Abstract
Background:
Exposure to toxic metals (TMs) such as lead can cause lifelong neurodevelopmental impairment and other adverse outcomes. TMs enter drinking water from human activity, geogenic contamination, and corrosion of water system components. Several studies report TM contamination in piped systems and private wells in high-income countries (HICs). However, few robust studies report on TM contamination in low- and middle-income countries (LMICs).
Objectives:
We characterized the occurrence and investigated sources of TM contamination in 261 rural water systems in three West African LMICs to inform prevention and management.
Methods:
Water samples were collected from 261 community water systems (handpumps and public taps) across rural Ghana, Mali, and Niger. Scrapings were collected from accessible components of a subset of these systems using a drill with acid-washed diamond-tipped bits. Samples were analyzed by inductively coupled plasma (ICP) mass spectrometry or ICP optical emission spectroscopy.
Results:
Of the TMs studied, lead most frequently occurred at levels of concern in sampled water system components and water samples. Lead mass fractions exceeded International Plumbing Code (IPC) recommended limits (0.25% wt/wt) for components in 82% (107/130) of systems tested; brass components proved most problematic, with 72% (26/36) exceeding IPC limits. Presence of a brass component in a water system increased expected lead concentrations in drinking-water samples by 3.8 times. Overall, lead exceeded World Health Organization (WHO) guideline values in 9% (24/261) of drinking-water samples across countries; these results are broadly comparable to results observed in many HICs. Results did not vary significantly by geography or system type.
Discussion:
Ensuring use of lead-free () components in new water systems and progressively remediating existing systems could reduce drinking-water lead exposures and improve health outcomes for millions. However, reflexive decommissioning of existing systems may deprive users of sufficient water for health or drive them to riskier sources. Because supply chains for many water system components are global, TM monitoring, prevention, and management may be warranted in other LMICs beyond the study area as well. https://doi.org/10.1289/EHP7804
Introduction
The United Nations Sustainable Development Goal (SDG) Target 6.1 calls for “universal and equitable access to safe and affordable drinking-water for all” (United Nations 2015) International policy defines “safe” drinking water as free from pathogens and elevated levels of toxic chemicals (WHO 2011). This reflects a need to concurrently address pathogens and “key chemicals responsible for large-scale health effects through drinking-water exposure,” including toxic metals (TMs) such as “arsenic… lead… selenium, and uranium” (WHO 2011).
Exposure to various TMs has been associated with health problems including cancer, cardiovascular disease, hypertension, kidney disease, and adverse reproductive outcomes (Smith 1992; Järup 2003; Houston 2007; WHO 2011; Rauh and Margolis 2016; Chowdhury 2018). Lead, in particular, can cross the blood–brain barrier when ingested and has been associated with irreversible neurodevelopmental impairment—which is especially serious in children and developing fetuses (Järup 2003; Sanders et al. 2009; WHO 2011; Rauh and Margolis 2016). A considerable burden of disease exists as a result of exposure to TMs; an estimated 100,000 deaths and disability-adjusted life years each year can be attributed to environmental lead exposure alone (Lim et al. 2012).
TM exposure can occur through consumption of contaminated drinking water. TMs can originate directly from aquifers and surface waters or can be released from water system components (Deshommes et al. 2010). Environmental exposures occur through ingestion or inhalation of contaminated dust, air, and food; however, widespread bans of leaded gasoline and leaded paint and improved food safety mitigate these exposures (Brown and Margolis 2012).
The World Health Organization (WHO) has set health-based guidelines for TMs in drinking water (WHO 2011), and many countries have adopted similar standards (Ministry of Water Resources, Works, and Housing 2015; Ministere de L’Energie and des Mines et de L’Eau 2007; Republique du Niger 2005; WHO 2011) (Table 1). Manufacturing standards such as NSF International Standard 61 also set limits for acceptable leaching of several TMs, including lead from drinking-water system components (International Code Council 2017), whereas International Plumbing Codes (IPCs) establish maximum lead fraction by weight (0.25% wt/wt) in such components (NSF International 2018).
Table 1.
WHO Guideline Values and selected national standards for nine toxic metals in drinking water.
| Toxic metal | WHO Guideline Value () | Ghana National Standard (Ministry of Water Resources, Works, and Housing 2015) () | Mali National Standard (Ministere de L’Energie, des Mines et de L’Eau 2007) () | Niger National Standard (Republique du Niger 2005) () | Indicative detection limit for analysis in this study ()a |
|---|---|---|---|---|---|
| Antimony | 20 | 5 | 5 | 1 | 0.1 |
| Arsenic | 10 | 10 | 10 | NA | 0.5 |
| Cadmium | 3 | 3 | 3 | 5 | 0.1 |
| Chromium | 50 | 50 | 50 | 50 | 1 |
| Lead | 10 | 10 | 10 | 50 | 0.5 |
| Manganese | 400 | 400 | 500 | 100 | 2 |
| Mercury | 6 | 1 | 1 | 1 | 0.1 |
| Nickel | 70 | 20 | 20 | NA | 1 |
| Selenium | 40 | 10 | 10 | 10 | 10 |
Note: NA, not applicable; WHO, World Health Organization.
Detection limits varied by laboratory. The highest value reported by any of the three laboratories (typically the Ghana lab) is shown.
Despite these efforts, TM exposure occurs in drinking-water systems worldwide (WHO 2011). High-profile lead exposures have occurred in large piped systems in high-income countries (HICs), including cases in Flint, Michigan (Pieper et al. 2017) and Glasgow, UK (Addis and Moore 1974). These cases occurred in systems predating current regulations and often involve unsuitable water system components/materials in combination with corrosive water, treatment failures, or both (e.g., Flint).
TM exposure likewise occurs in small rural water systems, such as private wells and handpumps. Studies in Bangladesh in the 1990s found that 35% of tube wells were estimated to have of arsenic (Smith et al. 2000). A study based on over 20,000 U.S. water samples estimated that more than U.S. well users were likely consuming water exceeding allowable levels (i.e., ) of arsenic (Ayotte et al. 2017). A recent U.S. study in Virginia found that 20% of 2,146 private wells had lead concentrations exceeding U.S. standards (i.e., ). (Pieper et al. 2015).
Rural water systems are often less extensively regulated and monitored than urban networks (Trémolet 2013) and are less amenable to some of the technical remediation and corrosion control methods available to large utilities (Schock 1989). Rural water system users are thus disproportionately vulnerable to TM exposure through drinking water, with users in low-income and middle-income countries (LMICs) potentially at particular risk of undetected exposures (Chikaodili et al. 2017). In sub-Saharan Africa (SSA), over people use groundwater and an estimated use handpumps—many of them in rural settings (Cobbina et al. 2015; Tukura 2014). Rural water systems in SSA are often implemented by governments, nongovernmental organizations (NGOs), private enterprises, and individuals. This patchwork can limit coherent oversight, harmonization, and verification of appropriate implementation practices (Langenegger 1989). Monitoring, generally infrequent for rural water systems, is especially difficult in this region given limited laboratory capacity. Finally, IPCs or similar national standards have not been adopted in many SSA countries.
Although large populations may be exposed to TMs from drinking water, few studies (especially representative studies) have characterized widespread TM occurrence in drinking water in SSA. Many studies focus on industrial and anthropogenic point sources of TM contamination and report localized phenomena rather than conditions representative of national or substantive subnational populations (Cobbina et al. 2015; Langenegger 1989; Tukura 2014). The few studies that cover larger areas often report on metals of limited health concern such as iron and zinc (Langenegger 1989), report on groundwater samples collected prior to water system installation (thereby missing corrosion effects) (Lutz et al. 2013), or use methods such as flame atomic absorption spectroscopy, which are unable to detect many TMs at microgram-per-liter levels corresponding to health-based guidelines (Obiri-Danso et al. 2009). Many studies do not include a stagnation period before sampling (important to capture the effects of corrosion of water system components) or else analyze only filtered samples (thereby missing particulate lead—an important source of lead exposure). One high-quality study reported TM occurrence across Ethiopia (Reimann et al. 2003). To our knowledge, no high-quality multicountry studies have examined lead occurrence in drinking water in LMIC settings using suitable methods to detect lead at microgram-per-liter levels or associate its occurrence with potential sources of contamination.
To address these evidence gaps, we conducted a multicountry study of TM contamination in drinking water from rural community water systems in Ghana, Niger, and Mali. Our objective was to determine a) whether substantive TM contamination occurs in these settings and, if so; b) which TM contaminants exceeded applicable standards and guidelines most frequently; c) which determinants were associated with this contamination; and d) what actions might be appropriate for preventing TM exposure.
Methods
Sample Frame
Data were collected during a larger evaluation of rural water programs implemented by the international nongovernmental organization (INGO), World Vision (WV). The results of the larger evaluation comparing INGO programs to comparison areas are not presented here.
Briefly, a cluster-randomized, population-based study design was used to collect household data. Lists of subnational administrative sampling units (clusters) were obtained from the relevant national census bureau (Niger, Mali) or national statistics office (Ghana). Within each country, clusters of households were sampled in regions within which the sponsoring INGO had worked, stratified into subregional clusters in which INGO had (WV areas), or had not (comparison areas) implemented rural water supply activities. For each country, 56 WV clusters and 56 comparison clusters were randomly selected. Although not nationally representative, these samples collectively covered large areas of each country in which rural water supply activities are being implemented with high intensity by local and international NGOs, as well as local and national government implementers. In Ghana, clusters were identified within the Brong Ahafo, Northern, and Upper East regions; in Mali, clusters were identified within the Kayes, Koulikoro, Mopti, Segou, and Sikasso regions; and in Niger, clusters were identified within the Tillaberi, Dosso, Tahoua, Maradi, and Zinder regions.
Within each cluster, all water systems were identified, mapped, and compiled into a list. The list contained all functioning water systems containing metal parts (excluding, e.g., unimproved surface water sources and unimproved dug wells) within each cluster. From this list, one water system in each cluster was randomly selected for TM sampling.
Sample and Water System Survey Collection
Enumerators administered a water system survey at each water source visited. The survey was administered to a respondent with detailed knowledge of the water system, typically a community water committee member, caretaker, or community leader with knowledge of the water system. This survey included questions on the water source including water source type, system age, geolocation (direct GPS measurement), and reported implementer (to the best knowledge of the survey respondent). Sources were classified according to the WHO/United Nations International Children’s Fund Joint Monitoring Program for Water Supply, Sanitation, and Hygiene classifications of drinking-water system types (WHO and UNICEF 2006). No distinctions were made between public taps/standpipes based on the water source to which they were connected (e.g., surface water vs. groundwater, treated vs. untreated) because it was not anticipated that enumerators or respondents would be able to reliably make these distinctions in the field.
At each of the randomly selected water systems, enumerators visited the water system and observed a 1-h stagnation period, during which the water system was not used (, []). This 1-h stagnation period was selected, as opposed to a longer period (e.g., 6 h or overnight), for logistical reasons: It was not considered safe or feasible for enumerators to arrive at every water source before its first use in the morning because this would require extensive travel in the dark, on roads that are safest during daylight; likewise it was considered highly burdensome to ask communities members to abstain from using water sources for periods of several hours or longer. Enumerators collected a first-draw sample immediately after the stagnation period, using a new high-density polyethylene bottle. Samples were preserved with of trace metal–grade nitric acid per liter to achieve a final pH of . After preservation, duplicate aliquots were removed and samples were delivered to commercial laboratories in each country for analysis (with the exception of Niger, where samples were shipped to a laboratory in Ghana).
In Ghana and Mali, a second field campaign was conducted, in which a subset of water systems was revisited and drinking-water samples were collected again, as described above. Groundwater samples were collected by flushing one full well volume from each source (boreholes with handpumps), or flushing piped sources for at least 7 min with the tap fully open prior to collecting a sample. For boreholes, well volumes (Vs) were calculated based on reported well depth (H) and casing radius (r) as .
Metal scrapings were collected from water systems in WV program areas. For systems with handpumps, scrapings were collected from rising mains, foot valves, rods, and taps/spouts; for piped water systems, samples were collected from taps/faucets. These parts were chosen for their accessibility and because they span a range of materials (e.g., brass, galvanized steel, stainless steel) that could contribute to TM occurrence in water systems. For each selected part, of material was removed using a cordless electric drill fitted with a fresh acid-washed diamond-tipped drill bit. The scrapings were collected in a fresh GhostWipe positioned in a clean plastic container beneath the component being sampled. Once sampling was complete, the GhostWipe with metal scrapings was placed back in the trace metal–free GhostWipe collection tube for transport to an approved laboratory for analysis.
Between June 2017 and October 2018, 261 water systems were sampled in two field campaigns. For the subset of systems that were sampled twice (once in 2017 and once in 2018), the most recent results from the second field campaign have been presented in cross-sectional analyses and summary statistics.
Ethics
This work was conducted with approval from relevant ministries in each country and with support from WV. The INGO did not influence the design or analysis of this study. No sensitive or identifiable personal information was collected during these surveys, and all respondents were interviewed in their official capacity as committee members or community leaders, for example. The survey was approved by the University of North Carolina at Chapel Hill (UNC) institutional review board (#17-0663), the Ministry of Water Resources, Works, and Housing in Ghana (reference TJMSW), the University of Bamako Medical School in Mali (reference 2017/105/CE/FMPOS), and the Ministry of Water Resources in Niger (reference 000008/MH/A/DGH).
Materials
Unless stated otherwise, all chemicals and materials were reagent-grade and were used as received. Virgin high-density polyethylene (HDPE) wide-mouthed sample collection bottles, metal-free tubes (for storing duplicate sample aliquots), and metal-free low-density polyethylene transfer pipets (for adding acid to samples) were obtained from Thermo Fisher Scientific. Trace metal–grade nitric acid was sourced from Aqua Solutions. A random subset of the HDPE sample bottles was analyzed for TMs of interest to confirm that bottles did not contribute TMs at levels comparable to those of interest. Cordless drills were obtained from local suppliers in each country; diamond-tipped drill bits for water system materials sampling were obtained from SPTA, Inc. Drill bits were cleaned and washed with trace metal–grade nitric acid prior to each use. GhostWipe metal-free tubes and wipes for water system materials sampling were obtained from Environmental Express. Water quality testing field kits (for, e.g., field-level determination of pH and conductivity) were obtained from Aquagenx LLC. Mobile phones (Android) were procured locally and equipped with mWater mobile data collection software (version 11.0.2).
Sample Analysis
Acidified water samples were analyzed by inductively coupled plasma mass spectrometry [ICP-MS; Mali, Ghana (second field campaign)] or ICP optical emission spectroscopy [ICP-OES; Ghana (first field campaign), Niger] according to published standard methods [APHA 3020 B (Ghana, Niger), ISO 17294 (Mali)]. Briefly, for ICP-OES analysis, water samples were aspirated into argon plasma at 8,000–10,000K with a gas flow rate of , and emission was quantified at characteristic wavelengths for each analyte (e.g., for lead) as described in APHA Method 3020 B and U.S. Environmental Protection Agency Method 200.7. For ICP-MS analysis, water samples were aspirated into argon plasma at 6,000–10,000K with a gas flow rate of and a spray chamber temperature of 2–5°C. Elements were quantified by MS at characteristic m/z ratios for each analyte (e.g., 208, 207, 206 for lead) as described in ISO Method 17294. Water system component samples were digested in trace metal–grade nitric acid at 95°C for and diluted to a volume of in trace metal–grade water, then analyzed by the same methods as the water samples. In all countries, samples were analyzed for antimony, arsenic, cadmium, chromium, copper, iron, lead, manganese, mercury, nickel, selenium, tin, and zinc. In each country, laboratories were instructed to prepare and analyze daily laboratory blanks, duplicates, and calibration curves (see examples in Figure S1) and to document these in their reports. A subset of duplicate samples from each lab were analyzed at UNC by ICP-MS (Figure S1C).
Data Cleaning, Merging, and Analysis
Raw data files were cleaned and merged using Stata (version 14.2; Stata Corporation) and R (version 3.5.1; R Development Core Team). For each country, the water system survey and laboratory analysis data files were trimmed and cleaned (unused variables and duplicate results dropped, retained variables converted to a common format, and likely outliers excluded). A data point was considered a likely outlier if the reported value for three or more analytes within the same sample each exceeded 100 times the IQR for the respective analytes. A value of half the reported lower limit of detection (LLOD) for each laboratory was used as a continuity correction for nondetects. Where a laboratory reported an implausibly low LLOD (e.g., ), a value of was substituted as a conservative estimate of achievable LLOD. Water system survey data and TM laboratory analysis results were then merged for each country based on a unique sample ID, and the merged country data sets were then appended to create a three-country merged data set. For water system component samples, the concentration of each TM of interest was divided by the sum of the concentrations of all elements analyzed to obtain an indicative relative mass fraction. Given that TMs such as iron, zinc, copper, tin, manganese, and chromium were selected in part on the basis of comprising the anticipated majority constituents of common water system component materials (e.g., bronze, galvanized, stainless steels), the assumption was made that these relative mass fractions can serve as meaningful indicators of the approximate abundance of TMs of concern in water system components.
Summary statistics were calculated. The number and proportion of samples exceeding the applicable WHO Guideline Value (GV) and national standards (NS) were calculated for all samples and analytes. These exceedances were also disaggregated by country, source type, implementer, and source age quartile. In addition, univariable and multivariable ordinary least squares (OLS) regressions were calculated to determine the association between log TM concentration and country, source type, implementer, and/or system age, controlling for relevant covariates (e.g., stagnation period duration, water sample pH, conductivity). Log TM concentrations were used because concentrations of metals in drinking water are generally lognormally distributed, making a log-transformed dependent variable more suitable for OLS regression methods. Independent model variables included were those likely to relate to observable sources of TM contamination from water system corrosion or groundwater contamination (i.e., presence of water system materials of interest, such as brass, galvanized steel; or occurrence of lead in flushed groundwater samples), likely to influence the solubility/extent of corrosion of TMs of concern (pH, conductivity, stagnation time) or to be indicative of the occurrence of corrosion of materials of interest [e.g., copper (bronze, brass), zinc (brass, galvanized steel), tin (bronze)]. Regression diagnostics were used to identify collinearity. Influential observations and variables demonstrating multicollinearity were removed from models (although none were found). In addition, multivariable regressions were conducted to determine associations between the log-transformed concentrations of each TM of interest and all other TMs analyzed (controlling for relevant covariates). This was done to identify associations between occurrence of TMs that might provide insights into the relative influence of different potential sources of TM occurrence (e.g., the association of lead and copper occurrence is potentially indicative of the importance of brass or bronze corrosion, previously documented in other water systems). Longitudinal analyses were also conducted to determine the extent to which measured concentrations of lead and other TMs in an initial sample (e.g., TM concentration greater than WHO GV) predicted similar results for a second sample from the same site. Geospatial analyses were conducted using ArcGIS (version 10.6.1) to assess Moran’s I as a measure of spatial clustering. Statistical analyses were conducted using Stata (version 14.2; Stata Corporation). For all analyses, statistical significance was evaluated with a -value of 0.05 (95% confidence).
Results
Final Sample
Samples were obtained from 261 water systems in two sampling rounds across Ghana, Mali, and Niger. Water systems sampled were handpumps (either boreholes or hand-dug wells) or public taps, ranging from 1 to 61 years of age, as reported by the respondents (Table 2).
Table 2.
Water system sample sizes by country, water system type, handpump type, and water system age in a study on toxic metals in drinking water in West Africa.
| Variable | (%) |
|---|---|
| Country | |
| Ghana | 95 (36) |
| Mali | 90 (34) |
| Niger | 76 (29) |
| Water system type | |
| Handpump (borehole or hand-dug well) | 156 (60) |
| Public tap | 105 (40) |
| Handpump typea | |
| Afridev | 45 (29) |
| India Mark II | 72 (46) |
| Other | 25 (16) |
| Unknown | 14 (9) |
| Water system age (y) | |
| 0–3 | 40 (15) |
| 4–7 | 54 (21) |
| 8–15 | 56 (21) |
| 62 (24) | |
| Unknown | 49 (19) |
Percentages for this category are calculated of the 156 handpumps sampled instead of the 261 total water systems sampled. Other handpump types include Vergnet, Nira, Hydro India, and Kardia.
Summary Statistics and Exceedances
Drinking-water samples.
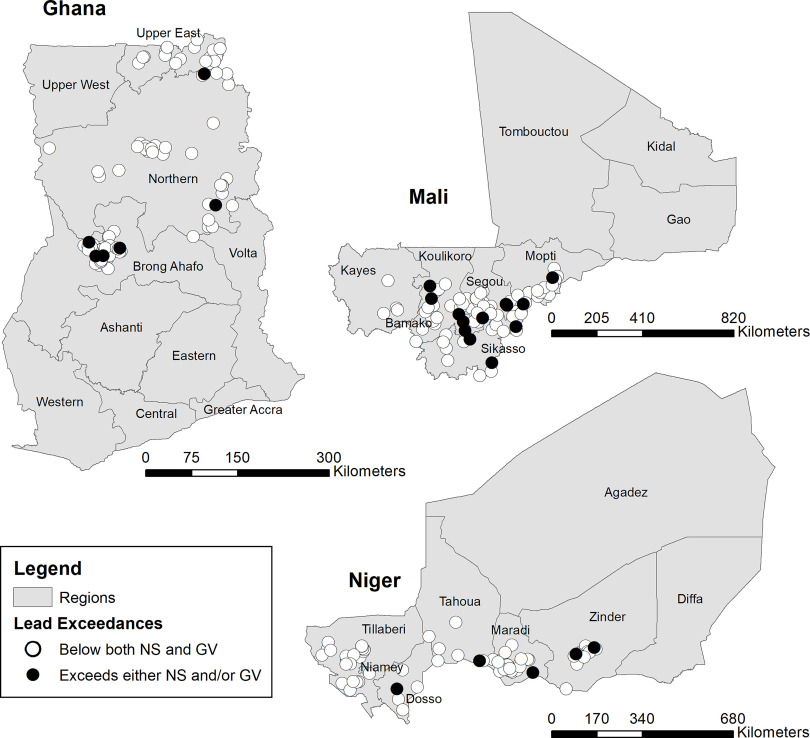
Measured concentrations of TMs were compared with WHO GVs and NSs (Table 1 presents a list GVs and NSs and associated references) of the three countries (Table 3). Of the tested TMs, lead had the highest proportion of exceedances, with water samples from 7.3% (19/261) of systems exceeding the NSs and samples from 9.2% (24/261) of systems exceeding the WHO GV. No significant geospatial clustering of lead exceedances was observed (Figure 1). Manganese, nickel, and selenium exceedances were observed in multiple countries. Antimony NS exceedances occurred in Niger, although no samples exceeded the antimony WHO GV. Full results are reported in Excel Table S2.
Table 3.
Number (%) of WHO guideline value and national standard exceedances for nine toxic metals in drinking-water samples collected after a 1-h stagnation period ().
| Standard | Antimony | Arsenic | Cadmium | Chromium | Lead | Manganese | Mercury | Nickel | Selenium | |
|---|---|---|---|---|---|---|---|---|---|---|
| National Standard Exceedances (see Table 1) | Ghana | 0 | 1 (1.1) | 0 | 1 (1.1) | 6 (6.3) | 3 (3.2) | 0 | 1 (1.1) | 1 (1.1) |
| Mali | 0 | 0 | 1 (1.6) | 0 | 13 (14.4) | 1 (1.1) | 0 | 8 (8.9) | 1 (1.1) | |
| Niger | 13 (17.1) | NA | 0 | 0 | 0 | 1 (1.3) | 1 (1.3) | NA | 0 | |
| Overall | 13 (5.0) | 1 (0.4) | 1 (0.4) | 1 (0.4) | 19 (7.3) | 5 (1.9) | 1 (0.4) | 9 (3.4) | 2 (0.8) | |
| WHO Guideline Value Exceedances (see Table 1) | Ghana | 0 | 1 (1) | 0 | 1 (1.1) | 6 (6.3) | 3 (3.2) | 0 | 1 (1.1) | 1 (1.1) |
| Mali | 0 | 0 | 1 (1.6) | 0 | 13 (14.4) | 1 (1.1) | 0 | 1 (1.1) | 0 | |
| Niger | 0 | 1 (1.3) | 0 | 0 | 5 (6.6) | 1 (1.3) | 0 | 0 | 0 | |
| Overall | 0 | 2 (0.8) | 1 (0.4) | 1 (0.4) | 24 (9.2) | 5 (1.9) | 0 | 2 (0.8) | 1 (0.4) | |
Note: NA, not applicable; WHO, World Health Organization.
Figure 1.
Maps of TM exceedances by metal, country, and source type for lead exceedances in Ghana, Mali, and Niger. Exceedances are defined as detection of a TM at concentrations exceeding the applicable drinking-water quality national standard (NS) and/or WHO Guideline Value (GV; see Table 1). Note: TM, toxic metal; WHO, World Health Organization.
Summary statistics were calculated for each TM (Table 4). Median concentrations for many TMs were equal to half the detection limit (continuity correction for nondetects), consistent with the substantive proportion of nondetects for each element. However, median concentrations of lead, manganese, and nickel were .
Table 4.
Mean, median, and maximum concentrations (95% CI) of nine toxic metals and four selected other indicator metals in drinking-water samples after a 1-h stagnation period.
| Metric | Toxic metals | Other metals | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimony | Arsenic | Cadmium | Chromium | Lead | Manganese | Mercury | Nickel | Selenium | Copper | Iron | Tin | Zinc | |
| Arithmetic Mean (, 95% CI) | 0.30 (0.22, 0.38) | 1.78 (0, 3.68) | 0.23 (0, 0.57) | 2.45 (1.54, 3.35) | 7.74 (0.46, 15.01) | 50.05 (25, 75) | 0.08 (0.06, 0.10) | 3.68 (2.23, 5.13) | 2.71 (2.18, 3.25) | 32.32 (21.60, 43.03) | 792.0 (155.7, 1,428) | 3.04 (1.89, 4.23) | 202.90 (11.2, 394.6) |
| Geometric Mean (, 95% CI) | 0.05 (0.04, 0.07) | 0.17 (0.12, 0.22) | 0.02 (0.015, 0.025) | 0.33 (0.24, 0.46) | 1.00 (0.80, 1.24) | 7.37 (5.76, 9.44) | 0.04 (0.029, 0.043) | 0.78 (0.59, 1.02) | 0.21 (0.14, 0.32) | 6.31 (4.65, 8.57) | 25.8 (18.0, 37.1) | 0.14 (0.09, 0.21) | 6.75 (4.84, 9.42) |
| Median () | 0.10 | 0.25 | 0.05 | 0.50 | 1.20 | 6.90 | 0.05 | 1.00 | 0.48 | 11.00 | 37.1 | 0.1 | 5.55 |
| Max () | 5.00 | 260.00 | 40.74 | 71.00 | 935.84 | 2,500.00 | 1.00 | 121.00 | 60.00 | 1,160.00 | 14,100 | 57.24 | 19,727.71 |
| WHO GV | 20 | 10 | 3 | 50 | 10 | 400 | 6 | 70 | 40 | 2,000 | 300a | NAb | NAb |
Note: A continuity correction of one half the method detection limit for each element was applied to nondetects for the purposes of calculating arithmetic mean, geometric mean, and median values. CI, confidence interval; GV, Guideline Value; NA, not applicable; WHO, World Health Organization.
Not a health-based GV.
No WHO GV established.
Predictive value of TM drinking-water sampling.
Test–retest data for systems visited twice during sampling activities showed a low positive predictive value (PPV) (Table 5, Table S1). This means that water systems with samples that exceeded the WHO GV for concentration of a given TM were unlikely to exceed the WHO GV in a subsequent sample. Meanwhile, the negative predictive value (NPV) was typically high, meaning that most tested nonexceedances were also nonexceedances upon retest. For example, lead had a PPV of 33% and an NPV of 95% (Table S1). Overall, 10% of systems (11/109) changed status with respect to lead exceedance status between test and retest.
Table 5.
Number of systems with samples exceeding WHO GV for lead on first and second test.
| Retest lead exceedance | |||
|---|---|---|---|
| Yes | No | ||
| Test lead exceedance | Yes | 3 | 6 |
| No | 5 | 95 | |
Note: GV, Guideline Value; WHO, World Health Organization.
Water system component scrapings.
In a subset of 61 water systems, system component scrapings were collected (Table S1, Excel Table S1) and compared with lead content recommendations under the IPC ( wt/wt). Overall, 80% of tested water systems (49/61 systems) contained at least one tested component exceeding 0.25% lead. Foot valves, handpump spouts, and tap spouts were more likely than rising mains and pump rods to exceed this value (Table 6, Table S3); brass components were more likely to exceed this threshold than other materials, with 72% of tested brass components (26/36) exceeding 0.25% lead (wt/wt; Table 6, Table S3; Excel Table S2).
Table 6.
Percentage and number of common water system components, materials exceeding 0.25% and 8% lead threshold by relative mass fraction (wt/wt).
| Result | Water system components | Materials | Systems | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Foot valve () | Pump spout () | Rising main () | Pump rod () | Tap spout () | Brass () | Galvanized steel () | Stainless steel () | Systems with any component over 0.25% lead () | |
| Samples exceeding 0.25% lead threshold | 19 [76 (55–91)] | 17 [59 (39–76)] | 9 [32 (16–52)] | 1 [4 (0–20)] | 13 [65 (41–85)] | 26 [72 (55–86)] | 44 [44 (34–54)] | 43 [41 (31–51)] | 107 [82 (75–88)] |
| Samples exceeding 8% lead thresholda | 17 [68 (46–85)] | 0 [0 (0–12)] | 5 [18 (6–39)] | 1 [4 (0–18)] | 1 [5 (0–2)] | 11 [31 (16–48)] | 23 [23 (15–32)] | 21 [20 (13–29)] | 76 [58 (49–67)] |
Although 0.25% is the currently recommended threshold, plumbing codes used to reference an 8% maximum lead threshold prior to 2014.
Predictors of Lead Concentration in Drinking Water
In the simplest of four multivariable models exploring factors associated with lead occurrence, log concentration of lead in water samples was associated with country and conductivity, but not with water system type, water system age, or pH, after controlling for variability in reported stagnation time [Table 7 (Model 1)]. Associations were observed between lead and other metals: specifically, chromium, copper, and zinc [Tables 7 (Model 2), Table 8]. When regression coefficients were normalized to the median concentration of each of these metals present in samples, the relative strength of association influences of chromium and copper on lead concentration were greatest (Table 8, Table S4; in other words, when correlation coefficients for significant associations between lead concentration and the concentration of other metals were normalized to the median concentration of those other metals, the resulting normalized coefficient values were greatest for chromium and copper). After controlling for the presence of other TMs, log lead was no longer associated with country [Table 7 (Model 2)]. Lead concentration in flushed samples (groundwater samples) was a predictor of lead concentration in stagnated first-draw drinking-water samples [Table 7 (Model 3), Table S5]. Finally, log lead concentration in water samples was associated with the presence of one or more brass parts in the water system [Table 7 (Model 4)], and after controlling for brass parts, the influence of flushed groundwater log lead concentrations on log lead in stagnated first-draw samples became nonsignificant. Regression diagnostics showed no problematic collinearity () for all variables in all models. Analysis of a random subset of duplicate samples from each lab by ICP-MS at UNC suggested poor comparability at low () concentrations but good agreement on the classification of samples as above/below applicable guidelines (Figure S1, Table S6).
Table 7.
Multivariable regressions of log lead concentration as a function of log copper, log lead in groundwater, and presence of at least one brass part, controlling for relevant covariates.
| Variable | Model 1 | Model 2 | Model 3 | Model 4 |
|---|---|---|---|---|
| Adjusted estimate (-value) | Adjusted estimate (-value) | Adjusted estimate (-value) | Adjusted estimate (-value) | |
| 212 | 212 | 104 | 51 | |
| 0.08 | 0.40 | 0.55 | 0.66 | |
| Country | ||||
| Ghana | Ref | Ref | Ref | Ref |
| Mali | 0.53 (0.30) | 0.41 (0.24) | 0.84 (0.29)** | 1.15 (0.45)* |
| Niger | 0.78 (0.33)* | 0.19 (0.28) | — | — |
| Water system type | ||||
| Handpump | Ref | Ref | Ref | Ref |
| Public tap | 0.17 (0.27) | (0.23) | (0.38) | (0.58)* |
| Age of water system | (0.012) | (0.010) | 0.001 (0.013) | (0.028) |
| Stagnation time | () | () | (0.002) | (0.002) |
| pH | 0.005 (0.004) | 0.002 (0.003) | 0.024 (0.18) | (0.26) |
| Conductivity | (0.0004)* | () | () | () |
| Log copper | — | 0.43 (0.04)*** | 0.39 (0.05)*** | 0.48 (0.09)*** |
| Log lead in flushed samples (groundwater) | — | — | 0.36 (0.14)* | (0.21) |
| Brass component | — | — | — | 1.34 (0.45)** |
Note: All models were run using the regress command in STATA. Model 1 (base model) is a simple linear regression of log lead concentration as a function of country, system type, system age, stagnation time, and water sample pH, and conductivity. Model 2 is based on Model 1 but also controls for log copper concentration in water samples. Model 3 is based on Model 2 but also controls for log lead concentration in flushed groundwater samples from sampled sources. Model 4 is based on Model 3 but includes a dummy variable for the presence of one or more brass components identified in the water system. In each model, the -value given is the -value associated with the -value calculated as the mean square model divided by the mean square residual. In each model, the comparator is designated by the abbreviation Ref. —, not applicable; Ref, referent. *; **; ***.
Table 8.
Multivariable linear regressions for associations between concentrations of TM of interest and selected other indicator metals (controlling for country, pH, conductivity, and stagnation time).
| Antimony | Arsenic | Cadmium | Chromium | Copper | Iron | Lead | Manganese | Mercury | Nickel | Selenium | Tin | Zinc | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Antimony | — | 0.002 | 0.515 | 0.048* | * | 0.377 | 0.0150 | *** | |||||
| Arsenic | 2.088 | — | 0.005 | 0.046 | 0.093 | ||||||||
| Cadmium | 0.014 | — | *** | *** | 0.002 | ** | 0.036 | 0.003 | 0.003 | ||||
| Chromium | 0.623* | *** | — | 0.013*** | 0.220*** | 0.002 | 1.060 | 0.008 | 0.023 | 0.020* | |||
| Copper | 4.102 | 0.024 | 161.556*** | 5.731*** | — | * | 3.157*** | * | 2.628*** | 0.124 | |||
| Iron | 678.785* | 120.454 | * | — | 47.051 | 9.852*** | 59.160 | 30.153 | 3.540*** | ||||
| Lead | 0.004 | 6.344 | 1.889*** | 0.062*** | — | 0.002 | 0.002** | ||||||
| Manganese | 0.005 | 186.202** | 2.846 | * | 0.011*** | 0.368 | — | 1.291 | 0.420 | 0.002 | |||
| Mercury | 0.023 | 0.079 | 0.005 | — | |||||||||
| Nickel | 6.585 | 0.043 | 0.310*** | 0.004 | 0.797 | — | 0.003 | ||||||
| Selenium | 0.951 | 5.71 | 0.110 | 0.728 | — | 0.077*** | |||||||
| Tin | *** | 0.061 | 1.247* | 0.017 | 0.014 | 6.952 | 0.040 | 0.976*** | — | 0.003 | |||
| Zinc | 0.448 | 479.987 | 0.161*** | 17.040** | 0.093 | 169.954 | 3.139 | — |
Note: Regressions were run using the regress command in STATA. Associations were evaluated for significance at the 95% confidence level based on the -value associated with the -value calculated as the mean square model divided by the mean square residual. Unadjusted (non-normalized) coefficients are presented in this table. In contrast, coefficients were normalized by the median concentration of the TM of interest for other comparisons to provide a more meaningful indication of the relative strength of association of different TMs with different median concentrations on a given analyte such as lead. —, not applicable; TM, toxic metal. *; **; ***.
Discussion
Toxic Metal Contamination in West African Water Systems
Unsuitable water system components were widely used in the 61 water systems for which components were tested: 82% of these systems (49/61 systems) contained at least one tested component exceeding IPC maximum lead content (0.25% wt/wt). Brass components were particularly likely to have elevated lead content (72% of such components exceeded 0.25% lead; 26/36). Although some manufacturers incorporate several percent lead by mass in brasses to improve machineability (Maas et al. 2005), such components release lead into drinking water as they corrode (Kimbrough 2007; Tam and Elefsiniotis 2009), making them unsafe for use in drinking-water systems. The association between lead and copper concentrations in drinking-water samples suggests that leaded brass (which contains copper) may be an important source of contamination. Drinking water from systems with identified brass components was predicted to have 3.8 times the lead content of drinking water from systems with no brass components (), further supporting the hypothesis that leaded brass components are important sources of lead in drinking water in West Africa.
Lead concentration in drinking water was associated with copper, chromium, and zinc. The association of lead with zinc may likewise implicate brass, but it could also arise from corrosion of galvanized steel. Brass is widely used in water system valves and taps, whereas galvanized and stainless steel are widely used in pipes, pumps, rods, and other fittings. Associations of lead with chromium may likewise be related to the presence of lead-containing steel components; however, the lack of an association with nickel may contradict this interpretation. Differences in redox potentials may also play a role in the relative detection of chromium vs. nickel ions under typical groundwater conditions. Associations of lead with different types of steels and different steel components may be complex, and further exploration is warranted.
Lead in drinking water was found to occur across countries and water system types. These associations may relate to differences in water systems components and materials across settings, differences in water chemistry, or a combination of factors, or they may be spurious (e.g., due to other, unobserved variables).
Lead in drinking-water samples was significantly associated with lead in flushed groundwater in Model 3; however this association decreased when controlling for copper in drinking water and disappeared when controlling for the presence of brass water system components. This suggests that lead in aquifers is not a main source of lead in drinking-water samples from these systems, which aligns with existing literature (WHO 2011). Although groundwater can contain lead from natural or anthropogenic point sources (WHO 2003), the WHO Guidelines for Drinking-Water Quality note that lead dissolution from natural sources is rare and that lead in drinking water primarily results from corrosion of plumbing systems (WHO 2011).
TMs, other than lead, were not detected in most samples, and WHO GV exceedances for these TMs were infrequent. WHO GV exceedances were recorded for arsenic, cadmium, chromium, manganese, nickel, and selenium; however, the proportion of GV exceedances was not more than 3.2% in any country for any of these TMs.
Some exceedances were well over the WHO GV: Maximum measurements of arsenic, cadmium, and manganese were recorded. These elements have been previously documented as primarily geogenic groundwater contaminants (Thompson et al. 2007).
The factors identified in this study may contribute to TM exposures elsewhere. Water system components are generally sourced from global supply chains, and corrosion of these components likely occurs at appreciable rates across settings. Geogenic TM contaminants such as arsenic and manganese are widespread, and many exposures may go undetected. Given the hundreds of millions of people relying on boreholes with handpumps and public taps in SSA, the public health impacts resulting from use of unsafe water system components in SSA are likely substantial.
The proportion of samples with lead exceeding reported in this study, although lower than proportions reported in some recent U.S. studies (GAO 2018; Pieper et al. 2015), is comparable to results observed in many HICs (Hayes and Skubala 2009; Levallois et al. 2014). The association between the occurrence of lead and copper in this study is also consistent with U.S. results from private wells in Virginia (Pieper et al. 2015).
Limitations
Samples from different countries were analyzed at different laboratories—a deliberate effort of the present study to align with scalable local monitoring options where possible. These laboratories varied in their analytical capabilities, instrumentation, methods, and personnel, thereby reducing comparability of results. Analysis of duplicate samples from each lab at UNC suggested that analytical precision and comparability of laboratories was inadequate for achieving submicrogram-per-liter quantitation; however, precision and repeatability appear to have been adequate for classifying samples with respect to exceedance of/conformity to applicable NSs and WHO GVs (Figure S1, Table S6).
In solid samples (scrapings), lead concentrations were normalized to the total concentrations of all metals analyzed, rather than to the total initial sample mass given that scrapings adhered to damp GhostWipe collection wipes and could not be accurately weighed before digestion. The error introduced by this limitation is likely small, given that major constituents of most brasses, bronzes, and steels were included in the analyzed metals, but future analyses might use methods such as sample collection on dry metal-free paper to improve precision.
The limited number of water system components sampled ( components from 61 systems) prevented more precise identification of significant relationships between system component samples and drinking-water samples. As some components cannot be readily accessed after installation or sampled in situ without risking damage to the system, scrapings were not collected from some water system components of interest (e.g., borehole casings, pipe solders, pump cylinders), and future work might examine these as well.
Survey data quality was limited by respondent recall or imperfect knowledge of water source characteristics, such as age. Recall was also likely imperfect for implementer, as well as occurrence and date of rehabilitation or repairs.
Further evidence might reduce uncertainty in estimates of lead and other TM occurrence in drinking water and water system components in these study settings, but it is unlikely to modify the finding that action is needed to prevent and reduce exposure. The benefits of taking action to prevent introducing leaded water components in new drinking-water systems substantively outweigh the risks and costs of such action.
Potential Next Steps
Preventing exposures to lead and other TMs would contribute to progress on UN SGD Target 6.1. Lead, and perhaps other TMs, should be addressed (alongside microbial and other priority chemical contaminants) in order to attain universal access to safe drinking water (United Nations 2015).
This work demonstrates that the majority of water systems in the study setting [80% (49/61 systems)] contain components with lead in excess of IPC guidance and that corrosion of such components is a major source of lead contamination in the study setting, contributing to lead in excess of WHO drinking-water guidelines in 5–10% of first-draw water samples. This contamination may be readily addressed through cost-effective preventive action, such as consistent use of components and materials compliant with IPC codes (Tracy et al. 2020). Supply chain improvements with verification of compliance would reduce the availability and use of unsuitable components, such as leaded brass parts, in drinking-water systems. Governments may develop or update regulations related to lead-free water system components and their implementation, including verification schemes (Tracy et al. 2020).
Because of the large number of existing systems containing unsafe components, targeted, progressive remediation might usefully supplement prevention. Such remediation might involve replacing noncompliant components with suitable ones during normal maintenance procedures and prioritizing systems for remediation based on remaining design life, known or suspected component materials, groundwater chemistry, and number of users served, among other factors (Tracy et al. 2020).
Unlike primary prevention, unsafe component replacement may not fully and immediately correct some lead exposure problems given that lead previously leached from source components, such as brass valves, may persist adsorbed to other surfaces within water systems (e.g., iron oxide particles on steel surfaces) and may mobilize into drinking-water supplies even after the original source of the contamination is replaced. Further study may be helpful to identify auxiliary measures, such as cleaning or filtration, to enhance the effectiveness of remediation.
Where TM-contaminated groundwater results from a point source of pollution such as mining, corrective measures for reducing such exposures, including strengthening pollution controls, environmental remediation, and source substitution are advisable (Tracy et al. 2020). These approaches may be complicated by high costs, logistical complexity, and/or regulatory challenges. Furthermore, TM contamination may linger after remediation activities have occurred. Further work should explore options for improving the implementation of solutions to address exogenous TM contamination (i.e., from sources other than water system corrosion) in rural LMIC settings. Although less broadly significant than corrosion as a source of lead exposure in the study setting, these measures may protect localized populations exposed to high TM concentrations in groundwater from, for example, industrial or geogenic contamination sources.
Although progressive correction of water systems containing unsuitable components is likely justified in many LMIC settings, countries should beware of overreacting to concerns about lead and TM contamination and should avoid taking precipitous actions unsupported by available evidence. Specifically, closing of otherwise functional and otherwise safe water systems is unlikely to be helpful and may be profoundly harmful in many cases because there are substantive adverse consequences of interrupting water availability (Hunter et al. 2009). The evidence supporting the benefits of adequate quantities of water is extensive (Howard and Bartram 2003).
Given the relatively low PPV of lead tests in water systems in this study, targeting individual water systems for remediation based on post-implementation water quality tests is likely to be ineffective. Primary prevention for new systems and repairs, coupled with testing of older water system components during routine maintenance and replacement of parts that are unsuitable, could be a more effective approach for progressive remediation. However, post-implementation monitoring of TM concentrations in water from a representative sample of drinking-water systems is likely to be valuable in assessing the effectiveness of corrective and preventive actions and to track progress in reducing lead contamination over a population of systems.
We further suggest that collaboration between governments, NGOs, researchers, and funders is likely to be instrumental in expanding and enhancing laboratory capacity to support robust, representative TM monitoring and surveillance. Likewise, component testing (e.g., prior to installation of new water systems and prior to repairs) can likely be made economical if centralized (i.e., for verification and supply chain improvement purposes), whereas field-based component and water testing as part of routine surveillance could potentially cover a subset of systems in any given year and track the impact of certification and testing schemes over time. Lower-cost methods for quantifying lead in drinking-water and water system components may also be explored. X-ray fluorescence methods have low per-sample costs and are potentially suitable for centralized supply chain verification and parts certification/testing (Weindorf et al. 2014). Low-cost field-based methods (e.g., lead test strips, automated portable anodic stripping voltammetry) may also be available or achievable, and further work is needed to characterize the suitability of these for monitoring or screening applications requiring microgram-per-liter sensitivity.
Further work characterizing the occurrence and determinants of lead and other TM contamination in water systems in SSA and other LMIC settings appears warranted. However, further justification of primary prevention is unnecessary. Preventive action is a no-regrets response to available evidence offering cost-effective public health benefits for millions.
To undertake such a response, governments might set or revisit standards for lead in drinking water and in drinking-water system components and act to require implementer compliance. To support such efforts, we suggest that NGOs and other implementers commit to using only components conforming to national and IPC guidance. Intergovernmental organizations (IGOs) could likewise reflect on the high prevalence of lead in drinking water documented in this work and by others, and ensure that policy and financing commitments reflect appropriate support and responses. Finally, we suggest that implementers of all types should move quickly on prevention and develop targeted remediation strategies. In so doing, countries, implementers, and funders should resist the urge to fixate on past actions, assign blame, or delay response pending greater certainty on all particulars, which would delay meaningful preventive action by years.
The evidence linking lead exposure to health outcomes is robust, and the present work echoes previous findings indicating that lead exposure in drinking water is widespread in LMICs. Multiple context-specific factors will contribute to the precise health effects of this widespread exposure. These include the facts that lead occurrence in drinking water from a given source is variable (as indicated by the low PPV of test–retest exceedance results for lead) and that no safe level of lead exposure has been identified [NSs and WHO’s GVs are pragmatically based on measurability (WHO 2003)] and do not represent safety, per se. Furthermore, lead and other TMs have complex interacting health effects, and metals mixtures may adversely and synergistically impact health and development (Sanders et al. 2015). Finally, nutritional and iron status modify the impacts of lead exposure (Goyer 1995), suggesting that the most vulnerable populations may experience the greatest harm. Further work to characterize these interacting factors may help elucidate the burden and distribution of preventable disease from lead in drinking water, but it will not affect the strength of the available evidence for recommending timely and effective preventive actions.
Supplementary Material
Acknowledgments
We gratefully acknowledge the support, assistance, and engagement of collaborators in Ghana, Niger, and Mali. We also gratefully acknowledge the support and assistance of P. Cable, K. Pieper, and many others whose efforts, contributions, and suggestions made this work possible. Finally, we gratefully acknowledge World Vision for financial support of this work and for invaluable support and assistance in carrying out this work.
References
- Addis G, Moore MR. 1974. Lead levels in the water of suburban Glasgow. Nature 252:120–121, 10.1038/252120a0. [DOI] [Google Scholar]
- Ayotte JD, Medalie L, Qi SL, Backer LC, Nolan BT. 2017. Estimating the high-arsenic domestic-well population in the conterminous United States. Environ Sci Technol 51(21):12443–12454, PMID: 29043784, 10.1021/acs.est.7b02881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown MJ, Margolis S. 2012. Lead in drinking water and human blood lead levels in the United States. MMWR Suppl 61(4):1–9, PMID: 22874873. [PubMed] [Google Scholar]
- Chikaodili EB, Iheanyichukwu OA, Kelechi NO, Ikechukwu NE. 2017. Geochemical and bacteriological analyses of water resources prone to contamination from solid waste dumpsites in Imo State, southeastern Nigeria. J Environ Sci Tech 10(6):325–343, 10.3923/jest.2017.325.343. [DOI] [Google Scholar]
- Chowdhury R, Ramond A, O’Keeffe LM, Shahzad S, Kunutsor SK, Muka T, et al. 2018. Environmental toxic metal contaminants and risk of cardiovascular disease: systematic review and meta-analysis. BMJ 362:k3310, PMID: 30158148, 10.1136/bmj.k3310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbina S, Duwiejuah A, Quansah R, Obiri S, Bakobie N. 2015. Comparative assessment of heavy metals in drinking water sources in two small-scale mining communities in northern Ghana. Int J Environ Res Public Health 12(9):10620–10634, PMID: 26343702, 10.3390/ijerph120910620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshommes E, Laroche L, Nour S, Cartier C, Prévost M. 2010. Source and occurrence of particulate lead in tap water. Water Res 44(12):3734–3744, PMID: 20546838, 10.1016/j.watres.2010.04.019. [DOI] [PubMed] [Google Scholar]
- GAO (U.S. Government Accountability Office). 2018. K-12 Education: Lead Testing of School Drinking Water Would Benefit from Improved Federal Guidance. GAO-18-382. https://www.gao.gov/assets/700/693280.pdf [accessed 7 April 2021].
- Goyer RA. 1995. Nutrition and metal toxicity. Am J Clin Nutr 61(suppl 3):646S–650S, PMID: 7879732, 10.1093/ajcn/61.3.646S. [DOI] [PubMed] [Google Scholar]
- Hayes CR, Skubala ND. 2009. Is there still a problem with lead in drinking water in the European Union? J Water Health 7(4):569–580, PMID: 19590124, 10.2166/wh.2009.110. [DOI] [PubMed] [Google Scholar]
- Houston MC. 2007. The role of mercury and cadmium heavy metals in vascular disease, hypertension, coronary heart disease, and myocardial infarction. Altern Ther Health Med 13(2):S128–S133, PMID: 17405690. [PubMed] [Google Scholar]
- Howard G, Bartram J. 2003. Domestic Water Quantity, Service Level and Health. Geneva, Switzerland: World Health Organization. https://www.who.int/water_sanitation_health/diseases/WSH03.02.pdf [accessed 7 April 2021]. [Google Scholar]
- Hunter PR, Zmirou-Navier D, Hartemann P. 2009. Estimating the impact on health of poor reliability of drinking water interventions in developing countries. Sci Total Environ 407(8):2621–2624, PMID: 19193396, 10.1016/j.scitotenv.2009.01.018. [DOI] [PubMed] [Google Scholar]
- International Code Council. 2017. 2018 International Plumbing Code. Country Club Hills, IL: International Code Council Inc. [Google Scholar]
- Järup L. 2003. Hazards of heavy metal contamination. Br Med Bull 68:167–182, PMID: 14757716, 10.1093/bmb/ldg032. [DOI] [PubMed] [Google Scholar]
- Kimbrough DE. 2007. Brass corrosion as a source of lead and copper in traditional and all‐plastic distribution systems. J Am Water Works Assoc 99(8):70–76, 10.1002/j.1551-8833.2007.tb08008.x. [DOI] [Google Scholar]
- Langenegger O. 1989. Groundwater quality—an important factor for selecting handpumps. Dev Water Sci 39:531–541. [Google Scholar]
- Levallois P, St-Laurent J, Gauvin D, Courteau M, Prévost M, Campagna C, et al. 2014. The impact of drinking water, indoor dust and paint on blood lead levels of children aged 1–5 years in Montréal (Québec, Canada). J Expo Sci Environ Epidemiol 24(2):185–191, PMID: 23361441, 10.1038/jes.2012.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SS, Vos T, Flaxman AD, Danaei G, Shibuya K, Adair-Rohani H, et al. 2012. A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380(9859):2224–2260, PMID: 23245609, 10.1016/S0140-6736(12)61766-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz A, Diarra S, Apambire WB, Thomas JM, Ayamsegna J. 2013. Drinking water from hand-pumps in Mali, Niger, and Ghana, West Africa: review of health effects. J Water Resour Prot 5(8A):13–20, 10.4236/jwarp.2013.58A002. [DOI] [Google Scholar]
- Maas RP, Patch SC, Morgan DM, Pandolfo TJ. 2005. Reducing lead exposure from drinking water: recent history and current status. Public Health Rep 120(3):316–321, PMID: 16134575, 10.1177/003335490512000317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ministere de L’Energie, des Mines et de L’Eau. 2007. Stratégie Nationale de Développement de l’Alimentation en Eau Potable au Mali. Rabat, Morocco: Republique du Mali. https://dnhmali.org/IMG/Strategie_AEPA.pdf [accessed 7 April 2021]. [Google Scholar]
- Ministry of Water Resources, Works, and Housing. 2015. National Drinking Water Quality Management Framework for Ghana. http://www.gwcl.com.gh/national_drinking_water_quality__management_framework.pdf [accessed 7 April 2021].
- NSF International. 2018. International Standard/American National Standard for Drinking Water Additives—drinking water system components—health effects (NSF/ANSI 61). Ann Arbor, MI: NSF International. [Google Scholar]
- Obiri-Danso K, Adjei B, Stanley KN, Jones K. 2009. Microbiological quality and metal levels in wells and boreholes water in some peri-urban communities in Kumasi, Ghana. Afr J Environ Sci Tech 3(1):59–66. [Google Scholar]
- Pieper KJ, Krometis LAH, Gallagher DL, Benham BL, Edwards M. 2015. Incidence of waterborne lead in private drinking water systems in Virginia. J Water Health 13(3):897–908, PMID: 26322775, 10.2166/wh.2015.275. [DOI] [PubMed] [Google Scholar]
- Pieper KJ, Tang M, Edwards MA. 2017. Flint water crisis caused by interrupted corrosion control: investigating “ground zero” home. Environ Sci Technol 51(4):2007–2014, PMID: 28145123, 10.1021/acs.est.6b04034. [DOI] [PubMed] [Google Scholar]
- Rauh VA, Margolis AE. 2016. Research review: environmental exposures, neurodevelopment, and child mental health—new paradigms for the study of brain and behavioral effects. J Child Psychol Psychiatry 57(7):775–793, PMID: 26987761, 10.1111/jcpp.12537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann C, Bjorvatn K, Frengstad B, Melaku Z, Tekle-Haimanot R, Siewers U. 2003. Drinking water quality in the Ethiopian section of the East African Rift Valley I—data and health aspects. Sci Total Environ 311(1–3):65–80, PMID: 12826384, 10.1016/S0048-9697(03)00137-2. [DOI] [PubMed] [Google Scholar]
- Republique du Niger. 2005. Fixant les normes de potabilite de I’eau de boisson. http://extwprlegs1.fao.org/docs/pdf/ner95228.pdf [accessed 10 December 2019].
- Sanders AP, Henn BC, Wright RO. 2015. Perinatal and childhood exposure to cadmium, manganese, and metal mixtures and effects on cognition and behavior: a review of recent literature. Curr Environ Health Rep 2(3):284–294, PMID: 26231505, 10.1007/s40572-015-0058-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders T, Liu Y, Buchner V, Tchounwou PB. 2009. Neurotoxic effects and biomarkers of lead exposure: a review. Rev Environ Health 24(1):15–45, PMID: 19476290, 10.1515/reveh.2009.24.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schock MR. 1989. Understanding corrosion control strategies for lead. J Am Water Works Assoc 81(7):88–100, 10.1002/j.1551-8833.1989.tb03244.x. [DOI] [Google Scholar]
- Smith AH, Hopenhayn-Rich C, Bates MN, Goeden HM, Hertz-Picciotto I, Duggan HM, et al. 1992. Cancer risks from arsenic in drinking water. Environ Health Perspect 97:259–267, PMID: 1396465, 10.1289/ehp.9297259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith AH, Lingas EO, Rahman M. 2000. Contamination of drinking-water by arsenic in Bangladesh: a public health emergency. Bull World Health Organ 78(9):1093–1103, PMID: 11019458. [PMC free article] [PubMed] [Google Scholar]
- Tam YS, Elefsiniotis P. 2009. Corrosion control in water supply systems: effect of pH, alkalinity, and orthophosphate on lead and copper leaching from brass plumbing. J Environ Sci Health A Tox Hazard Subst Environ Eng 44(12):1251–1260, PMID: 19847713, 10.1080/10934520903140009. [DOI] [PubMed] [Google Scholar]
- Thompson T, Fawell J, Kunikane S, Jackson D, Appleyard S, Callan P, et al. 2007. Chemical Safety of Drinking Water: Assessing Priorities for Risk Management. Geneva, Switzerland: World Health Organization. https://apps.who.int/iris/bitstream/handle/10665/43285/9789241546768_eng.pdf;sequence=1 [accessed 7 April 2021]. [Google Scholar]
- Tracy JW, Guo A, Liang K, Bartram J, Fisher M. 2020. Sources of and solutions to toxic metal and metalloid contamination in small rural drinking water systems: a rapid review. Int J Environ Res Public Health 17(19):7076, PMID: 32992630, 10.3390/ijerph17197076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trémolet S. 2013. Regulation in rural areas. Water services that last. IRC. https://www.ircwash.org/sites/default/files/084-201502triple-s_bn07defweb.pdf [accessed 10 December 2019]. [Google Scholar]
- Tukura B. 2014. Assessment of heavy metals in ground water from Nasarawa State, Middle Belt, Nigeria. Am Chem Sci J 4(6):798–812, 10.9734/ACSJ/2014/10553. [DOI] [Google Scholar]
- United Nations. 2015. Transforming our world: the 2030 Agenda for Sustainable Development. Resolution adopted by the General Assembly. https://sdgs.un.org/2030agenda [accessed 7 April 2021].
- Weindorf DC, Bakr N, Zhu Y. 2014. Advances in portable X-ray fluorescence (PXRF) for environmental, pedological, and agronomic applications. Adv Agronomy 128:1–45, 10.1016/B978-0-12-802139-2.00001-9. [DOI] [Google Scholar]
- WHO (World Health Organization). 2003. Lead in Drinking-Water: Background document for development of WHO Guidelines for Drinking-Water Quality. WHO/SDE/WSH/03.04/09/Rev/1. https://www.who.int/water_sanitation_health/dwq/chemicals/lead.pdf [accessed 7 April 2021].
- WHO. 2011. Guidelines for Drinking-Water Quality. Fourth Edition. 104–108. https://www.who.int/water_sanitation_health/publications/2011/9789241548151_toc.pdf?ua=1 [accessed 7 April 2021].
- WHO, UNICEF (United Nations Children’s Fund). 2006. Core Questions on Drinking Water and Sanitation for Household Surveys. https://apps.who.int/iris/bitstream/handle/10665/43489/9789241563260_eng.pdf;sequence=1 [accessed 7 April 2021].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.