To the Editor:
There are concerns that in-center maintenance hemodialysis (HD) patients may have higher risk for infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) because they take 3 or more round-trips a week to dialysis centers and spend several hours during dialysis close to fellow patients and clinic staff. This concern is supported by data from dialysis facilities in London, United Kingdom.1
During the first wave of coronavirus disease 2019 (COVID-19), New York City was one of the worst affected regions in the United States. Starting in August 2020, the New York City Department of Health has published regularly updated seropositivity rates for the general population for most 5-digit zip code areas.2 This allowed us to link zip code area seroprevalence data with results of 2 cross-sectional serosurveillance projects conducted in in-center maintenance HD patients and clinic staff in Manhattan dialysis facilities.
Between June and September 2020, we conducted a quality improvement project and an institutional review board (IRB)-approved clinical research study (Western IRB protocol # 20201875) to assess the prevalence of SARS-CoV-2 antibodies in in-center maintenance HD patients and clinic staff. The quality improvement project was approved by the clinic governing bodies and underwent legal and compliance review. Verbal consent was obtained from all patients. Written informed consent was obtained from staff before blood sampling. Blood samples were obtained and analyzed for immunoglobulin G (IgG) and IgM antibodies by Spectra Laboratories (Rockleigh, NJ) using the emergency use authorized kits with reported specificity and sensitivity of 98.7% and 100%.3
Zip code seropositivity rates were calculated by dividing the number of seropositive Renal Research Institute in-center maintenance HD patients by the number of all Renal Research Institute patients living in that area. We used simple weighted linear regression and Spearman rank correlation to estimate the correlation between general population and patient seroprevalence rates across all applicable zip codes.
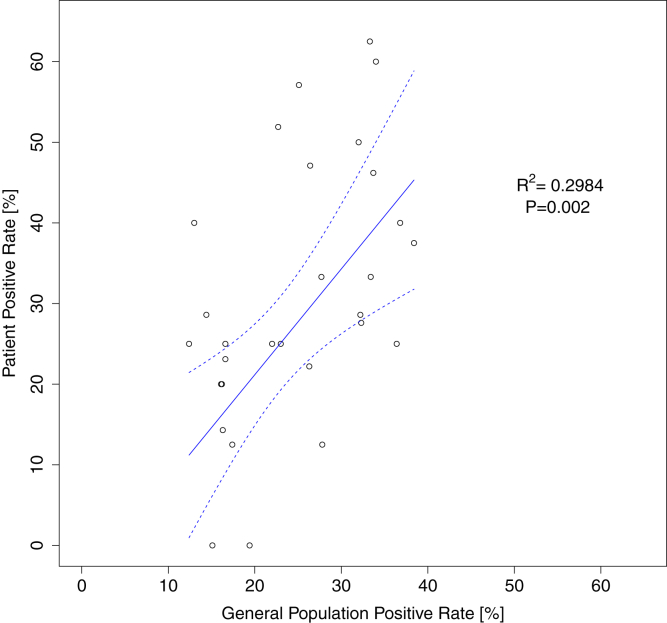
Serology data were available for 429 (86.1%) patients. Of these 429 patients, 130 (30.3%) had antibodies against SARS-CoV-2: 90 (69.2%) tested positive for IgG only; 39 (30%), for both IgG and IgM; and 1 (0.8%), for IgM only. These 429 patients resided in 87 zip code areas, and 418 of them lived in 78 zip code areas for which general population seropositivity estimates were available. Of these 429 patients, 339 (79%) lived in 29 zip code areas with 4 or more patients (average 11.7; range, 4-52) and were thus included in the correlational analysis. We found a significant correlation (R2 = 0.2984; P = 0.002) between zip code area seropositivity rates of the general and dialysis population (Fig 1). The Spearman rank correlation coefficient was 0.498 (P = 0.006).
Figure 1.
Association between seropositivity rates in the general population and dialysis patients. Each symbol represents a zip code area. Dashed lines are the 95% CI for the weighted linear regression. The equation of the fitted line is: Patient positive rate = 1.31 × General population positive rate - 5.07. The slope of the regression line does not differ significantly from the line of identify (95% CI of the slope, 0.52-2.11).
Serology data were available for 115 (72.3%) staff members. Of these, 19 (16.5%) had antibodies against SARS-CoV-2; 17 (89.5%) showed IgG only and 2 (10.5%) showed IgM only. None tested positive for both IgG and IgM antibodies. The staff lived in 88 zip code areas; only 2 areas comprised 4 or more staff members. The geographic spread of staff residencies in areas for which general population seropositivity estimates were not available prevented us from a zip code–level analysis comparing staff and general populations seropositivity rates.
In an aggregate analysis, the staff seropositivity rate of 16.5% was significantly lower than the 30.3% seropositivity observed in patients (P = 0.005). Analysis on a clinic level revealed a lower seropositivity rate in staff compared with patients in 2 clinics (clinics A and D) and no significant difference in clinics B and C (Table 1).
Table 1.
Seropositivity Rates in Hemodialysis Patients and Clinic Staff From 4 Dialysis Clinics (A, B, C, and D) Located in Manhattan, New York City, NY
| Facility | Seropositivity Rate |
Difference (95% CI) | P | |
|---|---|---|---|---|
| Patients | Staff | |||
| A | 58 (39.2%) | 6 (15.4%) | 23.8% (8.3 to 39.2) | 0.009 |
| B | 25 (24.0%) | 5 (21.7%) | 2.3% (−18.7 to 23.3) | 0.99 |
| C | 16 (32.0%) | 6 (27.3%) | 4.7% (−21.2 to 30.7) | 0.90 |
| D | 31 (24.4%) | 2 (6.5%) | 17.9% (4.5 to 31.4) | 0.05 |
| All | 130 (30.3%) | 19 (16.5%) | 13.8% (5.1 to 22.4) | 0.005 |
Our results indicate a significant correlation between general population and in-center HD patient seropositivity rates for SARS-CoV-2 infection and support the notion that COVID-19 infection in in-center maintenance HD patients is primarily driven by infections in patients’ residence areas.4 The lower seropositivity rates in dialysis staff compared with patients points toward the effectiveness of screening measures and efforts to limit transmission in dialysis centers.
Serosurveillance studies can shed light on infection rates and their correlates, providing key data to inform infection control. We found a seroprevalence rate of 30.3% among in-center maintenance HD patients residing in a region with a high COVID-19 prevalence. This finding corroborates a nationwide serosurveillance study in 1,300 dialysis clinics.5 An analysis per zip code in clinic staff was not possible due to the small number of staff residences in zip code areas with reported seroprevalence rates and the dispersion of staff residences across a large number of zip code areas, resulting in a small number of staff members per zip code. Of note, staff seroprevalence rates were lower compared with those of patients in 2 of 4 clinics.
We are aware of some limitations. First, general population seropositivity rates were either not available in all zip code areas or the number of patients and staff living in a specific area was too small. Second, more research is needed to investigate the relationship between socioeconomic indicators and COVID-19 seropositivity rates across different regions.
In summary, our results indicate an association between patient and general population seropositivity rates. Seropositivity rates were higher in patients compared with clinic staff.
Article Information
Authors’ Contributions
Study design and implementation: OT, NG, XY, LMTS, XY, HZ, and PK; data analysis: OT, HZ, XY, YW, and PK; supervision and mentorship: OT, NG. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
Research was partially supported by National Institute of Health/National Institute of Diabetes and Digestive and Kidney Diseases grant 1R01DK130067-01. The funder had no role in study design; data collection, analysis, or report; or the decision to submit for publication.
Financial Disclosure
The Manhattan Quality Improvement Project and IRB approved research project were planned, designed, conducted, and fully paid for by the Renal Research Institute. The Renal Research Institute is a wholly owned subsidiary of Fresenius Medical Care, North America. Dr Kotanko holds stock in Fresenius Medical Care, North America. The remaining authors declare that they have no relevant financial interests.
Peer Review
Received November 30, 2020. Evaluated by 1 external peer reviewer, with direct editorial input from the Statistical Editor and the Editor-in-Chief. Accepted in revised form February 15, 2021.
References
- 1.McCafferty K., Davari M., Price K., Rajakariar R., Cove-Smith A., Forbes S.H. COVID-19 prevalence and seroconversion in an urban hemodialysis unit in the United Kingdom. Hemodial Int. 2021;25(1):137–139. doi: 10.1111/hdi.12883. [DOI] [PubMed] [Google Scholar]
- 2.NYC Department of Health and Mental Hygiene Coronavirus data. https://github.com/nychealth/coronavirus-data/blob/master/totals/antibody-by-modzcta.csv
- 3.Suhandynata R.T., Hoffman M.A., Kelner M.J., McLawhon R.W., Reed S.L., Fitzgerald R.L. Longitudinal monitoring of SARS-CoV-2 IgM and IgG seropositivity to detect COVID-19. J Appl Lab Med. 2020;5(5):908–920. doi: 10.1093/jalm/jfaa079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cherif A., Willetts J.L., Usvyat L., Wang Y., Kotanko P. Comparative analysis of SARS-CoV-2 reproduction rates in the dialysis and general populations. J Am Soc Nephrol. 2021;32(4):791–794. doi: 10.1681/ASN.2020121691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anand S., Montez-Rath M., Han J. Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: a cross-sectional study. Lancet. 2020;396(10259):1335–1344. doi: 10.1016/S0140-6736(20)32009-2. [DOI] [PMC free article] [PubMed] [Google Scholar]