Abstract
Mitochondria are involved in a large number of essential roles related to neuronal function. Ca2+ handling by mitochondria is critical for many of these functions, including energy production and cellular fate. Conversely, mitochondrial Ca2+ mishandling has been related to a variety of neurodegenerative diseases. Investigating mitochondrial Ca2+ dynamics is essential for advancing our understanding of the role of intracellular mitochondrial Ca2+ signals in physiology and pathology. Improved Ca2+ indicators, and the ability to target them to different cells and compartments, have emerged as useful tools for analysis of Ca2+ signals in living organisms. Combined with state-of-the-art techniques such as multiphoton microscopy, they allow for the study of mitochondrial Ca2+ dynamics in vivo in mouse models of the disease. Here, we provide an overview of the Ca2+ transporters/ion channels in mitochondrial membranes, and the involvement of mitochondrial Ca2+ in neurodegenerative diseases followed by a summary of the main tools available to evaluate mitochondrial Ca2+ dynamics in vivo using the aforementioned technique.
Keywords: Mitochondria, calcium, multiphoton microscopy, Alzheimer’s disease, fluorescent proteins, GECIs
1. Introduction
Mitochondria are crucial and highly dynamic organelles known for providing energy to the cell in the form of adenosine triphosphate (ATP). They also regulate a multitude of cellular functions, including Ca2+ buffering, reactive oxygen species (ROS) production, cellular metabolism and apoptotic cell death [1]. ATP production is vital for maintenance of the proper neuronal function, because these cells have limited glycolytic capacity and do not have a backup system to provide energy as is possible in other cells [2]. Besides being energy suppliers of the cell, mitochondria play a central role in regulating intracellular Ca2+ signaling. By handling Ca2+, mitochondria control important physiological processes such as the synthesis of hormones, neurotransmitter metabolism or cardiac activity. However, excessive Ca2+ levels within the mitochondrial matrix, i.e., mitochondrial Ca2+ overload, could lead to an increase in the generation of ROS and the resulting apoptotic cell death that takes place during ischemic episodes and in neurodegenerative diseases such as Alzheimer’s disease (AD) and Parkinson’s disease (PD) [3].
Traditionally, most studies about the role of mitochondria in neurodegenerative diseases have been performed on cell lines, primary cells extracted from animal models, or in ex vivo samples from postmortem human tissue. Recently, the development of more compelling methods, like multiphoton microscopy and genetically targeted indicators, to evaluate mitochondrial dynamics, have ameliorated the study of the organelle’s dysfunction in the living brain. Understanding mitochondrial Ca2+ dynamics is critical to further understand the role of mitochondria in normal physiology but also in a pathological state. Here, we first briefly review the mitochondrial structure and the Ca2+ channels that participate in mitochondrial Ca2+ homeostasis. We then address the involvement of mitochondrial Ca2+ dysregulation in neurodegenerative diseases. Finally, we summarize recent progress in mitochondrial Ca2+ imaging using in vivo multiphoton microscopy and optical Ca2+ indicators targeted to mitochondria, followed by an example of using Yellow Cameleon (YC) to evaluate mitochondrial Ca2+ in vivo using this technique and a transgenic mouse model of AD.
2. Brief review of mitochondrial structure
Mitochondria are dynamic entities that extend from the soma to neurites, forming a network along the neuron. They undergo replication, fission and fusion. Depending on the metabolic demand, they migrate along axons and dendrites in anterograde or retrograde directions [4], become stationary in regions where the metabolic demand is higher, and move again depending on physiological changes within the cell. Because of this, they must adapt to any physiological or environmental change that occurs in the neurons [5]. Mitochondria are quasi-independent from the rest of the cell: they have their own DNA, different from the nuclear DNA, and thus synthesize some of their own proteins, which play vital roles in regulating cellular bioenergetics. The rest of their proteins are encoded by nuclear DNA and targeted to mitochondria via different transport mechanisms. Inherited mitochondrial DNA mutations are responsible for the etiology of several human diseases [6, 7]. The different mitochondrial functions are possible due to their compartmentalized organization. Two differentiated membranes are part of the mitochondria, the outer mitochondrial membrane (OMM) -which forms the outer-most border- and the inner mitochondrial membrane (IMM) -which encapsulates the mitochondrial matrix space. IMM has a much larger extension than the OMM due to its cristae (foldings in its internal space), specialized in the oxidative phosphorylation (OXPHOS) [8]. The space between OMM and IMM - the intermembrane space - contains enzymes, such as creatine kinase, and cytochromes, such as cytochrome c.
The OMM is permeable to ions and small molecules, due to the abundant expression of large channels on its surface, known as porins or voltage dependent anion channels (VDAC). VDAC was the first mitochondrial channel described [9]. Substrates needed to produce ATP such as pyruvate or ADP, and other metabolites such as Ca2+, Na+, and K+ go through this channel from the cytosol and across the OMM. The ATP produced in mitochondria can then reversely traverse the VDAC and reach the cytosol. The IMM on the other hand, is a non-permeable membrane, and only those solutes with specific transporters can cross it. It contains ion channels and transporters, like the mitochondrial Ca2+ uniporter (MCU) complex or the Na+/Ca2+ exchanger (NCLX), and mitochondrial enzyme systems like the electron transport chain (ETC). ETC is the molecular machinery used for energy production. It is comprised of complexes I-IV located in the IMM. The energy released by these complexes is used to pump H+ from the mitochondrial matrix into the intermembrane space, generating an electrochemical gradient (ΔμH) across the IMM. The return of H+ into the mitochondrial matrix following the gradient established is carried out by the F1-F0 ATP synthase (complex V), which drives phosphorylation of ADP to make ATP [10]. This huge potential difference provides the driving force for cytosolic Ca2+ to accumulate in the matrix via the MCU. The main mitochondrial Ca2+ transporters and channels are described in the following section and represented in Figure 1.
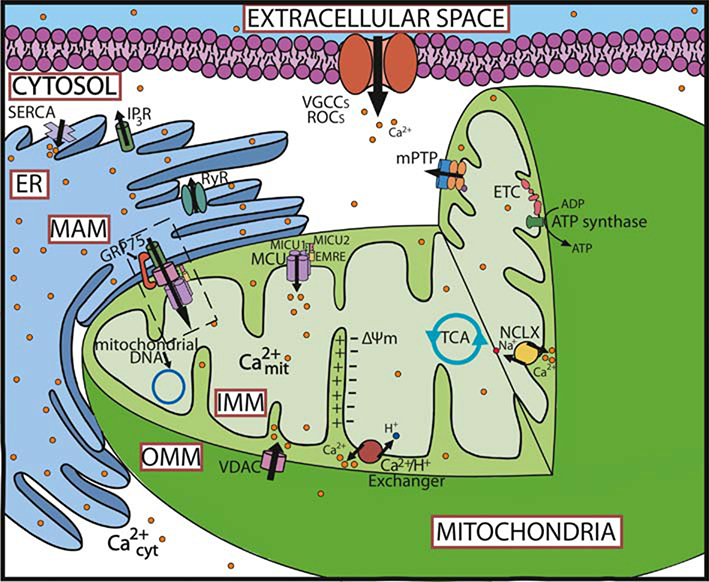
Fig. 1.
Main components of the mitochondrial Ca2+ homeostasis in neurons The main components of Ca2+ regulation in the cell are part of the plasma membrane (VGCCs and ROCs among other channels and pumps), endoplasmic reticulum, and mitochondria. In the mitochondria, the MCU complex is the part of the main Ca2+ influx pathway. VDAC contributes to this movement by allowing the flow of ions through the OMM. Ca2+ efflux is predominantly regulated by the NCLX and the H+/Ca2+ exchangers. In addition to this, the mPTP can also vent Ca2+ ions out of the cell. Mitochondria-ER communication is moderated through the MAMs (comprised of the MCU complex, VDAC, and IP3R among other proteins and tethers), and allow the flow of Ca2+ ions into the mitochondria from the ER for further regulation. In the ER, Ca2+ release is regulated through the RyR and IP3R pathways, while SERCA is involved in its extrusion from the cytosol. Abbreviations: ΔΨm (mitochondrial membrane potential), ER (endoplasmic reticulum), ETC (electron transport chain), GRP75 (glucose-regulated protein 75), H+/Ca2+ (H+/Ca2+ exchanger), IMM (inner mitochondrial membrane), IP3R (inositol trisphosphate receptor), MAM (mitochondrial associated membranes), MCU (mitochondrial Ca2+ uniporter), MICU (mitochondrial Ca2+ uptake proteins), (mtDNA (mitochondrial DNA), NCLX (Na+/Ca2+ Li+ permeable exchanger), mPTP (mitochondrial permeability transition pore), ROCs (receptor-operated Ca2+ channels), RyR (ryanodine receptor), SERCA (sarco/endoplasmic reticulum Ca2+-ATPase), TCA (tricarboxylic acid), VDAC (voltage dependent anion channel), VGCCs (voltage gated Ca2+ channels).
3. Mitochondrial Ca2+ homeostasis: transporters and channels
In the 60s and 70s, studies conducted in isolated mitochondria revealed that they were able to take up high amounts of Ca2+ [11]. Since then, it has been widely accepted that mitochondrial Ca2+ homeostasis plays an important role in physiology and pathophysiology.
3.1. Mitochondrial Ca2+ influx: The Mitochondrial Ca2+ Uniporter Complex
The MCU complex is the main route for Ca2+ uptake into the mitochondrial matrix, although a rapid uptake mode (Ram) for short Ca2+ pulses activated by lower external Ca2+ concentration has also been described [12].
In 2011, the groups of Mootha and Rizzuto independently identified the protein CCDC109A as necessary and sufficient for mitochondrial Ca2+ uptake in vitro and in vivo, which matched the features of the MCU [13, 14]. In the following years, numerous studies characterizing the uniporter arose. The MCU complex is a macromolecular complex of proteins and includes the pore forming and regulatory subunits. The MCU is the ion conducting pore. It is ubiquitously expressed throughout varying organisms and human tissues. MCU monomers oligomerize, likely as a tetramer, to form the pore [13]. Two other proteins participate in pore activity: the MCUb and the essential MCU regulator (EMRE). The MCUb shares 50% of similarity with the MCU and hetero-oligomerizes with it [15]. It does not conduct Ca2+ on its own, but its presence inhibits mitochondrial Ca2+ uptake. EMRE in an auxiliary protein that keeps the regulatory subunits mitochondrial Ca2+ uptake 1 (MICU1) and MICU2 (see below) attached to the MCU complex [16].
Mitochondrial Ca2+ levels in the matrix are similar to those of the cytoplasm (~100 nM) under resting conditions. The MCU is therefore inactive despite the driving force of the mitochondrial membrane potential (ΔΨm) that is produced by the ETC. Upon stimulation, the MCU activates and transports Ca2+ into the mitochondrial matrix instantaneously. This response is regulated by the MICU family, located in the intermembrane space. MICU1 was the first component of the uniporter complex to be reported [17], a few months before the identification of the MCU and after the completion of the protein inventory MitoCarta. MICU1 and MICU2, which constitute an obligate heterodimer [18], act as MCU Ca2+ sensors using their two Ca2+-binding EF-hand motifs, which confer the Ca2+ sensitivity. MICU1 and 2 are considered the gatekeepers of the MCU, as the combination of both proteins regulates the uniporter and prevents Ca2+ uptake at low extramitochondrial Ca2+ concentrations [19, 20]. MICU1 participates when the extramitochondrial Ca2+ concentration is high, activating the open channel state. At low concentrations, however, MICU2 is the main player, leading to minimal accumulation of Ca2+ within mitochondria [18], and preventing mitochondrial Ca2+ overload at resting conditions. MICU3, a paralogue of MICU1 and MICU2, is mainly expressed in the central nervous system (CNS) and enhances mitochondrial Ca2+ uptake in neurons [21]. Together, these regulators allow a short response rate to low extracellular Ca2+ (resting conditions) and a large capacity at high Ca2+ levels. This phenomenon explains the Ca2+ buffering function of mitochondria. In addition, the mitochondrial Ca2+ uniporter regulator 1 (MCUR1) [22], which is necessary for the MCU-mediated mitochondrial Ca2+ uptake, and the small calcium-binding mitochondrial carrier protein (SCaMC, also known as SLC25A23) [23], which interacts with MCU and MICU1, also participate in the regulation of the complex.
3.2. Mitochondrial Ca2+ efflux: NCLX and LETM1
Ca2+ efflux from the mitochondrial matrix depends on two main mechanisms: an electrogenic exchange of Na+/Ca2+ (3 or 4 Na+ ions per Ca2+) encoded by the NCLX gene, and an ubiquitous H+/Ca2+ exchange (likely an electroneutral process of 2 H+ per Ca2+).
The Na+-dependent Ca2+ release exchanger (NCLX) was first discovered by Ernesto Carafoli in 1974 in cardiac mitochondria [24]. In 2010, Sekler’s group identified the Na+/Ca2+ exchanger, expressed only in internal membranes and enriched in the mitochondrial fraction, specifically in the IMM [25]. It was named the Na+/Ca2+ Li+ permeable exchanger (NCLX), being characterized by its exchanging of Ca2+ with Na+ and Li+, the only member of its family to do so. The Ca2+ extrusion is coupled with the influx of Na+ into the mitochondrial matrix from the cytosol, which can be effectively replaced by Li+, and hence its name. This is different from other members of the Na+/Ca2+ exchangers, which do not transport Li+. This process is allosterically regulated by ΔΨm and by the channel’s phosphorylation [26]. Future investigations are needed to determine other regulatory factors and their regulation process.
The Leucine zipper and EF-hand containing transmembrane protein 1 (Letm1) is required for maintaining mitochondrial morphology and cristae structures [27]. Additionally, it has been suggested as a Ca2+ extrusion mechanism when Ca2+ levels are elevated in the mitochondrial matrix [28]. This protein was proposed by Clapham to catalyze the exchange Ca2+/2H+ [29]. However, other researchers have proposed Letm1 to be part of the K+/H+ antiporter [30], rather than being involved in mitochondrial Ca2+ extrusion [31], and its molecular nature is still debated.
3.3. Mitochondrial-associated ER membranes
Mitochondrial Ca2+ levels are tightly regulated by the ER via mitochondrial-associated ER membranes (MAMs). MAMs are contact points between juxtaposed ER and OMM. They are enriched with VDAC and the MCU on the mitochondrial side, and with inositol trisphosphate receptor (IP3R) on the ER. The structure is coupled by tethers such as the chaperone protein Grp75 [32] or mitofusin2 [33]. The most important roles of MAMs include intracellular trafficking of mitochondria and ER, energy metabolism, protein folding and cell autophagy [34–36]. In addition, they mediate Ca2+ transfer from the ER to mitochondria [37, 38]. These contact sites allow for the formation of Ca2+ hotspots that meet the low affinity threshold of MCU to take up Ca2+. The mitochondrial Ca2+ uptake from the ER allows for regulation of mitochondrial oxidative metabolism and ATP production [39]. However, excessive mitochondrial Ca2+ uptake compromises mitochondrial function, triggering the transient collapse of the mitochondrial membrane potential and leading to apoptosis or necrosis [40, 41]. ER-mitochondria association also regulate macroautophagy, and blocking Ca2+ transfer from the ER to mitochondria stimulates autophagy in response to the cell’s impaired bioenergetics [42]. Relatedly, mitochondria tethering to the plasma membrane and the Golgi compartment have also been observed [43, 44].
4. Mitochondrial Ca2+ and neurodegenerative diseases
Neurodegenerative diseases are a large group of heterogeneous disorders characterized by the selective death of neuronal subtypes. Mitochondrial dysfunction contributes to many neurodegenerative diseases, such as AD or PD [3]. When the brain demands high amounts of energy, the number of mitochondria increases mainly at synapses. Accumulation of misfolded or damaged proteins within neurons is a hallmark of most age-related neurodegenerative diseases. Protein accumulation inside mitochondria can lead to mitochondrial dysfunction and eventually threaten neuronal survival. An immediate consequence of dysfunctional mitochondria is the increase of ROS production. This which promotes oxidative damage to DNA, RNA, proteins and lipids, and eventually leads to cell death. Additionally, many studies suggest that alteration of Ca2+ homeostasis is a hallmark of many of these pathologies [45]. In the sections below, we review the main aspects of mitochondrial Ca2+ dysregulation in some of the most prevalent neurodegenerative diseases.
4.1. Mitochondria and their role in cell death
Besides ATP production, mitochondria along with mitochondrial Ca2+ are needed for initiating apoptotic cell death. An excess of Ca2+ taken up by mitochondria, due to impairment of mitochondrial Ca2+ influx or efflux, can lead to mitochondrial Ca2+ dysregulation and overload. This may cause mitochondrial swelling, respiration impairment, enhanced ROS production and trigger mitochondrial permeabilization and cell death. This process requires opening of the mitochondrial permeability transition pore (mPTP), which is believed to be the initial trigger for apoptosis and necrosis [46]. After mitochondrial permeabilization, different proapoptotic factors are released into the cytoplasm through the OMM. These include cytochrome c, apoptosis inducing factor (AIF), endonuclease (endo) G, and Smac/diablo, each one with different downstream targets. In addition to this, caspases get activated.
Mitochondria are the main intracellular source of ROS. ROS are reactive molecules with an unpaired electron, which can have detrimental effects to the cells. The main ROS produced by mitochondria is the anion superoxide (O2−), although other ROS are produced, such as hydrogen peroxide (H2O2), hydroxyl radical (HO−), nitric oxide (NO) and peroxynitrite (ONOO−). ROS are mainly produced in the mitochondrial matrix, as byproducts of the mitochondrial ETC. In normal conditions, antioxidant enzymes like superoxide dismutase (SOD), glutathione peroxidase (GPX) and catalase act as free radical scavengers and eliminate the excess of ROS. Oxidative stress takes place when there is an imbalance in the generation and detoxification of ROS. This leads to oxidation and damage of proteins, DNA and lipids. Whereas transiently elevated ROS can have physiological functions, such as cell signaling or promoting protein synthesis [47], chronically elevated mitochondrial ROS production can lead to cellular dysfunction. Since ROS exposure can lead to further intracellular ROS production, these molecules can have detrimental downstream effects such a mtDNA mutation. ROS is particularly damaging to already susceptible neurons, since those neurons require high energy and have large amounts of mitochondria. Upon oxidative stress-induced accumulation of ROS in neurons, proteins and toxic wastes are deposited in the brain, impairing brain homeostasis [48]. Combined with increased ROS production, diminished production of antioxidant agents can further induce cell death and neurodegeneration.
4.2. Mitochondrial Ca2+ dysregulation and AD
AD is the most prevalent neurodegenerative disorder. It is commonly associated with age, and leads to progressive cognitive impairment and dementia. Unfortunately, the direct cause of AD is still unknown. Most AD cases are sporadic, with a late onset, and the symptoms appearing after the age of 60. A small fraction of cases - less than 1% - are genetically inherited and characterized by an early onset. Three genes, the amyloid precursor protein (APP) and presenilin-1 and 2 (PSEN1 and PSEN2) have been identified as responsible for the familial form of AD (FAD). The main hallmarks of AD are (i) amyloid plaques, formed after increased accumulation of extraneuronal amyloid beta (Aβ) peptide, which assemble from monomeric and oligomeric intermediates, (ii) intraneurofibrillary tangles (NFTs), composed of hyperphosphorylated and misfolded tau, and (iii) severe neuronal loss that leads to memory impairment. Several hypotheses have been postulated to explain the origin of the disease. Since all genetic mutations that lead to FAD are involved in Aβ processing, the “amyloid cascade hypothesis” was proposed [49]. This hypothesis suggests that the origin of AD pathogenesis results from Aβ overproduction and/or the failure of Aβ clearance mechanisms. However, alternative hypotheses have recently gained momentum due to the lack of correlation between Aβ deposition and cognitive decline, and the negative outcomes of many clinical trials targeting Aβ [50, 51]. Among the most relevant of these are the “cholinergic hypothesis” [52], the “tau propagation hypothesis” [53], the “inflammatory hypothesis” [54] and the “glymphatic system hypothesis” [55]. The “Ca2+ hypothesis of AD” postulates that activation of the amyloidogenic pathway causes remodeling of the neuronal Ca2+ signaling pathway, altering Ca2+ homeostasis and impairing mechanisms involved in learning and memory [56]. Disrupted Ca2+ homeostasis has been found both in cells from sporadic and familial cases of AD [57–59]. Mitochondrial Ca2+ dyshomeostasis has also been related to AD, due to the neurotoxic effects of Aβ, tau, and other risk factors associated to AD (recently reviewed in [60]).
Both Aβ and tau have been found in mitochondria from human AD brain tissue and transgenic mouse models of AD [61, 62], and when they are exogenously applied to cells [63]. Moreover, some studies indicate that Aβ accumulation in mitochondria starts before the deposition of extracellular plaques [64]. Their import into mitochondria requires the participation of the translocase of the OMM (TOM) and IMM (TIM) import channels [65, 66]. Once in the mitochondrial matrix, they interact with specific intra-mitochondrial proteins, leading to degeneration of the organelle [62, 67].
Aβ itself impairs mitochondrial morphology and function. Using intravital multiphoton imaging, our group has previously shown that the structure and function of mitochondria are impaired in the living brain of transgenic mouse models of AD expressing amyloid deposits [68]. In the regions close to Aβ plaques, decreased mitochondrial number, reduced mitochondrial membrane potential and increased oxidative stress were observed. Altered mitochondria morphology and distribution have also been observed in neurons from AD patients [69] and in vitro after Aβ, APP or PS1 expression [70, 71].
Aβ oligomers have been shown to induce mitochondrial Ca2+ uptake and overload in primary cells in vitro [72], particularly in primary cells aged long-term in vitro [73], trigger Ca2+ transfer from ER to mitochondria [74], and enhance the Ca2+ amount transferred from the ER to mitochondria following an stimulus to release Ca2+ from ER (like acetylcholine or caffeine) [75]. Aβ-driven mitochondrial Ca2+ overload leads to mPTP activation, cytochrome c release and cell death via apoptosis or necrosis [72]. The use of mitochondrial uncouplers, such as FCCP or CCCP (cyanide-4-(trifluoromethoxy)phenylhydrazone and carbonyl cyanide chlorophenylhydrazone respectively), or NSAIDs (non-steroidal anti-inflammatory drugs), used at low concentrations, induces a mild mitochondrial depolarization, inhibiting mitochondrial Ca2+ overload elicited by Aβ without leading to an increase in cytosolic Ca2+ and preventing those events. This suggests that mitochondria itself plays a key role in the cell death induced by Aβ, and supports a different mechanism of neuroprotection for NSAIDs via mitochondrial depolarization and that is not related to their anti-inflammatory effect [59, 73]. Using multiphoton microscopy in vivo in a mouse model of cerebral amyloidosis (APP:PS1), our group recently demonstrated that a significative amount of mitochondria exhibit high levels of Ca2+ and mitochondrial Ca2+ overload in neurons compared to wild-type (Wt) controls, but only after plaque deposition [76, 77]. Furthermore, topical application of Aβ oligomers -naturally secreted soluble Aβ oligomers, known as conditioned media- onto the naïve Wt brain in vivo also increased the mitochondrial Ca2+ concentration. This effect could be prevented by using the MCU inhibitor Ru360 [76]. This result implied that an intact MCU is required for Aβ driven mitochondrial Ca2+ uptake. In addition, neurons with elevated mitochondrial Ca2+ in their neuronal soma died after a short period of time, thus linking mitochondrial Ca2+ overload with neuronal cell death in vivo.
Tau is a monomeric protein that stabilizes microtubules, allowing the axonal transport of organelles. In AD, tau becomes abnormally hyperphosphorylated and misfolded, detaching from microtubules and aggregating into neurofibrillary tangles. When this takes place, mitochondrial transport and therefore the cell’s energy supply get compromised, eventually leading to neurodegeneration [78]. It has been shown that, in addition to Aβ, pathological tau also disrupts mitochondrial Ca2+ homeostasis. In cells overexpressing tau and cells exposed to tau aggregates, mitochondrial Ca2+ buffering is defective [79, 80]. Cortical neurons exposed to extracellular tau and AD patient induced pluripotent stem cell (iPSC)-derived neurons expressing a tau mutation exhibit higher basal mitochondrial Ca2+ levels [81], likely due to the inhibition of NCLX by tau.
Additionally, RNA-seq and microarray analyses from human AD and control brains [82–84] of the expression of the genes involved in the mitochondrial Ca2+ transport were shown to be altered in AD. Expression of the genes involved in mitochondrial Ca2+ influx (the MCU complex) was downregulated, whereas the expression of the gene encoding the channel involved in mitochondrial Ca2+ efflux (NCLX) was upregulated [76]. In our opinion, this might suggest a compensatory effort to avoid mitochondrial Ca2+ overload in the human AD brain. However, using a different technique, others have found decreased expression of NCLX in human AD brains and in the 3xTg-AD mouse (which develops both plaques and neurofibrillary tangles), which could also explain the mitochondrial Ca2+ overload observed in vivo [85].
MAMs-associated proteins are also dysregulated in AD [86], and their expression has been reported to be upregulated in post-mortem AD brains, in mouse models of the disease and in primary neurons exposed to Aβ and tau [86]. In addition, it has been proposed that there is an optimal distance between ER and mitochondria, and if this optimal distance is disturbed, this can lead to higher levels of Ca2+ transfer from ER to mitochondria [75], which can trigger apoptosis. Increased MAM activity and ER-mitochondria contact points have been observed in human fibroblasts from AD patients [87], and in primary neurons exposed to Aβ and tau [62, 88].
4.3. Mitochondrial Ca2+ dysregulation and Parkinson’s disease
PD is a neurological locomotory disorder that involves rigidity, bradykinesia and a resting tremor. It is considered the most common movement disorder and the second most common neurodegenerative disorder after AD. The main histopathological hallmarks of PD are Lewy body inclusions containing α-synuclein, a small lipid-binding protein, and loss of pigmented dopaminergic neurons in a small and defined area of the brain involved in motor control called substantia nigra pars compacta [89]. The phenotypes of both sporadic and familiar forms of PD exhibit the same symptoms, suggesting that they might share common underlying mechanisms. PD belongs to a larger group of neurodegenerative diseases, known as synucleinopathies. On the cellular level, mitochondrial dysfunction and oxidative stress have been described in many studies investigating PD pathology [90–92]. In addition, chronic inflammation, aberrant protein folding and aggregation, and genetic and environmental factors have all been implicated in the etiology of PD. Familial hereditary PD involves mutated genes that negatively impact mitochondrial physiology, supporting the idea that mitochondrial signaling and homeostasis are implicated in PD pathology [93]. PD-risk genes include loss-of-function mutations in PTEN-induced putative kinase 1 (PINK1), which is involved in the neuronal stress-response pathway, Parkin, and Parkinsonism associated deglycase (PARK7, best known as DJ-1).
In PD, global Ca2+ dysregulation impairs neuronal signaling and damages mitochondria, resulting in cell death [94]. L-type voltage gated Ca2+ channels (VGCCs) are autonomously active, leading to increased extracellular Ca2+ influx and elevated cytosolic Ca2+ [95]. α-synuclein itself increases cytosolic Ca2+ by forming pores in the plasma membrane that allow Ca2+ entry into the cytosol [96, 97]. In addition, the increase of intracellular Ca2+ levels may promote α-synuclein aggregation, leading to a vicious cycle which further increases intracellular Ca2+ levels. α-synuclein has been found in mitochondria [98, 99] as well as in MAMs [100]. Defects in mitochondrial dynamics (i.e. fission, fusion and transport) and quality control are major contributors in PD pathology. Relatedly, it has been found that several PD-associated proteins (including α-synuclein, PINK1, DJ-1, Parkin and leucine-rich repeat kinase 2 (LRRK2)), which mutations are the most common causes of familial and sporadic forms of PD [101], directly participate in the regulation of mitochondrial dynamics and oxidative stress [102–104].
Although increased Ca2+ influx at the plasma membrane significantly contributes to the pathogenesis of PD, the organelles also exhibit Ca2+ dysregulation in this pathology. Particularly, mitochondrial Ca2+ overload and dysfunction have been observed in PINK1-deficient neurons [105, 106]. These neurons are sensitive to dopamine, which induce mitochondrial Ca2+ overload, triggering mPTP opening and eventual cell death [105]. This effect is thought to be a result of the impairment of mitochondrial Ca2+ extrusion. Mitochondrial Ca2+ efflux is also inhibited in cells from PD patients bearing PINK1 mutations [107]. In the absence of PINK1, NCLX activity is severely impaired, leading to mitochondrial Ca2+ overload. Pharmacological or genetic activation of NCLX corrected these defects, rescuing the mitochondrial phenotypes observed in PD models [107]. This pointed to NCLX as a novel therapeutic strategy in PD. Additionally, inhibition of mitochondrial Ca2+ uptake decreased the oxidative stress observed in dopaminergic neurons, suggesting that oxidative stress could also be a consequence of mitochondrial Ca2+ overload in PD [108]. Knocking out MCU and inhibiting Ca2+ influx into the mitochondrial matrix rescues dopaminergic neuronal loss in PINK1 mutant cells in zebrafish models of PD [109].
The link between Ca2+ dyshomeostasis and mitochondrial dysfunction in PD progression is further supported by the fact that both α-synuclein and DJ-1 interact with MAMs via Grp75 [110]. This interaction promotes MAMs assembly and function by controlling ER-mitochondria Ca2+ transfer and lipid homeostasis [100, 111, 112]. α-synuclein disrupts these ER-mitochondria contacts, impairing Ca2+ exchange between the two organelles and affecting the PD phenotype [113]. This suggests that MAMs homeostasis can also play a role in the pathogenesis of PD. Together, these studies imply that proper mitochondrial Ca2+ homeostasis is crucial for the correct homeostasis of dopaminergic neurons, and that anti-PD treatments could include compounds that prevent mitochondrial Ca2+ overload.
4.4. Mitochondrial Ca2+ dysregulation and Amyotrophic lateral sclerosis
Amyotrophic lateral sclerosis (ALS) is a neurodegenerative disease that is hallmarked by the progressive loss of upper or lower motor neurons in the spinal cord and brain, leading to fatal paralysis and death within 5 years. Following the initial dysfunction, neurodegeneration induced by inflammatory agents and eventual loss of trophic support take place. Most cases are sporadic, with only 10% being familial. Several mutations in proteins were identified to be causally related to familial forms of ALS. Mutations in the Cu/Zn-superoxide dismutase (SOD1) gene – encoding the antioxidant enzyme SOD – are among the most frequent, accounting for the 20% of the familial cases [114]. Other mutations include TAR DNA binding protein (TARDBP; TDP-43), Fused in sarcoma (FUS) and C9orf72 [115]. Oxidative stress is one of the major contributors to ALS pathology. Mutations in SOD1 lead to enhanced ROS production [116]. Accumulation of damaged proteins also takes place in the CNS of ALS patients, which interferes with neuronal transport of mitochondria in the axons, reducing ATP production and impairing the synaptic transmission by altering Ca2+ signaling [117]. Furthermore, mitochondrial degeneration in motor neurons has been proposed to trigger ALS onset in familial mouse models of the disease expressing mutant SOD1 [118]. Oxidative stress, proteasome dysfunction and excitotoxicity have also been proposed at the onset of the sporadic disease. Despite this, its definitive etiology remains elusive [119–121].
Vulnerable neurons in ALS, such as spinal and brain stem neurons, have very low Ca2+ buffering capacity. They exhibit reduced levels of Ca2+ buffering proteins, like parvalbumin and calbindin amongst others [122]. Low cytosolic Ca2+ buffering capacity is therefore a primary risk factor for motor neuron decline, whereas enhancing Ca2+ buffering capacity protects motor neurons from degeneration [123, 124]. These excessive Ca2+ levels due to diminished Ca2+ buffering capacity lead to mitochondrial degeneration in motor neurons. Further, these neurons are enriched in Ca2+ permeable glutamate AMPA receptors. On top of this, the presence of mutant SOD1 increases AMPA receptor permeability to Ca2+, thus increasing Ca2+ levels even more [125]. Following stimulation, they mediate glutamate-excitotoxicity, resulting in neuronal degeneration and cell loss [126, 127].
Mitochondrial dysfunction is also involved in the pathology of ALS, particularly in ALS subtypes associated with SOD1 mutation and in sporadic cases of the disease. This includes the generation of free radical species, changes in mitochondrial morphology, and activity of its complexes, especially in the upper and lower motor neurons, frontal cortex and spinal cord [128–130]. Defects in mitochondrial function, including mitochondrial swelling and vacuolization, have been found both in sporadic and familial forms of ALS, through histopathological observations in ALS patients and transgenic mouse models of the disease [130, 131].
Mitochondrial regulation of Ca2+ levels is particularly important in motor neurons, where Ca2+ uptake by mitochondria is greater than in other cell types. This makes motor neuronal mitochondria more susceptible to Ca2+ mediated damage [127, 132]. Mitochondrial Ca2+ overload might result from the increased activity and permeability of AMPA receptors in ALS motor neurons [127], which can lead to increased ROS production and oxidative stress. Mutant SOD1 transgenic mouse models show decreased mitochondrial Ca2+ buffering capacity in the CNS, even before the disease onset, pointing to an early loss of the Ca2+ buffering as a cause of the disease [133]. Furthermore, reduced MCU Ca2+ uptake has been observed in motor neurons [134]. Interestingly, mitochondria might be strategically located close to the ER or plasma membranes to effectively buffer Ca2+ in the motor neurons in order to compensate for their lower levels of Ca2+ buffering protein expression [135]. Impaired MAM function can lead to reduced mitochondrial Ca2+ uptake, and increased cytosolic Ca2+ levels after triggering ER Ca2+ release [136, 137]. MAM disruption has been reported in mutant SOD1, Sig1R, TDP-43 and FUS-related ALS [134, 136, 137], suggesting that disrupted ER-mitochondria communication might be a common characteristic in ALS, and a primary cause of motor neuron death in the disease.
5. Tools to study mitochondrial Ca2+ dynamics in vivo
As discussed above, mitochondria are dynamic organelles, with the capacity to move, divide and fuse, to travel along the cell and to take up Ca2+. The research of these events has become an important tool for researchers in the investigation of mitochondrial abnormalities related to neurodegenerative diseases. The study of these events in vivo became possible after the development of fluorescence reporters along with live cell microscopy techniques. Just until a few years ago, most of the studies investigating mitochondrial structure and function were developed in vitro or with the use of biochemical assays. Unavoidably, with the new techniques of fluorescent imaging technology, such as multiphoton microscopy, most recent research is aimed at applying these measurements in vivo. Multiphoton microscopy allows greater tissue penetration and less phototoxicity, making it possible to use the imaging approaches established in isolated cells in intact sections of tissue or the living brain. The selection of the best probe will depend on the question that is being asked. Ideally, the Ca2+ indicator should localize specifically into the target, (i.e., mitochondria), and show robust fluorescence, increased signal to noise ratio and fast kinetics. Additionally, its affinity for Ca2+ should be suitable for detecting the expecting Ca2+ changes in the organelle. Finally, it should not be toxic or interfere with the mitochondrial environment. The two main methods for studying mitochondrial Ca2+ homeostasis are fluorescent dyes and genetically encoded Ca2+ indicators (GECIs). Whereas fluorescent dyes are electrically retained in the mitochondrial matrix, GECIs can be maintained in the mitochondrial intermembrane space or in the external surface of the OMM. A variety of reporters to measure free mitochondrial Ca2+ levels are listed in Table 1.
Table 1.
Main reporters to measure free mitochondrial Ca2+ concentration in vitro and in vivo.
| Name | Intensiometric/ratiometric | Excitation/emission (nm) single photon | Kd for Ca2+ | Ref. |
|---|---|---|---|---|
| Fluorescent dyes | ||||
| Rhod2 | Intens | Ex: 540; Em: 580 | 320 μM | [141] |
| Rhod-5N | Intens | Ex: 551; Em: 576 | 0.5 mM | [191] |
| Rhod-FF | Intens | Ex; 550; Em: 580 | 19 μM | [192] |
| Fluorescent proteins | ||||
| mtGCaMP5G | Intens | Ex; 497; Em: 515 | 460 nM | [193] |
| mtGCaMP6s | Intens | Ex; 497; Em: 515 | 144 nM | [193] |
| 4mtD3cpv | FRET | Ex: 433; Eml: 483; Em2: 534 | 760 nM | [194] |
| 2mtYC3.6 | FRET | Ex: 433; Eml: 483; Em2: 534 | 4.21 μM | [76] |
| mito-GEM- GECOl | Ratio | Ex: 377; Eml: 447; Em2: 520 | 340 nM | [177] |
| mito-R-GECOl | Intens | Ex; 562; Em: 624 | 482 nM | [177] |
| Mito-CAR- GECO | Intens | Ex; 560; Em: 609 | 490 nM | [195] |
| mito-LAR- GECOl.2 | Intens | Ex; 561; Em: 620 | 12 μM | [196] |
| CEPIA2mt | Intens | Ex; 488; Em: 508 | 160 nM | [183] |
| CEPIA3mt | Intens | Ex: 488; Em: 508 | 11 μM | [183] |
| CEPLA4mt | Intens | Ex: 488; Em: 508 | 56 μM | [183] |
| R-CEPIA3mt | Intens | Ex: 565; Em: 641 | 3.7 μM | [197] |
| R-CEPIA4mt | Intens | Ex: 565; Em: 641 | 26.9 μM | [197] |
| R-Pericam mt | Ratio | Exl: 415; Ex2: 494; Em: 517 | 11 μM | [170] |
| 2mt8PR | FRET | Exl: 415; Ex2: 494; Em: 517 | 1.7 μM | [185] |
5.1. Chemically engineered Ca2+ indicators: Fluorescent dyes
The use of fluorophores as a dye to target organelles is a broad technique used for investigators. These molecules change their fluorescence properties upon binding to Ca2+. The uptake of these fluorescent dyes into mitochondria depends on the negative mitochondrial membrane potential across the inner membrane. The fluorophores, however, are usually lipophilic and positively charged. They are frequently used as markers of mitochondrial localization and membrane potential, but also as an indicator for mitochondrial Ca2+ uptake. Fluorescent dyes need to be prepared as acetoxymethyl ester (AM), allowing for their diffusion into the cytoplasm [138]. Once in the cytoplasm, they get hydrolyzed by non-specific but ubiquitous esterases and the Ca2+ sensitive moiety is generated [139]. However, accumulation within mitochondria is highly variable and depends on the system and procedure used. A potential drawback is the toxicity of the AM hydrolyzed products (acetate and formaldehyde), but these effects have been largely minimized [140].
In the nineties, Rhod-2 was first introduced [141], as a Ca2+-dependent molecule for longer wavelength Ca2+ imaging [142]. Since then, it has been observed that Rhod-2 accumulates in mitochondria and has been used in several studies assessing mitochondrial Ca2+ of cultured cells and slice preparations, such as glia [143] or neuronal cells [144–146]. Several rhod derivates are commercially available, including Rhod-2, Rhod-5N, X-rhod-1, X-rhod-5F, X-rhod-FF and X-rhod-5N, each with different excitation or emission wavelengths. They are mainly used as cell-permeant AM esters, presenting a delocalized positive charge, and they accumulate within the mitochondrial matrix, where they are hydrolyzed and therefore trapped [141, 147]. However, it has been demonstrated that these constructs are no truly specific for targeting mitochondria, since they can sometimes be found in the cytosol or extramitochondrial localizations, causing inaccuracy during long term duration experiments [148]. One of the other main drawbacks is that they are intensity-based Ca2+ sensors, and unlikely other ratiometric Ca2+ sensors such as fura-2 or indo-1, quantitative measurements of mitochondrial Ca2+ may be inaccurate. When imaging in vivo, other problems may include the potential toxicity in the AM hydrolysis as well as the surgical method to deliver the dye to the tissue of interest itself [140].
5.2. Genetically encoded Ca2+ indicators: Fluorescent and bioluminescent proteins
In the early 1990s, the green fluorescent protein (GFP) from the jellyfish Aequorea Victoria was discovered and cloned [149]. The advances in genetic manipulation allowed for targeting proteins selectively to different cellular locations including organelles. This has allowed for the study of the mitochondria as well as the dynamics of specific mitochondrial proteins. Aside from just GFP, a broader toolkit of fluorescent proteins (FP) for in vivo imaging has been produced [150, 151]. Several molecular parameters within mitochondria can be assessed by using FPs. They include mitochondrial membrane potential, pH, Ca2+ (GECIs) or Cl− levels. GECIs show reduced toxicity. They display a varying range of affinities for Ca2+ (different Kd), and can be targeted selectively to different locations including mitochondria [152–154]. Their main drawbacks are the requirement of genetic manipulation, the sensitivity that some of them have to mitochondrial pH, and the effects that the mitochondrial matrix environment as opposed to the cytoplasm can have on their affinity for Ca2+. GECIs can be either bioluminescent (Aequorin-based GECIs) or fluorescent (GFP-based GECIs).
Bioluminescent GECIs derive from the photoprotein aequorin, which is isolated from the Aequorea Victoria jellyfish [155]. Aequorins were the first mitochondria targeted GECI [156], called mtAEQ. It was composed of the mitochondrial targeting sequence (cytochrome c oxidase polypeptide VIII (COX8)) and the native aequorin. The use of mtAEQ helped to provide the first evidence that mitochondria were able to accumulate Ca2+ in living cells [157]. When aequorin binds to Ca2+ in the presence of the external cofactor coelenterazine, a photon of light is produced. One of its main limitations is that once the reaction takes place, the indicator is consumed. The main advantages of using aequorin, however, is the lack of excitation illumination, making the measurements autofluorescence and phototoxicity free, and completely free of background noise. A drawback of the aequorin-based indicators is their low brightness relative to other GECIs [158]. Due to their low photon emission rates, obtaining subcellular resolution is very challenging. Additionally, they do not allow for long-term imaging, since aequorin gets consumed during the course of the reaction [159].
Fluorescent GECIs were developed in the nineties [160, 161]. They are comprised of a Ca2+ sensing polypeptide, which triggers a change in the fluorescence fused protein. There are two types of fluorescent GECIs: Föster resonance energy transfer (FRET)-based GECIs and single-wavelength fluorescent GECIs.
FRET-based GECIs use the energy transfer potential of two fluorophores with partially overlapping excitation/emission spectra. This way, the donor fluorophore transfers non-radiative energy to allow fluorescence of the second fluorescent acceptor. Both molecules are usually linked via a Ca2+-binding domain [162], since FRET only occurs when both proteins are in close proximity. The first and most well-known family of FRET-based Ca2+ indicators are cameleons. These are formed by a blue and yellow fluorescent protein linked by calmodulin fused to a kinase-binding peptide (M13) [160, 161]. Cameleons are ratiometric sensors, and therefore they provide an approximate absolute free Ca2+ concentration. In addition, motion artifacts and differences in sensor expression can be eliminated, as this is an important advantage while performing imaging in a living breathing animal [163–165]. The main drawbacks of these reporters are their lower signal-to-noise ratio, decreased brightness and slower kinetics than their single-fluorophore GECI counterparts [166]. Cameleons, however, have been extensively improved since the first generations, and the new versions show decreased sensitivity to pH, higher fluorescence intensity and less photobleaching [167].
On the other hand, single-wavelength fluorescent GECIs show large changes in fluorescence intensity. They were developed after finding that insertion of proteins into GFP still gives it the ability to fluoresce [168]. The first single-wavelength GECI developed was the camgaroo family [169], followed by pericams [170]. The most known example of single-fluorophore GECIs is the GCaMP family. As for other GECIs, the first generations of GCaMP exhibited low brightness, poor expression, and temperature and pH sensitivity [171]. However, the most recent versions (GCaMP5, GCaMP6 and GCaMP7) have improved brightness, dynamic range and Ca2+ affinity [172–174]. GCaMPs rely of the circular permutation of GFP, fused to a M13 and calmodulin-binding domains [175]. When Ca2+ binds to the calmodulin, it results in the increase of fluorescence intensity. Compared to FRET-based indicators, single-wavelength fluorescent GECIs exhibit a generally greater dynamic range, mostly due to their lower basal fluorescence, and are less likely to photobleach in long-term assays. However, as they are based in fluorescence intensity, they do not provide measures of free absolute Ca2+ concentration [176].
A new family of GECIs based on the red fluorescent protein (RFP) named GECO was developed a few years ago [177, 178]. They are intesiometric and exhibit improved signal-to-noise ratios. GECOs have been targeted to measure Ca2+ in different organelles or mitochondrial compartments simultaneously. GECOs emission fluorescence falls in longer wavelengths, allowing for multiplexing and Ca2+ imaging in different compartments (or cell populations) at the same time. Additionally, red-shifted indicators allow for greater tissue penetration, reduced phototoxicity and less scattering, clear advantages for in vivo imaging [179, 180]. These indicators include RCaMP, jRCaMP1a, jRCaMP1b and jRGECO1a [181, 182], and exhibit different affinities for Ca2+ with different dynamic ranges. Recently, low affinity indicators targeted to ER and mitochondria have been developed for the measurement of free Ca2+ levels in these organelles, which are known as CEPIA, with CEPIAmt being specific to mitochondria [183].
Our group has recently targeted Yellow Cameleon 3.6 [184] to mitochondria. We introduced the mitochondrial targeting sequence COX8A to the N terminus, duplicated in tandem in order to enhance the specificity of its location [185]. 2mtYC3.6 was subcloned into an associated adenovirus (AAV) hsyn (the synapsin promoter for neurons), for a final product of AAV.hSyn.2mtYC3.6. The expression cassette contained the following components: (1) human synapsin 1 gene promoter, (2) 2mt (mitochondrial targeting sequence), (3) YC3.6, (4) WPRE and (5) Simian virus 40 (SV40) [76]. This virus was injected in the somatosensory cortex of mice, and a cranial window was performed in the skull (Figure 2). Multiphoton microscopy and this reporter allowed us to evaluate the free Ca2+ concentration in neuronal mitochondria and to compare the Tg mouse (APP:PS1, a mouse model of AD) and their Wt controls in vivo [76]. An example of the images obtained can be observed in Figure 3. The use of this ratiometric indicator targeted to mitochondria allowed us to observe in the living brain the different mitochondrial Ca2+ levels in vivo in different compartments (somas and neurites) and ages (pre- and post-plaque), and follow neurons longitudinally with elevated Ca2+ concentration [76]. Using this technique, we directly observed and demonstrated elevated Ca2+ levels in neuronal mitochondria in the Tg mouse in vivo (see results above).
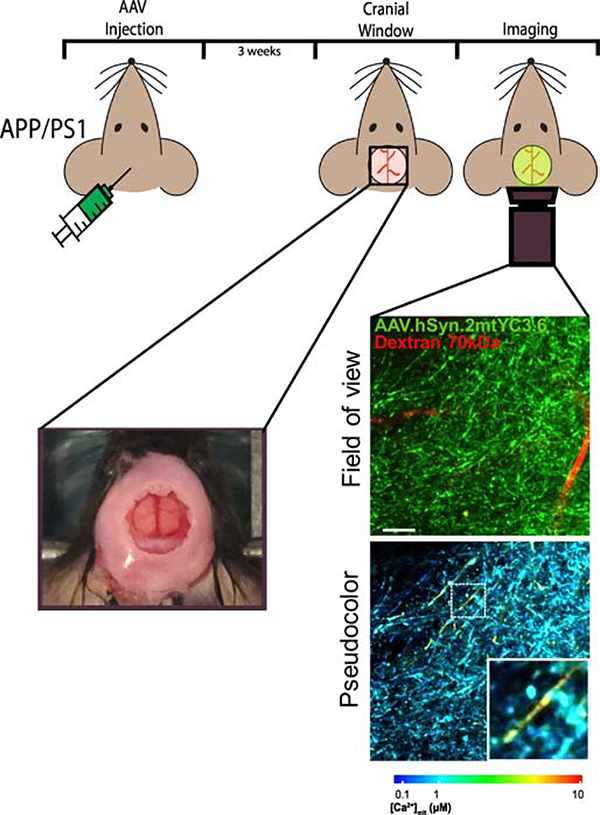
Figure 2. Schematic of the study of mitochondrial function in vivo.
The use of fluorophores in conjunction with in vivo imaging to evaluate intracellular function can be summarize as such: (1) a fluorescent indicator is injected into the cortex of the mouse model, (2) and after 3 weeks viral expression should be sufficient for imaging. (3) At this point a cranial widow is surgically implanted and (4) in vivo imaging with multiphoton microscopy may commence. Left image represents a cranial window in the mouse skull. Field of view shows the reporter in neuronal mitochondria (green) and the blood vessels labeled with fluorescent Dextran Texas Red (red). Pseudocolor images represent the color coded (according to the lower bar) mitochondrial Ca2+ concentrations for the corresponding neurites. Warm colors represent high Ca2+, whereas blue colors represent low Ca2+ concentration. Scale bar represents 15 μm.
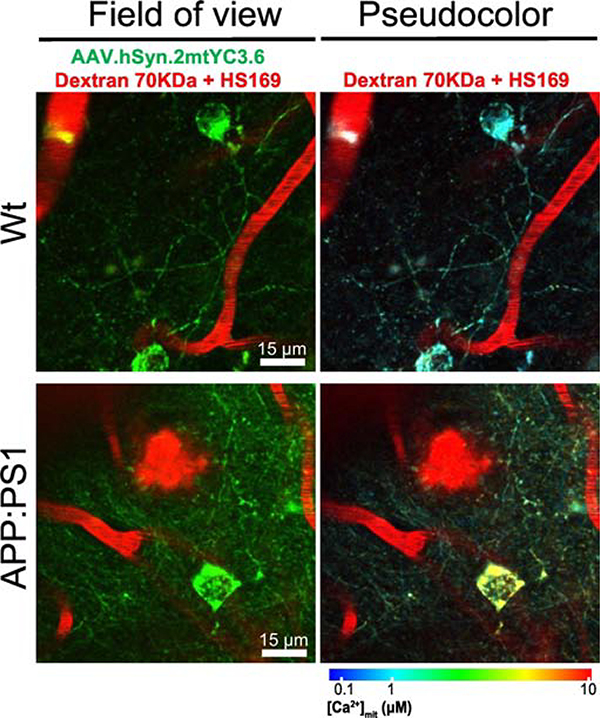
Figure 3. In vivo multiphoton microscopy images of mitochondrial Ca2+ in neurons in Wt and APP/PS1 mice.
APP/PS1 transgenic (Tg) mice and wild-type (Wt) mice were injected with the mitochondrial Ca2+ reporter AAV.hSyn.2mtYC3.6 and a cranial window was implanted. Mitochondrial Ca2+ was assessed with multiphoton microscopy. Pictures represent Wt (top) and APP/PS1 Tg mice (bottom). Field of view shows the reporter in neuronal mitochondria (green), the blood vessels labeled with fluorescent Dextran Texas Red (red) and amyloid plaques labeled with the dye HS169 (red, [198]). Pseudocolor images represent the color coded (according to the lower bar) mitochondrial Ca2+ concentrations for the corresponding neurons. Warm colors represent high Ca2+, whereas blue colors represent low Ca2+ concentration. Scale bar represents 15 μm.
6. Conclusion and future perspectives
Neurodegenerative diseases are likely multifactorial and can arise from mutation of different genes. However, Ca2+ dyshomeostasis and particularly mitochondrial Ca2+ impairment seem to be a common characteristic. Neurodegenerative diseases have been long associated with oxidative stress and impaired respiration. Mitochondria are critical for the maintenance of appropriate Ca2+ levels, and mitochondrial Ca2+ overload might be the cause that leads to mitochondrial dysfunction, oxidative stress and cell death. Altered mitochondrial Ca2+ homeostasis might result from a dysfunctional MCU complex (increased influx), altered mitochondrial Ca2+ extrusion (impaired NCLX) mechanisms, altered capacity of Ca2+ buffer or increased Ca2+ transfer from ER to mitochondria (MAMs). This might result in greater mitochondrial and cytosolic Ca2+ levels. Interventions aimed at reducing mitochondrial Ca2+ uptake or to enhance mitochondrial Ca2+ release could be effective for the treatment of these diseases. However, it might also represent a great challenge. Therefore, more research efforts are needed to elucidate the role of mitochondrial Ca2+ dyshomeostasis in this type of pathogenesis. Ca2+ imaging has been instrumental for the study of neurodegenerative diseases in animal models, mostly in vitro, in cell cultures or in brain slices. The combination of Ca2+ indicators and novel optical imaging techniques with cellular resolution that emerged in the recent years have allowed a better characterization of mitochondrial Ca2+ dynamics, and the patho-physiological mechanisms involved in the neurodegenerative diseases. Therefore, Ca2+ imaging is a powerful approach to perform functional studies in neuronal populations in the healthy and diseased brain in vivo. Recent advances in optical techniques, such as multiphoton microscopy, are more adequate for the observation of different cell types or protein aggregates in the cortex of the mouse brain (such as in AD). However, due to depth-penetration limitations, this technique is not suitable for the exploration of deeper brain regions (as in the case of PD). In vivo two photon imaging experiments are still limited to the superficial layers of the neocortex, cerebellum and olfactory bulb. The evaluation of deeper brain structures requires different technologies, such as two-photon imaging coupled to Gradient Index (GRIN) lenses, which provides optical interface to the brain, allowing for endoscopy imaging of deep brain regions [186, 187]. Other techniques include single fiber volumetric recordings [188] or fiber-bundle-bases confocal imaging [189, 190]. The development of these other techniques will give access to the in vivo study of diseases in which deeper brain regions are impaired. Understanding the complexity of mitochondrial Ca2+ dynamics in vivo will help understanding the role of mitochondrial Ca2+ dysfunction in these and other neurodegenerative diseases. To date, the MCU subunit of the mitochondrial uniporter complex is one of the best studied. Further research is required to ascertain the therapeutic candidacy of the other subunits of the uniporter complex, of NCLX and the MAMs. Overexpressing and knock-out (KO) mouse models, and virally-mediated gene transfer approaches will be necessary to validate the findings, as well as to evaluate their clinical relevance. With these results in hand and with the knowledge on the molecular mechanisms linking disrupted mitochondrial Ca2+ homeostasis to neurodegenerative diseases broadening, more specific therapeutic agents targeting these channels will be developed, providing hope for the treatment of these diseases.
Acknowledgements
This work was supported by NIHR01AG0442603, S10 RR025645 and R56AG060974 (BJB), and by the Tosteson & Fund for Medical Discovery and the BrightFocus Foundation A2019488F (MCR).
Footnotes
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Conflict of interest
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Wang C, Youle RJ, The role of mitochondria in apoptosis*, Annual review of genetics, 43 (2009) 95–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Attwell D, Laughlin SB, An energy budget for signaling in the grey matter of the brain, Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 21 (2001) 1133–1145. [DOI] [PubMed] [Google Scholar]
- [3].Manfredi G, Beal MF, The role of mitochondria in the pathogenesis of neurodegenerative diseases, Brain pathology, 10 (2000) 462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Hollenbeck PJ, Saxton WM, The axonal transport of mitochondria, Journal of cell science, 118 (2005) 5411–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Woods LC, Berbusse GW, Naylor K, Microtubules Are Essential for Mitochondrial Dynamics-Fission, Fusion, and Motility-in Dictyostelium discoideum, Frontiers in cell and developmental biology, 4 (2016) 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Dimmer KS, Navoni F, Casarin A, Trevisson E, Endele S, Winterpacht A, Salviati L, Scorrano L, LETM1, deleted in Wolf-Hirschhorn syndrome is required for normal mitochondrial morphology and cellular viability, Human molecular genetics, 17 (2008) 201–214. [DOI] [PubMed] [Google Scholar]
- [7].Taylor RW, Turnbull DM, Mitochondrial DNA mutations in human disease, Nature reviews. Genetics, 6 (2005) 389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Frey TG, Renken CW, Perkins GA, Insight into mitochondrial structure and function from electron tomography, Biochimica et biophysica acta, 1555 (2002) 196–203. [DOI] [PubMed] [Google Scholar]
- [9].Moran O, Sciancalepore M, Sandri G, Panfili E, Bassi R, Ballarin C, Sorgato MC, Ionic permeability of the mitochondrial outer membrane, European biophysics journal : EBJ, 20 (1992) 311–319. [DOI] [PubMed] [Google Scholar]
- [10].Stock D, Leslie AG, Walker JE, Molecular architecture of the rotary motor in ATP synthase, Science, 286 (1999) 1700–1705. [DOI] [PubMed] [Google Scholar]
- [11].Deluca HF, Engstrom GW, Calcium uptake by rat kidney mitochondria, Proceedings of the National Academy of Sciences of the United States of America, 47 (1961) 1744–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Buntinas L, Gunter KK, Sparagna GC, Gunter TE, The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria, Biochimica et biophysica acta, 1504 (2001) 248–261. [DOI] [PubMed] [Google Scholar]
- [13].Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, Bao XR, Strittmatter L, Goldberger O, Bogorad RL, Koteliansky V, Mootha VK, Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter, Nature, 476 (2011) 341–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R, A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter, Nature, 476 (2011) 336–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Foskett JK, Philipson B, The mitochondrial Ca(2+) uniporter complex, Journal of molecular and cellular cardiology, 78 (2015) 3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Sancak Y, Markhard AL, Kitami T, Kovacs-Bogdan E, Kamer KJ, Udeshi ND, Carr SA, Chaudhuri D, Clapham DE, Li AA, Calvo SE, Goldberger O, Mootha VK, EMRE is an essential component of the mitochondrial calcium uniporter complex, Science, 342 (2013) 1379–1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, Mootha VK, MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake, Nature, 467 (2010) 291–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Patron M, Checchetto V, Raffaello A, Teardo E, Vecellio Reane D, Mantoan M, Granatiero V, Szabo I, De Stefani D, Rizzuto R, MICU1 and MICU2 finely tune the mitochondrial Ca2+ uniporter by exerting opposite effects on MCU activity, Molecular cell, 53 (2014) 726–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Fan M, Zhang J, Tsai CW, Orlando BJ, Rodriguez M, Xu Y, Liao M, Tsai MF, Feng L, Structure and mechanism of the mitochondrial Ca(2+) uniporter holocomplex, Nature, 582 (2020) 129–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Xing Y, Wang M, Wang J, Nie Z, Wu G, Yang X, Shen Y, Dimerization of MICU Proteins Controls Ca(2+) Influx through the Mitochondrial Ca(2+) Uniporter, Cell reports, 26 (2019) 1203–1212 e1204. [DOI] [PubMed] [Google Scholar]
- [21].Plovanich M, Bogorad RL, Sancak Y, Kamer KJ, Strittmatter L, Li AA, Girgis HS, Kuchimanchi S, De Groot J, Speciner L, Taneja N, Oshea J, Koteliansky V, Mootha VK, MICU2, a paralog of MICU1, resides within the mitochondrial uniporter complex to regulate calcium handling, PloS one, 8 (2013) e55785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, Csordas G, Madireddi P, Yang J, Muller M, Miller R, Kolesar JE, Molgo J, Kaufman B, Hajnoczky G, Foskett JK, Madesh M, MCUR1 is an essential component of mitochondrial Ca2+ uptake that regulates cellular metabolism, Nature cell biology, 14 (2012) 1336–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Hoffman NE, Chandramoorthy HC, Shanmughapriya S, Zhang XQ, Vallem S, Doonan PJ, Malliankaraman K, Guo S, Rajan S, Elrod JW, Koch WJ, Cheung JY, Madesh M, SLC25A23 augments mitochondrial Ca(2)(+) uptake, interacts with MCU, and induces oxidative stress-mediated cell death, Molecular biology of the cell, 25 (2014) 936–947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Carafoli E, Tiozzo R, Lugli G, Crovetti F, Kratzing C, The release of calcium from heart mitochondria by sodium, Journal of molecular and cellular cardiology, 6 (1974) 361–371. [DOI] [PubMed] [Google Scholar]
- [25].Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, Nolte C, Fishman D, Shoshan-Barmatz V, Herrmann S, Khananshvili D, Sekler I, NCLX is an essential component of mitochondrial Na+/Ca2+ exchange, Proceedings of the National Academy of Sciences of the United States of America, 107 (2010) 436–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kostic M, Katoshevski T, Sekler I, Allosteric Regulation of NCLX by Mitochondrial Membrane Potential Links the Metabolic State and Ca(2+) Signaling in Mitochondria, Cell reports, 25 (2018) 3465–3475 e3464. [DOI] [PubMed] [Google Scholar]
- [27].Nakamura S, Matsui A, Akabane S, Tamura Y, Hatano A, Miyano Y, Omote H, Kajikawa M, Maenaka K, Moriyama Y, Endo T, Oka T, The mitochondrial inner membrane protein LETM1 modulates cristae organization through its LETM domain, Communications biology, 3 (2020) 99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Jiang D, Zhao L, Clapham DE, Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter, Science, 326 (2009) 144–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Tsai MF, Jiang D, Zhao L, Clapham D, Miller C, Functional reconstitution of the mitochondrial Ca2+/H+ antiporter Letm1, The Journal of general physiology, 143 (2014) 67–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].McQuibban AG, Joza N, Megighian A, Scorzeto M, Zanini D, Reipert S, Richter C, Schweyen RJ, Nowikovsky K, A Drosophila mutant of LETM1, a candidate gene for seizures in Wolf-Hirschhorn syndrome, Human molecular genetics, 19 (2010) 987–1000. [DOI] [PubMed] [Google Scholar]
- [31].De Marchi U, Santo-Domingo J, Castelbou C, Sekler I, Wiederkehr A, Demaurex N, NCLX protein, but not LETM1, mediates mitochondrial Ca2+ extrusion, thereby limiting Ca2+-induced NAD(P)H production and modulating matrix redox state, The Journal of biological chemistry, 289 (2014) 20377–20385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, Nagy AI, Balla T, Rizzuto R, Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels, The Journal of cell biology, 175 (2006) 901–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].de Brito OM, Scorrano L, Mitofusin 2 tethers endoplasmic reticulum to mitochondria, Nature, 456 (2008) 605–610. [DOI] [PubMed] [Google Scholar]
- [34].Gomez-Suaga P, Paillusson S, Stoica R, Noble W, Hanger DP, Miller CCJ, The ER-Mitochondria Tethering Complex VAPB-PTPIP51 Regulates Autophagy, Current biology : CB, 27 (2017) 371–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hamasaki M, Furuta N, Matsuda A, Nezu A, Yamamoto A, Fujita N, Oomori H, Noda T, Haraguchi T, Hiraoka Y, Amano A, Yoshimori T, Autophagosomes form at ER-mitochondria contact sites, Nature, 495 (2013) 389–393. [DOI] [PubMed] [Google Scholar]
- [36].van Vliet AR, Verfaillie T, Agostinis P, New functions of mitochondria associated membranes in cellular signaling, Biochimica et biophysica acta, 1843 (2014) 2253–2262. [DOI] [PubMed] [Google Scholar]
- [37].Rizzuto R, Pinton P, Carrington W, Fay FS, Fogarty KE, Lifshitz LM, Tuft RA, Pozzan T, Close contacts with the endoplasmic reticulum as determinants of mitochondrial Ca2+ responses, Science, 280 (1998) 1763–1766. [DOI] [PubMed] [Google Scholar]
- [38].Szabadkai G, Simoni AM, Rizzuto R, Mitochondrial Ca2+ uptake requires sustained Ca2+ release from the endoplasmic reticulum, The Journal of biological chemistry, 278 (2003) 15153–15161. [DOI] [PubMed] [Google Scholar]
- [39].Griffiths EJ, Rutter GA, Mitochondrial calcium as a key regulator of mitochondrial ATP production in mammalian cells, Biochimica et biophysica acta, 1787 (2009) 1324–1333. [DOI] [PubMed] [Google Scholar]
- [40].Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R, Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis, Oncogene, 27 (2008) 6407–6418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Bononi A, Bonora M, Marchi S, Missiroli S, Poletti F, Giorgi C, Pandolfi PP, Pinton P, Identification of PTEN at the ER and MAMs and its regulation of Ca(2+) signaling and apoptosis in a protein phosphatase-dependent manner, Cell death and differentiation, 20 (2013) 1631–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Cardenas C, Miller RA, Smith I, Bui T, Molgo J, Muller M, Vais H, Cheung KH, Yang J, Parker I, Thompson CB, Birnbaum MJ, Hallows KR, Foskett JK, Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria, Cell, 142 (2010) 270–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Hoth M, Fanger CM, Lewis RS, Mitochondrial regulation of store-operated calcium signaling in T lymphocytes, The Journal of cell biology, 137 (1997) 633–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Pizzo P, Lissandron V, Capitanio P, Pozzan T, Ca(2+) signalling in the Golgi apparatus, Cell calcium, 50 (2011) 184–192. [DOI] [PubMed] [Google Scholar]
- [45].Arundine M, Tymianski M, Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity, Cell calcium, 34 (2003) 325–337. [DOI] [PubMed] [Google Scholar]
- [46].Bernardi P, Di Lisa F, Fogolari F, Lippe G, From ATP to PTP and Back: A Dual Function for the Mitochondrial ATP Synthase, Circulation research, 116 (2015) 1850–1862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Sena LA, Chandel NS, Physiological roles of mitochondrial reactive oxygen species, Molecular cell, 48 (2012) 158–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Andersen JK, Oxidative stress in neurodegeneration: cause or consequence?, Nat Med, 10 Suppl (2004) S18–25. [DOI] [PubMed] [Google Scholar]
- [49].Hardy JA, Higgins GA, Alzheimer’s disease: the amyloid cascade hypothesis, Science, 256 (1992) 184–185. [DOI] [PubMed] [Google Scholar]
- [50].Panza F, Solfrizzi V, Imbimbo BP, Logroscino G, Amyloid-directed monoclonal antibodies for the treatment of Alzheimer’s disease: the point of no return?, Expert opinion on biological therapy, 14 (2014) 1465–1476. [DOI] [PubMed] [Google Scholar]
- [51].Selkoe DJ, Hardy J, The amyloid hypothesis of Alzheimer’s disease at 25 years, EMBO molecular medicine, 8 (2016) 595–608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Davies P, Maloney AJ, Selective loss of central cholinergic neurons in Alzheimer’s disease, Lancet, 2 (1976) 1403. [DOI] [PubMed] [Google Scholar]
- [53].Frost B, Jacks RL, Diamond MI, Propagation of tau misfolding from the outside to the inside of a cell, The Journal of biological chemistry, 284 (2009) 12845–12852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].McGeer PL, Rogers J, Anti-inflammatory agents as a therapeutic approach to Alzheimer’s disease, Neurology, 42 (1992) 447–449. [DOI] [PubMed] [Google Scholar]
- [55].Tarasoff-Conway JM, Carare RO, Osorio RS, Glodzik L, Butler T, Fieremans E, Axel L, Rusinek H, Nicholson C, Zlokovic BV, Frangione B, Blennow K, Menard J, Zetterberg H, Wisniewski T, de Leon MJ, Clearance systems in the brain-implications for Alzheimer disease, Nature reviews. Neurology, 11 (2015) 457–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Khachaturian ZS, The role of calcium regulation in brain aging: reexamination of a hypothesis, Aging, 1 (1989) 17–34. [DOI] [PubMed] [Google Scholar]
- [57].Boada M, Antunez C, Lopez-Arrieta J, Galan JJ, Moron FJ, Hernandez I, Marin J, Martinez-Lage P, Alegret M, Carrasco JM, Moreno C, Real LM, Gonzalez-Perez A, Tarraga L, Ruiz A, CALHM1 P86L polymorphism is associated with late-onset Alzheimer’s disease in a recessive model, Journal of Alzheimer’s disease : JAD, 20 (2010) 247–251. [DOI] [PubMed] [Google Scholar]
- [58].Tolar M, Keller JN, Chan S, Mattson MP, Marques MA, Crutcher KA, Truncated apolipoprotein E (ApoE) causes increased intracellular calcium and may mediate ApoE neurotoxicity, The Journal of neuroscience : the official journal of the Society for Neuroscience, 19 (1999) 7100–7110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zatti G, Ghidoni R, Barbiero L, Binetti G, Pozzan T, Fasolato C, Pizzo P, The presenilin 2 M239I mutation associated with familial Alzheimer’s disease reduces Ca2+ release from intracellular stores, Neurobiology of disease, 15 (2004) 269–278. [DOI] [PubMed] [Google Scholar]
- [60].Calvo-Rodriguez M, Bacskai BJ, Mitochondria and Calcium in Alzheimer’s Disease: From Cell Signaling to Neuronal Cell Death, Trends in neurosciences, (2020). [DOI] [PubMed] [Google Scholar]
- [61].Chen JX, Yan SD, Amyloid-beta-induced mitochondrial dysfunction, Journal of Alzheimer’s disease : JAD, 12 (2007) 177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Cieri D, Vicario M, Vallese F, D’Orsi B, Berto P, Grinzato A, Catoni C, De Stefani D, Rizzuto R, Brini M, Cali T, Tau localises within mitochondrial sub-compartments and its caspase cleavage affects ER-mitochondria interactions and cellular Ca(2+) handling, Biochimica et biophysica acta. Molecular basis of disease, 1864 (2018) 3247–3256. [DOI] [PubMed] [Google Scholar]
- [63].Cha MY, Han SH, Son SM, Hong HS, Choi YJ, Byun J, Mook-Jung I, Mitochondria-specific accumulation of amyloid beta induces mitochondrial dysfunction leading to apoptotic cell death, PloS one, 7 (2012) e34929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Caspersen C, Wang N, Yao J, Sosunov A, Chen X, Lustbader JW, Xu HW, Stern D, McKhann G, Yan SD, Mitochondrial Abeta: a potential focal point for neuronal metabolic dysfunction in Alzheimer’s disease, FASEB journal : official publication of the Federation of American Societies for Experimental Biology, 19 (2005) 2040–2041. [DOI] [PubMed] [Google Scholar]
- [65].Devi L, Prabhu BM, Galati DF, Avadhani NG, Anandatheerthavarada HK, Accumulation of amyloid precursor protein in the mitochondrial import channels of human Alzheimer’s disease brain is associated with mitochondrial dysfunction, The Journal of neuroscience : the official journal of the Society for Neuroscience, 26 (2006) 9057–9068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hansson Petersen CA, Alikhani N, Behbahani H, Wiehager B, Pavlov PF, Alafuzoff I, Leinonen V, Ito A, Winblad B, Glaser E, Ankarcrona M, The amyloid beta-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 13145–13150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Manczak M, Reddy PH, Abnormal interaction of VDAC1 with amyloid beta and phosphorylated tau causes mitochondrial dysfunction in Alzheimer’s disease, Human molecular genetics, 21 (2012) 5131–5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Xie H, Guan J, Borrelli LA, Xu J, Serrano-Pozo A, Bacskai BJ, Mitochondrial alterations near amyloid plaques in an Alzheimer’s disease mouse model, The Journal of neuroscience : the official journal of the Society for Neuroscience, 33 (2013) 17042–17051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Hirai K, Aliev G, Nunomura A, Fujioka H, Russell RL, Atwood CS, Johnson AB, Kress Y, Vinters HV, Tabaton M, Shimohama S, Cash AD, Siedlak SL, Harris PL, Jones PK, Petersen RB, Perry G, Smith MA, Mitochondrial abnormalities in Alzheimer’s disease, The Journal of neuroscience : the official journal of the Society for Neuroscience, 21 (2001) 3017–3023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Wang X, Su B, Siedlak SL, Moreira PI, Fujioka H, Wang Y, Casadesus G, Zhu X, Amyloid-beta overproduction causes abnormal mitochondrial dynamics via differential modulation of mitochondrial fission/fusion proteins, Proceedings of the National Academy of Sciences of the United States of America, 105 (2008) 19318–19323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Pigino G, Morfini G, Pelsman A, Mattson MP, Brady ST, Busciglio J, Alzheimer’s presenilin 1 mutations impair kinesin-based axonal transport, The Journal of neuroscience : the official journal of the Society for Neuroscience, 23 (2003) 4499–4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Sanz-Blasco S, Valero RA, Rodriguez-Crespo I, Villalobos C, Nunez L, Mitochondrial Ca2+ overload underlies Abeta oligomers neurotoxicity providing an unexpected mechanism of neuroprotection by NSAIDs, PloS one, 3 (2008) e2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Calvo-Rodriguez M, Garcia-Durillo M, Villalobos C, Nunez L, Aging Enables Ca2+ Overload and Apoptosis Induced by Amyloid-beta Oligomers in Rat Hippocampal Neurons: Neuroprotection by Non-Steroidal Anti-Inflammatory Drugs and R-Flurbiprofen in Aging Neurons, Journal of Alzheimer’s disease : JAD, 54 (2016) 207–221. [DOI] [PubMed] [Google Scholar]
- [74].Ferreiro E, Oliveira CR, Pereira CM, The release of calcium from the endoplasmic reticulum induced by amyloid-beta and prion peptides activates the mitochondrial apoptotic pathway, Neurobiology of disease, 30 (2008) 331–342. [DOI] [PubMed] [Google Scholar]
- [75].Calvo-Rodriguez M, Hernando-Perez E, Nunez L, Villalobos C, Amyloid beta Oligomers Increase ER-Mitochondria Ca(2+) Cross Talk in Young Hippocampal Neurons and Exacerbate Aging-Induced Intracellular Ca(2+) Remodeling, Frontiers in cellular neuroscience, 13 (2019) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Calvo-Rodriguez M, Hou SS, Snyder AC, Kharitonova EK, Russ AN, Das S, Fan Z, Muzikansky A, Garcia-Alloza M, Serrano-Pozo A, Hudry E, Bacskai BJ, Increased mitochondrial calcium levels associated with neuronal death in a mouse model of Alzheimer’s disease, Nature communications, 11 (2020) 2146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Calvo-Rodriguez M, Bacskai BJ, High mitochondrial calcium levels precede neuronal death in vivo in Alzheimer’s disease, Cell stress, 4 (2020) 187–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Stamer K, Vogel R, Thies E, Mandelkow E, Mandelkow EM, Tau blocks traffic of organelles, neurofilaments, and APP vesicles in neurons and enhances oxidative stress, The Journal of cell biology, 156 (2002) 1051–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Quintanilla RA, Matthews-Roberson TA, Dolan PJ, Johnson GV, Caspase-cleaved tau expression induces mitochondrial dysfunction in immortalized cortical neurons: implications for the pathogenesis of Alzheimer disease, The Journal of biological chemistry, 284 (2009) 18754–18766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Gomez-Ramos A, Diaz-Hernandez M, Rubio A, Miras-Portugal MT, Avila J, Extracellular tau promotes intracellular calcium increase through M1 and M3 muscarinic receptors in neuronal cells, Molecular and cellular neurosciences, 37 (2008) 673–681. [DOI] [PubMed] [Google Scholar]
- [81].Britti E, Ros J, Esteras N, Abramov AY, Tau inhibits mitochondrial calcium efflux and makes neurons vulnerable to calcium-induced cell death, Cell calcium, 86 (2020) 102150. [DOI] [PubMed] [Google Scholar]
- [82].De Jager PL, Ma Y, McCabe C, Xu J, Vardarajan BN, Felsky D, Klein HU, White CC, Peters MA, Lodgson B, Nejad P, Tang A, Mangravite LM, Yu L, Gaiteri C, Mostafavi S, Schneider JA, Bennett DA, A multi-omic atlas of the human frontal cortex for aging and Alzheimer’s disease research, Scientific data, 5 (2018) 180142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Hodes RJ, Buckholtz N, Accelerating Medicines Partnership: Alzheimer’s Disease (AMP-AD) Knowledge Portal Aids Alzheimer’s Drug Discovery through Open Data Sharing, Expert opinion on therapeutic targets, 20 (2016) 389–391. [DOI] [PubMed] [Google Scholar]
- [84].Wang M, Beckmann ND, Roussos P, Wang E, Zhou X, Wang Q, Ming C, Neff R, Ma W, Fullard JF, Hauberg ME, Bendl J, Peters MA, Logsdon B, Wang P, Mahajan M, Mangravite LM, Dammer EB, Duong DM, Lah JJ, Seyfried NT, Levey AI, Buxbaum JD, Ehrlich M, Gandy S, Katsel P, Haroutunian V, Schadt E, Zhang B, The Mount Sinai cohort of large-scale genomic, transcriptomic and proteomic data in Alzheimer’s disease, Scientific data, 5 (2018) 180185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Jadiya P, Kolmetzky DW, Tomar D, Di Meco A, Lombardi AA, Lambert JP, Luongo TS, Ludtmann MH, Pratico D, Elrod JW, Impaired mitochondrial calcium efflux contributes to disease progression in models of Alzheimer’s disease, Nature communications, 10 (2019) 3885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Hedskog L, Pinho CM, Filadi R, Ronnback A, Hertwig L, Wiehager B, Larssen P, Gellhaar S, Sandebring A, Westerlund M, Graff C, Winblad B, Galter D, Behbahani H, Pizzo P, Glaser E, Ankarcrona M, Modulation of the endoplasmic reticulum-mitochondria interface in Alzheimer’s disease and related models, Proceedings of the National Academy of Sciences of the United States of America, 110 (2013) 7916–7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Area-Gomez E, Del Carmen Lara Castillo M, Tambini MD, Guardia-Laguarta C, de Groof AJ, Madra M, Ikenouchi J, Umeda M, Bird TD, Sturley SL, Schon EA, Upregulated function of mitochondria-associated ER membranes in Alzheimer disease, The EMBO journal, 31 (2012) 4106–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Zampese E, Fasolato C, Kipanyula MJ, Bortolozzi M, Pozzan T, Pizzo P, Presenilin 2 modulates endoplasmic reticulum (ER)-mitochondria interactions and Ca2+ cross-talk, Proceedings of the National Academy of Sciences of the United States of America, 108 (2011) 2777–2782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Gandhi S, Vaarmann A, Yao Z, Duchen MR, Wood NW, Abramov AY, Dopamine induced neurodegeneration in a PINK1 model of Parkinson’s disease, PloS one, 7 (2012) e37564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Schapira AH, Cooper JM, Dexter D, Clark JB, Jenner P, Marsden CD, Mitochondrial complex I deficiency in Parkinson’s disease, Journal of neurochemistry, 54 (1990) 823–827. [DOI] [PubMed] [Google Scholar]
- [91].Zhang J, Perry G, Smith MA, Robertson D, Olson SJ, Graham DG, Montine TJ, Parkinson’s disease is associated with oxidative damage to cytoplasmic DNA and RNA in substantia nigra neurons, The American journal of pathology, 154 (1999) 1423–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Dias V, Junn E, Mouradian MM, The role of oxidative stress in Parkinson’s disease, Journal of Parkinson’s disease, 3 (2013) 461–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Luoma P, Melberg A, Rinne JO, Kaukonen JA, Nupponen NN, Chalmers RM, Oldfors A, Rautakorpi I, Peltonen L, Majamaa K, Somer H, Suomalainen A, Parkinsonism, premature menopause, and mitochondrial DNA polymerase gamma mutations: clinical and molecular genetic study, Lancet, 364 (2004) 875–882. [DOI] [PubMed] [Google Scholar]
- [94].Zaichick SV, McGrath KM, Caraveo G, The role of Ca(2+) signaling in Parkinson’s disease, Disease models & mechanisms, 10 (2017) 519–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Surmeier DJ, Schumacker PT, Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease, The Journal of biological chemistry, 288 (2013) 10736–10741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zakharov SD, Hulleman JD, Dutseva EA, Antonenko YN, Rochet JC, Cramer WA, Helical alpha-synuclein forms highly conductive ion channels, Biochemistry, 46 (2007) 14369–14379. [DOI] [PubMed] [Google Scholar]
- [97].Angelova PR, Ludtmann MH, Horrocks MH, Negoda A, Cremades N, Klenerman D, Dobson CM, Wood NW, Pavlov EV, Gandhi S, Abramov AY, Ca2+ is a key factor in alpha-synuclein-induced neurotoxicity, Journal of cell science, 129 (2016) 1792–1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Devi L, Raghavendran V, Prabhu BM, Avadhani NG, Anandatheerthavarada HK, Mitochondrial import and accumulation of alpha-synuclein impair complex I in human dopaminergic neuronal cultures and Parkinson disease brain, The Journal of biological chemistry, 283 (2008) 9089–9100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Pozo Devoto VM, Dimopoulos N, Alloatti M, Pardi MB, Saez TM, Otero MG, Cromberg LE, Marin-Burgin A, Scassa ME, Stokin GB, Schinder AF, Sevlever G, Falzone TL, alphaSynuclein control of mitochondrial homeostasis in human-derived neurons is disrupted by mutations associated with Parkinson’s disease, Scientific reports, 7 (2017) 5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Guardia-Laguarta C, Area-Gomez E, Rub C, Liu Y, Magrane J, Becker D, Voos W, Schon EA, Przedborski S, alpha-Synuclein is localized to mitochondria-associated ER membranes, The Journal of neuroscience : the official journal of the Society for Neuroscience, 34 (2014) 249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Tolosa E, Vila M, Klein C, Rascol O, LRRK2 in Parkinson disease: challenges of clinical trials, Nature reviews. Neurology, 16 (2020) 97–107. [DOI] [PubMed] [Google Scholar]
- [102].Exner N, Lutz AK, Haass C, Winklhofer KF, Mitochondrial dysfunction in Parkinson’s disease: molecular mechanisms and pathophysiological consequences, The EMBO journal, 31 (2012) 3038–3062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Pickrell AM, Youle RJ, The roles of PINK1, parkin, and mitochondrial fidelity in Parkinson’s disease, Neuron, 85 (2015) 257–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Das Banerjee T, Dagda RY, Dagda M, Chu CT, Rice M, Vazquez-Mayorga E, Dagda RK, PINK1 regulates mitochondrial trafficking in dendrites of cortical neurons through mitochondrial PKA, Journal of neurochemistry, 142 (2017) 545–559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Gandhi S, Wood-Kaczmar A, Yao Z, Plun-Favreau H, Deas E, Klupsch K, Downward J, Latchman DS, Tabrizi SJ, Wood NW, Duchen MR, Abramov AY, PINK1-associated Parkinson’s disease is caused by neuronal vulnerability to calcium-induced cell death, Molecular cell, 33 (2009) 627–638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Schapira AH, Calcium dysregulation in Parkinson’s disease, Brain : a journal of neurology, 136 (2013) 2015–2016. [DOI] [PubMed] [Google Scholar]
- [107].Kostic M, Ludtmann MH, Bading H, Hershfinkel M, Steer E, Chu CT, Abramov AY, Sekler I, PKA Phosphorylation of NCLX Reverses Mitochondrial Calcium Overload and Depolarization, Promoting Survival of PINK1-Deficient Dopaminergic Neurons, Cell reports, 13 (2015) 376–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Guzman JN, Sanchez-Padilla J, Wokosin D, Kondapalli J, Ilijic E, Schumacker PT, Surmeier DJ, Oxidant stress evoked by pacemaking in dopaminergic neurons is attenuated by DJ-1, Nature, 468 (2010) 696–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Soman SK, Bazala M, Keatinge M, Bandmann O, Kuznicki J, Restriction of mitochondrial calcium overload by mcu inactivation renders a neuroprotective effect in zebrafish models of Parkinson’s disease, Biology open, 8 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Jin J, Li GJ, Davis J, Zhu D, Wang Y, Pan C, Zhang J, Identification of novel proteins associated with both alpha-synuclein and DJ-1, Molecular & cellular proteomics : MCP, 6 (2007) 845–859. [DOI] [PubMed] [Google Scholar]
- [111].Cali T, Ottolini D, Negro A, Brini M, alpha-Synuclein controls mitochondrial calcium homeostasis by enhancing endoplasmic reticulum-mitochondria interactions, The Journal of biological chemistry, 287 (2012) 17914–17929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Ottolini D, Cali T, Negro A, Brini M, The Parkinson disease-related protein DJ-1 counteracts mitochondrial impairment induced by the tumour suppressor protein p53 by enhancing endoplasmic reticulum-mitochondria tethering, Human molecular genetics, 22 (2013) 2152–2168. [DOI] [PubMed] [Google Scholar]
- [113].Paillusson S, Gomez-Suaga P, Stoica R, Little D, Gissen P, Devine MJ, Noble W, Hanger DP, Miller CCJ, alpha-Synuclein binds to the ER-mitochondria tethering protein VAPB to disrupt Ca(2+) homeostasis and mitochondrial ATP production, Acta neuropathologica, 134 (2017) 129–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O’Regan JP, Deng HX, et al. , Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis, Nature, 362 (1993) 59–62. [DOI] [PubMed] [Google Scholar]
- [115].Mejzini R, Flynn LL, Pitout IL, Fletcher S, Wilton SD, Akkari PA, ALS Genetics, Mechanisms, and Therapeutics: Where Are We Now?, Frontiers in neuroscience, 13 (2019) 1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Tan W, Pasinelli P, Trotti D, Role of mitochondria in mutant SOD1 linked amyotrophic lateral sclerosis, Biochimica et biophysica acta, 1842 (2014) 1295–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Magrane J, Manfredi G, Mitochondrial function, morphology, and axonal transport in amyotrophic lateral sclerosis, Antioxidants & redox signaling, 11 (2009) 1615–1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Kong J, Xu Z, Massive mitochondrial degeneration in motor neurons triggers the onset of amyotrophic lateral sclerosis in mice expressing a mutant SOD1, The Journal of neuroscience : the official journal of the Society for Neuroscience, 18 (1998) 3241–3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Foran E, Trotti D, Glutamate transporters and the excitotoxic path to motor neuron degeneration in amyotrophic lateral sclerosis, Antioxidants & redox signaling, 11 (2009) 1587–1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Webster CP, Smith EF, Shaw PJ, De Vos KJ, Protein Homeostasis in Amyotrophic Lateral Sclerosis: Therapeutic Opportunities?, Frontiers in molecular neuroscience, 10 (2017) 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Ilieva EV, Ayala V, Jove M, Dalfo E, Cacabelos D, Povedano M, Bellmunt MJ, Ferrer I, Pamplona R, Portero-Otin M, Oxidative and endoplasmic reticulum stress interplay in sporadic amyotrophic lateral sclerosis, Brain : a journal of neurology, 130 (2007) 3111–3123. [DOI] [PubMed] [Google Scholar]
- [122].Fahandejsaadi A, Leung E, Rahaii R, Bu J, Geula C, Calbindin-D28K, parvalbumin and calretinin in primate lower motor neurons, Neuroreport, 15 (2004) 443–448. [DOI] [PubMed] [Google Scholar]
- [123].Van Den Bosch L, Schwaller B, Vleminckx V, Meijers B, Stork S, Ruehlicke T, Van Houtte E, Klaassen H, Celio MR, Missiaen L, Robberecht W, Berchtold MW, Protective effect of parvalbumin on excitotoxic motor neuron death, Experimental neurology, 174 (2002) 150–161. [DOI] [PubMed] [Google Scholar]
- [124].Beers DR, Ho BK, Siklos L, Alexianu ME, Mosier DR, Mohamed AH, Otsuka Y, Kozovska ME, McAlhany RE, Smith RG, Appel SH, Parvalbumin overexpression alters immune-mediated increases in intracellular calcium, and delays disease onset in a transgenic model of familial amyotrophic lateral sclerosis, Journal of neurochemistry, 79 (2001) 499–509. [DOI] [PubMed] [Google Scholar]
- [125].Spalloni A, Albo F, Ferrari F, Mercuri N, Bernardi G, Zona C, Longone P, Cu/Zn-superoxide dismutase (GLY93-->ALA) mutation alters AMPA receptor subunit expression and function and potentiates kainate-mediated toxicity in motor neurons in culture, Neurobiology of disease, 15 (2004) 340–350. [DOI] [PubMed] [Google Scholar]
- [126].Ikonomidou C, Qin Qin Y, Labruyere J, Olney JW, Motor neuron degeneration induced by excitotoxin agonists has features in common with those seen in the SOD-1 transgenic mouse model of amyotrophic lateral sclerosis, Journal of neuropathology and experimental neurology, 55 (1996) 211–224. [DOI] [PubMed] [Google Scholar]
- [127].Carriedo SG, Sensi SL, Yin HZ, Weiss JH, AMPA exposures induce mitochondrial Ca(2+) overload and ROS generation in spinal motor neurons in vitro, The Journal of neuroscience : the official journal of the Society for Neuroscience, 20 (2000) 240–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].von Lewinski F, Keller BU, Ca2+, mitochondria and selective motoneuron vulnerability: implications for ALS, Trends in neurosciences, 28 (2005) 494–500. [DOI] [PubMed] [Google Scholar]
- [129].Sasaki S, Warita H, Murakami T, Abe K, Iwata M, Ultrastructural study of mitochondria in the spinal cord of transgenic mice with a G93A mutant SOD1 gene, Acta neuropathologica, 107 (2004) 461–474. [DOI] [PubMed] [Google Scholar]
- [130].Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL, An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria, Neuron, 14 (1995) 1105–1116. [DOI] [PubMed] [Google Scholar]
- [131].Jaarsma D, Rognoni F, van Duijn W, Verspaget HW, Haasdijk ED, Holstege JC, CuZn superoxide dismutase (SOD1) accumulates in vacuolated mitochondria in transgenic mice expressing amyotrophic lateral sclerosis-linked SOD1 mutations, Acta neuropathologica, 102 (2001) 293–305. [DOI] [PubMed] [Google Scholar]
- [132].Rao SD, Yin HZ, Weiss JH, Disruption of glial glutamate transport by reactive oxygen species produced in motor neurons, The Journal of neuroscience : the official journal of the Society for Neuroscience, 23 (2003) 2627–2633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Damiano M, Starkov AA, Petri S, Kipiani K, Kiaei M, Mattiazzi M, Flint Beal M, Manfredi G, Neural mitochondrial Ca2+ capacity impairment precedes the onset of motor symptoms in G93A Cu/Zn-superoxide dismutase mutant mice, Journal of neurochemistry, 96 (2006) 1349–1361. [DOI] [PubMed] [Google Scholar]
- [134].Fuchs A, Kutterer S, Muhling T, Duda J, Schutz B, Liss B, Keller BU, Roeper J, Selective mitochondrial Ca2+ uptake deficit in disease endstage vulnerable motoneurons of the SOD1G93A mouse model of amyotrophic lateral sclerosis, The Journal of physiology, 591 (2013) 2723–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].David G, Mitochondrial clearance of cytosolic Ca(2+) in stimulated lizard motor nerve terminals proceeds without progressive elevation of mitochondrial matrix [Ca(2+)], The Journal of neuroscience : the official journal of the Society for Neuroscience, 19 (1999) 7495–7506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Stoica R, Paillusson S, Gomez-Suaga P, Mitchell JC, Lau DH, Gray EH, Sancho RM, Vizcay-Barrena G, De Vos KJ, Shaw CE, Hanger DP, Noble W, Miller CC, ALS/FTD-associated FUS activates GSK-3beta to disrupt the VAPB-PTPIP51 interaction and ER-mitochondria associations, EMBO reports, 17 (2016) 1326–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Stoica R, De Vos KJ, Paillusson S, Mueller S, Sancho RM, Lau KF, Vizcay-Barrena G, Lin WL, Xu YF, Lewis J, Dickson DW, Petrucelli L, Mitchell JC, Shaw CE, Miller CC, ER-mitochondria associations are regulated by the VAPB-PTPIP51 interaction and are disrupted by ALS/FTD-associated TDP-43, Nature communications, 5 (2014) 3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Paredes RM, Etzler JC, Watts LT, Zheng W, Lechleiter JD, Chemical calcium indicators, Methods, 46 (2008) 143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P, A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals, Cell calcium, 28 (2000) 213–223. [DOI] [PubMed] [Google Scholar]
- [140].Pozzan T, Rudolf R, Measurements of mitochondrial calcium in vivo, Biochimica et biophysica acta, 1787 (2009) 1317–1323. [DOI] [PubMed] [Google Scholar]
- [141].Minta A, Kao JP, Tsien RY, Fluorescent indicators for cytosolic calcium based on rhodamine and fluorescein chromophores, The Journal of biological chemistry, 264 (1989) 8171–8178. [PubMed] [Google Scholar]
- [142].Hajnoczky G, Robb-Gaspers LD, Seitz MB, Thomas AP, Decoding of cytosolic calcium oscillations in the mitochondria, Cell, 82 (1995) 415–424. [DOI] [PubMed] [Google Scholar]
- [143].Tjalkens RB, Zoran MJ, Mohl B, Barhoumi R, Manganese suppresses ATP-dependent intercellular calcium waves in astrocyte networks through alteration of mitochondrial and endoplasmic reticulum calcium dynamics, Brain research, 1113 (2006) 210–219. [DOI] [PubMed] [Google Scholar]
- [144].Peng TI, Greenamyre JT, Privileged access to mitochondria of calcium influx through N-methyl-D-aspartate receptors, Molecular pharmacology, 53 (1998) 974–980. [PubMed] [Google Scholar]
- [145].Peng TI, Jou MJ, Sheu SS, Greenamyre JT, Visualization of NMDA receptor-induced mitochondrial calcium accumulation in striatal neurons, Experimental neurology, 149 (1998) 1–12.9454610 [Google Scholar]
- [146].Muriel MP, Lambeng N, Darios F, Michel PP, Hirsch EC, Agid Y, Ruberg M, Mitochondrial free calcium levels (Rhod-2 fluorescence) and ultrastructural alterations in neuronally differentiated PC12 cells during ceramide-dependent cell death, The Journal of comparative neurology, 426 (2000) 297–315. [DOI] [PubMed] [Google Scholar]
- [147].Tsien RY, A non-disruptive technique for loading calcium buffers and indicators into cells, Nature, 290 (1981) 527–528. [DOI] [PubMed] [Google Scholar]
- [148].Del Nido PJ, Glynn P, Buenaventura P, Salama G, Koretsky AP, Fluorescence measurement of calcium transients in perfused rabbit heart using rhod 2, The American journal of physiology, 274 (1998) H728–741. [DOI] [PubMed] [Google Scholar]
- [149].Chalfie M, Tu Y, Euskirchen G, Ward WW, Prasher DC, Green fluorescent protein as a marker for gene expression, Science, 263 (1994) 802–805. [DOI] [PubMed] [Google Scholar]
- [150].Chudakov DM, Lukyanov S, Lukyanov KA, Fluorescent proteins as a toolkit for in vivo imaging, Trends in biotechnology, 23 (2005) 605–613. [DOI] [PubMed] [Google Scholar]
- [151].Shaner NC, Steinbach PA, Tsien RY, A guide to choosing fluorescent proteins, Nature methods, 2 (2005) 905–909. [DOI] [PubMed] [Google Scholar]
- [152].Demaurex N, Calcium measurements in organelles with Ca2+-sensitive fluorescent proteins, Cell calcium, 38 (2005) 213–222. [DOI] [PubMed] [Google Scholar]
- [153].Suzuki J, Kanemaru K, Iino M, Genetically Encoded Fluorescent Indicators for Organellar Calcium Imaging, Biophysical journal, 111 (2016) 1119–1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Williams DA, Monif M, Richardson KL, Compartmentalizing genetically encoded calcium sensors, Methods in molecular biology, 937 (2013) 307–326. [DOI] [PubMed] [Google Scholar]
- [155].Shimomura O, Johnson FH, Saiga Y, Extraction, purification and properties of aequorin, a bioluminescent protein from the luminous hydromedusan, Aequorea, Journal of cellular and comparative physiology, 59 (1962) 223–239. [DOI] [PubMed] [Google Scholar]
- [156].Robert V, Pinton P, Tosello V, Rizzuto R, Pozzan T, Recombinant aequorin as tool for monitoring calcium concentration in subcellular compartments, Methods in enzymology, 327 (2000) 440–456. [DOI] [PubMed] [Google Scholar]
- [157].Rizzuto R, Simpson AW, Brini M, Pozzan T, Rapid changes of mitochondrial Ca2+ revealed by specifically targeted recombinant aequorin, Nature, 358 (1992) 325–327. [DOI] [PubMed] [Google Scholar]
- [158].Ottolini D, Cali T, Brini M, Methods to measure intracellular Ca(2+) fluxes with organelle-targeted aequorin-based probes, Methods in enzymology, 543 (2014) 21–45. 6 [DOI] [PubMed] [Google Scholar]
- [159].Bonora M, Giorgi C, Bononi A, Marchi S, Patergnani S, Rimessi A, Rizzuto R, Pinton P, Subcellular calcium measurements in mammalian cells using jellyfish photoprotein aequorin-based probes, Nature protocols, 8 (2013) 2105–2118. [DOI] [PubMed] [Google Scholar]
- [160].Miyawaki A, Llopis J, Heim R, McCaffery JM, Adams JA, Ikura M, Tsien RY, Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin, Nature, 388 (1997) 882–887. [DOI] [PubMed] [Google Scholar]
- [161].Romoser VA, Hinkle PM, Persechini A, Detection in living cells of Ca2+-dependent changes in the fluorescence emission of an indicator composed of two green fluorescent protein variants linked by a calmodulin-binding sequence. A new class of fluorescent indicators, The Journal of biological chemistry, 272 (1997) 13270–13274. [DOI] [PubMed] [Google Scholar]
- [162].Zhang J, Campbell RE, Ting AY, Tsien RY, Creating new fluorescent probes for cell biology, Nature reviews. Molecular cell biology, 3 (2002) 906–918. [DOI] [PubMed] [Google Scholar]
- [163].Lutcke H, Murayama M, Hahn T, Margolis DJ, Astori S, Zum Alten Borgloh SM, Gobel W, Yang Y, Tang W, Kugler S, Sprengel R, Nagai T, Miyawaki A, Larkum ME, Helmchen F, Hasan MT, Optical recording of neuronal activity with a genetically-encoded calcium indicator in anesthetized and freely moving mice, Frontiers in neural circuits, 4 (2010) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [164].Rose T, Goltstein PM, Portugues R, Griesbeck O, Putting a finishing touch on GECIs, Frontiers in molecular neuroscience, 7 (2014) 88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [165].Tian L, Hires SA, Looger LL, Imaging neuronal activity with genetically encoded calcium indicators, Cold Spring Harbor protocols, 2012 (2012) 647–656. [DOI] [PubMed] [Google Scholar]
- [166].Ai HW, Fluorescent-protein-based probes: general principles and practices, Analytical and bioanalytical chemistry, 407 (2015) 9–15. [DOI] [PubMed] [Google Scholar]
- [167].Palmer AE, Qin Y, Park JG, McCombs JE, Design and application of genetically encoded biosensors, Trends in biotechnology, 29 (2011) 144–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [168].Baird GS, Zacharias DA, Tsien RY, Circular permutation and receptor insertion within green fluorescent proteins, Proceedings of the National Academy of Sciences of the United States of America, 96 (1999) 11241–11246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [169].Griesbeck O, Baird GS, Campbell RE, Zacharias DA, Tsien RY, Reducing the environmental sensitivity of yellow fluorescent protein. Mechanism and applications, The Journal of biological chemistry, 276 (2001) 29188–29194. [DOI] [PubMed] [Google Scholar]
- [170].Nagai T, Sawano A, Park ES, Miyawaki A, Circularly permuted green fluorescent proteins engineered to sense Ca2+, Proceedings of the National Academy of Sciences of the United States of America, 98 (2001) 3197–3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [171].Tallini YN, Ohkura M, Choi BR, Ji G, Imoto K, Doran R, Lee J, Plan P, Wilson J, Xin HB, Sanbe A, Gulick J, Mathai J, Robbins J, Salama G, Nakai J, Kotlikoff MI, Imaging cellular signals in the heart in vivo: Cardiac expression of the high-signal Ca2+ indicator GCaMP2, Proceedings of the National Academy of Sciences of the United States of America, 103 (2006) 4753–4758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [172].Akerboom J, Chen TW, Wardill TJ, Tian L, Marvin JS, Mutlu S, Calderon NC, Esposti F, Borghuis BG, Sun XR, Gordus A, Orger MB, Portugues R, Engert F, Macklin JJ, Filosa A, Aggarwal A, Kerr RA, Takagi R, Kracun S, Shigetomi E, Khakh BS, Baier H, Lagnado L, Wang SS, Bargmann CI, Kimmel BE, Jayaraman V, Svoboda K, Kim DS, Schreiter ER, Looger LL, Optimization of a GCaMP calcium indicator for neural activity imaging, The Journal of neuroscience : the official journal of the Society for Neuroscience, 32 (2012) 13819–13840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [173].Chen TW, Wardill TJ, Sun Y, Pulver SR, Renninger SL, Baohan A, Schreiter ER, Kerr RA, Orger MB, Jayaraman V, Looger LL, Svoboda K, Kim DS, Ultrasensitive fluorescent proteins for imaging neuronal activity, Nature, 499 (2013) 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Dana H, Sun Y, Mohar B, Hulse BK, Kerlin AM, Hasseman JP, Tsegaye G, Tsang A, Wong A, Patel R, Macklin JJ, Chen Y, Konnerth A, Jayaraman V, Looger LL, Schreiter ER, Svoboda K, Kim DS, High-performance calcium sensors for imaging activity in neuronal populations and microcompartments, Nature methods, 16 (2019) 649–657. [DOI] [PubMed] [Google Scholar]
- [175].Nakai J, Ohkura M, Imoto K, A high signal-to-noise Ca(2+) probe composed of a single green fluorescent protein, Nature biotechnology, 19 (2001) 137–141. [DOI] [PubMed] [Google Scholar]
- [176].Whitaker M, Genetically encoded probes for measurement of intracellular calcium, Methods in cell biology, 99 (2010) 153–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Zhao Y, Araki S, Wu J, Teramoto T, Chang YF, Nakano M, Abdelfattah AS, Fujiwara M, Ishihara T, Nagai T, Campbell RE, An expanded palette of genetically encoded Ca(2)(+) indicators, Science, 333 (2011) 1888–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [178].Shen Y, Dana H, Abdelfattah AS, Patel R, Shea J, Molina RS, Rawal B, Rancic V, Chang YF, Wu L, Chen Y, Qian Y, Wiens MD, Hambleton N, Ballanyi K, Hughes TE, Drobizhev M, Kim DS, Koyama M, Schreiter ER, Campbell RE, A genetically encoded Ca(2+) indicator based on circularly permutated sea anemone red fluorescent protein eqFP578, BMC biology, 16 (2018) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [179].Rodriguez EA, Campbell RE, Lin JY, Lin MZ, Miyawaki A, Palmer AE, Shu X, Zhang J, Tsien RY, The Growing and Glowing Toolbox of Fluorescent and Photoactive Proteins, Trends in biochemical sciences, 42 (2017) 111–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [180].Oheim M, van ‘t Hoff M, Feltz A, Zamaleeva A, Mallet JM, Collot M, New red-fluorescent calcium indicators for optogenetics, photoactivation and multi-color imaging, Biochimica et biophysica acta, 1843 (2014) 2284–2306. [DOI] [PubMed] [Google Scholar]
- [181].Inoue M, Takeuchi A, Horigane S, Ohkura M, Gengyo-Ando K, Fujii H, Kamijo S, Takemoto-Kimura S, Kano M, Nakai J, Kitamura K, Bito H, Rational design of a high-affinity, fast, red calcium indicator R-CaMP2, Nature methods, 12 (2015) 64–70. [DOI] [PubMed] [Google Scholar]
- [182].Dana H, Mohar B, Sun Y, Narayan S, Gordus A, Hasseman JP, Tsegaye G, Holt GT, Hu A, Walpita D, Patel R, Macklin JJ, Bargmann CI, Ahrens MB, Schreiter ER, Jayaraman V, Looger LL, Svoboda K, Kim DS, Sensitive red protein calcium indicators for imaging neural activity, eLife, 5 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [183].Suzuki J, Kanemaru K, Ishii K, Ohkura M, Okubo Y, Iino M, Imaging intraorganellar Ca2+ at subcellular resolution using CEPIA, Nature communications, 5 (2014) 4153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [184].Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A, Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins, Proceedings of the National Academy of Sciences of the United States of America, 101 (2004) 10554–10559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [185].Filippin L, Abad MC, Gastaldello S, Magalhaes PJ, Sandona D, Pozzan T, Improved strategies for the delivery of GFP-based Ca2+ sensors into the mitochondrial matrix, Cell calcium, 37 (2005) 129–136. [DOI] [PubMed] [Google Scholar]
- [186].Barretto RP, Messerschmidt B, Schnitzer MJ, In vivo fluorescence imaging with high-resolution microlenses, Nature methods, 6 (2009) 511–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [187].Werner CT, Williams CJ, Fermelia MR, Lin DT, Li Y, Circuit Mechanisms of Neurodegenerative Diseases: A New Frontier With Miniature Fluorescence Microscopy, Frontiers in neuroscience, 13 (2019) 1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [188].Adelsberger H, Garaschuk O, Konnerth A, Cortical calcium waves in resting newborn mice, Nature neuroscience, 8 (2005) 988–990. [DOI] [PubMed] [Google Scholar]
- [189].Krivitski NM, Kislukhin VV, Snyder JW, MacGibbon DR, Kuznetsova OA, Reasons AM, Depner TA, In vivo measurement of hemodialyzer fiber bundle volume: theory and validation, Kidney international, 54 (1998) 1751–1758. [DOI] [PubMed] [Google Scholar]
- [190].Davenne M, Custody C, Charneau P, Lledo PM, In vivo imaging of migrating neurons in the mammalian forebrain, Chemical senses, 30 Suppl 1 (2005) i115–116. [DOI] [PubMed] [Google Scholar]
- [191].de la Fuente S, Fonteriz RI, Montero M, Alvarez J, Dynamics of mitochondrial [Ca(2+)] measured with the low-Ca(2+)-affinity dye rhod-5N, Cell calcium, 51 (2012) 65–71. [DOI] [PubMed] [Google Scholar]
- [192].Billups B, Forsythe ID, Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses, The Journal of neuroscience : the official journal of the Society for Neuroscience, 22 (2002) 5840–5847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [193].Li H, Wang X, Zhang N, Gottipati MK, Parpura V, Ding S, Imaging of mitochondrial Ca2+ dynamics in astrocytes using cell-specific mitochondria-targeted GCaMP5G/6s: mitochondrial Ca2+ uptake and cytosolic Ca2+ availability via the endoplasmic reticulum store, Cell calcium, 56 (2014) 457–466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Palmer AE, Tsien RY, Measuring calcium signaling using genetically targetable fluorescent indicators, Nature protocols, 1 (2006) 1057–1065. [DOI] [PubMed] [Google Scholar]
- [195].Wu J, Liu L, Matsuda T, Zhao Y, Rebane A, Drobizhev M, Chang YF, Araki S, Arai Y, March K, Hughes TE, Sagou K, Miyata T, Nagai T, Li WH, Campbell RE, Improved orange and red Ca(2)+/− indicators and photophysical considerations for optogenetic applications, ACS chemical neuroscience, 4 (2013) 963–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [196].Wu J, Prole DL, Shen Y, Lin Z, Gnanasekaran A, Liu Y, Chen L, Zhou H, Chen SR, Usachev YM, Taylor CW, Campbell RE, Red fluorescent genetically encoded Ca2+ indicators for use in mitochondria and endoplasmic reticulum, The Biochemical journal, 464 (2014) 13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Kanemaru K, Suzuki J, Taiko I, Iino M, Red fluorescent CEPIA indicators for visualization of Ca(2+) dynamics in mitochondria, Scientific reports, 10 (2020) 2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Calvo-Rodriguez M, Hou SS, Snyder AC, Dujardin S, Shirani H, Nilsson KPR, Bacskai BJ, In vivo detection of tau fibrils and amyloid beta aggregates with luminescent conjugated oligothiophenes and multiphoton microscopy, Acta neuropathologica communications, 7 (2019) 171. [DOI] [PMC free article] [PubMed] [Google Scholar]