Introduction
The World Health Organization declared the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) to be a pandemic on March 11, 2020.1 As of February 25, 2021, there have been approximately 112 million cases and 2.5 million confirmed deaths attributable to COVID-19 disease.2 The physical, biochemical, and imaging characteristics of SARS-CoV-2 infection are well described.3 , 4 Recent data implicate SARS-CoV-2 involvement in a wide range of central nervous system (CNS) manifestations of the disease.5 SARS-CoV-2 is postulated to enter the CNS via translocation from the cribriform plate to the orbitofrontal cortex6 or via hematologic spread.7 The potential mechanisms for the neurological and neuropsychiatric effects of the virus are many and include direct viral encephalitis, as well as less direct effect of the infection, including inflammation, hypoxia, hypercoagulability, postinfectious auto-immunity, or effects of immunomodulatory treatments.8
Psychiatric symptoms have also been reported, though data are limited. A recent review and meta-analysis by Krishnamoorthy, et al.9 found that of 50 studies examining mental health outcomes, only 4 were focused on outcomes among patients infected with SARS-CoV-2. All studies were cross-sectional, and the majority measured self-reported symptoms, typically using online surveys. Existing data describe depression, anxiety, “psychological distress,” post-traumatic symptoms, “poor sleep quality,” and insomnia among healthcare workers, the general population, and COVID-19 patients.9
Catatonia is a potentially fatal psychomotor condition characterized by neurovegetative and motor tone changes, as well as psychological echoing.10 Rich descriptions of catatonic symptoms in COVID-19 disease are largely absent from the literature. Here, we report catatonic features among 3 patients with SARS-CoV-2 infection presenting to a tertiary care hospital in a major metropolitan area. All patients were positive for SARS-CoV-2 on polymerase chain reaction testing (nucleic acid amplification test) conducted via nasopharyngeal swab. We review the extant literature describing catatonia in the setting of COVID-19 disease and highlight commonalities among the cases.
Literature Search Strategy
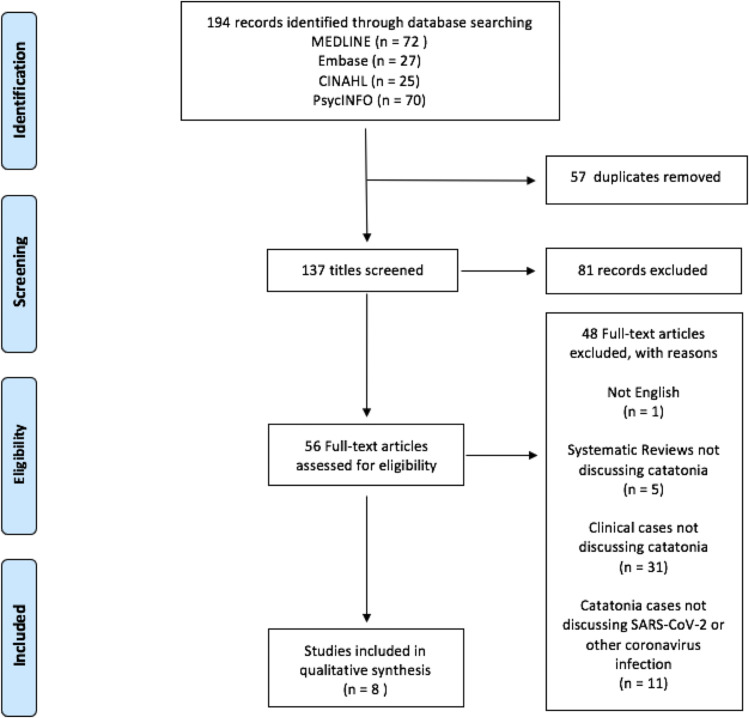
We sought to systematically review all reported cases of catatonia attributable to SARS-CoV-2 infection. We queried OVID Medline ® (1948 – Feb 9, 2021), PsycINFO, CINAHL, and Embase for applicable reports through December 31, 2020 using a combination of MeSH or keyword search terms targeted to recent coronavirus pandemics (including MERS, SARS, and COVID-19) and catatonia. For MEDLINE we used the following terms: (‘Middle East Respiratory Syndrome Coronavirus’/exp or ‘coronaviridae’/exp or ‘coronaviridae infections’/exp or ‘coronavirus’/exp or ‘pneumonia, viral’/exp or ‘coronavirus infections’/exp or coronavir∗.mp. or ‘infectious bronchitis virus’/exp or (COVID or COVID-19).mp.) AND (‘catatonia’/exp or catatoni∗.mp. OR ‘psychotic disorders’/exp or psychosis.mp.). A similar search strategy was implemented for each database (see Supplement 1).
Duplicate records were excluded automatically and manually using EndNote. Titles were reviewed for relevance and irrelevant titles or non-English articles were excluded. We then assessed full-text articles; articles were excluded if they were irrelevant, not a clinical report, a systematic review or meta-analysis not discussing catatonia, or case reports that discussed psychotic or manic illness in the context of COVID-19 but without a relevant finding of catatonic features (see Figure 1 ). Eight records are presented in the discussion as a review of current literature.
Figure 1.
Study Selection. CINAHL = Cumulative Index to Nursing and Allied Health Literature.
From Moher D, Liberati A, Tezlaff J, Altman DG, The PRISMA Group (2009). Preferred Reporting Items for Systematic Reviews and Meta Analyses: The PRISMA Statement. PLoS Med 6(6): e1000097. https://doi.org/10.1371/journal.pmed1000097.34
Case Presentations
Case 1
Ms. A is a female in her 50s with no known psychiatric history presenting to the emergency department (ED) with self-inflicted stab wounds. On presentation, she was tearful and withdrawn. Physical examination was significant for tachycardia to 100 bpm, hypertension to 150s/80s mmHg and multiple small lacerations to the right neck, left chest, abdomen, and both wrists. Initial labs showed a leukocytosis of 14 × 109 cells/L, mildly elevated lactate dehydrogenase of 292 U/L, C-reactive protein of 13.9 mg/L, and positive SARS-CoV-2 nasopharyngeal swab. Urine drug screen and serum toxicology screen were negative.
Psychiatry was consulted on hospital day (HD) #2 following emergent exploratory laparoscopy and laceration repair to evaluate whether the injuries were driven by suicidal ideation. Ms. A had poor participation on initial interview. Collateral from family revealed a medical history remarkable only for hypertension and osteoarthritis. She was in her normal state of health until presenting to an outside facility for cough, myalgias, and fatigue 11 days before admission, where she was diagnosed with SARS-CoV-2 infection and discharged home. The night prior to admission, other family members called Ms. A's daughter stating the patient wandered from the home saying she was “tired.” Ms. A was found several hours later with self-inflicted injuries and sent to the ED.
Physical examination by the consultation-liaison psychiatry team showed a Bush Francis Catatonia Rating Scale (BFCRS)11 severity score of 11 with prominent symptoms of stupor, mutism, staring, mundane posturing, negativism, and withdrawal. She was challenged with 2 mg of IV lorazepam with lysis of catatonia (BFCRS severity = 0) after approximately 45 minutes. Ms. A later reported a 10-day history of anxiety, low appetite, and depressed mood about her recent COVID-19 diagnosis. She could not recall her catatonic episode or whether she experienced suicidal ideation, but did believe that her catatonic symptoms preceded her self-inflicted injury. She was oriented without alterations in consciousness and denied auditory or visual hallucinations, paranoia, or delusions. Her thought process was well-organized, and she did not appear to be responding to internal stimuli.
Ms. A was treated with 1 mg lorazepam by mouth (PO) every 8 hours for catatonia and 6 mg melatonin PO nightly to restore the sleep/wake cycle. While inpatient, she denied symptoms of coronavirus infection including fever, myalgia, fatigue, respiratory or gastrointestinal symptoms, which remained stable over the course of her admission. Following several days of observation, lorazepam was changed and tapered to clonazepam 0.5 mg PO daily, and mirtazapine was initiated for depression and appetite stimulation and titrated to 30 mg nightly. She discharged to home on HD#8 with outpatient psychiatric follow-up where benzodiazepines were discontinued at 3 weeks, and she had no recurrence of symptoms at 5 weeks.
Case 2
Ms. B is a female in her 50s with a history of schizophrenia and chronic kidney disease stage 3 who was brought to the ED from a local jail twice for “refusal to eat.” On assessment by psychiatry, she reported her refusal to eat was volitional to secure hospital admission. She did not appear psychotic, suicidal, or homicidal on either visit and discharged back to jail. Ms. B presented a third time for “refusal to leave” jail property after release. She was electively mute and only said “let me lay down” in a whispered voice. Her behavior was felt to be volitional, and she was again discharged. The following day, bystanders reported she had been lying on the sidewalk since her discharge, raising suspicion for acute medical illness.
Ms. B was returned to the ED by paramedics and received intramuscular haloperidol, lorazepam, and diphenhydramine due to combativeness. Collateral obtained from Ms. B's previous personal care homeowner revealed a 50-pound weight loss over 2 months. At baseline, the patient was pleasant and able to care for herself. Labs showed an elevated creatinine of 1.5 mg/dL, glucose 59 mg/dL, and elevated white blood cell count of 19.8 × 109 cells/L. She was admitted to the medical floor for workup. Serum inflammatory markers were elevated and peaked at lactate dehydrogenase 385 U/L, C-reactive protein 45.9 mg/L, and D-dimer 7433 ng/mL. A computed tomography scan of the head without contrast showed an age-indeterminant left basal ganglia infarct, pneumomediastinum, and pneumopericardium. A computed tomography with aortic dissection protocol was concerning for an esophageal rupture. Stool Clostridium (C.) difficile antigen and polymerase chain reaction showed active infection. SARS-CoV-2 swab was positive. Urinalysis indicated an active urinary tract infection. Pertinent negative labs included HIV, rapid plasma reagin, thyroid stimulating hormone, urine drug screen, and ethanol level. Ms. B denied fatigue, cough, headaches, and respiratory symptoms.
On HD#6, she was started on 250 mg IV valproic acid every 12 hours for irritability and titrated to 500 mg twice daily. Ms. B was deemed not to be a surgical candidate for esophageal rupture repair and was treated with IV antibiotics including vancomycin, cefepime, metronidazole, ketoconazole, and anidulafungin for 4 days for esophageal rupture followed by oral vancomycin and amoxicillin-clavulanate for 14 days for C. difficile infection. She was disengaged from care and appeared to be sleeping most of the day, however remained oriented without alterations in consciousness. Electrolyte abnormalities and hypoglycemia were corrected as needed due to poor oral intake.
On HD#14, the primary medical team suspected Ms. B's withdrawal was a catatonic symptom. The primary team calculated a BFCRS severity of 12, with symptoms including withdrawal, mutism, staring, and negativism, and administered a lorazepam challenge of 2 mg intramuscular with good response (BFCRS severity = 2). Following the challenge, Ms. B reported auditory hallucinations, depression, insomnia, anorexia, anergia, amotivation, tearfulness, hopelessness, guilt, and suicidal ideation with plan to cut or shoot herself but without intent. She reported nonadherence to antipsychotic medications for auditory hallucinations. She reported that earlier attempts to pull out her IV catheter were suicide attempts. She had no prior history of suicide attempts or catatonic features.
Ms. B remained inpatient for an additional 25 days. Lorazepam was tapered and switched to clonazepam, which was discontinued after 14 days. As her catatonia resolved, she was switched to oral extended-release valproic acid and initiated on olanzapine, titrated to 10 mg nightly. At discharge, Ms. B had resolution of all psychotic, depressive, and catatonic symptoms. Since discharge, she has had multiple presentations to the ED for unrelated medical complaints.
Case 3
Ms. C is a female in her 20s with a history of bipolar disorder (type 1) who presented to the ED after exhibiting bizarre behavior. She was discharged from a 4-day inpatient psychiatric hospitalization 1 day before the current presentation. During that admission, she was stabilized for an acute manic episode without psychotic symptoms with extended-release oral lithium 900 mg nightly and returned near her baseline mental status. She tested negative for SARS-CoV-2 at that time and had no prior history of catatonic features. According to the patient's mother, following discharge, she showed paranoia towards family and refused to accept food or water for fear that it had been tampered with. Ms. C went to another family member's house at her own request where she ate, but did not sleep, and called her mother early the next morning requesting to return to the hospital. On the car ride to the hospital, she was noted to be staring and mute.
On re-evaluation in the psychiatric ED, Ms. C had limited participation with the interview, staring, thought blocking, and perseverative speech. She was withdrawn, lying in the same position for an extended period while staring at the ceiling. Physical examination showed tachycardia to 108 bpm. Initial labs were largely within normal limits, including normal leukocyte count, urine drug screen and toxicology screen, and lithium level of 0.6 mEq/L. Before acceptance to a psychiatric unit, Ms. C's repeat SARS-CoV-2 swab resulted as positive, and she was admitted to medicine for isolation.
Physical examination showed a BFCRS severity score of 14 with prominent symptoms of stupor, mutism, mundane posturing, rigidity, negativism, echopraxia, and withdrawal. Ms. C received 2 mg intramuscular lorazepam with lysis of catatonia (BFCRS severity = 3) after approximately 30 minutes. She became acutely agitated, with perseverative speech, and impoverished thought content, however remained oriented and without symptoms of delirium. Ms. C continued to exhibit verbigeration, mutism, and had poor memory of recent events. She said that she felt “foggy” but was unable to provide further details. She denied fever, chills, malaise, myalgias, sore throat, cough, nausea, vomiting, or diarrhea.
She was started on 1 mg oral lorazepam 3 times daily. Extended-release lithium was titrated to 1500 mg PO nightly to target a serum concentration of 1.0 mEq/L. Following transfer to the psychiatric inpatient unit, she displayed paranoia, pseudocyesis, and appeared to be responding to internal stimuli, and aripiprazole 10 mg daily was initiated. Ms. C had resolution of catatonic symptoms (BFCRS = 0) on HD#20 and was discharged on HD#26 without psychotic symptoms. She was subsequently lost to follow-up.
Discussion
Literature Review
We identified 8 records reporting catatonia in the context of the SARS-CoV-2 pandemic (see Table 1 ). Seven were detailed case reports, 6 of which described patients with SARS-CoV-2 infection and one which described catatonic symptoms attributed to the psychological stress of the pandemic. Caan et al.12 first reported the case of a 43-year-old male without past psychiatric or medical history who presented to the ED several times following his COVID-19 diagnosis with complaints of anxiety and insomnia that eventually progressed to akinetic catatonia. This was followed by a case report of an 80-year-old male presenting with moderately severe COVID-19 pneumonia who later developed akinetic catatonia.13 Importantly, the authors demonstrated a rise in pro-inflammatory markers typically seen in COVID-19 disease (lymphocyte count, C-reactive protein, D-dimer, ferritin, lactate dehydrogenase, and procalcitonin) concurrent with the onset of catatonia, that suggested an immune-mediated or inflammatory mechanism.
Table 1.
Cases of Catatonia Associated With the SARS-CoV-2 Pandemic
| Reference | Age, gender, location | Psychiatric symptoms | Nonpsychiatric COVID-19 symptoms | Psychiatric history | Medical history | Serum inflammatory markers | Initial treatment | Clinical course/outcome | Potential sources of bias |
|---|---|---|---|---|---|---|---|---|---|
| Present case (Ms. A) | Early 50s, Female, USA | Stupor, mutism, staring, mundane posturing, negativism, withdrawal, self-harm by laceration BFCRS score 11 |
Myalgia and fatigue 11 d before presentation, resolved by time of presentation | None | HTN, osteoarthritis SARS-CoV-2 infection |
WBC count 14 × 109 cells/L, LDH 292 U/L, CRP 13.9 mg/L | Lorazepam 2 mg IV once with lysis of catatonia (BFCRS = 0) after 45 min | Lorazepam 1 mg PO q8 h for 7 d and then changed to clonazepam 0.5 mg PO daily and discontinued at 3-wk follow-up appointment; mirtazapine 30 mg PO nightly; melatonin 6 mg PO nightly No recurrence of symptoms at 5 wk. |
Ascertainment – No CSF studies |
| Present case (Ms. B) | Early 50s, Female, USA | Withdrawal, mutism, staring, negativism BFCRS score 12 |
Diarrhea | Schizophrenia, disorganized type, without catatonic features | Cerebrovascular infarct, age indeterminant, esophageal rupture SARS-CoV-2 infection |
WBC count 19.8 × 109 cells/L, LDH 385 U/L, CRP 45.9 mg/L, D-dimer 7433 ng/mL | Valproic acid 500 mg IV BID Lorazepam 2 mg IM once with lysis of catatonia (BFCRS = 2) after 1 h |
Lorazepam 2 mg PO q8 h, tapered and discontinued on day 14; olanzapine 10 mg PO nightly; Extended-release valproic acid 250 mg PO BID Multiple ED presentations for medical complaints over several months, no apparent catatonic symptoms |
Ascertainment – No CSF studies Causality – Underlying SMI. – Other medical illness – Use of medications which may provoke psychosis |
| Present case (Ms. C) | Early 20s, Female, USA | Stupor, mutism, mundane posturing, rigidity, negativism, echopraxia, withdrawal, paranoia, pseudocyesis BFCRS score 14 |
None | Bipolar disorder, type 1, MRE manic | SARS-CoV-2 infection | None | Lorazepam 2 mg IM once with lysis of catatonia (BFCRS = 3) and residual agitation, perseverative speech | Lorazepam 3 mg PO TID, reduced to 1.5 mg PO TID by discharge on HD#10; Lithium 1500 mg PO nightly; Aripiprazole 10 mg PO daily Catatonic symptoms resolved by discharge, lost to follow-up. |
Ascertainment – No CSF studies Causality – Underlying SMI. |
| Caan et al., 202012 | 43, Male, USA | Anxiety, withdrawal, response to internal stimuli, rigidity, mutism, posturing, auditory hallucinations, paranoia. BFCRS not reported on initial examination. Authors note likely severity score of 12. |
Fever, tachycardia, cough 15 d prior to catatonic episode Diaphoresis on presentation for catatonia |
None | SARS-CoV-2 infection | Platelet count 551 TH/μL | Lorazepam 1 mg IV TID | Lorazepam tapered to 1 mg PO BID by discharge on HD#10; No recurrence of catatonic symptoms as of post-discharge day #6. Residual anhedonia, sadness, insomnia treated with lorazepam 1 mg PO nightly and 6 mg melatonin nightly. | Ascertainment – No CSF studies |
| Gouse et al., 202013 | Elderly, Male, USA | Mutism, staring, posturing grimacing, echolalia, verbigeration, stereotypy, rigidity, waxy flexibility, automatic obedience BFCRS score 18 |
Fatigue, headache, hypoxia, fever, progressing to hypoxic respiratory failure and death on HD#7 | Schizophrenia | COPD, interstitial lung disease, DM2, HTN, atrial fibrillation, essential tremor SARS-CoV-2 infection |
Ferritin 1400 ng/mL, CRP 85.20 mg/L, D-dimer 1200 ng/mL, LDH 600 U/L, Pro-calcitonin > 0.3 ng/mL | Lorazepam 1 mg IV with improvement of catatonia (BFCRS = 9) | Lorazepam 1 mg IV TID, reduced to 0.5 mg IV TID due to worsening respiratory failure Patient expired on HD#7. |
Ascertainment – No CSF studies Causality – Underlying SMI. – Other medical illness |
| Zandifar and Badrfam, 202014 | 61, Male, Iran | Auditory hallucinations, Capgras delusion, paranoia progressing to mutism, stupor, posturing, negativism, rigidity BFCRS score not reported |
Lethargy, nausea, seizure. Hyponatremia (Na 120 mg/L) |
Schizophrenia | SARS-CoV-2 infection | WBC count 15.7 × 109 cells/L | Prior to catatonic symptoms: haloperidol 10 PO daily, biperiden 3 mg PO daily; medications held at time of catatonia diagnosis Lorazepam 2 mg PO TID |
Authors do not report whether lorazepam was continued on discharge; restarted on haloperidol 10 mg PO daily, biperiden 3 mg PO daily; resolution of catatonia after 24 h. Authors note a marked reduction in psychiatric symptoms at discharge. |
Ascertainment – No CSF studies – BFCRS not used Causality – Underlying SMI – Other medical illness |
| Amouri et al., 202015 | 70, Female, USA | HD#3 – confusion HD#4- somnolence, disorientation, confusion which continued through HD#12. HD#12 – immobility, mutism, grimacing, catalepsy, echolalia, stereotypy, verbigeration, rigidity, negativism, waxy flexibility, automatic obedience, gegenhalten BFCRS score 21 |
Five day history of cough, fatigue, fever. Hospital course complicated by acute hypoxemic respiratory failure, NSTEMI, physical deconditioning, fever, delirium. |
None | End-stage renal disease, DM2, HTN, Coronary artery disease, NSTEMI, heart failure with preserved ejection fraction, hypothyroidism, TIA SARS-CoV-2 infection |
Inpatient laboratory results not reported in detail. Blood cultures noted to be negative. | Lorazepam 0.5 mg IM with improvement in catatonia (BFCRS = 12). | Lorazepam 0.5 mg PO or IV q8 h, tapered to 0.5 mg PO or IV BID, discontinued by HD #16; “Broad spectrum antibiotics” from HD#5-HD#8; Catatonic symptoms improved Patient discharged to rehabilitation facility. |
Ascertainment – No CSF studies Causality – Underlying SMI. – Other medical illness |
| Deocleciano de Araujo et al., 202016 | 50, Male, Brazil | Disorganization, rigidity, negativism, withdrawal. BFCRS score not reported. | Fever, Tachypnea, tachycardia, hypoxia. Course complicated by aspiration pneumonia. | Intellectual disability, mild | Childhood epilepsy SARS-CoV-2 infection | CK 8819 U/L, WBC count 20.8 × 109 cells/L, Platelet count 544,000 mm3/L | Pneumonia treatments: Azithromycin, amoxicillin/clavulanate, piperacillin/tazobactam, meropenem, vancomycin, dexamethasone 6 mg daily Diazepam 10 mg IV QID with poor response; switched to lorazepam 2 mg PO TID on HD#4 |
Sertraline 25 mg PO daily and olanzapine 5 mg PO daily added on HD #18. Transferred to psychiatry unit for ECT on HD#19. Received bilateral stimulus at 30% with immediate partial response (improved mutism). Remission after 10 sessions. Discharged after 50 d with unspecified dose of sertraline and olanzapine. |
Ascertainment – BFCRS not used Causality – Other medical illness – Use of medications which may provoke psychosis |
| Sarli et al., 202017 | 59, Male, Italy | Anxiety, depression, hopelessness, anhedonia, apathy, anorexia, insomnia, delusion of pauperization, mutism, stupor, waxy flexibility. BFCRS score not reported. |
Patient was not diagnosed with SARS-CoV-2 infection | None | None | Inpatient labs not reported. | Lorazepam 4 mg “vial” (route not specified, presumably IM or IV), olanzapine 5 mg. Response not reported. | Lorazepam tapered to 2.5 mg PO nightly, olanzapine increased to 7.5 mg PO nightly, and sertraline 100 mg PO daily. The patient was noted to have “slow improvement.” | Ascertainment – BFCRS not used Causality – Patient was not diagnosed with SARS-CoV-2 infection. |
| Huarcaya-Victoria et al., 202018 | 23, Female, Peru | Anxiety, insomnia, religious delusions, delusions of reference, auditory hallucinations, agitation, impaired attention, stereotypy, catalepsy, verbigeration. BFCRS score not reported. | Fever | None | SARS-CoV-2 infection | Platelet count 329,000 mm3/L | Midazolam IV at an outside facility with “little effect,” ziprasidone 40 mg (route and frequency not reported) | Olanzapine 15 mg PO daily Patient had “remission of psychotic symptoms.” |
Ascertainment – No CSF studies – BFCRS not used Causality – Typical catatonia challenge protocol not used Reporting – Clinical course not described in detail |
| Varatharaj et al., 202019 | “One patient with catatonia”, UK | Case details not available. | Not reported | Not reported | SARS-CoV-2 infection | Not reported | Not reported | Not reported | Reporting – No case details available; high risk of bias |
BFCRS = Bush-Francis catatonia rating scale; BID = twice daily; COPD = chronic obstructive pulmonary disease; CRP = C-reactive protein; CSF = cerebrospinal fluid; DM2 = diabetes mellitus, type 2; ECT = electroconvulsive therapy; HD = hospital day; HTN = hypertension; IM = intramuscular; IV = intravenous; LDH = lactate dehydrogenase; MRE = most recent episode; Na = sodium; NSTEMI = non-ST elevation myocardial infarction; PO = per mouth; QID = 4 times daily; SMI = serious mental illness; TIA = transient ischemic attack; TID = 3 times daily; WBC = white blood cell.
Catatonia is also reported in patients with other medically complex COVID-19 disease. Zandifar and Badrfram14 discuss about a 61-year-old male with a history of schizophrenia who was admitted to psychiatry and became symptomatic with COVID-19 disease and hyponatremia on HD#2, that progressed to seizures and akinetic catatonia. Amouri et al.15 reported a 70-year-old female with critical hypoxic respiratory failure due to COVID-19 pneumonia and delirium who subsequently developed akinetic catatonia, noting the increased risk of catatonia due to delirium as well as SARS-CoV-2. A striking case by Deocleciano de Araujo et al.16 similarly details a 50-year-old male with mild intellectual disability who was admitted with akinetic catatonia in the setting of a friend's recent suicide and was initially found to have asymptomatic SARS-CoV-2 infection with elevated pro-inflammatory markers. This case was complicated by aspiration pneumonia and acute hypoxic respiratory failure which required ICU admission and electroconvulsive therapy.
Authors have also reported akinetic catatonia in a noninfected Italian patient with no prior psychiatric history, presumably secondary to the psychological stress of lockdown,17 and a 23-year-old previously healthy Peruvian female who had otherwise asymptomatic SARS-CoV-2 infection that presented with psychosis and akinetic catatonic features in the setting of elevated serum inflammatory markers.18
The eighth report comes from compiled data of 157 patients submitted to United Kingdom researchers via an online data-reporting scheme for providers early in the pandemic.19 While these authors note that a concurrent case of catatonia and SARS-CoV-2 infection was reported, details are not available.
Contextualization
It is surprising there are few reported cases of catatonia in SARS-CoV-2 infection. Up to 20% of catatonia is caused by medical illness,20 its nonpsychiatric correlates are well described,21 and catatonia has been associated with other viral pandemics in the 19th and 20th centuries. Encephalitis lethargica, characterized by profound lethargy and abnormal movements, was used to describe catatonic symptoms that followed infection with influenza in the pandemic of 1918.22 Given the lack of robust observational data on COVID-19 associated catatonia, there is significant debate about its pathogenesis. Existing literature and our cases suggest both inflammation and anxiety may play a role in the development of catatonia among COVID-19 patients.
While we did not exhaustively review cases of psychosis without catatonic features, selected reports provide helpful examples of predominant anxious features alongside the catatonia cases. Anxiety was a predominant feature in 6 publications: 2 cases of new-onset psychosis cases reported by Ferrando et al.,23 2 cases of new-onset psychosis reported by Martin,24 a decompensated patient with paranoid-type schizophrenia,25 the case of malignant catatonia in the patient with critical SARS-CoV-2 pneumonia,16 and 1 asymptomatic SARS-CoV-2 positive patient requiring inpatient treatment for extreme anxiety.26 Of note, in the aforementioned case of catatonia in an Italian patient without SARS-CoV-2 by Sarli et al.17 the patient initially presented with anxiety about financial decline. His symptoms progressed to depression with delusions of pauperization and catatonic symptoms. Several of the patients in the above cases had premorbid serious mental illness, were otherwise asymptomatic from their SARS-CoV-2 infection, and in some cases, catatonia quickly resolved with anxiolytics. These data suggest catatonia may develop secondary to COVID-19 primarily due to psychological factors.27
However, the aforementioned reports also suggest an inflammatory basis for COVID-19-associated catatonia. We highlight the work of Gouse et al.13 in the current pandemic, who first demonstrated a correlation between the development of catatonic symptoms in COVID-19 patients and the concordant rise in serum pro-inflammatory markers. In general, there is also a co-occurrence of catatonic symptoms with nonviral inflammatory or autoimmune conditions, and with biochemical inflammatory markers in the neuroleptic malignant syndrome.20 The co-occurrence of hyperactive delirium and catatonia,15 or neuroleptic malignant syndrome and SARS-CoV-2 infection28 provide additional support of a systemic inflammatory response. While delirium appears to be an important sequela of infection with SARS-CoV-2, it is unclear whether delirium seen in COVID-19 targets subcortical structures or if it indicates a severe systemic illness in a vulnerable patient.29
Our cases provide additional evidence that catatonia in COVID-19 disease is multifactorial. The case of Ms. A demonstrates the role that anxiety, isolation, and SARS-CoV-2 had in the development of catatonia culminating in self-harm in a patient without a psychiatric history and mildly elevated serum inflammatory markers. Ms. B and Ms. C's courses highlight the possibility that SARS-CoV-2, or other infection, may produce worsened symptoms than previously observed patients with chronic mental illness. Ms. B's case is confounded by co-morbid C. difficile infection and brief treatment with cefepime. Second-generation cephalosporins have been implicated in catatonia, particularly in patients with reduced renal clearance, though fifth-generation cephalosporins have not.30 That Ms. B exhibited catatonic symptoms prior to receiving any cephalosporins argues against cephalosporin neurotoxicity which may mimic catatonia. We also note that Ms. A and Ms. B both had elevated serum inflammatory markers, which is consistent with findings by Gouse et al.13 and suggests systemic inflammation may be a driving factor. Finally, of the 10 detailed cases currently available (including our own), all share the feature of akinetic mutism. Whether this has clinical or pathophysiological relevance is currently unknown.
Limitations
A description of potential sources of bias in the reported cases is provided in Table 1. In general, the cases are limited by the co-occurrence of confounding patient factors such as pre-morbid serious mental illness, or co-morbid serious medical illness, which may present with catatonic features. This raises the possibility that SARS-CoV-2 infection was merely coincidental. Additionally, cerebrospinal fluid (CSF) studies demonstrating the presence of SARS-CoV-2 during catatonic symptoms have not been reported in any patient. This could be helpful in confirming the presence of SARS-CoV-2 and assessing the role of inflammatory markers in the CNS. However, 3 of 10 cases with full descriptions occurred in patients without any psychiatric history and mild COVID-19 symptoms, and 7 of 10 had some degree of elevated inflammatory markers, suggesting that SARS-CoV-2 has a contributing role. This question clearly requires larger observational studies.
Finally, publication bias is likely, such that only remarkable cases have been reported. We note that several cases did not use a standardized symptom scale, such as the BFCRS. Standardized reporting among authors may help identify additional subtle cases and add richness to the phenomenology of catatonia in COVID-19 disease.
Conclusion
Our cases and the literature highlight 2 issues. The first is that SARS-CoV-2 may have a role in causing catatonia via inflammation or CNS action. This is particularly salient for inpatient providers caring for COVID-19 patients who have entered the inflammatory phase of illness. Given the morbidity and mortality associated with catatonia, inpatient and consulting psychiatrists should keep a high index of suspicion for catatonia when consulted for abnormal behavior or altered mental status in COVID-19 patients.
The second key point is the importance of screening for and prompt treatment of anxiety in patients with less severe COVID-19 disease. Ms. A's course demonstrates that prompt recognition and treatment of acute depression and anxiety may have prevented self-harm. However, limited access to outpatient psychiatric services during the COVID-19 pandemic remains an ongoing issue.31 We emphasize the importance of the psychological stress of the SARS-CoV-2 pandemic. Pandemics in general,32 and COVID-1933 have caused increased morbidity among patients with serious mental illness, the public, and healthcare workers. Much of the existing literature demonstrates post-traumatic stress symptoms, anxiety, and depression.32
We suggest future research should include more robust observational data of catatonia among patients with SARS-CoV-2 infection and explore the roles of CNS inflammatory markers in COVID-19 disease, and treatment options specific to the systemic inflammatory response.
Footnotes
Conflicts of Interest: The authors declare that they have no conflict of interest.
Informed Consent: Ms. A gave verbal informed consent to publish her case. Ms. B could not be reached to discuss informed consent due to lack of contact information on her chart. Ms. C was unable to participate in an informed consent discussion and verbal consent was given by her surrogate (mother).
Funding: This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jaclp.2021.04.003.
Supplementary Data
References
- 1.Adhanom T. World Health Organization. Who Director-General’s Opening Remarks at the Media Briefing on COVID-19. 2020. https://www.who.int/director-general/speeches/detail/who-director-general-s-opening-remarks-at-the-media-briefing-on-covid-19---11-march-2020 Available from:
- 2.WHO Coronavirus Disease (COVID-19) Dashboard. World Health Organization. 2020. https://covid19.who.int/ Available from:
- 3.Salehi S., Abedi A., Balakrishnan S., Gholamrezanzhad A. Coronavirus disease 2019 (COVID-19): a systematic review of imaging findings in 919 patients. Am J Roentgenol. 2020;215:87–93. doi: 10.2214/AJR.20.23034. [DOI] [PubMed] [Google Scholar]
- 4.D’Errico S., Zanon M., Montanaro M., et al. More than pneumonia: distinctive features of SARS-CoV-2 infection. From autopsy findings to clinical implications: a systematic review. Microorganisms. 2020;8:1642. doi: 10.3390/microorganisms8111642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Divani A.A., Andalib S., Biller J., et al. Central nervous system manifestations associated with COVID-19. Curr Neurol Neurosci Rep. 2020;20:60. doi: 10.1007/s11910-020-01079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Guennec L., Devianne J., Jalin L., et al. Orbitofrontal involvement in a neuroCOVID-19 patient. Epilepsia. 2020;61:e90–e94. doi: 10.1111/epi.16612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host-virus interaction, and proposed neurotropic mechanisms. ACS Chem Neurosci. 2020;11:995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 8.Troyer E.A., Kohn J.N., Hong S. Are we facing a crashing wave of neuropsychiatric sequelae of COVID-19? Neuropsychiatric symptoms and potential immunologic mechanisms. Brain Behav Immun. 2020;87:34–39. doi: 10.1016/j.bbi.2020.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krishnamoorthy Y.J., Nagarajan R., Saya G.K., Menon V. Prevalence of psychological morbidities among general population, healthcare workers, and COVID-19 patients amidst the COVID-19 pandemic: a systematic review and meta-analysis. Psychiatry Res. 2020;293:113382. doi: 10.1016/j.psychres.2020.113382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Francis A. Catatonia: diagnosis, classification, and treatment. Curr Psychiatry Rep. 2010;12:180–185. doi: 10.1007/s11920-010-0113-y. [DOI] [PubMed] [Google Scholar]
- 11.Bush G., Fink M., Petrides G., Dowling F., Francis A. Catatonia. I. Rating scale and standardized examination. Acta Psychiatr Scand. 1996;93:129–136. doi: 10.1111/j.1600-0447.1996.tb09814.x. [DOI] [PubMed] [Google Scholar]
- 12.Caan P.M., Lim C.T., Howard M. A case of catatonia in a man with COVID-19. Psychosomatics. 2020;61:556–560. doi: 10.1016/j.psym.2020.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gouse B.M., Spears W.E., Archibald A.N., Montalvo C. Catatonia in a hospitalized patient with COVID-19 and proposed immune-mediated mechanism. Brain Behav Immun. 2020;89:529–530. doi: 10.1016/j.bbi.2020.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zandifar A., Badrfam R. Exacerbation of psychosis accompanied by seizure and catatonia in a patient with COVID-19: a case report. Psychiatry Clin Neurosci. 2021;75:63–64. doi: 10.1111/pcn.13174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Amouri J., Andrews P.S., Heckers S., Ely E.W., Wilson J.E. A case of concurrent delirium and catatonia in a woman with coronavirus disease 2019. Psychosomatics. 2020;62:109–114. doi: 10.1016/j.psym.2020.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deocleciano de Araujo C., Schlittler L.X.C., Sguario R.M., Tsukumo D.M., Dalgalarrondo P., Banzato C.E.M. Life-threatening catatonia associated with coronavirus disease 2019. Psychosomatics. 2020 doi: 10.1016/j.psym.2020.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sarli G., Polidori L., Lester D., Pompili M. COVID-19 related lockdown: a trigger from the pre-melancholic phase to catatonia and depression: a case report of a 59-year-old man. BMC Psychiatry. 2020;20:558. doi: 10.1186/s12888-020-02978-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huarcaya-Victoria J., Meneses-Saco A., Luna-Cuadros M.A. Psychotic symptoms in COVID-19 infection: a case series from Lima, Peru. Psychiatry Res. 2020;293:113378. doi: 10.1016/j.psychres.2020.113378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varatharaj A., Thomas N., Ellul M.A., et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: a UK-wide surveillance study. Lancet Psychiatry. 2020;7:875–882. doi: 10.1016/S2215-0366(20)30287-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rogers J.P., Pollak T.A., Blackman G., David A.S. Catatonia and the immune system: a review. Lancet Psychiatry. 2019;6:620–630. doi: 10.1016/S2215-0366(19)30190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rustad J.K., Landsman S., Ivkovic A., Finn C.T., Stern T.A. Catatonia: an approach to diagnosis and treatment. Prim Care Companion CNS Disord. 2018;20:17. doi: 10.4088/PCC.17f02202. [DOI] [PubMed] [Google Scholar]
- 22.Cooper J.J., Ross D.A. COVID-19 catatonia – would we even know? Biol Psychiatry. 2020;88:e19–e21. doi: 10.1016/j.biopsych.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ferrando S.J., Klepacz L., Lynch S., et al. COVID-19 psychosis: a potential new neuropsychiatric condition triggered by novel coronavirus infection and the inflammatory response? Psychosomatics. 2020;61:551–555. doi: 10.1016/j.psym.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Martin E.B. Brief psychotic disorder triggered by fear of coronavirus? Psychiatr Times. 2020;37 [Google Scholar]
- 25.Fischer M., Coogan A.N., Faltraco F., Throne J. COVID-19 paranoia in a patient suffering from schizophrenic psychosis – a case report. Psychiatry Res. 2020;288:113001. doi: 10.1016/j.psychres.2020.113001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mader E., Dori G. Patients with the novel SARS-Cov-2 disease require a novel standard of care—med-psych. Psychosomatics. 2020;61:578–579. doi: 10.1016/j.psym.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ng Q.X., Yeo W.S., Lim D.Y., Chee K.T. Re-examining the association between COVID-19 and psychosis. Psychosomatics. 2020;61:853–855. doi: 10.1016/j.psym.2020.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kajani R., Apramian A., Vega A., Ubhayakar N., Xu P., Liu A. Neuroleptic malignant syndrome in a COVID-19 patient. Brain Behav Immun. 2020;88:28–29. doi: 10.1016/j.bbi.2020.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beach S.R., Praschan N.C., Hogan C., et al. Delirium in COVID-19: a case series and exploration of potential mechanisms for central nervous system involvement. Gen Hosp Psychiatry. 2020;65:47–53. doi: 10.1016/j.genhosppsych.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deshays S., Coquerel A., Verdon R. Neurological adverse effects attributable to β-lactam antibiotics: a literature review. Drug Saf. 2017;40:1171–1198. doi: 10.1007/s40264-017-0578-2. [DOI] [PubMed] [Google Scholar]
- 31.Shiozawa P., Uchida R.R. An updated systematic review on the coronavirus pandemic: lessons for psychiatry. Braz J Psychiatry. 2020;32:330–331. doi: 10.1590/1516-4446-2020-0975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neelam K., Duddu V., Anyim N., Neelam J., Lewis S. Pandemics and pre-existing mental illness: a systematic review and metanalysis. Brain Behav Immun Health. 2021;10:100177. doi: 10.1016/j.bbih.2020.100177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talevi D., Socci V., Carai M., et al. Mental health outcomes of the COVID-19 pandemic. Riv Psichiar. 2020;55:137–144. doi: 10.1708/3382.33569. [DOI] [PubMed] [Google Scholar]
- 34.Moher D., Liberati A., Tetzlaff J., Altman D.G., The PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.