Abstract
Introduction
Studies have revealed hypocalcemia and low vitamin D levels in severe covid-19 that warrant further research.
Objective
Our study investigates the correlation between calcium levels at presentation as a primary endpoint and pre-existing calcium levels as a secondary endpoint to the severity of disease presentation and progression.
Method
Observational cohort study in adults admitted with COVID-19 from March utill September 2020. Multiple clinical scales and laboratory parameters were used to correlate corrected calcium and vitamin D associations with risk factors and outcomes.
Results
Four hundred and forty five patients were included in the study. Hypocalcemic patients had more abnormal laboratory parameters and longer hospitalization duration. Hypocalcemia was in 60–75% of all age groups (p-value 0.053), for which 77.97% were ICU admissions (p-value 0.001) and 67.02% were diabetic (p-value 0.347). There were non-significant correlations between Vitamin D and almost all the parameters except for chronic respiratory diseases, which had a P-value of 0.024.
Conclusion
It can be concluded that hypocalcemia is a significant and reliable marker of disease severity and progression regardless of underlying comorbidities. Vitamin D levels fail to reflect correlation with severity of COVID-19 infections.
Keywords: COVID-19, Oman, Hypocalcemia, Vitamin D, Prognosis, Disease marker
Introduction
Coronaviruses (CoVs) are enveloped single‐stranded RNA viruses with a highly diverse nature. Over the past two decades, two novel viruses, severe acute respiratory syndrome CoV (SARS‐CoV) and Middle East respiratory syndrome CoV (MERS‐CoV), emerged to cause severe human disease. They were found to cause multiple symptomatic effects in respiratory, enteric, hepatic, and neurological systems in humans and animals. The Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV- 2) is a newly emerging zoonotic coronavirus discovered in Wuhan, China, in December 2019 and has been identified as Coronavirus Disease 2019 (COVID-19). The virus’s main manifestations in patients include fever, cough, and shortness of breath that can progress rapidly in some cases to severe pneumonia, acute respiratory distress syndrome (ARDS), and septic shock (Khamis et al., 2020). The World Health Organization (WHO) declared coronavirus disease 2019 (COVID-19) as a public health emergency of grave international concern (Kešeľová et al., 2020). Although the clinical picture of SARS, MERS, and COVID-19 seems similar, differences were noted since early reports at the beginning of the pandemic. Hence a proper characterization of the complete pathophysiology of this disease, clinical progression, and possible sequelae are critical to battle its detrimental effects.
The National Health Commission of China has issued a series of diagnosis and treatment recommendations and suggested classifying the disease into four grades: mild, moderate, severe, and critical (Health Commission of PRC, 2019). Recent studies have reported the clinical characteristics and prognosis of COVID-19 with varying severity (Guan et al., 2020, Huang et al., 2020, Wang et al., 2020, Xu et al., 2020, Zhu et al., 2020). The underlying pathophysiology of this virus leading to disease progression and organ dysfunction remains to be further explored.
As of November 1st, 2020, we had 115,734 confirmed cases, 105,700 recoveries, and 1,246 deaths reported in our country. Worldwide cases stood at a staggering 46,632,558 cases, with 17,715,649 recoveries and 1,201,927 deaths (as per the COVID-19 Dashboard by the Center for Systems Science and Engineering, Johns Hopkins University). This unopposed disease progression has hampered the best healthcare systems worldwide, with unparalleled rapidity and calamitous economic effects. So far, evidence for definitive treatment regimens has proved anecdotal at best (Dong et al., 2020).
Due to the high mortality and the lack of effective treatments in critical patients, early identification of possible parameters that may predict clinical progression would be crucial to stratify care required for patients (Guan et al., 2020, Maclaren et al., 2020). This is particularly of significance to identify patients that are most likely to require early intensive care (Lipsitch et al., 2020).
Huang et al. reported that patients admitted into the intensive care unit (ICU) had more severe clinical symptoms and more abnormal serum parameters (Huang et al., 2020). Throughout this pandemic, there has been a panoply of biochemical parameters suggested in the literature that are inextricably linked to the clinical progression of patients in different populations (Di Filippo et al., 2020).
There have been a few published studies regarding the significance of calcium levels as a predictor of disease severity, amongst other biomarkers like D-Dimer, CRP, and Ferritin (Di Filippo et al., 2020, Millet and Whittaker, 2018, Sun et al., 2020). This study aims to investigate this link in our population of COVID patients, namely calcium correlated with the progression of our patients through a particular set of biochemical and clinical parameters. As for vitamin D levels, there have been a few studies that investigated the levels of vitamin D in previous disease outbreaks like H1N1 and SARS (Grant et al., 2020). The secondary endpoint in our study is to provide evidence that the correlation of clinical progression of our COVID cases is not linked to Vitamin D levels compared to corrected calcium levels.
Methods
This is an observational cohort study conducted at the Royal Hospital, a tertiary healthcare hospital in Muscat. The data was extracted from our hospital's COVID registry established for all patients since the start of the pandemic in our country. The registry includes multiple parameters such as epidemiological characteristics, clinical symptoms, comorbid diseases, and laboratory parameters. The laboratory parameters included corrected calcium and vitamin D levels. Our lab uses the Siemens Atellica® chemistry system (CH930), Germany. This study defined hypocalcemia as corrected calcium levels below 2.1 mmol/L and a low vitamin D level as <30 nmol/L. This study included all patients >15 years of age admitted to the hospital with COVID-19 infection.
The assessment of clinical condition on admission and progression during hospital stay was measured using several clinical tools. The WHO Ordinal scale of clinical improvement was used on admission and discharge (Please refer to Table 1 ) (World Health Organization, 2020). This scale ranks patients in meaningful categories but does not differentiate between underlying causes (Siegerink and Rohmann, 2018). The CURB-65 score was also used for admission evaluation (Lim et al., 2003).
Table 1.
Ordinal scale for clinical improvement for COVID-19.
| 0 | No clinical or virological evidence of infection |
| 1 | No limitation of activities |
| 2 | Limitation of activities |
| 3 | Hospitalized, no oxygen therapy |
| 4 | Oxygen by mask or nasal prongs |
| 5 | NIV or high flow oxygen |
| 6 | Intubation and mechanical ventilation |
| 7 | Ventilation + additional organ support - ino/RRT/ECMO |
| 8 | Death |
Data analysis was performed using STATA statistical software version 13.0, USA. The significance level was set at α = .05, and all tests were 2-tailed. The original scores of the four measurement tools are not normally distributed and so are presented as medians with interquartile ranges (IQRs). The ranked data derived from the counts of each level of diagnosed Calcium level and Vitamin D are presented as numbers and percentages. The nonparametric Mann–Whitney U test was used for continuous variables, multivariable logistic regression analysis was used to compare different variables against our calcium and vitamin D levels, and the associations between risk factors and outcomes were presented as odds ratios (ORs) and 95% CIs. A p-value of <0.05 was considered statistically significant in our analyses.
Results
A total of 445 hospitalized patients were included in the study. Baseline characteristics of our patients are summarized in Table 2 .
Table 2.
Baseline characteristics of study population.
| Number | Range | % | ||
|---|---|---|---|---|
| DEMOGRAPHICS | ||||
| Total | 445 | |||
| Age mean (years) | 50.8 | 15–94 | ||
| <30 yrs | 30 | 6.7% | ||
| 30–60 yrs | 282 | 63.4% | ||
| >60 yrs | 133 | 29.9% | ||
| Gender | ||||
| Male | 276 | 62.0% | ||
| Female | 169 | 38.0% | ||
| Nationality | Omani | 248 | 55.7% | |
| Non Omani | 197 | 44.3% | ||
| Region | Muscat | 326 | 73.3% | |
| Outside Muscat | 119 | 26.7% | ||
| Number | Range | % | ||
|---|---|---|---|---|
| ADMISSION INDICES | ||||
| Hospitalization Duration (days) | Mean | 11.8 | 1–92 | |
| Median (IQR) | 8.0 | |||
| Males (mean) | 12.4 | |||
| Females (mean) | 10.9 | |||
| Ordinal scale on admission | Males | Females | ||
| 0–4 | 58.6% | 41.4% | 66.4% | |
| 5–8 | 68.2% | 31.8% | 33.6% | |
| Ordinal scale on discharge | Males | Females | ||
| 0–4 | 61.4% | 38.6% | 82.6% | |
| 5–8 | 63.0% | 37.0% | 17.4% | |
| CURB Score on admission | Males (%) | Females (%) | ||
| 0 | 65.0% | 35.0% | 37.4% | |
| 1 | 59.3% | 40.7% | 33.3% | |
| 2 | 54.0% | 46.0% | 20.0% | |
| 3 | 76.7% | 23.3% | 6.9% | |
| 4 | 66.7% | 33.3% | 2.1% | |
| 5 | 100.0% | 0.0% | 0.5% | |
| Mean | 1.0436 | |||
| Standard Deviation | 1.0528 | |||
| HID/ICU Hospitalization needed | No | 259 | 58.2% | |
| Yes | 186 | 41.8% | ||
| SOFA Score on Admission | Mean | 4.1 | ||
| Median (IQR) | 4 | |||
| HID/ICU Hospitalization needed | Males | Females | 41.8% | |
| 66.7% | 33.3% | |||
| % | |
|---|---|
| CO-MORBIDITIES | |
| DM | 46.1% |
| Hypertension | 46.7% |
| Dyslipidemia | 24.0% |
| Respiratory diseases | 11.0% |
| Heart diseases | 18.4% |
| Liver diseases | 8.3% |
| CKD (eGFR < 70) | 20.2% |
| Number | Range | % | ||
|---|---|---|---|---|
| LABORATORY RESULTS | ||||
| Total White Cell Counts | Mean | 8.1 | 0.7–32.9 | |
| <2.2 | 2.9% | |||
| Lymphocyte count | Mean | 1.2 | 0–20.5 | |
| <1.2 | 68.5% | |||
| CRP | Mean | 118.1 | 1–362 | |
| >10 | 92.5% | |||
| Ferritin | Mean | 1458.4 | 2–71391 | |
| >708 | 55.6% | |||
| Corrected Calcium | Mean | 2.1 | 1.6–2.68 | |
| <2.1 | M = 65.6% | F = 34.4% | 68.8% | |
| Vitamin D | Mean | 66.6 | 17–128 | |
| <30 | 5.0% | |||
| Troponin | Mean | 311.5 | 2–32124 | |
| >14 | 53.0% | |||
| D-dimer | Mean | 5.1 | 0.05–80 | |
| >0.5 | 76.9% | |||
| Alanine Aminotransferase | Mean | 70.3 | 4–3300 | |
| >49 | 37.9% | |||
| Lactate Dehydrogenase | Mean | 515.6 | 126–4500 | |
| >246 | 88.3% | |||
| Number | Range | % | ||
|---|---|---|---|---|
| CLINICAL PROGRESSION | ||||
| Oxygen needed on admission | Mean | 6.2 | 0–15 | |
| 1–14 L/min | 35.9% | |||
| >15 L/min | 30.3% | |||
| ARDS | 29.0% | |||
| Sepsis | 8.6% | |||
| Intubated | 36.6% | |||
| NIV | 18.7% | |||
| Deceased | 17.7% | |||
Multiple clinical scales and laboratory values were used to classify patient’s severity on admission and discharge. The ordinal scale on admission showed 66.4% scaled (0–4) and 33.6% were (5–8). On the contrary, the scale on discharge showed 82.6% scaled (0–4) and 17.1% were (5–8).
As per the CURB 65 score on admission 37.4% were (0), 33.3% were (1),20% were (2),6.9% were (3),2.1% were (4) and only 0.5% were (5). Males were noted predominantly more severe than females, as detailed in the table.
As per the laboratory severity markers on admission, low total WBC count (<2.2 × 10*9) was seen in 2.9%, particularly lymphopenia present in 68.5%. Hypocalcemia (<2.1 mm/L) as predicted was seen in 68.8% (Males 65.6% & Females 34.4%), CRP was >10 mg/L in 92.5%, ferritin >708 mcg/L in 55.6%, Troponin was >49 pg/mL in 53%, ALT was >49 iU/L in 37.9%, D-dimer was >0.5 mg/L FEU in 76.9% and LDH > 246 iU/L in 88.3%. Vitamin D was only deficient in 5% of our population as expected, with a mean of 66.6 and range 17–128 nmol/L. Diabetes and hypertension were the most frequent comorbidities, totaling 46.1% and 46.7%, respectively.
Upon admission, 35.9% of our patients required 1–14 L/min oxygen, and 30.3% required >15 L/min; 36.6% required intubation, and 18.7% required Non-Invasive ventilation. The number of patients requiring ICU/HID admissions was 186 out of the 445 (41.8%), of which 29% were admitted with an impression of Acute Respiratory Distress Syndrome; nly 8.6% had sepsis. The overall mortality rate was 17.7%.
Univariate analysis (Table 3 ) showed the mean age group with hypocalcemia was 49.74 years (SD 14.779, 95% CI [47.99–51.48], P-value = 0.0111). The mean ordinal scale on admission for hypocalcemia patients was 4.49 (SD 1.45, 95% CI [4.31–4.66]), P-value 0.0018. Mean lymphopenia in hypocalcemic patients was 1.306 (SD 1.93, 95% CI [1.07–1.53], P-value = 0.32. CRP mean value among hypocalcemia patients was 127.28 (SD 86.09, 95% [114.08–134.4]), P-value = 0.134. The mean need for oxygen amongst hypocalcemic patients was 7.132 L/min (SD 6.44, 95% CI [6.36–7.90], with a significant P-value of 0.0034. Hospitalization period (days) among hypocalcemic patients was higher than patients with normal calcium, mean of 13.23 days (SD 11.50,95% CI [11.88–14.65], P-value = 0.0037. LDH mean value was 570 (SD 474.3, 95% CI [512.9–628.77], P-value = 0.002. Other parameters as shown in the table like ferritin, ALT, D-dimer, and Vitamin D showed non-significant P-values (>0.3).
Table 3.
Univariate analysis of Calcium and Vitamin D against parameters in study.
| Variables | Coef.Ca | P-value (Calcium) |
95% Conf. Interval (Calcium) | Coef.VitD | P-value (Vit D) |
95% Conf. Interval (Vitamin D) | ||
|---|---|---|---|---|---|---|---|---|
| Age, years | 0.00137 | 0.004 | 0.00044 | 0.00231 | −0.02470 | 0.906 | −0.43901 | 0.38960 |
| ALT | −0.00004 | 0.278 | −0.00011 | 0.00003 | 1.48162 | 0.797 | −9.93334 | 12.89658 |
| ALT (CAT) | −0.03740 | 0.010 | −0.06569 | −0.00911 | −0.02991 | 0.333 | −0.09089 | 0.03108 |
| ARDS CAT | −0.05282 | 0.001 | −0.08509 | −0.02054 | −15.09758 | 0.035 | −29.10400 | −1.09116 |
| Cardiac diseases | 0.18317 | 0.325 | −0.01822 | 0.05486 | −6.32452 | 0.429 | −22.13776 | 9.48872 |
| Citizen | −0.03420 | 0.018 | −0.06239 | −0.00600 | 24.86082 | 0.267 | −19.33794 | 69.05959 |
| CKD | 0.03413 | 0.055 | −0.00074 | 0.06899 | −3.43495 | 0.611 | −16.79497 | 9.92508 |
| CRP | −0.00069 | 0.410 | −0.00023 | 0.00010 | 2.43221 | 0.771 | −14.07374 | 18.93816 |
| CRP (CAT) | −0.84086 | 0.003 | −0.13931 | −0.02886 | 5.73446 | 0.419 | −8.29447 | 19.76339 |
| CURB score on admission | −0.00924 | 0.184 | −0.22860 | 0.00439 | 10.03615 | 0.184 | −4.85238 | 24.92469 |
| D-dimer | −0.01332 | 0.444 | −0.04752 | 0.02088 | −0.00171 | 0.966 | −0.08022 | 0.07680 |
| D-dimer | −0.00014 | 0.800 | −0.00120 | 0.00092 | 24.26167 | 0.084 | −3.29549 | 51.81882 |
| Death | 0.00935 | 0.684 | −0.03579 | 0.05449 | −0.26472 | 0.924 | −5.77898 | 5.24954 |
| DLP | 0.01638 | 0.321 | −0.01647 | 0.04881 | 0.08513 | 0.815 | −0.63346 | 0.80372 |
| DM | 0.01394 | 0.334 | −0.01439 | 0.04227 | −0.05032 | 0.995 | −15.55004 | 15.44941 |
| Ferritin | −0.00002 | 0.417 | −0.00053 | 0.00002 | 14.78333 | 0.310 | −43.74075 | 14.17408 |
| Gender | 0.01307 | 0.381 | −0.01622 | 0.04272 | 2.32861 | 0.737 | −11.40180 | 16.05903 |
| HDU/ICU Hospitalization | −0.56449 | 0.000 | −0.84410 | −0.02849 | 4.53853 | 0.487 | −8.35492 | 17.43199 |
| HDU/ICU Rehospitalization | −0.41170 | 0.068 | −0.85476 | 0.00313 | −0.00183 | 0.438 | −0.00649 | 0.00284 |
| Hospitalization period | −0.00200 | 0.002 | −0.00327 | −0.00072 | −4.86630 | 0.165 | −11.76788 | 2.03527 |
| HTN | 0.03626 | 0.012 | 0.00816 | 0.06436 | −9.46364 | 0.151 | −22.44561 | 3.51833 |
| Intubation CAT | −0.05943 | 0.000 | −0.08783 | −0.03102 | −9.36324 | 0.179 | −23.08427 | 4.35780 |
| LDH | −0.00007 | 0.000 | −0.00010 | −0.00003 | 7.43951 | 0.457 | −12.31127 | 27.19029 |
| LDH (CAT) | −0.17756 | 0.000 | −0.16329 | −0.07184 | 0.17703 | 0.543 | −0.39846 | 0.75253 |
| Liver diseases | −0.00415 | 0.869 | −0.05382 | 0.04551 | 6.11268 | 0.348 | −6.75617 | 18.98153 |
| Lymphocytes | −0.00499 | 0.256 | −0.01362 | 0.00363 | −9.36906 | 0.170 | −22.79974 | 4.06163 |
| Lymphocytes (CAT) | −0.02198 | 0.133 | −0.05065 | 0.00670 | −0.01530 | 0.088 | −0.03290 | 0.00230 |
| Max O2 needed | −0.00315 | 0.006 | −0.00538 | −0.00092 | −23.88712 | 0.021 | −44.11384 | −3.66041 |
| Max O2 needed (CAT) | −0.02553 | 0.005 | −0.04328 | −0.00777 | 3.42200 | 0.809 | −24.52815 | 31.37215 |
| NIV CAT | −0.05741 | 0.001 | −0.09264 | −0.02218 | −2.82411 | 0.474 | −10.62051 | 4.97229 |
| Ordinal scale on admission | −0.01810 | 0.000 | −0.28190 | −0.00800 | −3.43899 | 0.611 | −16.79898 | 9.92099 |
| Region | 0.01759 | 0.275 | −0.01407 | 0.04924 | 0.08374 | 0.882 | −1.03190 | 1.19938 |
| Respiratory diseases | 0.01715 | 0.458 | −0.28245 | 0.06254 | 3.74411 | 0.427 | −5.56706 | 13.05528 |
| Sepsis CAT | 0.03407 | 0.219 | −0.02032 | 0.08846 | 1.42142 | 0.843 | −12.77861 | 15.62144 |
| SOFA Score | 0.00028 | 0.934 | −0.00627 | 0.00682 | −4.41950 | 0.088 | −9.50948 | 0.67047 |
| Troponin | −0.01222 | 0.496 | −0.04755 | 0.02311 | −5.73684 | 0.500 | −22.54391 | 11.07022 |
| Troponin | −0.00003 | 0.468 | −0.00001 | 0.00005 | 26.22131 | 0.002 | 3.61110 | 48.83151 |
| Vitamin D | 0.00048 | 0.267 | −0.00037 | 0.00132 | 0.66015 | 0.958 | −24.46627 | 25.78657 |
| Vitamin D (CAT) | 0.02861 | 0.077 | −0.00317 | 0.06038 | −0.17344 | 0.881 | −2.48023 | 2.13334 |
| WCC | 0.00068 | 0.639 | −0.00218 | 0.00355 | 0.00003 | 0.997 | −0.17326 | 0.01739 |
| WCC (CAT) | −0.01090 | 0.184 | −0.02699 | 0.00519 | 6.00973 | 0.427 | −8.95969 | 20.97915 |
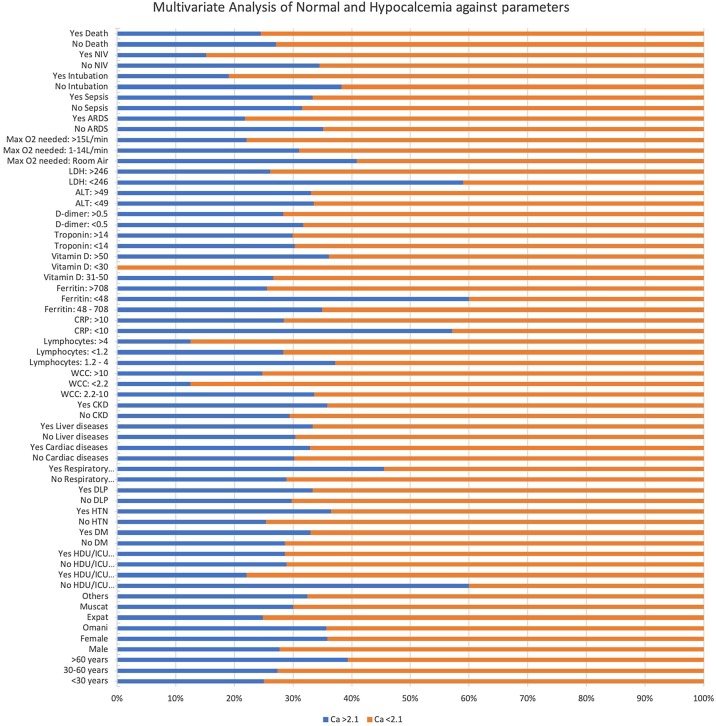
The multivariate analysis (Table 4 , Graph 1, Graph 2 ) revealed that hypocalcemia (<2.1 mm/L) was seen in 60%–75% among all age groups (P-value = 0.053), of which 72.33% were males and 64.20% were females (P-value of 0.088). Among Omani patients, 64.35% had hypocalcemia, while in non-Omanis, 75.14% had hypocalcemia (p-value = 0.02). The analysis showed a significant correlation between hypocalcemia and HDU/ICU hospitalization; 77.97% of HDU/ICU hospitalized patients had hypocalcemia (P-value = 0.001). Among hypocalcemic patients, 63.54% had hypertension, whereas 74.64% did not (P value = 0.016). Diabetes Mellitus was seen in 67.02% of the hypocalcemia group; 71.36% were non-diabetic (P value = 0.347). The P-value was 0.024 for chronic respiratory diseases, and 54.55% had hypocalcemia. Chronic liver diseases and chronic kidney diseases had a non-significant P-value of 0.717 and 0.262, respectively.
Table 4.
Multivariate analysis of Hypo against normal Calcium and Vitamin D.
| Parameter | Value | Ca >2.1 | Ca <2.1 | P value | Vit D <50 | Vit D >50 | P value |
|---|---|---|---|---|---|---|---|
| Age | <30 years | 25.00% | 75.00% | 0.053 | 28.57% | 71.43% | 1&2 = 0.891 1&3 = 0.953 2&3 = 0.690 All = 0.921 |
| 30–60 years | 27.31% | 72.69% | 31.08% | 68.92% | |||
| >60 years | 39.32% | 60.68% | 27.50% | 72.50% | |||
| Gender | Male | 27.67% | 72.33% | 0.088 | 30.14% | 69.86% | 0.909 |
| Female | 35.80% | 64.20% | 29.17% | 70.83% | |||
| Citizen | Omani | 35.65% | 64.35% | 0.02 | 36.36% | 63.64% | 0.035 |
| Expat | 24.86% | 75.14% | 18.18% | 81.82% | |||
| Region | Muscat | 30.00% | 70.00% | 0.636 | 33.71% | 66.29% | 0.112 |
| Others | 32.43% | 67.57% | 18.75% | 81.25% | |||
| HDU/ICU Hospitalization |
No | 60.00% | 40.00% | 0.001 | 30.67% | 69.33% | 0.779 |
| Yes | 22.03% | 77.97% | 28.26% | 71.74% | |||
| HDU/ICU Rehospitalization |
No | 28.88% | 71.12% | 0.964 | 32.67% | 67.33% | 0.128 |
| Yes | 28.57% | 71.43% | 13.33% | 86.67% | |||
| DM | No | 28.64% | 71.36% | 0.347 | 29.51% | 70.49% | 0.953 |
| Yes | 32.98% | 67.02% | 30.00% | 70.00% | |||
| HTN | No | 25.36% | 74.64% | 0.016 | 34.43% | 65.57% | 0.257 |
| Yes | 36.46% | 63.54% | 25.00% | 75.00% | |||
| DLP | No | 29.77% | 70.24% | 0.5 | 27.38% | 72.62% | 0.39 |
| Yes | 33.33% | 66.67% | 35.14% | 64.86% | |||
| Respiratory diseases |
No | 28.85% | 71.15% | 0.024 | 32.73% | 67.27% | 0.024 |
| Yes | 45.45% | 54.55% | 0.00% | 100.00% | |||
| Cardiac diseases | No | 30.18% | 69.82% | 0.652 | 28.57% | 71.43% | 0.558 |
| Yes | 32.88% | 67.12% | 34.78% | 65.22% | |||
| Liver diseases | No | 30.41% | 69.59% | 0.717 | 31.30% | 68.70% | 0.102 |
| Yes | 33.33% | 66.67% | 0.00% | 100.00% | |||
| CKD | No | 29.38% | 70.63% | 0.262 | 27.66% | 72.34% | 0.348 |
| Yes | 35.80% | 64.20% | 37.04% | 62.96% | |||
| WCC | 1) 2.2–10 | 33.57% | 66.43% | 1&2 = 0.211 1&3 = 0.096 2&3 = 0.433 |
24.42% | 75.58% | 1&2 = 0.100 1&3 = 0.130 2&3 = 0.347 All = 0.114 |
| 2) <2.2 | 12.50% | 87.50% | 66.67% | 33.33% | |||
| 3)>10 | 24.76% | 75.24% | 38.71% | 61.29% | |||
| Lymphocytes | 1) 1.2–4 | 37.19% | 62.81% | 1&2 = 0.079 1&3 = 0.158 2&3 = 0.326 All 0.112 |
21.05% | 78.95% | 0.183 |
| 2) <1.2 | 28.31% | 71.69% | 32.93% | 67.07% | |||
| 3) >4 | 12.50% | 87.50% | |||||
| CRP | <10 | 57.14% | 42.86% | 0.001 | 71.43% | 28.57% | 0.014 |
| >10 | 28.46% | 71.54% | 27.43% | 72.57% | |||
| Ferritin | 1) 48–708 | 34.90% | 65.10% | 1&2 = 0.110 1&3 = 0.056 2&3 = 0.017 all = 0.019 |
19.61% | 80.39% | 0.983 |
| 2) <48 | 60.00% | 40.00% | 20.00% | 80.00% | |||
| 3) >708 | 25.59% | 74.41% | |||||
| Vitamin D | 1) 31–50 | 26.67% | 73.33% | 1&2 = 0.151 1&3 = 0.346 2&3 = 0.070 All = 0.144 |
|||
| 2) <30 | 0.00% | 100.00% | |||||
| 3) >50 | 36.14% | 63.86% | |||||
| Troponin | <14 | 30.33% | 69.67% | 0.944 | 29.55% | 70.45% | 0.896 |
| >14 | 29.93% | 70.07% | 30.77% | 69.23% | |||
| D-Dimer | <0.5 | 31.71% | 68.29% | 0.553 | 37.04% | 62.96% | 0.235 |
| >0.5 | 28.31% | 71.69% | 25.29% | 74.71% | |||
| ALT | <49 | 33.47% | 66.53% | 0.073 | 23.46% | 76.54% | 0.029 |
| >49 | 33.03% | 66.97% | 43.24% | 56.76% | |||
| LDH | <246 | 58.97% | 41.03% | 0 | 14.29% | 85.71% | 0.228 |
| >246 | 26.13% | 73.87% | 29.70% | 70.30% | |||
| Max O2 needed | 1) Room Air | 40.80% | 59.20% | 1&2 = 0.095 1&3 = 0.001 2&3 = 0.098 All = 0.006 |
40.74% | 59.26% | 1&2 = 0.225 1&3 = 0.130 2&3 = 0.614 |
| 2) 1–14 L/min | 30.99% | 69.01% | 27.59% | 72.41% | |||
| 3) >15 L/min | 22.05% | 77.95% | 22.86% | 77.14% | |||
| ARDS | No | 35.15% | 64.85% | 0.012 | 30.67% | 69.33% | 0.497 |
| Yes | 21.82% | 78.18% | 24.24% | 75.76% | |||
| Sepsis | No | 31.51% | 68.49% | 0.838 | 29.29% | 70.71% | 0.244 |
| Yes | 33.33% | 66.67% | 11.11% | 88.89% | |||
| Intubation | No | 38.27% | 61.73% | 0 | 29.73% | 70.27% | 0.995 |
| Yes | 18.99% | 81.01% | 29.79% | 70.21% | |||
| NIV | No | 34.47% | 65.53% | 0.001 | 31.46% | 68.54% | 0.493 |
| Yes | 15.19% | 84.81% | 25.00% | 75.00% | |||
| Death | No | 27.05% | 72.95% | 0.72 | 35.00% | 65.00% | 0.448 |
| Yes | 24.44% | 75.56% | 22.22% | 77.78% |
Graph 1.
Multivariate analysis Calcium.
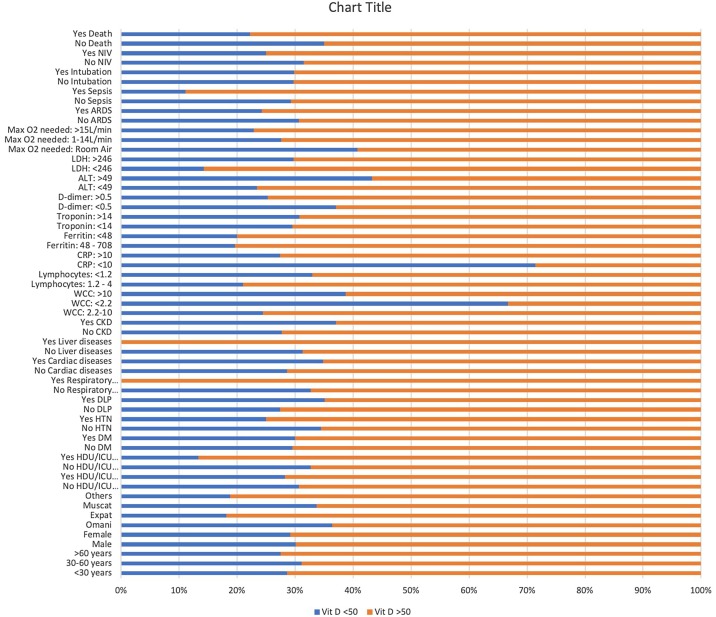
Graph 2.
Multivariate analysis Vitamin D.
Leukocytosis >10 × 109 combined with normal WCC (2.2–10 × 109) had a P-value of 0.096. Normal lymphocytes (1.2–4) and lymphopenia (<1.2) showed a P value of 0.079 in correlation with hypocalcemia, as 71.69% with normal Lymphocytes and 71.69% of lymphopenic patients had hypocalcemia. CRP was raised in 71.54% of the hypocalcemia group, representing a significant P-value of 0.001. High ferritin showed a P value of 0.019, correlating significantly with hypocalcemia. Other markers like ALT and LDH showed P values of 0.073 and <0.001, respectively. No significant correlation with Vitamin D was found (P value = 0.144).
Oxygen requirements were statistically significant with a P-value of 0.006, with 77.95% of hypocalcemia patients needing >15 L/min of O2. The need for NIV in hypocalcemic patients was 84.81% (P-value = 0.001). ARDS and intubation had P values of 0.012 and <0.001, respectively. Unexpectedly, sepsis and death had a non-significant correlation as the P-value was 0.0838 for sepsis and 0.72 for death.
Meta-analysis showed non-significant correlations between Vitamin D and almost all parameters except for chronic respiratory diseases with a P-value of 0.024, CRP with a P-value of 0.014, and ALT, where the P-value was 0.029. The Two-Sample T-Test of calcium and vitamin D against other parameters is displayed in Table 5 .
Table 5.
Two-sample T-test of Calcium and Vitamin D against parameters.
| C A L C I U M | ||||||
|---|---|---|---|---|---|---|
| Two sample T-test | Calcium | Mean | SD | 95% CI | P-value | |
| Age x Calcium |
Ca >2.1 | 53.8780 | 15.4254 | 51.1246 | 56.6314 | 0.0111 |
| Ca <2.1 | 49.7410 | 14.7799 | 47.9960 | 51.4860 | ||
| Ordinal adm x Calcium |
Ca >2.1 | 4.0165 | 1.2100 | 3.7985 | 4.2345 | 0.0018 |
| Ca <2.1 | 4.4909 | 1.4533 | 4.3180 | 4.6630 | ||
| SOFA x Calcium | Ca >2.1 | 4.1765 | 3.3269 | 3.2408 | 5.1121 | 0.9873 |
| Ca <2.1 | 4.1688 | 2.8487 | 3.7153 | 4.6223 | ||
| WCC x Calcium | Ca >2.1 | 8.1285 | 4.9899 | 7.2378 | 9.0191 | 0.8096 |
| Ca <2.1 | 8.2576 | 4.9195 | 7.6747 | 8.8406 | ||
| Lymphocytes x Calcium |
Ca >2.1 | 1.1301 | 0.6083 | 1.0215 | 1.2387 | 0.3227 |
| Ca <2.1 | 1.3065 | 1.9326 | 1.0783 | 1.5346 | ||
| CRP x Calcium | Ca >2.1 | 110.1215 | 87.7433 | 94.3282 | 125.9147 | 0.1345 |
| Ca <2.1 | 124.2819 | 86.0932 | 114.0801 | 134.4837 | ||
| Max O2 needed x Calcium |
Ca >2.1 | 5.1138 | 5.9934 | 4.0440 | 6.1836 | 0.0034 |
| Ca <2.1 | 7.1328 | 6.4421 | 6.3624 | 7.9033 | ||
| Hospitalization Period (days) x Calcium |
Ca >2.1 | 9.7500 | 9.6973 | 7.9971 | 11.5029 | 0.0037 |
| Ca <2.1 | 13.2687 | 11.5042 | 11.8851 | 14.6523 | ||
| Ferritin x Calcium | Ca >2.1 | 971.2252 | 946.7327 | 793.1440 | 1149.3060 | 0.1132 |
| Ca <2.1 | 1679.3710 | 4653.9570 | 1106.5530 | 2252.1890 | ||
| Vitamin D x Calcium | Ca >2.1 | 69.7206 | 29.8316 | 59.3118 | 80.1293 | 0.4618 |
| Ca <2.1 | 64.5563 | 35.1270 | 56.2419 | 72.8708 | ||
| Troponin x Calcium | Ca >2.1 | 95.4546 | 305.8532 | 26.0344 | 164.8747 | 0.3531 |
| Ca <2.1 | 362.6685 | 2509.8990 | −5.4561 | 730.7931 | ||
| D-dimer x Calcium | Ca >2.1 | 4.3630 | 12.1622 | 1.9742 | 6.7519 | 0.4630 |
| Ca <2.1 | 5.5401 | 14.1885 | 3.7727 | 7.3075 | ||
| ALT x Calcium | Ca >2.1 | 56.1897 | 89.4864 | 39.7319 | 72.6474 | 0.2894 |
| Ca <2.1 | 78.19259 | 215.4022 | 52.38339 | 104.0018 | ||
| LDH x Calcium | Ca >2.1 | 421.1818 | 266.0214 | 370.9109 | 471.4527 | 0.002 |
| Ca <2.1 | 570.85 | 474.3515 | 512.921 | 628.779 | ||
| V I T A M I N D | ||||||
|---|---|---|---|---|---|---|
| Two sample T-test | Vitamin D | Mean | SD | 95% CI | P-value | |
| Age x Vit D | <50 | 53 | 15.70259 | 47.68701 | 58.31299 | 0.819 |
| >50 | 52.28235 | 15.75528 | 48.88402 | 55.68069 | ||
| SOFA x Vit D | <50 | 3.684211 | 3.57542 | 1.960913 | 5.407508 | 0.5125 |
| >50 | 4.3 | 3.430059 | 3.325188 | 5.274812 | ||
| HDU/ICU Rehospitalization x Vit D |
<50 | 1.057143 | 0.235504 | 0.976244 | 1.138041 | 0.1301 |
| >50 | 1.160494 | 0.36935 | 1.078824 | 1.242164 | ||
| WCC x Vit D | <50 | 8.982857 | 6.495405 | 6.751607 | 11.21411 | 0.2327 |
| >50 | 7.703529 | 4.746438 | 6.679746 | 8.727312 | ||
| Lymphocytes x Vit D | <50 | 1.186111 | 1.255878 | 0.761183 | 1.611039 | 0.2664 |
| >50 | 1.010588 | 0.477599 | 0.907573 | 1.113604 | ||
| CRP x Vit D | <50 | 123.0833 | 99.60541 | 89.38171 | 156.785 | 0.9022 |
| >50 | 121.0905 | 72.11754 | 105.44 | 136.7409 | ||
| Max O2 needed x Vit D |
<50 | 5.228571 | 5.931401 | 3.191064 | 7.266079 | 0.1385 |
| >50 | 7.023529 | 6.015806 | 5.72595 | 8.321109 | ||
| Hospitalization Period (days) x Vit D |
<50 | 13.09091 | 11.93305 | 8.859633 | 17.32219 | 0.9412 |
| >50 | 13.26829 | 11.52137 | 10.73677 | 15.79982 | ||
| Ferritin x Vit D | <50 | 1691.939 | 2044.553 | 966.9725 | 2416.906 | 0.0152 |
| >50 | 980.4545 | 985.6189 | 756.7465 | 1204.163 | ||
| Troponin x Vit D | <50 | 132.069 | 444.7107 | −37.08992 | 301.2279 | 0.623 |
| >50 | 238.4308 | 1119.657 | −39.00658 | 515.8681 | ||
| D-dimer x Vit D | <50 | 2.743437 | 5.750296 | 0.670235 | 4.81664 | 0.2957 |
| >50 | 5.63525 | 15.10222 | 2.274415 | 8.996085 | ||
| ALT x Vit D | <50 | 68.94286 | 106.1309 | 32.48563 | 105.4001 | 0.6769 |
| >50 | 60.2561 | 101.6287 | 37.92583 | 82.58637 | ||
| LDH x Vit D | <50 | 582.7188 | 560.338 | 380.6954 | 784.7421 | 0.1243 |
| >50 | 464.0375 | 252.4008 | 407.8684 | 520.2066 | ||
Discussion
This study showed that 33% of patients in this cohort had an ordinal scale of 5-8 on admission, whereas it was 17% at discharge. A CURB score of two and above was present in one-third of patients and was more severe in males. Diabetes and hypertension were the main comorbidities occurring in 50% of participants. Hypocalcemia (<2.1 mm/L) was present in more than two-thirds of the participants, mainly in males. Hypocalcemia was associated with chronic respiratory diseases. Patients with hypocalcemia had a worse ordinal scale, lymphopenia, CRP, LDH, longer hospital stay, ICU admission, ARDS and intubation, and higher oxygen requirements. Hypocalcemia showed no significant association with death; however, there was a trend towards a significant statistical association with sepsis (P = 0.08).
A high incidence of hypocalcemia was observed in critically ill patients admitted in hospitals in Wuhan with Covid-19 during the beginning of the epidemic. Therefore, it was hypothesized that low serum calcium levels were associated with the severity of disease and prognosis in COVID-19. Hypocalcemia was detected in 60% of patients at hospital admission and 70% during hospitalization in a large group of SARS patients in North America (Booth et al., 2003). Data of patients with Ebola virus infection from the United States and European hospitals reported a similar incidence of hypocalcemia (Uyeki et al., 2016). Several studies have investigated the clinical and laboratory characteristics of COVID-19 patients, including inflammatory and organ injury biomarkers (Harries and Takarinda, 2020). Many cases of COVID-19 at presentation have been reported to have hypocalcemia (Bossoni et al., 2020). However, no detailed population data were available on calcium levels in COVID-19 (Puig-Domingo et al., 2020).
Several studies revealed a correlation between hypocalcemia, higher mortality, and poor clinical outcome in hospitalized critically ill patients (Akirov et al., 2017, Cheungpasitporn et al., 2018). Calcium plays a crucial role in the viral fusion of various enveloped viruses such as SARS-CoV, MERS-CoV, and Ebolavirus. It is known to directly interact with the fusion peptides of these viruses to promote their replication (Booth et al., 2003, Nathan et al., 2020, Straus et al., 2019). In patients with SARS CoV and Ebolavirus, hypocalcemia was a widespread laboratory finding. Despite its regularity, the underlying pathophysiology, clinical relevance, and prognostic significance were not thoroughly investigated (Nathan et al., 2020, Straus et al., 2019).
Calcium may prove to be a useful biochemical marker of disease severity. Since measuring calcium is a simple blood test that can be initiated upon presentation in emergency visits for most patients, it may prove a quick indicator for the clinician to discern the severity of the case. Whether hypocalcemia represents the pathophysiology of COVID-19, a dysregulation of calcium homeostasis, or perhaps linked to vitamin D levels as well is yet to be investigated.
Since a high incidence of hypocalcemia in COVID-19 patients may predict the severity of illness and the need for hospitalization, we suggest that calcium should always be assessed at initial hospital evaluation. Hypocalcemia may have a negative impact on cardiac function and may be even lethal when severe and acute. Monitoring and maintaining adequate calcium levels in all hospitalized patients with COVID-19 is recommended (Holick, 2007).
As a scientific postulate for the mechanism behind hypocalcemia in COVID-19 infections, it was found that these patients have elevated levels of unbound fatty acids and unsaturated fatty acids. The latter can bind to calcium with a favorable (−20KJ/mol) enthalpy and cause significant acute hypocalcemia. They can also induce cytokine storm and multiorgan system failure. In severe disease, hypoalbuminemia can also be induced by unsaturated fatty acids. This may affect corrected albumin measurements as was the case in our study. It was also found that if calcium is rapidly corrected, it may bind and neutralize these unsaturated fatty acids early in the disease, thereby preventing mitochondrial injury and the subsequent widespread cellular injury, organ failure, and sepsis that follows. Despite the logic underlying this explanation, it may be counterintuitive to ascertain that a single mechanism can explain such a widespread phenomenon in a very heterogeneous population with different degrees of severities, ethnic backgrounds, and manifestations worldwide (di Filippo et al., 2020, Singh et al., 2020).
As for Vitamin D, its metabolism and actions are well studied (Holick, 2007). Vitamin D3 is produced in the skin through the action of UVB radiation, forming 7-dehydrocholesterol, which is then followed by a thermal reaction. Vitamin D3 is then converted to 25(OH)D in the liver and then to the hormonal metabolite 1,25(OH)2D (calcitriol) in the kidneys. Most of the activity of vitamin D comes from calcitriol entering the vitamin D receptor in the nucleus. It is a DNA-binding protein that interacts directly with regulatory sequences near target genes. It recruits active chromatin complexes that participate genetically and epigenetically in modifying the transcriptional output (Pike and Christakos, 2017). Calcitriol then helps to regulate serum calcium concentrations by a negative feedback loop with parathyroid hormone (PTH) (Holick, 2007).
Several reviews considered the various possibilities by which vitamin D may reduce the risk of viral infections and death (Abhimanyu and Coussens, 2017, Beard et al., 2011, Gombart et al., 2020, Greiller and Martineau, 2015, Gruber-Bzura, 2018, Hewison, 2012, Lang and Aspinall, 2017, Rondanelli et al., 2018, Wei and Christakos, 2015). One review considering the role of vitamin D in reducing the risk of common cold, attempted to group these mechanisms into three categories: natural cellular immunity, adaptive immunity, and physical barrier (Rondanelli et al., 2018). Vitamin D maintains tight junctions, gap junctions, and adherens junctions (e.g., by E-cadherin) across the cellular structure (Schwalfenberg, 2011). Several articles have discussed the increase in infections by viruses and other organisms caused due to the disruption of these junction integrities (Chen et al., 2020, Kast et al., 2017, Rossi et al., 2020).
Vitamin D enhances innate cellular immunity partly through the induction of antimicrobial peptides, including 1,25-dihydroxy vitamin D defensins, human cathelicidin, and LL-37 cathelicidin-derived antimicrobial peptide (Adams et al., 2009, Laaksi, 2012, Liu et al., 2006). Cathelicidins demonstrate direct antimicrobial activities against an array of micro-organisms, Gram-positive and Gram-negative bacteria, enveloped and nonenveloped viruses, and fungi (Herr et al., 2007). These host-derived peptides destroy the invading pathogens by disrupting their cell membranes and neutralizing the biological actions of their endotoxins (Agier et al., 2015). In a mouse model, LL-37 significantly decreased influenza A virus replication (Barlow et al., 2011). In another laboratory study, 1,25(OH)2D reduced the replication of rotavirus, both in vitro and in vivo, by other processes (Zhao et al., 2019). A clinical trial has also reported a supplementation regimen with 4000 IU/d of vitamin D decreased dengue virus infection (Martínez-Moreno et al., 2020).
Vitamin D is also known to improve cellular immunity, partly by reducing the cytokine storm induced by the dysregulated innate immune system. This system generates both pro-inflammatory and anti-inflammatory cytokines in response to viral and bacterial infections and other chemical and oncological triggers (Huang et al., 2020). Vitamin D may reduce the production of pro-inflammatory Th1 cytokines, such as tumor necrosis factor and interferon (Zhao et al., 2019). Administering vitamin D may reduce the expression of pro-inflammatory cytokines and increase anti-inflammatory cytokines by macrophages like IL-10 (Zhao et al., 2019).
Vitamin D has known modulatory effects on adaptive immunity; 1,25(OH)2D3 suppresses responses mediated by the T helper cell type 1 (Th1) by mainly suppressing the production of inflammatory cytokines IL-2 and interferon-gamma (INF) (Cantorna, 2010, Lemire et al., 1984, Rondanelli et al., 2018). Additionally, 1,25(OH)2D3 promotes cytokine production by the T helper type 2 (Th2) cells, promoting indirect suppression of Th1 cells by complementing this with actions mediated by a congregation of other cell lines (Cantorna et al., 2015). Furthermore, 1,25(OH)2D3 promotes induction of the T regulatory cells, resulting in suppression of inflammatory processes (Jeffery et al., 2009).
Serum 25(OH)D concentrations usually tend to decrease with age, which may be significant in this COVID-19 pandemic, since case-fatality rates (CFRs) increase with age (CDC Weekly C, 2020, Vásárhelyi et al., 2011) . People in most countries in the Northern hemisphere spend less time in the sun and have a reduced production of vitamin D due to lower levels of 7-dehydrocholesterol in the skin (MacLaughlin and Holick, 1985). Additionally, some medications reduce serum 25(OH)D concentrations by activating the pregnane-X receptor (Gröber and Kisters, 2012). These medications include antihypertensives, antiepileptics, anti-inflammatory agents, endocrine drugs, antineoplastics, antibiotics, antiretrovirals, and some herbal medicines. This effect is compounded by trending polypharmacy due to more drug use as age increases.
Vitamin D supplementation also promotes the expression of genes associated with antioxidation (glutathione reductase and glutamate-cysteine ligase modifier subunit) (Lei et al., 2017). Increased glutathione production spares the use of ascorbic acid (vitamin C), which is known for its antimicrobial activities, and has been proposed as supplementation to decrease the effects of COVID-19 (Bâldea, 2020, Colunga Biancatelli et al., 2020, Mousavi et al., 2019).
While vitamin D levels vary widely in the planet's northern hemisphere, our regions fare differently and for different reasons. Studies in Oman have reported a high prevalence of vitamin D deficiency (87.5%) in healthy Omanis (Abiaka et al., 2013). The lack of exposure to sunlight is one of the leading causes of vitamin D deficiency (El-Hajj Fuleihan, 2009, Guan et al., 2020, Kast et al., 2017).
Given that most of our admissions were expatriate from mostly Asian nationalities, our study population presented a wide range of ethnic backgrounds to incorporate any inherent differences in vitamin D levels. The link between calcium levels and vitamin D levels may be of significance if proven with other worldwide studies (Meltzer et al., 2020).
As our data suggest, particularly from the multivariate analysis, hypocalcemia is a very reliable predictor for disease progression and is part of the disease's overall symptomatology. The worse the hypocalcemia, the more severe the clinical progression of patients with complications. Although death as an outcome in our univariate analysis had a p-value of 0.684, and in the multivariate analysis had a p-value of 0.72, this may be due to confounding factors, including differences in therapeutic regimens, which were not mentioned in our study.
There was a slight limitation in the corrected calcium measurements in our laboratory. Our laboratory uses the Siemens Atellica® chemistry system (CH930), Germany. Its method is based on the CPC method (O-Cresolphthalein complex one), a colorimetric method. There was a change in the albumin method, an element in the equation for corrected calcium. The change was from Bromocresol green (BCG) to Bromocresol purple (BCP). Both methods are colorimetric or dye-binding methods. They are both known to overestimate albumin concentration in hospitalized patients as the dye is reacting with acute phase reactants in these patients. The overestimation of albumin results in an underestimation of calcium, especially at albumin levels over 40 g/L. However, this effect is to a lesser extent in BCP compared to BCG. Our observation of non-COVID cases shows that the margin of error was negligible, as most non-COVID cases presented with normal corrected calcium values.
In conclusion, we highly recommend using corrected calcium levels as a predictor of possible clinical progression and initial stratifying parameters for the further need of intensive care. Also, given the possible theory behind its mechanism, rapid correction is advised to prevent further injury at the cellular level and stifle further provocation of the disease. Vitamin D levels represented no particular significance in our population to recommend correction or otherwise.
Conflict of interest, funding source, and ethical approval
We declare that this manuscript is original, has not been published before, and is not currently being considered for publication elsewhere.
We know of no conflicts of interest associated with this publication, and there has been no financial support for this work that could have influenced its outcome. As the first author, I confirm that the manuscript has been read and approved for submission by all the named authors. Ethical approval was granted by our hospital's Research Committee in July 2020. The current “Instructions to Authors” has been read by all authors, and we herewith ensure compliance with those instructions and accept the conditions imposed.
References
- Abhimanyu, Coussens A.K. The role of UV radiation and Vitamin D in the seasonality and outcomes of infectious disease. Photochem Photobiol Sci. 2017;16:314–338. doi: 10.1039/c6pp00355a. [DOI] [PubMed] [Google Scholar]
- Abiaka C., Delghandi M., Kaur M., Al-Saleh M. Vitamin D status and anthropometric indices of an Omani study population. Sultan Qaboos Univ Med J. 2013;13:224–233. doi: 10.12816/0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J.S., Ren S., Liu P.T., Chun R.F., Lagishetty V., Gombart A.F., et al. Vitamin D-directed rheostatic regulation of monocyte antibacterial responses. J Immunol. 2009;182:4289–4295. doi: 10.4049/jimmunol.0803736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agier J., Efenberger M., Brzezińska-Blaszczyk E. Cathelicidin impact on inflammatory cells. Cent Eur J Immunol. 2015;40:225–235. doi: 10.5114/ceji.2015.51359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirov A., Gorshtein A., Shraga-Slutzky I., Shimon I. Calcium levels on admission and before discharge are associated with mortality risk in hospitalized patients. Endocrine. 2017;57:344–351. doi: 10.1007/s12020-017-1353-y. [DOI] [PubMed] [Google Scholar]
- Bâldea I. What can we learn from the time evolution of COVID-19 epidemic in Slovenia? arXiv. 2020 doi: 10.1101/2020.05.25.20112938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow P.G., Svoboda P., Mackellar A., Nash A.A., York I.A., Pohl J., et al. Antiviral activity and increased host defense against influenza infection elicited by the human cathelicidin LL-37. PLoS One. 2011;6 doi: 10.1371/journal.pone.0025333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beard J.A., Bearden A., Striker R. Vitamin D and the anti-viral state. J Clin Virol. 2011;50:194–200. doi: 10.1016/j.jcv.2010.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C.M., Matukas L.M., Tomlinson G.A., Rachlis A.R., Rose D.B., Dwosh H.A., et al. Clinical features and short-term outcomes of 144 patients with SARS in the Greater Toronto area. J Am Med Assoc. 2003;289:2801–2809. doi: 10.1001/jama.289.21.JOC30885. [DOI] [PubMed] [Google Scholar]
- Bossoni S., Chiesa L., Giustina A. Severe hypocalcemia in a thyroidectomized woman with Covid-19 infection. Endocrine. 2020;68:253–254. doi: 10.1007/s12020-020-02326-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M.T. Mechanisms underlying the effect of vitamin D on the immune system. Proc Nutr Soc. 2010;69:286–289. doi: 10.1017/S0029665110001722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantorna M.T., Snyder L., Lin Y.D., Yang L. Vitamin D and 1,25(OH)2D regulation of T cells. Nutrients. 2015;7:3011–3021. doi: 10.3390/nu7043011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC Weekly C the epidemiological characteristics of an outbreak of 2019 novel coronavirus diseases (COVID-19) — China, 2020. China CDC Wkly. 2020;2:113–122. doi: 10.46234/ccdcw2020.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Leng K., Lu Y., Wen L., Qi Y., Gao W., et al. Epidemiological features and time-series analysis of influenza incidence in urban and rural areas of Shenyang, China, 2010-2018. Epidemiol Infect. 2020;148:e29. doi: 10.1017/S0950268820000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheungpasitporn W., Thongprayoon C., Mao M.A., Kittanamongkolchai W., Sakhuja A., Erickson S.B. Impact of admission serum calcium levels on mortality in hospitalized patients. Endocr Res. 2018;43:116–123. doi: 10.1080/07435800.2018.1433200. [DOI] [PubMed] [Google Scholar]
- Colunga Biancatelli R.M.L., Berrill M., Marik P.E. The antiviral properties of vitamin C. Expert Rev Anti Infect Ther. 2020;18:99–101. doi: 10.1080/14787210.2020.1706483. [DOI] [PubMed] [Google Scholar]
- Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20:533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Hajj Fuleihan G. Vitamin D deficiency in the Middle East and its health consequences for children and adults. Clin Rev Bone Miner Metab. 2009;7:77–93. doi: 10.1007/s12018-009-9027-9. [DOI] [Google Scholar]
- di Filippo L., Formenti A.M., Giustina A. Hypocalcemia: the quest for the cause of a major biochemical feature of COVID-19. Endocrine. 2020;70:463–464. doi: 10.1007/s12020-020-02525-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Filippo L., Formenti A.M., Rovere-Querini P., Carlucci M., Conte C., Ciceri F., et al. Hypocalcemia is highly prevalent and predicts hospitalization in patients with COVID-19. Endocrine. 2020;68:475–478. doi: 10.1007/s12020-020-02383-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gombart A.F., Pierre A., Maggini S. A review of micronutrients and the immune system–working in harmony to reduce the risk of infection. Nutrients. 2020;12:236. doi: 10.3390/nu12010236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant W.B., Lahore H., McDonnell S.L., Baggerly C.A., French C.B., Aliano J.L., et al. Evidence that vitamin D supplementation could reduce risk of influenza and covid-19 infections and deaths. Nutrients. 2020;12:1–19. doi: 10.3390/nu12040988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greiller C.L., Martineau A.R. Modulation of the immune response to respiratory viruses by vitamin D. Nutrients. 2015;7:4240–4270. doi: 10.3390/nu7064240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gröber U., Kisters K. Influence of drugs on vitamin D and calcium metabolism. Dermatoendocrinol. 2012;4:158–166. doi: 10.4161/derm.20731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruber-Bzura B.M. Vitamin D and influenza—prevention or therapy? Int J Mol Sci. 2018;19:2419. doi: 10.3390/ijms19082419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan W., Ni Z., Yu Hu, Liang W., Ou C., He J., et al. Clinical characteristics of 2019 novel coronavirus infection in China. N Engl J Med. 2020;2020 doi: 10.1101/2020.02.06.20020974. [DOI] [Google Scholar]
- Harries A., Takarinda K.C. Faculty opinions recommendation of presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Health Commission of PRC N Chinese guidelines for diagnosis and treatment of malignant lymphoma 2018 (English version) Chin J Cancer Res. 2019;31:557–577. doi: 10.21147/j.issn.1000-9604.2019.04.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr C., Shaykhiev R., Bals R. The role of cathelicidin and defensins in pulmonary inflammatory diseases. Expert Opin Biol Ther. 2007;7:1449–1461. doi: 10.1517/14712598.7.9.1449. [DOI] [PubMed] [Google Scholar]
- Hewison M. An update on vitamin D and human immunity. Clin Endocrinol (Oxf) 2012;76:315–325. doi: 10.1111/j.1365-2265.2011.04261.x. [DOI] [PubMed] [Google Scholar]
- Holick M.F. Medical progress: vitamin D deficiency. N Engl J Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeffery L.E., Burke F., Mura M., Zheng Y., Qureshi O.S., Hewison M., et al. 1,25-dihydroxyvitamin D 3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3. J Immunol. 2009;183:5458–5467. doi: 10.4049/jimmunol.0803217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kast J.I., McFarlane A.J., Głobińska A., Sokolowska M., Wawrzyniak P., Sanak M., et al. Respiratory syncytial virus infection influences tight junction integrity. Clin Exp Immunol. 2017;190:351–359. doi: 10.1111/cei.13042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kešeľová M., Šváb P., Korba P., Hovanec M. The impact of the covid-19 outbreak on aviation. Sci Pap Silesian Univ Technol – Organ Manag Ser. 2020;2020:253–262. doi: 10.29119/1641-3466.2020.148.19. [DOI] [Google Scholar]
- Khamis F., Al-Zakwani I., Al Naamani H., Al Lawati S., Pandak N., Omar M.B., et al. Clinical characteristics and outcomes of the first 63 adult patients hospitalized with COVID-19: an experience from Oman. J Infect Public Health. 2020;13:906–913. doi: 10.1016/j.jiph.2020.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laaksi I. Vitamin D and respiratory infection in adults. Proc Nutr Soc. 2012;71:90–97. doi: 10.1017/S0029665111003351. [DOI] [PubMed] [Google Scholar]
- Lang P.O., Aspinall R. Vitamin D status and the host resistance to infections: what it is currently (not) understood. Clin Ther. 2017;39:930–945. doi: 10.1016/j.clinthera.2017.04.004. [DOI] [PubMed] [Google Scholar]
- Lei G.S., Zhang C., Cheng B.H., Lee C.H. Mechanisms of action of vitamin D as supplemental therapy for Pneumocystis pneumonia. Antimicrob Agents Chemother. 2017;61 doi: 10.1128/AAC.01226-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemire J.M., Adams J.S., Sakai R., Fine R.N., Jordan S.C. 1, 25-dihydroxyvitamin D3 (1, 25(Oh)2D3) suppresses the in vitro proliferation and Im1Unoqabulin production by normal human peripheral blood cells. Pediatr Res. 1984;18 doi: 10.1203/00006450-198404001-00999. 259A–259A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim W.S., Van Der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I., et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19 — studies needed. N Engl J Med. 2020;382:1194–1196. doi: 10.1056/nejmp2002125. [DOI] [PubMed] [Google Scholar]
- Liu P.T., Stenger S., Li H., Wenzel L., Tan B.H., Krutzik S.R., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311:1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Maclaren G., Fisher D., Brodie D. Preparing for the most critically ill patients with COVID-19: the potential role of extracorporeal membrane oxygenation. JAMA. 2020;323:1245–1246. doi: 10.1001/jama.2020.2342. [DOI] [PubMed] [Google Scholar]
- MacLaughlin J., Holick M.F. Aging decreases the capacity of human skin to produce vitamin D3. J Clin Invest. 1985;76:1536–1538. doi: 10.1172/JCI112134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez-Moreno J., Hernandez J.C., Urcuqui-Inchima S. Effect of high doses of vitamin D supplementation on dengue virus replication, toll-like receptor expression, and cytokine profiles on dendritic cells. Mol Cell Biochem. 2020;464:169–180. doi: 10.1007/s11010-019-03658-w. [DOI] [PubMed] [Google Scholar]
- Meltzer D.O., Best T.J., Zhang H., Vokes T., Arora V., Solway J. Association of vitamin D status and other clinical characteristics with COVID-19 test results. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.19722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millet J.K., Whittaker G.R. Physiological and molecular triggers for SARS-CoV membrane fusion and entry into host cells. Virology. 2018;517:3–8. doi: 10.1016/j.virol.2017.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mousavi S., Bereswill S., Heimesaat M.M. Immunomodulatory and antimicrobial effects of vitamin C. Eur J Microbiol Immunol. 2019;9:73–79. doi: 10.1556/1886.2019.00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan L., Lai A.L., Millet J.K., Straus M.R., Freed J.H., Whittaker G.R., et al. Calcium ions directly interact with the Ebola virus fusion peptide to promote structure-function changes that enhance infection. ACS Infect Dis. 2020;6:250–260. doi: 10.1021/acsinfecdis.9b00296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike J.W., Christakos S. Biology and mechanisms of action of the vitamin D hormone. Endocrinol Metab Clin North Am. 2017;46:815–843. doi: 10.1016/j.ecl.2017.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puig-Domingo M., Marazuela M., Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondanelli M., Miccono A., Lamburghini S., Avanzato I., Riva A., Allegrini P., et al. Self-care for common colds: the pivotal role of vitamin D, vitamin C, zinc, and Echinacea in three main immune interactive clusters (physical barriers, innate and adaptive immunity) involved during an episode of common colds - practical advice on dosages. Evid Based Complement Altern Med. 2018;2018:1–36. doi: 10.1155/2018/5813095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi G.A., Fanous H., Colin A.A. Viral strategies predisposing to respiratory bacterial superinfections. Pediatr Pulmonol. 2020;55:1061–1073. doi: 10.1002/ppul.24699. [DOI] [PubMed] [Google Scholar]
- Schwalfenberg G.K. A review of the critical role of vitamin D in the functioning of the immune system and the clinical implications of vitamin D deficiency. Mol Nutr Food Res. 2011;55:96–108. doi: 10.1002/mnfr.201000174. [DOI] [PubMed] [Google Scholar]
- Siegerink B., Rohmann J.L. Impact of your results: beyond the relative risk. Res Pract Thromb Haemost. 2018;2:653–657. doi: 10.1002/rth2.12148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh V.P., Khatua B., El-Kurdi B., Rood C. Mechanistic basis and therapeutic relevance of hypocalcemia during severe COVID-19 infection. Endocrine. 2020;70:461–462. doi: 10.1007/s12020-020-02530-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straus M.R., Tang T., Lai A.L., Flegel A., Bidon M., Freed J.H., et al. Ca2+ ions promote fusion of Middle East Respiratory Syndrome coronavirus with host cells and increase infectivity. J Virol. 2020;94(13) doi: 10.1128/JVI.00426-20. e00426-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J.-K., Zhang W.-H., Zou L., Liu Y., Li J.-J., Kan X.-H., et al. 2020. Serum calcium as a biomarker of clinical severity and prognosis in patients with coronavirus disease 2019: a retrospective cross-sectional study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyeki T.M., Mehta A.K., Davey R.T., Liddell A.M., Wolf T., Vetter P., et al. Clinical management of Ebola virus disease in the United States and Europe. N Engl J Med. 2016;374:636–646. doi: 10.1056/nejmoa1504874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vásárhelyi B., Sátori A., Olajos F., Szabó A., Beko G. Alacsony D-vitamin-szint a Semmelweis Egyetem betegei körében: a központi laboratóriumban egy év alatt meghatározott D-vitamin-szintek retrospektív értékelése. Orv Hetil. 2011;152:1272–1277. doi: 10.1556/OH.2011.29187. [DOI] [PubMed] [Google Scholar]
- Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei R., Christakos S. Mechanisms underlying the regulation of innate and adaptive immunity by vitamin D. Nutrients. 2015;7:8251–8260. doi: 10.3390/nu7105392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization WHO R&D blueprint novel coronavirus COVID-19 therapeutic trial synopsis. World Heal Organ. 2020:1–9. [Google Scholar]
- Xu X.W., Wu X.X., Jiang X.G., Xu K.J., Ying L.J., Ma C.L., et al. Clinical findings in a group of patients infected with the 2019 novel coronavirus (SARS-Cov-2) outside of Wuhan, China: retrospective case series. BMJ. 2020;368:m606. doi: 10.1136/bmj.m606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Ran Z., Jiang Q., Hu N., Yu B., Zhu L., et al. Vitamin D alleviates rotavirus infection through a microrna-155-5p mediated regulation of the TBK1/IRF3 signaling pathway in vivo and in vitro. Int J Mol Sci. 2019;20:3562. doi: 10.3390/ijms20143562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., et al. A novel coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/nejmoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]