Salvage radiation therapy (SRT) for prostate cancer (PCa) recurrence after radical prostatectomy (RP) can offer long-term biochemical control in approximately 50–60% of patients [1]. SRT is commonly initiated in patients with serum prostate-specific antigen (PSA) of 0.1–1 ng/ml and recent phase 3 trial data show that early SRT is equivalent to adjuvant RT [2]. However, standard-of-care imaging is insufficiently sensitive for anatomic localization of recurrence. Therefore, SRT target volumes are usually drawn in the absence of radiographically visible disease [3,4].
Prostate-specific membrane antigen (PSMA) is highly overexpressed by PCa cells and represents a relevant target for PCa imaging and therapy. PSMA positron emission tomography (PET) using small radiolabeled ligands is highly sensitive, even at low PSA levels, and may offer early localization of PCa biochemical recurrence (BCR) [3,5].
In an international multicenter retrospective study, we found that PSMA PET had a major impact on SRT planning in 52 of 270 patients (19%) with early BCR (PSA <1.0 ng/ml) by revealing lesions not covered by the standard radiation fields, including both the prostate bed and pelvic lymph nodes, defined on computed tomography (CT) [6]. We hypothesized that this major impact of PSMA PET on patient selection and RT planning would translate into better outcomes from PSMA PET–guided SRT.
The purpose of the PSMA-SRT trial NCT03582774 is to evaluate the success rate of SRT for post-RP recurrence of PCa with and without planning based on PSMA PET [7]. This is a multicenter, prospective, randomized, controlled, open-label phase 3 clinical imaging trial conducted at University of California, Los Angeles (lead) and University of California, San Francisco that is powered for clinical outcome at 5 yr. The study is investigator-initiated and self-funded. There is no external funding source.
Patients scheduled for SRT of recurrence after primary RP and with PSA ≥0.1 ng/ml at the time of enrollment were eligible (no PSA upper limit). On the basis of our prior study, we hypothesized that PSMA PET would detect extrapelvic M1 disease in 13% of the patients [6]. These patients would probably not undergo SRT and therefore would not be included in the primary endpoint analysis. Indeed, the primary endpoint is the SRT success rate at 5 yr for patients who actually received SRT. We hypothesized that incorporation of PSMA PET in SRT planning will improve 5-yr biochemical progression–free survival (bPFS) by 20%: 60% in the control arm and 80% in the intervention arm at 5 yr. According to the sample size calculation, 90 patients are needed in each group to reach sufficient statistical power. A total sample size of 193 patients is needed assuming that 13 patients randomized to the intervention arm (n = 103) would experience extrapelvic disease and would not receive SRT.
The primary endpoint of the trial is the SRT success rate at 5 yr among patients who actually received SRT, measured as bPFS (with biochemical progression defined as PSA ≥0.2 ng/ml and rising after completion of SRT). Secondary endpoints include a subgroup analysis of the primary endpoint for patients with baseline PSA ≥0.5 ng/ml, metastasis free-survival, and change in the initial treatment intent after randomization.
Enrollment is complete: 193 patients were enrolled from September 6, 2018 to August 17, 2020 (Fig. 1). Seven of the 90 patients in the control arm (9%) dropped out of the study because they underwent PSMA PET at another institution, while one/103 patients in the intervention arm (1%) dropped out because of COVID-19-related complications. Median PSA at enrollment was 0.32 ng/ml (interquartile range [IQR] 0.17–1.35) in the control arm and 0.22 ng/ml (IQR 0.14–0.50) in the PSMA arm. Patients in the control group were staged using fluciclovine PET (27/83, 33%), computed tomography (30/83, 36%), bone scan (14/83 17%), magnetic resonance imaging (22/83 27%), or fluorodeoxyglucose PET (one/83 1%), while 28/83 had no imaging (34%; Table 1). In the intervention group, PSMA PET was positive in 38/102 patients (37%): nine/108 (9%) had PCa outside the pelvis (M1), 20/102 (20%) in pelvic nodes with or without concurrent recurrence in the prostate bed, and 13/102 (13%) in the prostate fossa only (Table 2).
Fig. 1 –
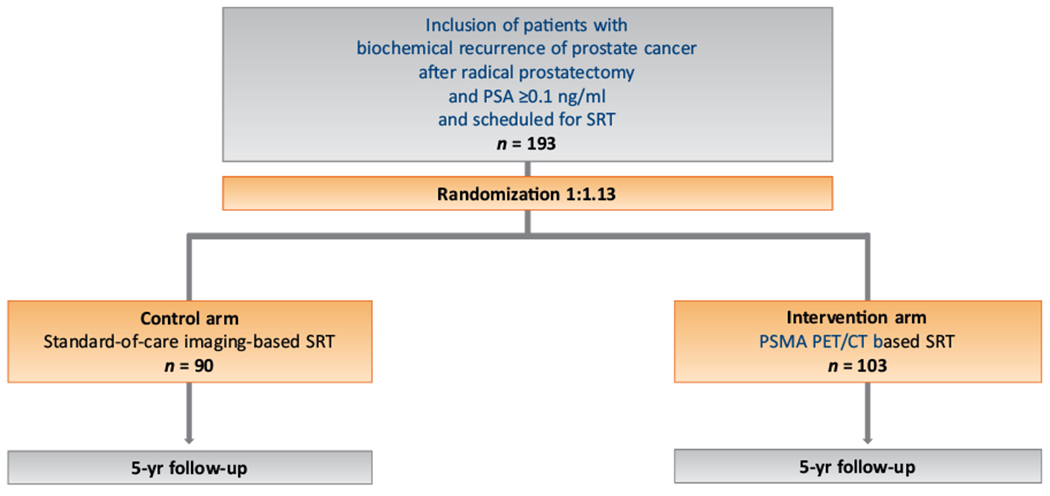
Study flowchart.
CT = computed tomography; PET = positron emission tomography; PSA = prostate-specific antigen; PSMA = prostate-specific membrane antigen; SRT = salvage radiotherapy.
Table 1 –
Imaging modalities used in the control group (n = 83)
| Imaging | Patients, n (%) |
|---|---|
| Fluciclovine PET only | 15 (18.1) |
| Fluciclovine PET + bone scan | 1 (1.2) |
| Fluciclovine PET + FDG PET | 1 (1.2) |
| Fluciclovine PET + MRI | 2 (2.4) |
| Fluciclovine PET + MRI + bone scan | 2 (2.4) |
| Fluciclovine PET + MRI + CT | 5 (6) |
| Fluciclovine PET + MRI + CT + bone scan | 1 (1.2) |
| Bone scan only | 1 (1.2) |
| Bone scan + MRI + CT | 4 (4.8) |
| CT only | 10 (12) |
| CT + bone scan | 5 (6) |
| CT + MRI | 5 (6) |
| MRI only | 3 (3.6) |
| None | 6 (7.2) |
| N/A | 22 (26.5) |
CT = computed tomography; FDG = fluorodeoxyglucose; MRI = magnetic resonance imaging; N/A = PET = positron emission tomography.
Table 2 –
Prostate-specific membrane antigen positron emission tomography findings and staging in the intervention group (n = 102)
| Stage | Patients, n (%) |
|---|---|
| T+ | 18 (17.6) |
| N1 | 20 (19.6) |
| M1 | 9 (8.8) |
| M1a | 3 (2.9) |
| M1b | 8 (7.8) |
| M1c | 1 (1) |
| T0 N0 M0 | 64 (62.7) |
| T+ N0 M0 | 13 (12.7) |
| T+ N1 M0 | 12 (11.8) |
| T0 N1 M0 | 4 (3.9) |
| T+ N0 M1 | 5 (4.9) |
| T0 N0 M1 | 3 (2.9) |
| T0 N1 M1 | 1 (1) |
In this prospective randomized phase 3 study, PSMA PET localized PCa in more than one-third of patients. PET showed lesions outside the pelvis in 9% of patients in the intervention group. Follow-up is ongoing to assess whether PSMA PET disease localization eventually translates into better patient outcomes.
Acknowledgments:
We thank all the patients and their referring physicians whose willingness to participate made this study possible. We thank the whole staff team of the University of California, Los Angeles Nuclear Medicine and Theranostics Division whose hard work made this study possible.
Conflicts of interest: Jeremie Calais reports prior consulting activities for Advanced Accelerator Applications, Blue Earth Diagnostics, Curium Pharma, GE Healthcare, Janssen Pharmaceuticals, Progenics Pharmaceuticals, Radiomedix, and Telix Pharmaceuticals outside the submitted work. Wolfgang P. Fendler is a consultant for Endocyte, Ipsen, and BTG, and has received fees from RadioMedix, Bayer, and Parexel outside the submitted work. Johannes Czernin is a founder and board member of and holds equity in Sofie Biosciences and Trethera Therapeutics, has served on the medical advisory board of Actinium, was a member of the VISION trial steering committee, participated in a clinical trial sponsored by Endocyte, and reports research funding from Endocyte and Ipsen, all outside the submitted work. Thomas A. Hope reports grants from Advanced Accelerator Applications and prior consulting activities for Blue Earth Diagnostics, Curium Pharma, Ipsen, GE Healthcare, and Progenics Pharmaceuticals outside the submitted work. Amar Upadhyaya Kishan reports research funding from ViewRay and personal fees from Varian Medical Systems, ViewRay, and Janssen Pharmaceuticals outside the submitted work. Nicholas George Nickols reports research funding from Janssen, Progenics, and Bayer outside the submitted work. The remaining authors have nothing to disclose.
Funding: There was no external funding source for this study. This was an investigator-initiated trial with institutional academic funding (Ahmanson Translational Theranostics Division, Department of Molecular and Medical Pharmacology, UCLA). Jeremie Calais is the recipient of grants from the ERF-SNMMI (2019–2021 Molecular Imaging Research Grant for Junior Academic Faculty), the Prostate Cancer Foundation (2020 Young Investigator Award 20YOUN05), the Philippe Foundation Inc. (New York, USA), and the ARC Foundation (France; International Mobility Award SAE20160604150). Johannes Czernin is the recipient of grants from the Prostate Cancer Foundation (19CHAL09, 17CHAL02) and the National Cancer Institute (P50 CA092131). Nicholas George Nickols is the recipient of grants from the Prostate Cancer Foundation and VA Office of Research and Development. Thomas A. Hope is the recipient of grants from the Prostate Cancer Foundation and the National Cancer Institute (R01CA235741). Amar Upadhyaya Kishan is the recipient of a grant from the Prostate Cancer Foundation.
References
- [1].Carrie C, Magné N, Burban-Provost P, et al. Short-term androgen deprivation therapy combined with radiotherapy as salvage treatment after radical prostatectomy for prostate cancer (GETUG-AFU 16): a 112-month follow-up of a phase 3, randomised trial. Lancet Oncol 2019;20:1740–9. [DOI] [PubMed] [Google Scholar]
- [2].Vale CL, Fisher D, Kneebone A, et al. Adjuvant or early salvage radiotherapy for the treatment of localised and locally advanced prostate cancer: a prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020;396:1422–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Calais J, Cao M, Nickols NG. The utility of PET/CT in the planning of external radiation therapy for prostate cancer. J Nucl Med 2018;59:557–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Michalski JM, Lawton C, El Naqa I, et al. Development of RTOG consensus guidelines for the definition of the clinical target volume for postoperative conformal radiation therapy for prostate cancer. Int J Radiat Oncol Biol Phys 2010;76:361–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Fendler WP, Calais J, Eiber M, et al. Assessment of 68 Ga-PSMA-11 PET accuracy in localizing recurrent prostate cancer: a prospective single-arm clinical trial. JAMA Oncol 2019;5:856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Calais J, Czernin J, Cao M, et al. 68 Ga-PSMA-11 PET/CT mapping of prostate cancer biochemical recurrence after radical prostatectomy in 270 patients with a PSA level of less than 1.0 ng/mL: impact on salvage radiotherapy planning. J Nucl Med 2018;59:230–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Calais J, Czernin J, Fendler WP, Elashoff D, Nickols NG. Randomized prospective phase III trial of 68Ga-PSMA-11 PET/CT molecular imaging for prostate cancer salvage radiotherapy planning [PSMA-SRT]. BMC Cancer 2019;19:18. [DOI] [PMC free article] [PubMed] [Google Scholar]


