Abstract
To overwinter, animals must detect constant cold temperatures before adapting their behavior accordingly. A new study in Drosophila describes a circuit mechanism — from sensory neurons to higher brain centers — that encodes and relays persistent, absolute cold stimuli to modulate sleep.
Animals reach and maintain their body temperatures via different means: endotherms rely on metabolic heat for warming, while ectotherms draw instead on environmental heat [1]. Regardless of their thermoregulatory mechanisms, most animals adapt physiologically and behaviorally to the prolonged cold temperatures encountered during seasonal shifts to winter. For endotherms, many species of birds and mammals reduce energy expenditure by entering hypometabolic states of cold-lethargy, or torpor [2]. Consecutive torpor bouts lasting up to weeks characterizes hibernation, a form a seasonal dormancy related to higher rates of overwinter survival for mammals [3]. Even non-hibernating endotherms may still modify sleep patterns in the cold, and readers might personally relate to a harder time waking on darker and colder winter mornings.
In contrast, most insects are ectothermic poikilotherms which conform their temperature to ambient environments [1], and whose small body sizes render them especially sensitive to persistent cold. By adopting seasonal lifestyles, insects generally avoid or resist cold thermal extremes through regulating their behaviors and the timing of development: specifically, they may reduce their overall activity, migrate to warmer areas, or rely on diapause to overwinter [4]. Diapause is a hormonally programmed suppression of development which conserves energy through low metabolic activity and initiates dormancy that subsequently confers resistance to severe cold [4]. Temperature, along with daylength, are reliable indicators of seasonal changes, and both are highly influential cues for the onset of diapause [5]. For example, female Drosophila melanogaster undergoes ovarian diapause; the expression of this reproductive dormancy is induced by short daylength and low temperature [6].
While overwintering strategies have been broadly observed, it is less clear how animals actively sense the persistent cold temperatures which signify the seasonal onset of winter. Despite the remarkable advances in recent decades regarding how mammalian and insect primary sensory neurons detect temperature cues [7,8], stimulus paradigms relying on slow cooling ramps or fluctuating temperature cycles have not identified thermosensory neurons that are tuned to absolute cold. Therefore, it remains an open question how long-term, seasonal cold temperatures are coded into neural signals.
In this issue of Current Biology, Alpert and colleagues [9] implemented elegant genetic, functional, and behavioral approaches to describe a complete neural circuit linking thermosensory input to central circadian neurons in the fruitfly Drosophila melanogaster. Specifically, the authors characterized key nodes, from peripheral sensory neurons to higher brain centers, which encode persistent cold stimuli into prolonged neural signaling. To distinguish whether sensory neurons respond to cooling or absolute cold temperatures, they employed a stimulation protocol with extended stable cold conditions flanked by rapid temperature drops and rises. At the sensory periphery, the authors identified a novel population of thermosensory receptor neurons (TRNs), located in the first chamber of the antenna’s sacculus structure, which shows persistent activation to temperatures lower than the fruitfly’s preferred 25°C (Figure 1, bottom panel).
Figure 1. Neural circuit linking cold thermosensory inputs to circadian regulation.
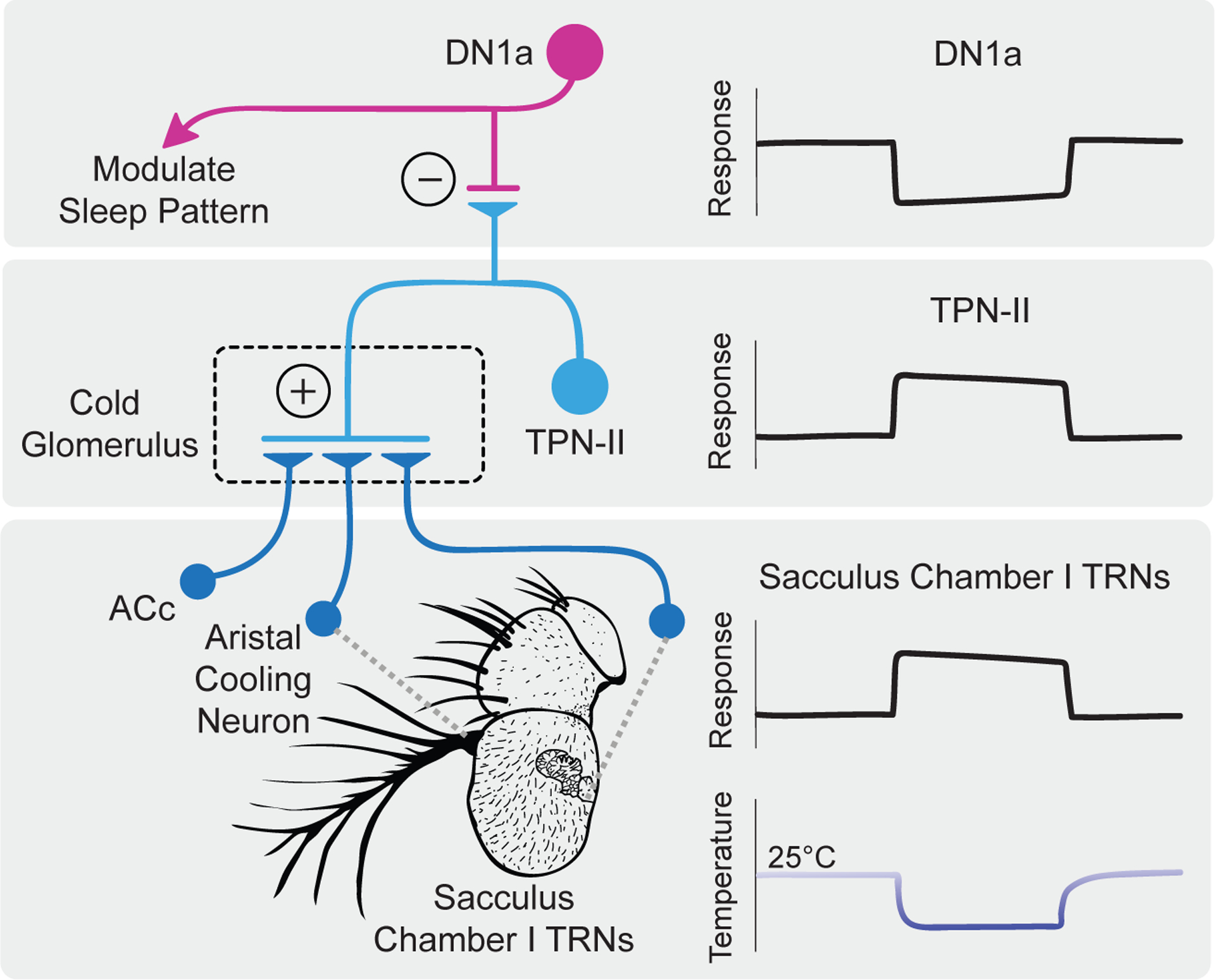
Bottom panel: The Anterior Cold cells (ACc, left), aristal cooling neurons (center), and sacculus chamber I thermosensory receptor neurons (TRNs, right) all project to the cold glomerulus of the posterior antennal lobe. The sacculus TRNs show sustained response to persistent cold stimulation below 25°C.
Middle panel: TPN-II thermosensory projection neurons receive input from the three peripheral TRN populations, and also show sustained activation to persistent cold.
Top panel: DN1a circadian neurons are inhibited by TPN-IIs, and show sustained inactivation to persistent cold. DN1a activity modulates the patterns and structure of sleep.
Illustration by Cora Xing.
This and two other types of TRNs project to the cold glomerulus of the posterior antennal lobe (PAL) and synapse onto previously identified second-order TPN-II thermosensory projection neurons [10]. Strikingly, TPN-IIs exhibit little adaptation, with persistent responses to continuous cold stimuli (Figure 1, middle panel). Furthermore, this sustained activation is dosage-dependent and only observed at stable temperatures below 25°C, indicating that TPN-II activity levels reflect the degree of absolute coldness.
What are the targets of TPN-IIs? These projection neurons form GABAergic inhibitory synapses with the 1a cluster of “Dorsal Neurons” (DN1a), which are parts of a circadian clock network implicated in sleep regulation [11]. The authors found that constant cold stimulation silences DN1a spike activity in a sustained manner, mirroring the tonic activation of TPN-IIs (Figure 1, top panel).
Given that cold temperatures reduce DN1a activity, might the TPN-II/DN1a circuit also link temperature with sleep? In agreement with this notion, cold temperature was found to reduce overall activity, increase morning sleep, and restructure both afternoon and evening sleep. Importantly, DN1a silencing mimics the cold condition, resulting in increased morning sleep even when the temperature is kept at 25°C. Consistent with this effect, TPN-II silencing occludes the restructuring of afternoon and evening sleep in response to cold stimuli. These observations indicate a modulatory role of the TPN-II/DN1a circuit in the structuring of sleep.
The authors further showed that in the absence of light, cold temperature prevents flies from waking from night sleep, while genetic silencing of DN1a is able to mimic this effect. Interestingly, they found that DN1as can be activated by light through input from small ventral lateral neurons (sLNVs), which were previously identified as regulators of morning activity [12]. In colder temperatures, light-activation of DN1a can therefore compensate for and override its cold-inhibition, allowing flies to still wake up with morning light. Overall, DN1a activity is modulated by both light and persistent cold, allowing for the integration of these two key sensory cues to regulate sleep and waking.
Notably, DN1a silencing does not completely abolish the impact of cold on sleep regulation, suggesting that other circadian components are also involved. Consistent with this notion, a different subset of DN1 neurons, named DN1p, is reported to exhibit sensitivity to temperature cycles and is likely involved in thermo-entrainment of the circadian clock [13]. Moreover, other DN1 subgroups are activated at the opposite temperature extreme (30°C), which in turn promotes sleep in response to warmth above the preferred 25°C temperature [11]. Therefore, it appears that distinct DN1 neuronal subtypes are sensitive to different aspects of thermosensory input to modulate circadian rhythm and sleep. The current study by Alpert et al. has thus furthered the understanding of this complex neuronal population.
The question of how persistent, low temperature is detected has now been answered by the identification of sacculus TRNs which exclusively sense absolute cold with sustained responses. Intriguingly, these tonically-responding TRNs are not the only presynaptic partners of TPN-IIs: two other types of TRNs — activated by cooling with distinct kinetics — also form connections (Figure 1, bottom panel). Why then do the non-adapting TPN-IIs receive input from both tonic and phasic peripheral thermosensory neurons? Are the sacculus TRNs required for all aspects of absolute cold sensing? Can the sacculus input be routed to other nodes of the circadian circuit? Furthermore, what could be the receptor of the novel sacculus TRNs? Previously identified cold-sensing receptors include the thermoTRP channels [7,8,14,15], and the kainate-type glutamate receptors [16]. Members of the insect Ionotropic Receptor (IR) family have also been implicated in sensing temperatures [17,18]. Future work should aim to address whether the receptors from the newly identified sacculus TRNs belong to these identified receptor classes, or to a novel receptor family. These are just a few of the many interesting questions and research directions stimulated by the current study.
It will also be important to examine whether sustained TPN-II activation contributes to other physiological adaptations that comprise the fruitfly’s overwintering strategies. For example, might tonic TPN-II input contribute to the onset of females’ ovarian diapause? Beyond fruitflies, whether the TPN-II/DN1a circuit is conserved across diapausing insect species awaits future research; this line of investigation is expected to provide insights into how sensory circuits are functionally adapted in species which occupy different temperature niches. Finally, it will be interesting to determine whether endotherms employ a similar circuit logic that allows absolute cold temperatures to regulate circadian neurophysiology.
References
- 1.Prosser CL, and Nelson DO (1981). The role of nervous systems in temperature adaptation of poikilotherms. Annu. Rev. Physiol 43, 281–300. Available at: http://www.ncbi.nlm.nih.gov/pubmed/7011185. [DOI] [PubMed] [Google Scholar]
- 2.Ruf T, and Geiser F (2015). Daily torpor and hibernation in birds and mammals. Biol. Rev 90, 891–926. Available at: http://doi.wiley.com/10.1111/brv.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Turbill C, Bieber C, and Ruf T (2011). Hibernation is associated with increased survival and the evolution of slow life histories among mammals. Proc. R. Soc. B Biol. Sci 278, 3355–3363. Available at: https://royalsocietypublishing.org/doi/10.1098/rspb.2011.0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tauber CA, and Tauber MJ (1981). Insect Seasonal Cycles: Genetics and Evolution. Annu. Rev. Ecol. Syst 12, 281–308. Available at: http://www.annualreviews.org/doi/10.1146/annurev.es.12.110181.001433. [Google Scholar]
- 5.Bale JS, and Hayward SAL (2010). Insect overwintering in a changing climate. J. Exp. Biol 213, 980–994. Available at: http://jeb.biologists.org/cgi/doi/10.1242/jeb.037911. [DOI] [PubMed] [Google Scholar]
- 6.Saunders DS, Henrich VC, and Gilbert LI (1989). Induction of diapause in Drosophila melanogaster: photoperiodic regulation and the impact of arrhythmic clock mutations on time measurement. Proc. Natl. Acad. Sci 86, 3748–3752. Available at: http://www.pnas.org/cgi/doi/10.1073/pnas.86.10.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Julius D (2013). TRP channels and pain. Annu Rev Cell Dev Biol 29, 355–384. Available at: http://www.ncbi.nlm.nih.gov/pubmed/24099085. [DOI] [PubMed] [Google Scholar]
- 8.Barbagallo B, and Garrity PA (2015). Temperature sensation in Drosophila. Curr. Opin. Neurobiol 34, 8–13. Available at: 10.1016/j.conb.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Alpert M, Frank D, Kaspi E, Flourakis M, Zaharieva E, Allada R, Para A, and Gallio M (2020). A circuit encoding absolute cold temperature in Drosophila. Curr. Biol [DOI] [PMC free article] [PubMed]
- 10.Frank DD, Jouandet GC, Kearney PJ, Macpherson LJ, and Gallio M (2015). Temperature representation in the Drosophila brain. Nature 2, 358–361. Available at: 10.1038/nature14284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo F, Yu J, Jung HJ, Abruzzi KC, Luo W, Griffith LC, and Rosbash M (2016). Circadian neuron feedback controls the Drosophila sleep-activity profile. Nature 536, 292–297. Available at: 10.1038/nature19097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoleru D, Peng Y, Agosto J, and Rosbash M (2004). Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature 431, 862–868. Available at: http://www.nature.com/articles/nature02926. [DOI] [PubMed] [Google Scholar]
- 13.Yadlapalli S, Jiang C, Bahle A, Reddy P, Meyhofer E, and Shafer OT (2018). Circadian clock neurons constantly monitor environmental temperature to set sleep timing. Nature 555, 98–102. Available at: 10.1038/nature25740. [DOI] [PubMed] [Google Scholar]
- 14.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, et al. (2002). A TRP Channel that Senses Cold Stimuli and Menthol. Cell 108, 705–715. Available at: https://linkinghub.elsevier.com/retrieve/pii/S0092867402006529. [DOI] [PubMed] [Google Scholar]
- 15.Arenas OM, Zaharieva EE, Para A, Vásquez-Doorman C, Petersen CP, and Gallio M (2017). Activation of planarian TRPA1 by reactive oxygen species reveals a conserved mechanism for animal nociception. Nat. Neurosci 20, 1686–1693. Available at: http://www.nature.com/articles/s41593-017-0005-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong J, Liu J, Ronan EA, He F, Cai W, Fatima M, Zhang W, Lee H, Li Z, Kim GH, et al. (2019). A Cold-Sensing Receptor Encoded by a Glutamate Receptor Gene. Cell 178, 1375–1386.e11. Available at: 10.1016/j.cell.2019.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen C, Buhl E, Xu M, Croset V, Rees JS, Lilley KS, Benton R, Hodge JJL, and Stanewsky R (2015). Drosophila Ionotropic Receptor 25a mediates circadian clock resetting by temperature. Nature 527, 516–520. Available at: 10.1038/nature16148. [DOI] [PubMed] [Google Scholar]
- 18.Ni L, Klein M, Svec KV, Budelli G, Chang EC, Ferrer AJ, Benton R, Samuel ADT, and Garrity PA (2016). The ionotropic receptors IR21a and IR25a mediate cool sensing in Drosophila. Elife 5, 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]


