Abstract
Background
Observational studies have suggested a higher risk of thrombotic events in patients with coronavirus disease 2019 (COVID-19). Moreover, elevated D-dimer levels have been identified as an important prognostic marker in COVID-19 directly associated with disease severity and progression. Prophylactic anticoagulation for hospitalized COVID-19 patients might not be enough to prevent thrombotic events; therefore, therapeutic anticoagulation regimens deserve clinical investigation.
Design
ACTION is an academic-led, pragmatic, multicenter, open-label, randomized, phase IV clinical trial that aims to enroll around 600 patients at 40 sites participating in the Coalition COVID-19 Brazil initiative. Eligible patients with a confirmed diagnosis of COVID-19 with symptoms up to 14 days and elevated D-dimer levels will be randomized to a strategy of full-dose anticoagulation for 30 days with rivaroxaban 20 mg once daily (or full-dose heparin if oral administration is not feasible) vs standard of care with any approved venous thromboembolism prophylaxis regimen during hospitalization. A confirmation of COVID-19 was mandatory for study entry, based on specific tests used in clinical practice (RT-PCR, antigen test, IgM test) collected before randomization, regardless of in the outpatient setting or not. Randomization will be stratified by clinical stability at presentation. The primary outcome is a hierarchical analysis of mortality, length of hospital stay, or duration of oxygen therapy at the end of 30 days. Secondary outcomes include the World Health Organization's 8-point ordinal scale at 30 days and the following efficacy outcomes: incidence of venous thromboembolism , acute myocardial infarction, stroke, systemic embolism, major adverse limb events, duration of oxygen therapy, disease progression, and biomarkers. The primary safety outcomes are major or clinically relevant non-major bleeding according to the International Society on Thrombosis and Haemostasis criteria.
Summary
The ACTION trial will evaluate whether in-hospital therapeutic anticoagulation with rivaroxaban for stable patients, or enoxaparin for unstable patients, followed by rivaroxaban through 30 days compared with standard prophylactic anticoagulation improves clinical outcomes in hospitalized patients with COVID-19 and elevated D-dimer levels.
The first cases of coronavirus disease 2019 (COVID-19), which is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), were reported in December 2019; less than 3 months later it was declared a global pandemic by the World Health Organization (WHO).1 COVID-19 was initially identified as a syndrome with primarily respiratory manifestations. However, the first series of published cases in patients with COVID-19 showed that other systems were affected including the cardiovascular system, which has proven to be an important marker of disease progression and mortality.2
Initial evidence indicates that the increased systemic inflammatory response and coagulation system disorders that occur with COVID-19 infection directly impact the cardiovascular system,3 , 4 and are associated with adverse cardiovascular events such as myocardial ischemia, myocarditis, arrhythmias, and thromboembolic phenomena. Serum troponin and D-dimer have been identified as prognostic markers for cardiovascular events.2 , 5 Current guidance recommends monitoring patients with COVID-19 using echocardiography, electrocardiography, and monitoring levels of biomarkers like troponin and D-dimer.3
The ongoing COVID-19 pandemic is a global health emergency. In this scenario, clinical studies should be implemented to address different medical questions simultaneously as a collaborative effort to control the consequences of the spread of the disease and improve patient outcomes.6., 7., 8., 9. Efficacy and safety of any given intervention should be assessed through a randomized clinical trial in order to provide reliable information to guide clinical practice.6., 7., 8., 9.
Coalition COVID-19 Brazil
Coalition COVID-19 Brazil is an initiative developed by Brazilian investigators and sites to test therapies for different targets in order to quickly identify the efficacy and safety of treatments for COVID-19 that have been used during the pandemic in an empirical manner but with no proven benefit. Hydroxychloroquine and azithromycin were tested and did not show benefit in hospitalized patients with mild-to-moderate COVID-19.10 This project encompassed other randomized studies involving patients with a range of severity of COVID-19, including the addition of azithromycin to hydroxychloroquine in patients with severe disease which also did not show clinical benefit.11 In addition, treatments for complications of the infection were also addressed in the Coalition initiative and focused on 2 main pathways related to adverse outcomes of COVID-19: inflammatory response12 , 13 and coagulation system. In the Coalition III study,12 we assessed the efficacy and safety of dexamethasone in patients with moderate or severe acute respiratory distress syndrome (ARDS) caused by COVID-19, and this intervention improved the number of days alive and free from mechanical ventilation during the first 28 days as compared with standard of care alone. The current study (ACTION) is the Coalition IV trial and will compare a strategy of full anticoagulation with a direct and selective factor Xa inhibitor with a prophylactic anticoagulation strategy in patients hospitalized with COVID-19 and an elevated D-dimer level.
Study rationale
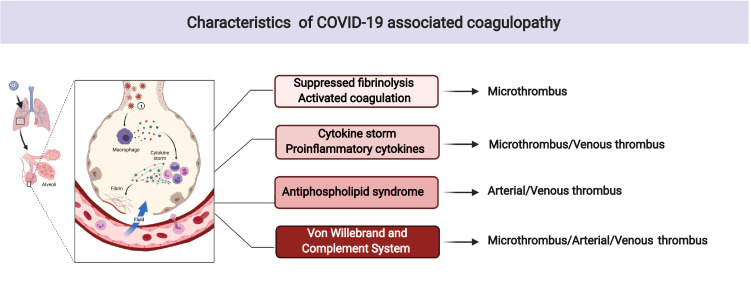
There are many reports of patients with COVID-19 having diverse cardiovascular and thrombotic clinical and laboratory manifestations that are of great prognostic implication. Abnormal levels of coagulation biomarkers like D-dimer, fibrinogen, and PAI 1 are commonly identified in patients with COVID-19.2 , 14 Clinical and laboratory features of COVID-19-associated coagulopathy partially overlap with sepsis-induced coagulopathy, disseminated intravascular coagulation, hemophagocytic syndrome/hemophagocytic lymphohistiocytosis, antiphospholipid syndrome, and thrombotic microangiopathy; however, it does not perfectly match with any other specific coagulopathies (Figure 1 ).15 Among the risk markers for mortality, the serum level of D-dimer has shown important and independent prognostic relevance.2 , 16 , 17
Figure 1.
Characteristics of COVID-19 associated coagulopathy. Legend: The pathogenesis of COVID-19- associated coagulopathy (CAC) is multifactorial and continues to be determined. CAC with suppressed fibrinolysis and activated coagulation resembles sepsis-induced coagulopathy and disseminated intravascular coagulation. The prothrombotic state due to cytokine storm and proinflammatory cytokines have some aspects of hemophagocytic syndrome and hemophagocytic lymphohistiocytosis. The activation of complement system and von Willebrand factor partially overlap with antiphospholipid syndrome, and thrombotic microangiopathy but CAC has unique features that may be defined as a new category of coagulopathy.
Considering that the risk of disease progression or mortality is directly related to the level of such thrombotic markers, there is a strong rationale for the potential benefit of anticoagulation in these patients. In addition, case series have shown that patients with COVID-19 commonly present with intra-vascular microthrombi,18 especially in the pulmonary vasculature, which frequently evolves to symptomatic venous thromboembolism (VTE) and results in arterial thrombotic events.19 A case series from the Netherlands showed that 31% of patients admitted to the intensive care unit presented with symptomatic acute pulmonary embolism, deep vein thrombosis, ischemic stroke, myocardial infarction, or systemic arterial embolism.19 Observational data showed that the use of anticoagulants in patients with elevated D-dimer levels was associated with lower mortality when compared with no anticoagulation.17 A retrospective analysis of 4,389 patients showed that compared with no anticoagulation, both therapeutic and prophylactic anticoagulation were associated with approximately 50% lower in-hospital mortality and 30% less intubation.20 When initiated ≤48 hours from admission, there was no statistically significant difference between therapeutic vs prophylactic anticoagulation (adjusted hazard ratio [HR] 0.86, 95% confidence interval [CI] 0.73–1.02; P = .08).20 On the other hand, 89 patients (2%) had major bleeding adjudicated by clinician review, with 27 of 900 patients (3.0%) on therapeutic anticoagulation, 33 of 1959 (1.7%) on prophylactic anticoagulation, and 29 of 1530 (1.9%) on no anticoagulation.20 Despite these interesting results, observational data regarding a medical intervention are subject to confounding and should be considered hypothesis generating at best.21 Nevertheless, despite the biological rationale and promising observational data for such patients, well-designed randomized clinical trials are lacking. It is time for high quality evidence in this field since thousands of patients are treated daily for COVID-19 and are exposed to different empirical anticoagulation treatments for which the risks and benefits remain unknown.21
Methods
Study overview
ACTION (NCT04394377) is an investigator-initiated, academic-led, pragmatic, phase IV, multicenter, randomized trial. The study is open-label with blinded outcomes adjudication (PROBE) and will include approximately 600 patients with a confirmed diagnosis of COVID-19 with symptoms up to 14 days and elevated D-dimer levels.
The ACTION trial is part of the Coalition COVID-19 Brazil initiative. The study protocol was approved by the National Commission for Research Ethics from the Brazilian Ministry of Health. The study will be conducted in compliance with the protocol and adheres fully to the ethical principles of the International Council for Harmonization and Good Clinical Practice. The ACTION trial began enrolling patients on June 21, 2020 and finished enrollment on February 28, 2021. The protocol requires that each patient must provide informed consent before any study procedure is initiated.
Primary objective
The primary objective of the trial is to evaluate, among hospitalized patients with COVID-19 and elevated D-dimer levels, the clinical impact of the strategy of routine full-dose anticoagulation compared with standard of care on the following outcomes in a hierarchical analysis: mortality, length of hospital stay, or duration of oxygen therapy at 30 days.
Secondary objectives
The secondary objectives are to evaluate the impact of the strategy of routine full-dose anticoagulation at 30 days in hospitalized patients with COVID-19 and elevated D-dimer levels using the following 8-point ordinal COVID-19 outcome scale developed by the World Health Organization22 , 23: death; invasive mechanical ventilation and support for another organ dysfunction; invasive mechanical ventilation alone; noninvasive ventilation/high-flow oxygen; hospitalized on supplemental oxygen; hospitalized not requiring supplemental oxygen; not hospitalized, limitation on activities and/or requiring home oxygen; not hospitalized, no limitations on activities.
The impact of the intervention will also be assessed in the following efficacy end points: VTE, acute myocardial infarction, stroke, systemic embolism, major adverse limb events, time using oxygen therapy, time using noninvasive ventilation, time using mechanical ventilation, progression of the disease, troponin, and inflammatory and coagulation biomarkers. The primary safety outcome will be major and clinically relevant non-major bleeding as defined using International Society on Thrombosis and Haemostasis (ISTH) criteria.24
Study population
All patients hospitalized with a confirmed diagnosis of COVID-19 with symptoms up to 14 days and elevated D-dimer levels are potentially eligible if they provide written (or oral, if applicable) informed consent after detailed explanation of the protocol. Since this is a study of hospitalized patients, most patients will experience progression of the disease warranting hospital admission by around 1 week. The 14-day period from symptom onset used as an inclusion criterion in the ACTION trial is not only targeting the most common period of hospitalization, but also provides an additional guarantee that the hospitalization is related to COVID-19.
Exclusion criteria are detailed below in groups according to the main reason for exclusion:
-
1.
A clear evidence-based indication or contraindication for full-dose anticoagulation.
-
2.
A condition associated with high-risk of bleeding in which it would not be appropriate to test a full-dose anticoagulation therapy (eg, platelets <50,000/mm3; international normalized ratio >1.5; use of acetylsalicylic acid >100 mg/day or P2Y12 inhibitor; chronic use of NSAIDs; sustained uncontrolled systolic blood pressure (BP) ≥180 mm Hg or diastolic BP ≥100 mm Hg; disseminated intravascular coagulation; active cancer; or history of conditions related to intracranial bleeding).
-
3.
Common limitations to study drug, such as hypersensitivity to rivaroxaban or heparin; use of strong inhibitors or inducers of cytochrome P450 3A4 and/or P-glycoprotein; known HIV infection; creatinine clearance <30 mL/min; and pregnancy or breastfeeding.
The use of full-dose anticoagulation prior to study entry is allowed as long as the duration of therapy before randomization was ≤48 hours. In addition, the time from the last dose of anticoagulant prior to randomization to the first dose of anticoagulant post-randomization should be at least 24 hours. Full inclusion and exclusion criteria are shown in Table I .
Table I.
Inclusion and exclusion criteria for ACTION trial
| Inclusion criteria |
|
|
|
|
|
| Exclusion criteria |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AF indicates atrial fibrillation; ASA, acetylsalicylic acid; BP, blood pressure; COVID-19, coronavirus 2019; DIC, disseminated intravascular coagulation; HIV, human immunodeficiency virus; NSAIDs, nonsteroidal anti-inflammatory drugs; P-gp, P-glycoprotein; ULN, upper limit of normal; VTE, venous thromboembolism.
Randomization and allocation concealment
Concealed randomization will be performed using a central, automated, electronic web-based system. Randomization will be stratified according to the patient's clinical condition at the time of randomization: unstable or stable. Unstable patients will be categorized by site investigators as having COVID-19–related critical illness, suffering from a life-threatening condition requiring mechanical ventilation, use of vasopressors, and/or unable to take an oral medication due to degree of illness.25 These patients will be treated with subcutaneous enoxaparin 1 mg/kg twice daily; unfractionated heparin is the preferred treatment option for patients with decreasing creatinine clearance <30 ml/m2 and/or disseminated intravascular coagulation. When they become stable, these patients will be transitioned to oral anticoagulation with rivaroxaban. All patients in the intervention group will continue therapeutic anticoagulation until 30 days after randomization.
Eligible patients will be randomized using a 1:1 ratio to either full-dose anticoagulation therapy for 30 days (Group 1) or standard of care (Group 2). Patients randomized to Group 1 will follow the strategy of routine full-dose anticoagulation for 30 days (standard therapeutic regimen of oral anticoagulation with rivaroxaban 20 mg once daily or subcutaneous enoxaparin 1 mg/kg twice daily if oral route is not feasible). Group 2 will follow the standard usual care with in-hospital prophylactic anticoagulation (without routine full-dose anticoagulation).
Study intervention (Group 1)
Considering a strategy of routine full-dose anticoagulation, the types of anticoagulants may vary according to clinical status. For patients who present to the hospital and are stable during the early phase (outside of the intensive care unit), the use of oral anticoagulants, like rivaroxaban, is an attractive option to potentially avoid clinical deterioration that commonly occurs between 7 to 10 days after the appearance of symptoms.10 , 26 Thus, the main question that requires investigation is whether a strategy of routine full-dose anticoagulation (with an oral agent or parenteral when indicated) improves patient outcomes when compared with standard of care in patients with COVID-19.
In this group, full-dose anticoagulation therapy will be maintained for 30 days. Depending on the patient's clinical condition, there will be 2 possible routes of administration—oral or parenteral.
Oral administration
Oral administration of anticoagulation is the default and should be the initial way to start study medication for most patients. The regimen to be used in the study is rivaroxaban 20 mg once daily (dose adjusted to 15 mg once daily if creatinine clearance [calculated using the Cockroft-Gault equation] is 30–49 mL/min and/or with concomitant use of azithromycin). The dose of rivaroxaban will be adjusted to 15 mg once daily with concomitant use of oral azithromycin because it increases levels of rivaroxaban by affecting hepatic/intestinal enzyme CYP3A4 metabolism. There is no weight limitation to using rivaroxaban. If a patient develops acute kidney injury with creatinine clearance <30 mL/min, the protocol recommends withdrawing rivaroxaban until renal function improves or switching to parenteral full-dose anticoagulation, at the discretion of the treating physician.
Parenteral administration
Patients using vasoactive agents (especially at high doses) and/or on invasive ventilatory support may participate in the trial or switch to full parenteral anticoagulation (at the discretion of the treating physician) using one of the options outlined in the protocol:
Parenteral 1: Enoxaparin 1 mg/kg every 12 hours subcutaneously (dose adjusted to 0.75 mg/kg every 12 hours if patient age is ≥75 years); or
Parenteral 2: Unfractionated heparin (preferred option for patients worsening with disseminated intravascular coagulation after randomization without prohibitive bleeding risk).
The default therapeutic strategy for the study is oral anticoagulation with rivaroxaban 20 mg once daily. Parenteral anticoagulation might be used for patients who are on mechanical ventilation and/or using vasoactive drugs and are unable to receive the oral anticoagulant at the time of inclusion in the study. In these instances, patients can start on the parenteral anticoagulation strategy and, once stable, should be converted to oral anticoagulation. Enrolment of patients entering the study using the parenteral strategy (enoxaparin as standard) will be capped at no more than 30% of the trial sample.
Additionally, stable patients randomized to the full anticoagulation strategy arm who start with rivaroxaban may develop clinical instability which can affect the absorption of oral medications. Therefore, it is permissible for these patients to be switched to full-dose heparin (unfractionated or low molecular weight) and continue on the full-dose anticoagulation strategy. Patients with creatinine clearance decreasing to <30 mL/min or disseminated intravascular coagulation who do not present a prohibitive risk of bleeding could continue to receive full anticoagulation with unfractionated heparin, according to the algorithm provided by the study leadership and at the discretion of the treating physician. Regardless of the route of administration, patients in Group 1 should continue using oral anticoagulation until the end of the study (30 days after randomization). Thus, our study is testing more than just a specific drug, but a therapeutic strategy of routine full-dose anticoagulation for 30 days vs standard of care, which will be prophylactic anticoagulation during hospitalization. If patients are randomized to the full-dose anticoagulation strategy and discharged before 30 days, all patients should continue on rivaroxaban 20 mg once daily for 30 days after randomization.
Standard of care (Group 2)
Patients in the standard of care group will receive the usual standard management and currently have no indication for full-dose anticoagulation. The protocol recommends the use of VTE prophylaxis in Group 2 (usual standard care) during hospitalization, but the extended prophylaxis beyond hospital discharge might be used in selected cases according to the decision of the treating physician.
For VTE prophylaxis, all patients should receive an approved dose of a parenteral anticoagulant for use in medically ill patients (Table II ).27 This pragmatic approach will strengthen the results, increasing the generalizability of the study findings.
Table II.
Suggested prophylactic scheme
| CrCl | BMI | Enoxaparin | Fondaparinux | UFH |
|---|---|---|---|---|
| ≥30 | <40 | 40 mg SC every 24h | 2.5 mg SC daily | 5000 units SC every 8 to 12 hours |
| ≥40 | 60 mg SC every 24h or 40 mg SC every 12 hours | Not recommended | 7500 units SC every 8 to 12 hours | |
| <30 | <40 | UFH 5000 units SC every 8 to 12 hours | ||
| ≥40 | UFH 7500 units SC every 8 to 12 hours | |||
BMI indicates body mass index in kg/m2; CrCl, creatinine clearance in mL/min; SC, subcutaneous; UFH, unfractionated heparin.
All other procedures and concomitant therapies will be at the discretion of the treating physician and should be based on current guidelines and local standards of care without systematic use of additional experimental therapies. Thus, the only difference between the groups will be in relation to the full anticoagulation strategy. Patients in Group 2 (standard of care) should only use full-dose anticoagulation if a new clinical indication arises after randomization (eg, VTE, atrial fibrillation, etc.).27 , 28
Study procedures
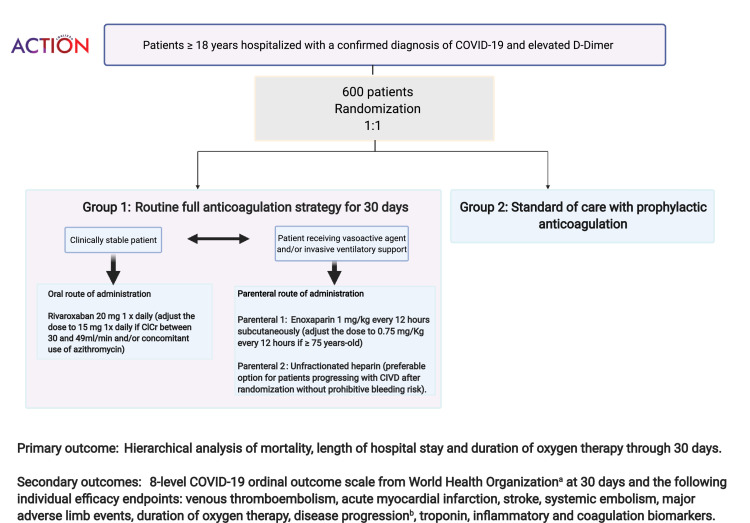
The data collected will include demographics, cardiovascular risk factors, relevant medical history, clinical characteristics, concomitant medications, and laboratory data. Data collection will be performed during hospitalization until discharge and a phone call at 30 days will capture clinical events after discharge. The flowchart including all study procedures is presented in Figure 2 . The detailed information collected during each study visit is provided in the Supplementary Appendix.
Figure 2.
Design of the ACTION trial (study flowchart). Legend: A) 8-point ordinal COVID-19 outcome: death; invasive mechanical ventilation and support for another organ dysfunction; invasive mechanical ventilation alone; noninvasive ventilation/high-flow oxygen; hospitalized on supplemental oxygen; hospitalized not requiring supplemental oxygen; not hospitalized, limitation on activities and/or requiring home oxygen; not hospitalized, no limitations on activities. B) Disease progression: Mild defined as cases without criteria to be classified within the “moderate” or “severe” groups; Moderate: Oxygen saturation <94% or pulmonary infiltrates> 50% or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300; Severe: Respiratory failure or hemodynamic instability or multiple organ dysfunction.
Clinical outcomes
Primary outcome
The primary end point is a hierarchical outcome consisting of time to death, length of hospital stay, and number of days on oxygen support, all evaluated at 30 days.
Secondary end points
Secondary efficacy end points
The 8-point ordinal scale of outcomes will be evaluated as a secondary end point and will identify the clinical condition of the patient at 30 days in the following order: death; invasive mechanical ventilation and support for another organ dysfunction; invasive mechanical ventilation alone; noninvasive ventilation/high-flow oxygen; hospitalized on supplemental oxygen; hospitalized not requiring supplemental oxygen; not hospitalized, limitation on activities and/or requiring home oxygen; not hospitalized, no limitations on activities (Table III ).22 , 23
Table III.
World Health Organization ordinal scale22,23
| Score | Patient state |
|---|---|
| 1 | Not hospitalized, no limitation of activities |
| 2 | Not hospitalized, limitation of activities |
| 3 | Hospitalized, no oxygen therapy |
| 4 | Oxygen by mask or nasal prongs |
| 5 | Non-invasive mechanical ventilation or high-flow oxygen |
| 6 | Intubation and mechanical ventilation |
| 7 | Ventilation + additional organ support – pressors, RRT, ECMO |
| 8 | Death |
ECMO indicates extra-corporeal membrane oxygenation; RRT, renal replacement therapy.
Cardiovascular outcomes include the incidence of VTE, acute myocardial infarction, stroke, systemic embolism, and major adverse limb events individually and in a composite outcome of thrombotic events. All thrombotic events will also be combined with all-cause mortality in an overall composite assessment of efficacy. Although the protocol does not mandate routine screening for these outcomes, the clinical events classification (CEC) committee will look for specific triggers (based on laboratory values, reports of adverse events, unknown cause of death, changes in therapy) in order to actively search for potentially under-reported events. Thus, all suspected events will be adjudicated by an independent CEC committee whose members are blinded to the trial treatment assignment.
Duration of oxygen therapy and/or noninvasive or mechanical ventilation are outcomes of special interest because of the potential benefit of anticoagulation in preventing pulmonary microthrombosis that could be associated with a greater need for oxygen therapy and/or invasive or mechanical ventilation. The same rationale applies to disease progression, which is classified as mild, moderate, and severe.29 , 30 Mild disease includes cases without criteria to be classified within the “moderate” or “severe” groups. Moderate disease is characterized by oxygen saturation <94% or pulmonary infiltrates >50% or ratio of partial pressure of arterial oxygen to fraction of inspired oxygen <300. Severe disease is defined as respiratory failure or hemodynamic instability or multiple organ dysfunction. Inflammatory and coagulation biomarkers and troponin level will also be collected as components of the efficacy end points. Since this is a pragmatic study, most laboratory tests will be performed based on practices at each site and will be assessed in an exploratory fashion. Among these biomarkers, the following coagulation and inflammatory tests are planned to be measured at baseline and at the end of the hospitalization: D-dimer (inclusion criteria), fibrinogen, prothrombin time, activated partial thromboplastin time, platelets, and C-reactive protein.
Key safety end points
The primary safety end point is major and clinically relevant non-major bleeding according to ISTH criteria. An additional composite outcome of net benefit will combine thrombotic events and all-cause mortality with major and clinically relevant non-major bleeding.
Other safety end points
Major and clinically relevant non-major bleeding will be also evaluated by an independent committee whose members are blinded to the treatment assignment (full anticoagulation or not), and will be classified according to the Thrombolysis in Myocardial Infarction (TIMI), Global Use of Strategies to Open Occluded Arteries (GUSTO), Bleeding Academic Research Consortium (BARC), and ISTH criteria.31 Hemoglobin values and number of blood transfusions will also be collected as components of the safety end points. Thrombocytopenia (included heparin induced) will be reported by the site investigator and platelet levels will also be collected in order to identify low levels regardless of reports from the investigators.
Sample size calculation
The initial planned sample size for ACTION is around 600 patients. Details of the simulations for the sample size calculation based on a hierarchical outcome using the Win Ratio statistic will be described in the statistical analysis plan. Briefly, based on simulations of scenarios assuming the following outcomes in the control group from Coalition I10 (mortality rate of 7%, 6 days standard deviation of time out of hospital until 30 days, 5 days standard deviation of days free of oxygen support and assuming treatment should improve mortality rate in 2%, improve mean days out of hospital by 1.5 days and mean days of oxygen support in 1.5 days) with a sample of 600 patients, the Win Ratio will have approximately 90% power.
Beyond the initial sample size calculation, a formal interim analysis by the Data and Safety Monitoring Board (DSMB) evaluating primarily safety may recommend modifications in the sample size. The first formal analysis will be performed when the first 300 patients have been enrolled and have completed the 30-day follow-up visit.
Statistical analysis
In order to maintain the benefits of randomization, the primary analysis will follow the intention-to-treat principle.
The primary outcome will be analyzed using the Win Ratio proposed by Wang and Pocock32 and will consider the treatment as a fixed effect stratified by the clinical condition of the patient (unstable and stable patients). The null hypothesis is H0: Ψ=1 (win ratio = 1): The rate of all winner comparisons between the groups (full anticoagulation or not) is the same. The alternative hypothesis is H1: Ψ≠1 (win ratio ≠ 1): The rate of all winner comparisons between the groups (full anticoagulation or not) is different.
Using this method, all patients in the treatment group are compared with all patients in the control group. Initially, the pairs will be compared for time until death truncated at 30 days. If both died, the “winner” of the comparison will be the one who has a longer time between the time of randomization and the date of death (at least 1 day later). If the patients are tied (they died with the same follow-up time or both remained alive until the 30-day visit), the pair will be compared for the length of hospital stay and the one with the shortest length of stay will be declared the “winner” (considering a difference higher than 2 days). Finally, if a second tie occurs, patients will be compared for the days of oxygen-free support until the 30-day visit and the one with the longest time without oxygen support will be declared the “winner” (considering a difference higher than 2 days). If contact with the patient is not obtained during the 30-day visit and the follow-up time to assess mortality is censored, comparisons paired with this individual will be made until the moment of censorship. However, it is expected that the vital condition of all patients will be obtained within 30 days.
Finally, we defined the test statistic (Win Ratio) as the number of pair wins in each group (full-dose anticoagulation vs. control) weighted by the size of each stratum (stratum of unstable and stable patients who were randomized and would start with parenteral or oral drug administration, respectively). The results will be presented with the respective 95% confidence intervals and the final test will use a significance level of 5%, which will not be corrected for multiplicity since the interim analyses use restrictive decision limits.
Each component of the hierarchical outcome will be compared individually using the same method. Binary secondary outcomes such as thromboembolic events at 30 days, and cardiovascular events at 30 days will be compared using log binomial models, reporting relative risks with the respective 95% confidence intervals.
WHO ordinal scale and disease progression will be compared using cumulative proportional odds ratio models.
Interaction tests for the primary outcome will be performed for specific subgroups that include: age >60 vs ≤60 years; days of symptoms (divided into tertiles); initial D-dimer level (divided into tertiles); use of antiviral vs not; stable vs unstable at randomization; use of mechanical ventilation vs no mechanical ventilation at randomization; use of mechanical ventilation vs noninvasive oxygen support vs without oxygen support at randomization; parenteral anticoagulation prior to randomization vs no; use of corticosteroids at baseline vs no; history of myocardial infarction vs no; history of stroke vs no; history of heart failure vs no; BMI (≤30 kg/m² or >30kg/m²); and severity of patients at the time of presentation (mild vs moderate vs severe). Additional analysis of subgroups (eg, according to WHO classification) could be performed in order to evaluate potential interaction of the intervention according to disease progression.
The details of the statistical analysis will be described in the Statistical Analysis Plan. All analyses will be performed using R software in its current version.
Organizational structure
Funding and trial oversight
This trial was funded by the hospitals and research institutes leading the Coalition COVID-19 Brazil. Bayer S.A. provided partial funding and study drug for the trial, but was not involved in the study design, conduct, analysis, or decision to publish the results.
The executive and steering committees, together with the operational teams from the Brazilian Clinical Research Institute (BCRI), will coordinate and oversee the medical, scientific, and operational conduct of the study. Study operations are being coordinated by the BCRI and the regulatory proceedings of the trial with the support from the academic research organization from Hospital Israelita Albert Einstein (HIAE). The BCRI will be responsible for data management, site management, clinical events adjudication, safety surveillance, and all statistical analyses of the study. The academic research organization from HIAE will be responsible for regulatory affairs and will perform an independent confirmatory statistical analysis of the primary results of the study. The executive and steering committee members are responsible for the reporting of the results and the drafting and editing of this and forthcoming manuscripts. The authors are solely responsible for the design and conduct of this study, all study analyses, and the drafting and editing of the paper and its final contents.
Data and Safety Monitoring Board
An independent DSMB will monitor safety data on an ongoing basis with access to unblinded data. The DSMB will review results from a formal interim analysis once 300 patients have completed the study. The DSMB will use a P-value < .001 in the interim analysis to declare an overwhelming statistically significant difference in efficacy and/or safety outcomes between study groups to guide their recommendations.30 , 31 An overall p-value <.05 will be used to declare statistical significance at the end of the study. A detailed DMSB charter will provide stopping rules for the trial for efficacy and safety issues. There will be no interruption for futility.
Discussion
The first reports of fatal cases of COVID-19 identified macro and microvascular thrombosis as a main factor associated with death.18 , 20 , 33 The prognostic relevance of thrombosis in patients with COVID-19 occurs both because of the intrinsic risk related to this condition alone (regardless of COVID-19 infection) and also because this condition is much more common and intense in patients with COVID-19.34 , 35 In all cases of sepsis, regardless of the etiology, there is thrombotic activation and a risk of coagulopathy due to consumption of coagulation factors commonly called sepsis-induced coagulopathy.36 Many different therapies were tested targeting the coagulation pathway in patients with sepsis36; however, until now, no robust evidence of benefit has been identified using full-dose anticoagulation therapy in patients with sepsis due to diverse etiologies.33
Despite the absence of clear benefit of anticoagulation in patients with sepsis, COVID-19 infection presents unique aspects that should be mentioned. The incidence and magnitude of thrombosis in patients with COVID-19 is much higher when compared with other infections like H1N1.34 , 35 The clinical and pathological evaluation identified a complete thrombotic syndrome including arterial and venous events and micro and macro complications.18 , 20 , 33., 34., 35., 36. The thrombosis represents a direct cause of death, even in patients without clinical suspicion of a thrombotic event. Finally, prophylaxis during hospitalization may not prevent events after discharge and a high residual risk of thrombotic events in these patients has been reported.37 Thus, there is a strong rationale for the potential benefit of full-dose anticoagulation in these patients.20 Nevertheless, there is also a well-known risk of anticoagulation related to bleeding and, as a consequence, equipoise should be evaluated in a clinical trial.21
The choice of rivaroxaban among all direct oral anticoagulants is supported by the posological convenience (once daily) and the benefit shown in different thrombotic scenarios such as atrial fibrillation,38 , 39 acute coronary syndrome,40 chronic coronary artery disease,41 peripheral artery disease,41 , 42 and in the prophylaxis and treatment of VTE.43 , 44 The dose of 20 mg once daily (adjusted for creatinine clearance) was chosen because of the efficacy and safety shown in patients with an indication for full-dose anticoagulation to reduce thromboembolic events (ie, the indication that will be evaluated in this study).
Currently, other clinical trials addressing the anticoagulation pathway are being conducted but most of them evaluate the use of parenteral anticoagulation during hospitalization.45 ACTION is different not only because of the duration of anticoagulation therapy, but also because it will assess the concept of whether full-dose anticoagulation, regardless of the route of administration, is beneficial or not. The final results of the study will also test the interaction in this prespecified subgroup of critical patients.
Despite being an open-label study, ACTION has a primary composite hierarchical end point that includes only objective information: mortality, days of hospitalization (after randomization), and days of oxygen support (after hospitalization). This patient-centered outcome represents the main goal of every patient with a condition like COVID19—to be alive and out of the hospital with no need for oxygen support. Since many different complications could occur due to COVID-19 and/or the study intervention, days of hospitalization is the second hierarchical end point and encompasses the potential effect of any relevant medical complications. If there is no difference comparing mortality and days of hospitalization, time of oxygen therapy will be assessed to evaluate potential benefit of anticoagulation in the prevention of pulmonary microthrombosis that could be associated with a greater need for oxygen therapy. All end points are objectively assessed and considered to be patient-centered and should help inform physicians in clinical practice. In addition, other end points of interest, such as thromboembolic complications and bleeding, will be reviewed by a CEC committee whose members are blinded to treatment assignment.
Conclusion
ACTION is a multicenter, randomized trial in hospitalized patients with COVID-19 and elevated D-dimer levels that aims to determine whether full-dose anticoagulation with rivaroxaban compared with standard of care improves clinical outcomes. The study findings will provide contemporary and high quality randomized evidence to guide medical decisions in patients hospitalized with COVID-19 and should impact clinical practice.
Footnotes
ClinicalTrials.gov NCT04394377.
Disclosures: RDL: Research support from Bristol-Myers Squibb, GlaxoSmithKline, Medtronic, Pfizer; Consulting fees from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, GlaxoSmithKline, Medtronic, Merck, Pfizer, Portola. PGMBS: Research support from Pfizer, Bayer and Roche Diagnostics; Consulting fees from Pfizer, Bayer and Roche Diagnostics. RHMF: Research grants and personal fees from AstraZeneca, Bayer; and Servier; and research grants from Pfizer, EMS, Aché, Brazilian Ministry of Health, and University Health Network. AVSM: Consulting fees from Pfizer, Bayer, Novartis, Daiichi-Sankyo, Zodiac and Roche. ER: Speaker's bureau for Bayer, BMS/PFE, Aspen, BI, Daiichi Sankyo. Consultant/advisor for Pfizer, BMS, Bayer, Sanofi, Amgen, Daiichi-Sankyo, Cristalia and Aspen.LPD: No conflict of interests. BB: No conflict of interests. ABC: Grants from Bayer, Bactiguard, Johnson & Johnson, do Brasil, Hemaclear, Hillrom, and Pfizer.RGR: No conflict of interests. LCPA: Research support from Aché, consulting fees from Halex-Istar and personal fees from Baxter. VCV: No conflict of interests. FRM: No conflict of interests. LER: Consulting fees from Pfizer. PAM: No conflict of interests. JHA: Research Support from Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, CryoLife, CSL Behring, Ferring, GlaxoSmithKline, U.S. FDA, U.S. NIH, XaTek and consulting fees or honoraria from AbbVie, Atricure, Bristol-Myers Squibb, CryoLife, GlaxoSmithKline, Janssen, Pfizer, Portola, and the U.S. VA CSP. AA: Research grants from Bayer, Sanofi-Pasteur, Population Health Research Institute. OB: Grants from AstraZeneca, Novartis, Servier, Bayer, Amgen, and Boehringer-Ingelheim.
References
- 1.World Health Organization. Coronavirus disease 2019 (COVID-19): situation report, 51. Available at: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200311-sitrep-51-covid-19.pdf?sfvrsn=1ba62e57_10 . Published: March 11, 2020. Accessed August 14, 2020.
- 2.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult in patients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lippi G, Lavie CJ. Sanchis-Gomar F. Cardiac troponin I in patients with coronavirus disease 2019 (COVID-19): Evidence from a meta-analysis. Prog Cardiovasc Dis. 2020;63:390–391. doi: 10.1016/j.pcad.2020.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zheng Y-Y, Ma Y-T, Zhang J-Y, et al. COVID-19 and the cardiovascular system. Nat Rev Cardiol. 2020;17:259–260. doi: 10.1038/s41569-020-0360-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi S, Qin M, Shen B, et al. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020;5:802–810. doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dean NE, Gsell P-S, Brookmeyer R, et al. Creating a framework for conducting randomized clinical trials during disease outbreaks. N Engl J Med. 2020;382:1366–1369. doi: 10.1056/NEJMsb1905390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bothwell LE, Podolsky SH. The emergence of the randomized, controlled trial. N Engl J Med. 2016;375:501–504. doi: 10.1056/NEJMp1604635. [DOI] [PubMed] [Google Scholar]
- 8.Ellenberg SS, Keusch GT, Babiker AG, et al. Rigorous clinical trial design in public health emergencies is essential. Clin Infect Dis. 2018;66:1467–1469. doi: 10.1093/cid/cix1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fanaroff AC, Califf RM, Harrington RA, et al. Randomized trials versus common sense and clinical observation: JACC review topic of the week. J Am Coll Cardiol. 2020;76:580–589. doi: 10.1016/j.jacc.2020.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cavalcanti AB, Zampieri FG, Rosa RG, et al. Hydroxychloroquine with or without Azithromycin in mild-to-moderate Covid-19. N Engl J Med. 2020;383:2041–2052. doi: 10.1056/NEJMoa2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furtado RHM, Berwanger O, Fonseca HA, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomised clinical trial. Lancet. 2020;396:959–967. doi: 10.1016/S0140-6736(20)31862-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tomazini BM, Maia IS, Cavalcanti AB, et al. Effect of Dexamethasone on days alive and ventilator-free in patients with moderate or severe acute respiratory distress syndrome and COVID-19: the CoDEX randomized clinical trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Veiga VC, Prats JAGG, Farias DLC, et al. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomised controlled trial. BMJ. 2021;372:84. doi: 10.1136/bmj.n84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin S, Huang M, Li D, et al. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J Thromb Thrombolysis. 2020 doi: 10.1007/s11239-020-02105-8. Online ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iba T, Levy JH, Connors JM, et al. The unique characteristics of COVID-19 coagulopathy. Crit Care Med. 2020;48:1358–1364. doi: 10.1186/s13054-020-03077-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang N, Li D, Wang X, et al. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844–847. doi: 10.1111/jth.14768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tang N, Bai H, Chen X, et al. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J Thromb Haemost. 2020;18:1094–1099. doi: 10.1111/jth.14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thromb Haemost. 2020;18:1517–1519. doi: 10.1111/jth.14844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klok FA, Kruip MJHA, van der Meer NJM, et al. Incidence of thrombotic complications in critically ill ICU patients with COVID-19. Thromb Res. 2020;191:145–147. doi: 10.1016/j.thromres.2020.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nadkarni GN, Lala A, Bagiella E, et al. Anticoagulation, mortality, bleeding and pathology among patients hospitalized with COVID-19: a single health system study. J Am Coll Cardiol. 2020;76:1815–1826. doi: 10.1016/j.jacc.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes RD, Fanaroff AC. Anticoagulation in COVID-19: it is time for high-quality evidence. J Am Coll Cardiol. 2020;76:1827–1829. doi: 10.1016/j.jacc.2020.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.World Health Organization. COVID-19 therapeutic trial synopsis. Available at: https://www.who.int/publications/i/item/covid-19-therapeutic-trial-synopsis . Published: February 18,2020. Accessed February 17, 2021.
- 23.WHO R&D Blueprint novel Coronavirus COVID-19 Therapeutic Trial Synopsis. Available at internet at: https://www.who.int/blueprint/priority-diseases/key-action/COVID19_Treatment_Trial_Design_Master_Protocol_synopsis_Final_18022020.pdf ; acessed on Apri 4th, 2021.
- 24.Kaatz S, Ahmad D, Spyropoulos AC, et al. Subcommittee on Control of Anticoagulation. Definition of clinically relevant non-major bleeding in studies of anticoagulants in atrial fibrillation and venous thromboembolic disease in non-surgical patients: communication from the SSC of the ISTH. J Thromb Haemost. 2015;11:2119–2126. doi: 10.1111/jth.13140. [DOI] [PubMed] [Google Scholar]
- 25.COVID-19 and VTE/Anticoagulation: Frequently Asked Questions. Available at: https://www.hematology.org/covid-19/covid-19-and-vte-anticoagulation . Acessed April 4, 2021.
- 26.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moores LK, Tritschler T, Brosnahan S, et al. Prevention, diagnosis, and treatment of VTE in patients with coronavirus disease 2019: CHEST Guideline and Expert Panel Report. Chest. 2020;158:1143–1163. doi: 10.1016/j.chest.2020.05.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Smythe MA, Priziola J, Dobesh PP, et al. Guidance for the practical management of the heparin anticoagulants in the treatment of venous thromboembolism. J Thromb Thrombolysis. 2016;41:165–186. doi: 10.1007/s11239-015-1315-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lopes RD, Macedo AVS, Barros PGB, et al. Effect of discontinuing vs continuing angiotensin-converting enzyme inhibitors and angiotensin ii receptor blockers on days alive and out of the hospital in patients admitted with COVID-19: a randomized clinical trial. JAMA. 2021;325:254–264. doi: 10.1001/jama.2020.25864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Centers for Disease Control and Prevention. Interim clinical guidance for management of patients with confirmed coronavirus disease (COVID-19). Available at: https://www.cdc.gov/coronavirus/2019-ncov/hcp/clinical-guidance-management-patients.html . Updated: February 16, 2021. Accessed February17, 2021.
- 31.Mehran R, Rao SV, Bhatt DL, et al. Standardized bleeding definitions for cardiovascular clinical trials: a consensus report from the Bleeding Academic Research Consortium. Circulation. 2011;123:2736–2747. doi: 10.1161/CIRCULATIONAHA.110.009449. [DOI] [PubMed] [Google Scholar]
- 32.Wang D, Pocock S. A win ratio approach to comparing continuous non-normal outcomes in clinical trials. Pharm Stat. 2016;15:238–245. doi: 10.1002/pst.1743. [DOI] [PubMed] [Google Scholar]
- 33.Wichmann D, Sperhake JP, Lütgehetmann M, et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19: A Prospective Cohort Study. Ann Intern Med. 2020;173:268–277. doi: 10.7326/M20-2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llitjos JF, Leclerc M, Chochois C, et al. High incidence of venous thromboembolic events in anticoagulated severe COVID-19 patients. J Thromb Haemost. 2020;18:1743–1746. doi: 10.1111/jth.14869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med. 2020;383:120–128. doi: 10.1056/NEJMoa2015432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Iba T, Levy JH, Raj A, et al. Advance in the management of sepsis-induced coagulopathy and disseminated intravascular coagulation. J Clin Med. 2019;8:728. doi: 10.3390/jcm8050728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patell R, Bogue T, Koshy A, et al. Postdischarge thrombosis and hemorrhage in patients with COVID-19. Blood. 2020;136:1342–1346. doi: 10.1182/blood.2020007938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883–891. doi: 10.1056/NEJMoa1009638. [DOI] [PubMed] [Google Scholar]
- 39.Guimarães HP, Lopes RD, de Barros E, Silva PGM, et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med. 2020;383:2117–2126. doi: 10.1056/NEJMoa2029603. [DOI] [PubMed] [Google Scholar]
- 40.Mega JL, Braunwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 41.Eikelboom JW, Connolly SJ, Bosch J, et al. Rivaroxaban with or without Aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330. doi: 10.1056/NEJMoa1709118. [DOI] [PubMed] [Google Scholar]
- 42.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382:1994–2004. doi: 10.1056/NEJMoa2000052. [DOI] [PubMed] [Google Scholar]
- 43.Büller HR, Prins MH, Lensin AW, et al. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287–1297. doi: 10.1056/NEJMoa1113572. [DOI] [PubMed] [Google Scholar]
- 44.Spyropoulos AC, Lipardi C, Xu J, et al. Improved benefit risk profile of rivaroxaban in a subpopulation of the MAGELLAN study. Clin Appl Thromb Hemost. 2019;25 doi: 10.1177/1076029619886022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tritschler T, Mathieu ME, Skeith L, et al. Anticoagulant interventions in hospitalized patients with COVID-19: a scoping review of randomized controlled trials and call for international collaboration. J Thromb Haemost. 2020;18:2958–2967. doi: 10.1111/jth.15094. [DOI] [PMC free article] [PubMed] [Google Scholar]