVID-19. Complement Ther Clin Pract. 2020;39:101132. doi: 10.1016/j.ctcp.2020.101132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vitale JA, Perazzo P, Silingardi M, et al. Is disruption of sleep quality a consequence of severe Covid-19 infection? A case-series examination. Chronobiol Int. 2020;37(7):1110–14. doi: 10.1080/07420528.2020.1775241. [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JBW. The PHQ-15: Validity of a new measure for evaluating the severity of somatic symptoms. Psychosom Med. 2002;64(2):258–66. doi: 10.1097/00006842-200203000-00008. [DOI] [PubMed] [Google Scholar]
- 21.Gierk B, Kohlmann S, Kroenke K, et al. The somatic symptom scale-8 (SSS-8): A brief measure of somatic symptom burden. JAMA Intern Med. 2014;174(3):399–407. doi: 10.1001/jamainternmed.2013.12179. [DOI] [PubMed] [Google Scholar]
- 22.Guan WJ, Ni ZY, Hu Y, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382(18):1708–20. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li T, Lu H, Zhang W. Clinical observation and management of COVID-19 patients. Emerg Microbes Infect. 2020;9(1):687–90. doi: 10.1080/22221751.2020.1741327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang D, Hu B, Hu C, et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–69. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carney S, Koetters T, Cho M, et al. Differences in sleep disturbance parameters between oncology outpatients and their family caregivers. J Clin Oncol. 2011;29(8):1001–6. doi: 10.1200/JCO.2010.30.9104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crum-Cianflone NF, Roediger MP, Moore DJ, et al. Prevalence and factors associated with sleep disturbances among early-treated HIV-infected persons. Clin Infect Dis. 2012;54(10):1485–94. doi: 10.1093/cid/cis192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ghabril M, Jackson M, Gotur R, et al. Most Individuals with advanced cirrhosis have sleep disturbances, which are associated with poor quality of life. Clin Gastroenterol Hepatol. 2017;15(8):1271–1278. e1276. doi: 10.1016/j.cgh.2017.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.National Health Commission of the People’s Republic of China. 6th edition of Diagnosis and Treatment Guideline of COVID-19. 2020. http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2/files/b218cfeb1bc54639af227f922bf6b817.pdf[in Chinese] [DOI] [PMC free article] [PubMed]
- 29.Shorofsky M, Bourbeau J, Kimoff J, et al. Impaired sleep quality in COPD Is associated with exacerbations: The CanCOLD Cohort Study. Chest. 2019;156(5):852–63. doi: 10.1016/j.chest.2019.04.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Xu H, Jiang H, et al. Clinical features and outcomes of discharged coronavirus disease 2019 patients: A prospective cohort study. QJM. 2020;113(9):657–65. doi: 10.1093/qjmed/hcaa178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang L, He W, Yu X, et al. Coronavirus disease 2019 in elderly patients: Characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–45. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.World Health Organization. Weekly epidemiological update – 5 January 2021. https://www.who.int/publications/m/item/weekly-epidemiological-update---5-january-2021.
- 33.Bo HX, Li W, Yang Y, et al. Posttraumatic stress symptoms and attitude toward crisis mental health services among clinically stable patients with COVID-19 in China. Psychol Med. 2020 doi: 10.1017/S0033291720000999. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhang J, Lu H, Zeng H, et al. The differential psychological distress of populations affected by the COVID-19 pandemic. Brain Behav Immun. 2020;87:49–50. doi: 10.1016/j.bbi.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geneen LJ, Moore RA, Clarke C, et al. Physical activity and exercise for chronic pain in adults: An overview of Cochrane Reviews. Cochrane Database Syst Rev. 2017;4(4):CD011279. doi: 10.1002/14651858.CD011279.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan S, Xiang Y, Fang W, et al. Clinical features and treatment of COVID-19 patients in northeast Chongqing. J Med Virol. 2020;92(7):797–806. doi: 10.1002/jmv.25783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mao L, Jin H, Wang M, et al. Neurologic manifestations of hospitalized patients with Coronavirus disease 2019 in Wuhan, China. JAMA Neurol. 2020;77(6):1–9. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1.
The Somatic Symptom Scale-8 (SSS8).
| Not at all | A little bit | Somewhat | Quite a bit | Very much | |
|---|---|---|---|---|---|
| 1. Stomach or bowel problems | 0 | 1 | 2 | 3 | 4 |
| 2. Back pain | 0 | 1 | 2 | 3 | 4 |
| 3. Pain in your arms, legs, or joints | 0 | 1 | 2 | 3 | 4 |
| 4. Headaches | 0 | 1 | 2 | 3 | 4 |
| 6. Dizziness | 0 | 1 | 2 | 3 | 4 |
| 5. Chest pain or shortness of breath | 0 | 1 | 2 | 3 | 4 |
| 7. Feeling tired or having low energy | 0 | 1 | 2 | 3 | 4 |
| 8. Trouble sleeping | 0 | 1 | 2 | 3 | 4 |
Supplementary Table 2.
The modified Medical Research Council scale (mMRC).
| Severity | Grade | |
|---|---|---|
| Dyspnea only with strenuous exercise | No dyspnea | 0 |
| Dyspnea when hurrying or walking up a slight hill | Slight dyspnea | 1 |
| Walks slower than people of the same age because of dyspnea or has to stop for breath when walking at own pace | Moderate dyspnea | 2 |
| Stops for breath after walking 100 yards (91m) or after a few minutes | Severe dyspnea | 3 |
| Too dyspneic to leave house or breathless when dressing | Very severe dyspnea | 4 |
Supplementary Table 3.
Pittsburgh Sleep Quality Index (PSQI).
|
Instructions: The following questions were designed to investigated your sleep quality during your hospitalization and self-quarantine period. Please answer all questions. 1. During the past month, what time have you usually gone to bed at night? ________________________________________ 2. During the past month, how long (in minutes) has it usually taken you to fall asleep each night? ______________________ 3. During the past month, what time have you usually gotten up in the morning? ____________________________________ 4. During the past month, how many hours of actual sleep did you get at night? (This may be different than the number of hours you spent in bed.) __________________________________________________________________________________________ | ||||
| None | Less than once a week | Once or twice a week | Three or more times a week | |
| 5. During hospitalization/self-quarantine period, how often have you had trouble sleeping because you… | ||||
| a. Cannot get to sleep within 30 minutes | ||||
| b. Wake up in the middle of the night or early morning | ||||
| c. Have to get up to use the bathroom | ||||
| d. Cannot breathe comfortably | ||||
| e. Cough or snore loudly | ||||
| f. Feel too cold | ||||
| g. Feel too hot | ||||
| h. Have bad dreams | ||||
| i. Have pain | ||||
| j. Other reason(s), please describe: | ||||
| 6. During hospitalization/self-quarantine period, how often have you taken medicine to help you sleep (prescribed or “over the counter”)? | ||||
| 7. During hospitalization/self-quarantine period, how often have you had trouble staying awake while eating meals? | ||||
| No problem at all | Only a very slight problem | Somewhat of a problem | A very big problem | |
| 8. During hospitalization/self-quarantine period, how much of a problem has it been for you to keep up enough enthusiasm to get things done? | ||||
| Very good | Fairly good | Fairly bad | Very bad | |
| 9. During hospitalization/self-quarantine period, how would you rate your sleep quality overall? | ||||
| No bed partner or room mate | Partner/room mate in other room | Partner in same room but not same bed | Partner in same bed | |
| 10. Do you have a bed partner or roommate? | ||||
| None | Less than once a week | Once or twice a week | Three or more times a week | |
| If you have a roommate or bed partner, ask him/her how often in hospitalization/self-quarantine period, you have had: | ||||
| a. Loud snoring | ||||
| b. Long pauses between breaths while asleep | ||||
| c. Legs twitching or jerking while you sleep | ||||
| d. Episodes of disorientation or confusion during sleep | ||||
| e. Other restlessness while you sleep, please describe: | ||||