Abstract
Introduction
Chlamydia psittaci infection is a zoonotic infectious disease, which mainly inhaled through the lungs when exposed to the secretions of poultry that carry pathogenic bacteria. The traditional respiratory specimens or serological antibody testing is slow, and the false-negative rate is high. Metagenomic next-generation sequencing (mNGS) gives a promising rapid diagnosis tool.
Methods
We retrospectively summarized the clinical characteristics of five C. psittaci pneumonia patients diagnosed by mNGS, conducted a literature review summarizing the clinical characteristics of patients with C. psittaci pneumonia reported since 2010.
Results
Five C. psittaci pneumonia patients confirmed by mNGS aged from 36 to 66 years with three males. About 60% of patients had a history of contact with avian or poultry. All patients had a high fever over 38.5 °C, cough, hypodynamia, hypoxemia, and dyspnea on admission. Two patients had invasive ventilator support and extracorporeal membrane oxygenation support. Inflammatory index levels on admission and follow-up were all higher than normal values. Doxycycline or moxifloxacin and their combination therapy were used in patients. Four patients improved and were discharged, and one patient died due to multiple organ failures and disseminated intravascular coagulation. We summarized 19 articles including 69 C. psittaci pneumonia patients and patients in 11 publications were identified by mNGS, and most patients are treated with tetracycline and quinolone with good outcomes.
Conclusion
mNGS is a promising rapid diagnosis tool, which may increase the detection rate and shorten the diagnosis time of C. psittaci pneumonia. Further case-control studies are needed to confirm.
Keywords: Chlamydia psittaci, pneumonia, psittacosis, chlamydia, mNGS
Introduction
Chlamydia psittaci pneumonia is caused by Chlamydia psittaci (C. psittaci), which can lead to severe pneumonia, adult respiratory distress syndrome, and even death.1 According to the sequence difference of C. psittaci outer membrane protein A gene (ompA), it can be divided into 10 genotypes, namely A-G, WC, E/B, and M56, among which genotype A is the main genotype that causes human infection.2 About 70% of respiratory tract infections caused by C. psittaci are asymptomatic or only with mild symptoms, but 30% of them are severe respiratory illnesses such as community-acquired pneumonia with atypical symptoms, bronchitis, and upper respiratory tract infections.3 Contacting with birds or poultry is regarded as the main risk factor for psittacosis.1 The clinical symptoms of C. psittaci infection are quite different and lack specificity, which ranges in severity from mild to severe. Since the clinical manifestations of C. psittaci are similar to influenza symptoms, and the extrapulmonary manifestations are similar to Legionella, it needs to be differentiated from influenza and Legionella.4 Recent publications also reported the co-infection of SARS-CoV-2 with Chlamydia,5–7 which makes infectious diseases more complex.
The culture of C. psittaci from respiratory secretions in special media is possible but difficult, and it is mainly performed in specialized laboratories only because of the high infectivity of this pathogen. Specific diagnostic testing is serological, which is regarded as the gold standard for C. psittaci pneumonia. Moreover, the micro-immunofluorescence test (MIF) is the most accurate serologic test for C. psittaci pneumonia but is also performed only in specialized laboratories. Polymerase chain reaction assay (PCR) is used to confirm the strong clinical suspicion of a possible diagnosis of psittacosis especially, to distinguish it from other chlamydial species.8 And complement fixation (CF) is also an acceptable diagnostic method. Detection methods for C. psittaci, such as PCR, CF, and MIF are not routinely available in most hospitals in China. Because of its non-specific symptoms and the limitations of traditional tests, C. psittaci pneumonia is easily underdiagnosed and misdiagnosed.9
Metagenomic next-generation sequencing (mNGS) can quickly and accurately identify potential pathogens, whether they are bacteria, fungi, viruses, or parasites.10 It is increasingly used for the diagnosis of infectious diseases, especially when traditional diagnostic methods have limitations.11 Studies have shown that mNGS is the most promising comprehensive diagnosis method for infection, especially for severe pneumonia.12 Recently, several studies have reported the application of mNGS in diagnosing C. psittaci pneumonia, two case reports describing 5 and 9 cases of C. psittaci diagnosed by mNGS13,14.
We retrospectively summarized the clinical characteristics of five C. psittaci pneumonia patients diagnosed by mNGS in our hospital. Besides, we conducted a literature review of patients with C. psittaci pneumonia reported since 2010, with the attention to summarize the diagnostic methods and anti-infective drugs. We also summarized the clinical outcome and history of exposure to avian or poultry of these infection patients to provide a reference for future C. psittaci pneumonia infection patients’ diagnosis and treatment. We present the following article following the CARE reporting checklist.
Case Presentations
Patients’ Information
We carried out a retrospective case series analysis of five patients admitted to the Second Xiangya Hospital of Central South University since 2018. We collected the clinical data of all patients confirmed to have C. psittaci pneumonia. Sex, age, clinical examination indexes such as procalcitonin (PCT), C-reactive protein (CRP), erythrocyte sedimentation rate (ESR), comprehensive computed tomography (CT), and arterial blood gas analysis were extracted from electronic medical records. The treatment of antibiotics, outcomes, and any relevant follow-up data were also collected.
The Ethics Committees of the Second Xiangya Hospital of Central South University (LYF-2020021) approved this study. Informed consent was obtained from patients and guardians. This study was carried out by the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All data were anonymized before analysis.
As the symptoms of the patients were sudden, some patients had no sputum and their blood oxygen saturation was low, we did not perform bronchoscopy for them on admission. The blood was used for performing mNGS testing on admission for all five patients, and only case 5 performed bronchoalveolar lavage fluid (BAFL) mNGS testing on admission. All five patients were positive for C. psittaci DNA fragments in the blood mNGS test. Moreover, BAFL mNGS of case 5 result showed Klebsiella DNA fragments. The median duration from admission to the mNGS pathogenic diagnosis of the 5 cases was 3 days (range from 2 to 4). The median of mNGS detection sequence number of C. psittaci was 217 (range from 175 to 289). No pathogenic microorganisms were found in the sputum, BAFL, and blood culture of the patient after admission to our hospital. We also conducted serological verification and PCR for five cases after an atypical pathogen was identified by mNGS. Serological detection of antibody to C. pneumoniae was positive only in case 2 and the PCR of C. pneumoniae was negative in all patients. While the serological detection of antibody, PCR, MIF, and CF of C. psittaci cannot be performed in our hospital.
There were three male and two female patients with a median age of 51 years (range from 36 to 66). 60% of patients had type 2 diabetes. Three of five patients had a history of contact with avian or poultry. All patients had a high fever over 38.5 °C, cough, hypodynamia, hypoxemia, and dyspnea. Two of five patients had a headache. In the course of treatment, two patients had invasive ventilator support and Extracorporeal Membrane Oxygenation (ECOM) support. The medium APACHE II was 17.6 (range from 8 to 22). Days from illness to respiratory failure were 4.8 (2–8) days.
Laboratory Test
mNGS was conducted using the following operational steps according to the company’s operating procedures (The Beijing Genomics Institute, Beijing, China). Briefly, clinical samples (blood or BALF)) were collected by following the standards of aseptic processing procedures. Nucleic acid extraction was conducted and the extracted DNA was subjected to processes of interruption, end repair, library construction, and sequencing. The mapped data were processed for advanced data analysis. Lists of suspected pathogenic microorganisms were produced, which included the numbers of strictly mapped reads, coverage rates, and depth. The clinical diagnosis was determined by considering all the clinical manifestations, possible pathogens identified by mNGS, and other laboratory tests together. On admission and during the hospital, inflammatory markers such as PCT, CRP, ESR, kidney, and liver function index, CT, and X-ray data were detected according to the patients’ condition.
Laboratory inspection parameters of the five patients on admission were shown in Table 2. Four patients had lower hemoglobin and the levels were 108.6 (range 68–148) g/L. The levels of CRP, PCT, and ESR on admission were all higher than normal values. Oxygen partial pressure and partial pressure of carbon dioxide of all patients were significantly lower than normal values.
Table 2.
Laboratory Inspections Parameters of the Five Patients on Admission
| Inspection Items | Case 1 | Case 2 | Case 3 | Case 4 | Case5 | Reference Value |
|---|---|---|---|---|---|---|
| PH | 7.504 | 7.47 | 7.52 | 7.4 | 7.53 | 7.35–7.45 |
| Oxygen partial pressure (mmHg) | 64.6 | 42 | 53.1 | 59 | 58 | 80–100 |
| Partial pressure of carbon dioxide (mmHg) | 28.8 | 29 | 26.7 | 27 | 29 | 35–45 |
| White blood cell count (x 109/L) | 16.88 | 8.43 | 13.56 | 5.66 | 2.1 | 3.5–9.5 |
| Hemoglobin (g/L) | 128 | 127 | 68 | 148 | 72 | 130–175 |
| Platelets (x 109/L) | 183 | 129 | 325 | 83 | 100 | 125–350 |
| C reactive protein (mg/L) | 423 | 109.12 | 434 | 179.5 | 191.59 | 0–8 |
| Procalcitonin (ng/mL) | 18.17 | 8.1 | 13.89 | 58.5 | 2.75 | 0–0.05 |
| Erythrocyte sedimentation rate (mm/h) | 68 | 100 | – | 82 | 32 | 0–15 |
| Alanine aminotransferase (IU/L) | 146.7 | 91.7 | 237 | 33.4 | 79.2 | 9–50 |
| Aspartate aminotransferase (IU/L) | 139 | 115.8 | 587.6 | 60 | 246 | 15–40 |
| Total bilirubin (umol/L) | 20.4 | 8.9 | 16.3 | 12.6 | 5.8 | 3.4–17.1 |
| Serum creatinine (umol/L) | 71.4 | 79.7 | 48.6 | 338 | 53.5 | 44–133 |
| Outcome | Survival | Survival | Survival | Survival | Death |
After admission, follow-up laboratory testing results showed that the levels of inflammatory markers including WBC, percentage of neutrophils, CRP, PCT, ESR were all higher than normal values (Table 1). Moreover, the values of CK, lactate dehydrogenase (LDH), ALT, and AST were all higher than normal values in all five patients. Three patients had hypokalemia and the potassium levels were range from 2.7 to 3.3 mmol/L.
Table 1.
Clinical Characteristics of the Five C. Psittaci Pneumonia Cases
| Characteristics | Abnormal Patients/Total Patients, n (%) | Median Value (Range) |
|---|---|---|
| Demographics | ||
| Age, median (range, years) | 51 (36–66) | |
| History of exposure to avian or poultry | 3/5 (60) | |
| Underlying disease | 3/5 (60) | |
| Clinical manifestations | ||
| Fever > 38.5 °C | 5/5 (100) | 39.1 (38.7–40.5) |
| Cough, hypodynamia, dyspnea | 5/5 (100) | |
| Headache | 2/5 (40) | |
| Hypoxemia | 5/5 (100) | |
| Invasive ventilator support | 2/5 (40) | |
| ECOM support | 2/5 (40) | |
| APACHE II | 17.6 (8–22) | |
| Days from illness to respiratory failure | 4.8 (2–8) | |
| NGS detection sequence number | 217 (175–289) | |
| Laboratory testing | ||
| WBC (normal 4–10, × 109/L) | 4/5 (80) | 14.35 (10.4–20.08) |
| Neutrophil ratio (normal 45–75%) | 5/5 (100) | 93.03 (80.5–97.7) |
| CRP (normal 0–8 mg/L) | 5/5 (100) | 129.83 (16.8–423) |
| PCT (normal 0–0.05 ng/mL) | 5/5 (100) | 10.12 (0.058–100) |
| ESR (normal 0–15mm/h) | 5/5 (100) | 65.64 (25–100) |
| CK (normal 30–135 U/L) | 5/5 (100) | 2261.18 (14–8842.2) |
| LDH (normal 109–245 U/L) | 5/5 (100) | 737.96 (252.9–1433.1) |
| ALT (normal 9–50 U/L) | 5/5 (100) | 310.17 (65.7–2889.7) |
| AST (normal 15–40 U/L) | 5/5 (100) | 1554.11 (40.4–23,553.3) |
| Hypokalemia (normal 3.5–5.3 mmol/L) | 3/5 (80) | 3.1 (2.7–3.3)* |
| Hemoglobin | ||
| Treatment | ||
| Doxycycline | 2/5 (40) | |
| Moxifloxacin | 1/5 (20) | |
| Moxifloxacin+Doxycycline | 2/5 (40) | |
| The duration from admission to diagnosis (days) | 3 (2–4) | |
| Treatment result | ||
| Survive | 4/5 (80) | |
| Death | 1/5 (20) | |
Note: *The potassium levels just analyzed the values of hypokalemia patients.
Abbreviations: ECOM, extracorporeal membrane oxygenation; APACHE, The Acute Physiology and Chronic Health Evaluation; CK, creatine kinase; CRP, C-reactive protein; CT, computed tomography; LDH, lactate dehydrogenase; PCT, procalcitonin; ALT, alanine transaminase; AST, aspartate aminotransferase; WBC, white blood cell; ESR, erythrocyte sedimentation rate.
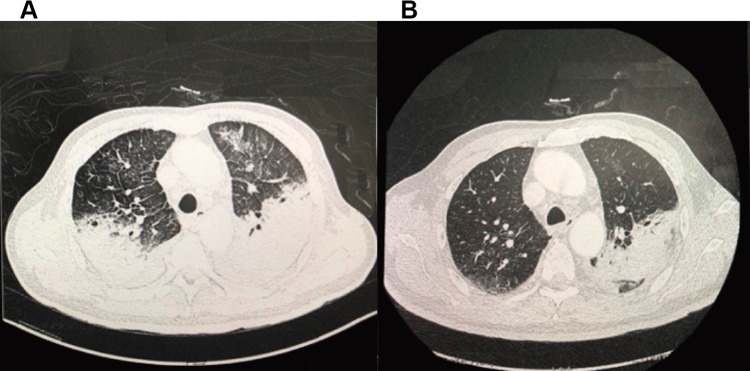
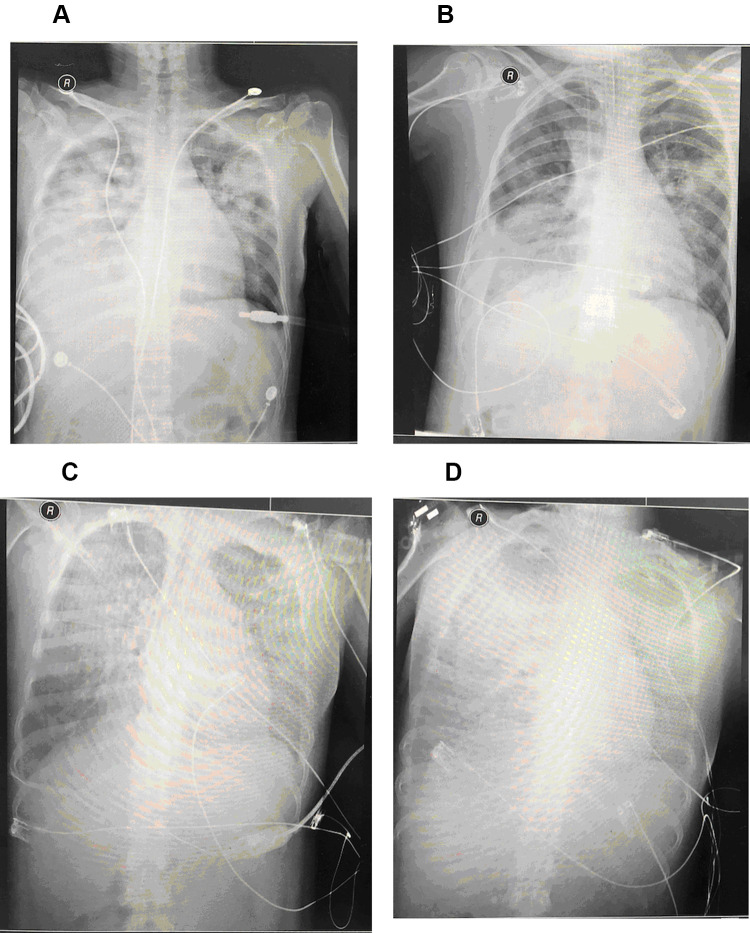
Chest CT and X-ray of patients showed that lungs were exuding consolidation foci, bilateral pectoral effusions, consolidation. After treatment, the image of four patients improved compared with those on admission, and the patients’ lung exudation, consolidation, and bilateral pleural effusion were less than before (Figures 1 and 2).
Figure 1.
Chest computed tomography (CT) scans of a 66-year-old man with C. psittaci pneumonia. Lungs were exuding consolidation foci, bilateral pectoral effusions, consolidation on the 8th day of hospitalization (A). After treatment, on the 18th day of hospitalization (B), the image of four patients improved, and the patients’ lung exudation, consolidation, and bilateral pleural effusion were less than before.
Figure 2.
Chest X-ray of a 36-year-old pregnant female patient with C. psittaci pneumonia and died of septic shock, DIC, and multiple organ failure. (A) on the first day of hospitalization, (B) on the third day of hospitalization, (C) on the sixth day of hospitalization, (D) on the seventh day of hospitalization.
Treatment and Outcome
After C. psittaci pneumonia was confirmed, one of the three male patients received symptomatic anti-infective treatment with moxifloxacin (400mg ivgtt qd) and with adjuvant non-invasive ventilator therapy. The other two male patients received symptomatic anti-infective therapy with doxycycline (100mg po q12h) and were treated with high flow nasal cannula therapy. All three male patients were improved and discharged after more than 10 days of treatment. Two female patients were transferred to our hospital after invasive ventilator adjuvant treatment with tracheal intubation from other hospitals. They were treated with moxifloxacin (400mg ivgtt qd) combined with doxycycline (100mg po q12h) for symptomatic anti-infective therapy. The two female patients were treated with ECOM and ventilator adjuvant therapy due to their critical illness. One patient was removed ECOM after five days of treatment with blood oxygen saturation and oxygen partial pressure was significantly improved. After 3 days of consecutive treatment, the invasive ventilator was removed. The patient continued to receive treatment and was discharged from the hospital. Another 35-years old female patient (case 5) was critically ill and the disease progressed rapidly and finally die. One week before admission, the B-mode ultrasound showed that the patient was in early intrauterine pregnancy with a size of about 6 weeks, with no germ and heartbeat. The patient was transferred to the respiratory ICU of our hospital after oral endotracheal intubation with a high fever of 39 °C and blood oxygen saturation of 65%. Chest radiograph showed multiple exudative lesions in both lungs and pleural effusion on the right side. After admission, she was given meropenem combined with moxifloxacin and ganciclovir. On the third day after admission, mNGS of blood indicated C. psittaci, with a sequence number of 533. mNGS of BAFL indicated Klebsiella pneumoniae, with a sequence number of 175. Then the patient was given an injection of doxycycline. On the 7th day of admission, the patient’s blood oxygen saturation was still 70% (through endotracheal intubation and invasive ventilator), and ECMO treatment was performed. On the 8th day of admission, the patient died due to septic shock, disseminated intravascular coagulation (DIC), and multiple organ failures.
Literature Review
We searched the databases including PubMed, EMBASE, Web of Science, Wanfang, and Chinese National Knowledge Infrastructure (CNKI) from 1st Jan. 2010 to 1st Oct. 2020. The searching strategy was “Chlamydia psittaci” or “Chlamydia psittaci pneumonia”. Dr. Jian Qu and Dr. Wen-Cheng Gong reviewed all relevant articles to identify potentially eligible studies. We conducted this literature review of patients with C. psittaci pneumonia to summarize the diagnostic methods and anti-infective drugs, and we also summarized the clinical outcome and history of exposure to avian or poultry of these infection patients to provide a reference for future C. psittaci pneumonia infection patients’ diagnosis and treatment. The data about authors, reported time, number of cases, ethnics, history of exposure to avian or poultry, anti-infective drugs regiment, and clinical outcome were collected. We found 794 publications. After excluded the full text could not be found or provided no information we needed about C. psittaci pneumonia or no original data or the Chlamydia was not specified. Finally, 19 articles were enrolled in further review.
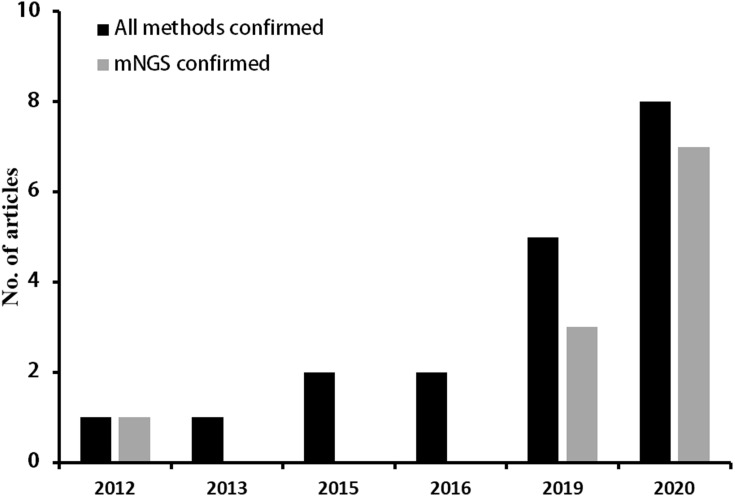
The summary of the detailed information was shown in Table 3. The articles were published from 2012 to 2020. With the development of detection technology, the number of articles reported tends to increase (Figure 3). Since 2019, there were 13 articles reported C. psittaci pneumonia and among them, 10 articles using mNGS to detect C. psittaci. There was a total of 69 C. psittaci pneumonia patients were reported and most patients had a history of exposure to avian or poultry. Most patients treated with doxycycline, moxifloxacin, meropenem, or their combinations, and three patients used ECOM support. Most of the patients’ treatment improved and four patients died.
Table 3.
Summary of Case Series and Case Report of C. psittaci Pneumonia
| Author | Reported Time | Number of Reported Cases | Reported Area | Methods | Anti-Infective Drugs | Clinical Outcome | History of Exposure to Avian or Poultry |
|---|---|---|---|---|---|---|---|
| Gacouin4 | 2012 | 13 | France | mNGS | Tetracycline ± Erythromycin/Levofloxacin | 11 patients improved, 2 patients died | Yes |
| Laroucau33 | 2013 | 8 | France | PCR | Macrolides + Cephalosporins | Improved | Yes |
| Chau34 | 2015 | 3 | Hong Kong | PCR | Doxycycline | Improved | Yes |
| Mair-Jenkins35 | 2015 | 4 | United Kingdom | Serology and PCR | Not stated | Improved | No history of direct contact, but someone raises pigeons near the office building |
| Spoorenberg36 | 2016 | 7 | Netherlands | PCR, MIF | 2 patients used Tetracycline, Macrolide or Quinolone; 1 patients used β-lactam | Improved | 6 patients had an exposure history |
| Cipriano37 | 2016 | 1 | Portugal | IIF | Amoxicillin/Clenauic Acid, Azithromycin | Improved | Yes |
| Qiu29 | 2019 | 1 | China | mNGS | Doxycycline + Moxifloxacin | Improved | No |
| Zhu38 | 2019 | 1 | China | mNGS | Moxifloxacin | Improved | Yes |
| Shi39 | 2019 | 1 | China | PM-seq | Piperacillin/tazobactam + Minocycline | Improved | Yes |
| Liu28 | 2019 | 1 | China | Antibody test | Moxifloxacin | Improved | Yes |
| Gu13 | 2019 | 5 | China | mNGS, IIF | 1 patient used Minocycline +Erythromycin | Improved | 4 patients had an exposure history |
| 2 patients used Doxycycline+ Moxifloxacin | |||||||
| 2 patients used Moxifloxacin | |||||||
| Chen14 | 2020 | 9 | China | mNGS | Minocycline | 8 patients improved, 1 patient died | 7 patients had an exposure history |
| He40 | 2020 | 1 | China | mNGS | Doxycycline | Improved | Yes |
| Chen30 | 2020 | 1 | China | mNGS | Moxifloxacin +Cefoperazone Sodium Sulbactam/Meropenem | Improved | Not stated |
| Katsura41 | 2020 | 1 | Japan | PCR | Meropenem+Gamma globulin | Died | Yes |
| Zhang42 | 2020 | 1 | China | mNGS | Doxycycline +Moxifloxacin | Improved | Yes |
| Zhang43 | 2020 | 1 | China | mNGS | Doxycycline + ceftazidime/meropenem | Improved | No |
| Fernández44 | 2020 | 5 | Murcia | mNGS | 3 patients used Levofloxacin | Improved | Yes |
| + Doxycycline | |||||||
| 2 patients used Cephalosporin | |||||||
| + Doxycycline | |||||||
| Luo45 | 2020 | 5 | China | mNGS | 2 patients used Doxycycline | Improved | Yes |
| 1 patients used Doxycycline + Meropenem | |||||||
| 1 patients used Doxycycline + Piperacillin/tazobactam | |||||||
| 1 patients used Doxycycline + Levofloxacin |
Abbreviations: mNGS, metagenomics next-generation sequencing; PCR, polymerase chain reaction; MIF, micro-Immunofluorescence; IIF, indirect immunofluorescence; PM-seq, pathogenic microorganisms sequencing.
Figure 3.
The summary of C. psittaci pneumonia literature review from 2010 to 2020 year.
Discussion
We retrospectively analyzed five cases of psittacosis pneumonia diagnosed using mNGS and summarized the clinical characteristics including disease progression, treatments, and outcomes, etc. Moreover, we also carried out a literature review that summarized the existing research and reports about C. psittaci pneumonia. In our study, five C. psittaci pneumonia patients confirmed by mNGS aged from 36 to 66 years with three males. 60% of patients had underlying diseases Type 2 diabetes. Three of these five patients had a history of contact with avian or poultry. All patients had a high fever over 38.5 °C, cough, hypodynamia, hypoxemia, and dyspnea on admission. Two patients had invasive ventilator support and ECOM support. The levels of CRP, PCT, and ESR on admission and follow-up were all higher than normal values. Doxycycline or moxifloxacin monotherapy was accounted for 1/5 (20%) and 2/5 (40%) patients, and combination therapy was accounted for 2/5 (40%) patients. Four patients improved and were discharged, and one patient died due to multiple organ failures and disseminated intravascular coagulation.
The lung manifestations are mainly cough, dry cough, shortness of breath, the rapid progress of lung disease, and occasionally acute respiratory distress syndrome (ARDS).15 In the five cases reported in this article, 3 patients had ARDS. And there were even case reports showing only abnormal liver function.16 In the initial auscultation, the lung lesions of C. psittaci pneumonia were often underestimated. The chest radiograph showed infiltrating patches with uneven density, which can seriously affect all lung lobes. CT of the lungs showed consolidation or ground glass-like changes, especially the lower lung, with pleural involvement and pleural effusion.17 After treatment, the patients’ cough and fever improved, but the oxygenation index recovered slowly. According to the reporting of literature, the absorption of the lesion was slow, with an average absorption time of 6 weeks, up to 20 weeks.18 The laboratory inspection parameters of the five patients on admission showed that two patients had WBC > 10×109/L, and three cases had PCT > 10 ng/mL, and the chest radiology presented mainly consolidation. The mNGS detection of case 5 also showed Klebsiella pneumoniae infection in addition to C. psittaci infection, but there were no other bacteria was isolated in the other four cases. Although other bacteria were not found in culture, other bacterial coinfections cannot be ruled out because of the high WBC and PCT value, and the chest radiology characterized by consolidation. Especially, some patients were carried out invasive ventilator support or ECOM. Therefore, patients are at risk for hospital-acquired pneumonia and ventilator-associated pneumonia infection. Before admission, the community-acquired pneumonia was also treated with antibiotics such as cephalosporins and quinolones in other hospitals before the diagnosis of C. psittaci pneumonia.
According to the control requirements of C. psittaci issued by the National Public Health Veterinary Association, the confirmed diagnosis of human C. psittaci pneumonia only needs to meet one of the following two standards: 1). Isolate C. psittaci from respiratory specimens (sputum, pleural fluid, tissue, etc.) or blood specimens 2). Serological examination: Measure the C. psittaci IgG antibody in the acute and convalescent phases during the interval of 2–4 weeks, the convalescent phase is more than four times higher than the acute phase. If the patient meets one of the following two standards, it may be infected: 1). Serum examination, C. psittaci IgM ≥ 32; 2). C. psittaci DNA can be detected through PCR amplification of respiratory specimens (sputum, pleural fluid, tissue, etc.).19 Since C. psittaci is strictly intracellular parasitic, its direct isolation and cultivation are very difficult and cannot be carried out routinely. At present, the clinical presentation and the positive serological result using MIF with paired sera are the most often used diagnostic methods of C. psittaci.1 MIF20 and PCR gene expansion detection have become auxiliary detection of molecular biological diagnosis due to their high sensitivity and specificity.21 Although MIF is more sensitive and specific than complement fixation (CF) tests, the test still displays cross-reactivity with other Chlamydia species in some instances.22 PCR testing is not clinical laboratory improvement amendments validated currently. mNGS can be used to detect pathogens that cannot be detected by traditional methods.23 Patients introduced in this article were all severely infected when they were admitted to our hospital, with respiratory failure, and dry cough without sputum. Due to the high risk of bronchoscopy and difficulty in taking respiratory tract specimens, the blood or BAFL samples of patients were sent out for mNGS testing and finally reported C. psittaci infection. Early pathogenic diagnosis can greatly benefit patients, and of the 5 patients reported in this article, 4 patients improved and were discharged. Our literature review also found that with the development of technology, the number of C. psittaci detected increased year by year, and the articles reported C. psittaci pneumonia via mNGS increased year by year (Figure 3 and Table 3).
Tetracyclines, macrolides, and quinolones can interfere with DNA and protein synthesis, therefore, these three kinds of antibiotics can be used to treat C. psittaci.24 At present, both in China and other country, tetracyclines are the first choice for the treatment of C. psittaci pneumonia including tetracycline, doxycycline, and minocycline.25,26 In severe cases, doxycycline can be administered intravenously. The treatment of the patient in case 2 with doxycycline is also effective. Macrolide drugs such as azithromycin and fluoroquinolones have been confirmed to have antibacterial activity against C. psittaci in vitro,26,27 In particular, moxifloxacin has strong antibacterial activity against Chlamydia, and there are case reports at home and abroad that the use of fluoroquinolone drugs is effective.28–31 Given the lack of experience in the use of tetracyclines in our hospital, Chlamydia trachomatis, which is the same species as C. psittaci in my country, is highly resistant to tetracycline,32 so the treatment for case 1 patient used moxifloxacin and it was effective. After five patients were treated, four patients were improved and discharged. Among them, one patient was treated with moxifloxacin, two patients were treated with doxycycline, and the other two patients were treated with moxifloxacin plus doxycycline. Current C. psittaci Pneumonia treatment guidelines recommend the addition of macrolide or quinolone to the initial regimen of severe C. psittaci in any case. According to current reports, it is unclear whether combination medication is more effective than single medication for patients. Further case-control studies with larger samples are needed to find the optimal treatment.
In this article, we have searched the relevant literature. At present, most cases of human infection with C. psittaci are reported in scattered cases and details are listed in Table 3. With the development of detection technology, mNGS became a routine examination for etiology. Therefore, more and more C. psittaci pneumonia was diagnosed and treated according to guidelines. Our literature review summarized 19 articles including 69 C. psittaci pneumonia patients, Patients in 11 articles were identified by mNGS, including 9 articles were reported in China. In recent years, the reports of mNGS for C. psittaci pneumonia diagnosis have increased, especially in China. We found that most patients had a history of exposure to avian or poultry. Therefore, epidemiological data combined with mNGS detection is helpful for the early diagnosis of C. psittaci pneumonia. Most patients are treated with doxycycline, moxifloxacin, or their combinations. Three patients used ECOM support and they are all improved. Among these 69 patients in our literature review, 65 patients of C. psittaci pneumonia improved and four patients died. In the future, further case-control studies with a large sample size are needed to find better diagnostic methods and better anti-infective drugs.
Conclusions
The history of contact with avian or poultry and the typical symptoms (high fever over 38.5 °C, cough, hypodynamia, hypoxemia, and dyspnea) are important for C. psittaci pneumonia diagnosis. Moxifloxacin, doxycycline, or their combinations are effective treatment options for C. psittaci pneumonia. mNGS is a promising rapid diagnosis tool, which may increase the detection rate and shorten the diagnosis time of C. psittaci pneumonia. Further case-control studies are needed to confirm.
Acknowledgments
We thank the patients enrolled in our study.
Funding Statement
No funding or sponsorship was received for this study or publication of this article.
Abbreviations
C. psittaci, Chlamydia psittaci; PCR, polymerase chain reaction assay; CF, complement fixation; mNGS, Metagenomic next-generation sequencing; PCT, procalcitonin; CRP, C-reactive protein; ESR, erythrocyte sedimentation rate; CT, computed tomography; ECOM, Extracorporeal Membrane Oxygenation; LDH, lactate dehydrogenase; DIC, disseminated intravascular coagulation; ARDS, acute respiratory distress syndrome.
Data Sharing Statement
Not applicable.
Ethics Approval and Informed Consent
The Ethics Committees of the Second Xiangya Hospital of Central South University (LYF-2020021) approved this study. Informed consents were obtained from patients and guardians.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work. All authors have read and approved the manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Balsamo G, Maxted AM, Midla JW, et al. Compendium of measures to control chlamydia psittaci infection among humans (Psittacosis) and Pet Birds (Avian Chlamydiosis), 2017. J Avian Med Surg. 2017;31(3):262–282. doi: 10.1647/217-265 [DOI] [PubMed] [Google Scholar]
- 2.Knittler MR, Berndt A, Bocker S, et al. Chlamydia psittaci: new insights into genomic diversity, clinical pathology, host-pathogen interaction and anti-bacterial immunity. Int J Med Microbiol. 2014;304(7):877–893. doi: 10.1016/j.ijmm.2014.06.010 [DOI] [PubMed] [Google Scholar]
- 3.Hahn DL, Azenabor AA, Beatty WL, Byrne GI. Chlamydia pneumoniae as a respiratory pathogen. Front Biosci. 2002;7:e66–e76. doi: 10.2741/hahn [DOI] [PubMed] [Google Scholar]
- 4.Gacouin A, Revest M, Letheulle J, et al. Distinctive features between community-acquired pneumonia (CAP) due to Chlamydophila psittaci and CAP due to Legionella pneumophila admitted to the intensive care unit (ICU). Eur J Clin Microbiol Infect Dis. 2012;31(10):2713–2718. doi: 10.1007/s10096-012-1618-6 [DOI] [PubMed] [Google Scholar]
- 5.Oliva A, Siccardi G, Migliarini A, et al. Co-infection of SARS-CoV-2 with Chlamydia or Mycoplasma pneumoniae: a case series and review of the literature. Infection. 2020;48:871–877. doi: 10.1007/s15010-020-01483-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ma L, Wang W, Le Grange JM, et al. Coinfection of SARS-CoV-2 and other respiratory pathogens. Infect Drug Resist. 2020;13:3045–3053. doi: 10.2147/IDR.S267238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lei JH, Xu Y, Jiang YF, Shi ZH, Guo T. Clustering cases of Chlamydia psittaci pneumonia in COVID-19 screening ward staff. Clin Infect Dis. 2020. doi: 10.1093/cid/ciaa1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cunha BA. The atypical pneumonias: clinical diagnosis and importance. Clin Microbiol Infect. 2006;12(Suppl 3):12–24. doi: 10.1111/j.1469-0691.2006.01393.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.de Gier B, Hogerwerf L, Dijkstra F, van der Hoek W. Disease burden of psittacosis in the Netherlands. Epidemiol Infect. 2018;146(3):303–305. doi: 10.1017/S0950268817003065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schlaberg R, Chiu CY, Miller S, et al. Validation of metagenomic next-generation sequencing tests for universal pathogen detection. Arch Pathol Lab Med. 2017;141(6):776–786. doi: 10.5858/arpa.2016-0539-RA [DOI] [PubMed] [Google Scholar]
- 11.Gu W, Miller S, Chiu CY. Clinical metagenomic next-generation sequencing for pathogen detection. Annu Rev Pathol. 2019;14:319–338. doi: 10.1146/annurev-pathmechdis-012418-012751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Langelier C, Kalantar KL, Moazed F, et al. Integrating host response and unbiased microbe detection for lower respiratory tract infection diagnosis in critically ill adults. Proc Natl Acad Sci U S A. 2018;115(52):E12353–E12362. doi: 10.1073/pnas.1809700115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gu L, Liu W, Ru M, et al. The application of metagenomic next-generation sequencing in diagnosing Chlamydia psittaci pneumonia: a report of five cases. BMC Pulm Med. 2020;20(1):65. doi: 10.1186/s12890-020-1098-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Cao K, Wei Y, et al. Metagenomic next-generation sequencing in the diagnosis of severe pneumonias caused by Chlamydia psittaci. Infection. 2020;48(4):535–542. doi: 10.1007/s15010-020-01429-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gough SL, Carrick J, Raidal SL, et al. Chlamydia psittaci infection as a cause of respiratory disease in neonatal foals. Equine Vet J. 2020;52(2):244–249. doi: 10.1111/evj.13170 [DOI] [PubMed] [Google Scholar]
- 16.Carella G, Marra L, Vallot T. [Hepatic psittacosis: a case of liver abnormality diagnosed by ultrasonography]. Presse Med. 1996;25(5):197–198. French. [PubMed] [Google Scholar]
- 17.Branley JM, Weston KM, England J, Dwyer DE, Sorrell TC. Clinical features of endemic community-acquired psittacosis. New Microbes New Infect. 2014;2(1):7–12. doi: 10.1002/2052-2975.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Fu J, Wang S, Zhang S, Sun G, Tang G. The chest radiological manifestation in psittacosis (in chinese). CHIN J RADIOL. 2005;39(11):1134–1137. [Google Scholar]
- 19.Smith KA, Campbell CT, Murphy J, Stobierski MG, Tengelsen LA. Compendium of measures to control chlamydophila psittaci infection among humans (Psittacosis) and Pet Birds (Avian Chlamydiosis), 2010 National Association of State Public Health Veterinarians (NASPHV). J Exotic Pet Med. 2011;20(1):32–45. doi: 10.1053/j.jepm.2010.11.007 [DOI] [Google Scholar]
- 20.Hogerwerf L, De Gier B, Baan B, Van Der Hoek W. Chlamydia psittaci (psittacosis) as a cause of community-acquired pneumonia: a systematic review and meta-analysis. Epidemiol Infect. 2017;145(15):3096–3105. doi: 10.1017/S0950268817002060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Forbes JD, Knox NC, Peterson CL, Reimer AR. Highlighting clinical metagenomics for enhanced diagnostic decision-making: a step towards wider implementation. Comput Struct Biotechnol J. 2018;16:108–120. doi: 10.1016/j.csbj.2018.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Persson K, Boman J. Comparison of five serologic tests for diagnosis of acute infections by Chlamydia pneumoniae. Clin Diagn Lab Immunol. 2000;7(5):739–744. doi: 10.1128/CDLI.7.5.739-744.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu Y, Zhang W. Clinical application of next-generation sequencing in etiological diagnosis of sepsis (in chinese). J Microbes Infect. 2018;13(2):97–101. [Google Scholar]
- 24.Kohlhoff SA, Hammerschlag MR. Treatment of Chlamydial infections: 2014 update. Expert Opin Pharmacother. 2015;16(2):205–212. doi: 10.1517/14656566.2015.999041 [DOI] [PubMed] [Google Scholar]
- 25.Beeckman DS, Vanrompay DC. Zoonotic Chlamydophila psittaci infections from a clinical perspective. Clin Microbiol Infect. 2009;15(1):11–17. doi: 10.1111/j.1469-0691.2008.02669.x [DOI] [PubMed] [Google Scholar]
- 26.Donati M, Rodriguez Fermepin M, Olmo A, D’Apote L, Cevenini R. Comparative in-vitro activity of moxifloxacin, minocycline and azithromycin against Chlamydia spp. J Antimicrob Chemother. 1999;43(6):825–827. doi: 10.1093/jac/43.6.825 [DOI] [PubMed] [Google Scholar]
- 27.Deb C, Dehollogne C, Dumont A, et al. Managing a cluster outbreak of psittacosis in Belgium linked to a pet shop visit in The Netherlands. Epidemiol Infect. 2016;144(8):1710–1716. doi: 10.1017/S0950268815003106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L, Wu W, Geng Y, et al. A case report of chlamydia psittaci pneumonia and literature review (in chinese). J Clin Pulm Med. 2015;(8):1543–1544. [Google Scholar]
- 29.Qiu C, Liu R, X H, Xiao X. A case of Chlamydia psittaci pneumonia detected by NGS (in chinese). J Gannan Med Univ. 2019;39(9):940–942. [Google Scholar]
- 30.Chen Q, Yu X, Chen J. Pharmaceutical care of a patient with chlamydia psittaci infection (in chinese). Chin J Clin Pharmacol. 2020;36(15):2320–2322. [Google Scholar]
- 31.Olivares-Gazca JC, Priesca-Marín JM, Ojeda-Laguna M, et al. Infusion of convalescent plasma is associated with clinical improvement in critically Ill Patients with COVID-19: a Pilot Study. Rev Invest Clin. 2020;72(3). doi: 10.24875/RIC.20000237. [DOI] [PubMed] [Google Scholar]
- 32.Li M, Zhang X, Huang K, et al. Presence of Chlamydia trachomatis and Mycoplasma spp., but not Neisseria gonorrhoeae and Treponema pallidum, in women undergoing an infertility evaluation: high prevalence of tetracycline resistance gene tet(M). AMB Express. 2017;7(1):206. doi: 10.1186/s13568-017-0510-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Laroucau K, Aaziz R, Meurice L, et al. Outbreak of psittacosis in a group of women exposed to Chlamydia psittaci-infected chickens. Euro Surveill. 2015;20(24). doi: 10.2807/1560-7917.ES2015.20.24.21155. [DOI] [PubMed] [Google Scholar]
- 34.Chau S, Tso EY, Leung WS, Fung KS. Three cases of atypical pneumonia caused by Chlamydophila psittaci. Hong Kong Med J. 2015;21(3):272–275. doi: 10.12809/hkmj144321 [DOI] [PubMed] [Google Scholar]
- 35.Mair-Jenkins J, Lamming T, Dziadosz A. et al. A psittacosis outbreak among english office workers with little or no contact with birds, August 2015. PLoS Curr;2018. 10. doi: 10.1371/currents.outbreaks.b646c3bb2b4f0e3397183f31823bbca6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spoorenberg SM, Bos WJ, van Hannen EJ, et al. Chlamydia psittaci: a relevant cause of community-acquired pneumonia in two Dutch hospitals. Neth J Med. 2016;74(2):75–81. [PubMed] [Google Scholar]
- 37.Cipriano A, Machado A, Santos FV, Abreu MA, Castro RS. [Human psittacosis: a case report]. Acta Med Port. 2019;32(2):161–164. Portuguese. doi: 10.20344/amp.10079 [DOI] [PubMed] [Google Scholar]
- 38.Zhu R, Luo R, Wang X. A case of severe community-acquired pneumonia caused by Chlamydia psittaci (in chinese). Chin J Tuberculosis Respir Dis. 2019;42(7):548–551. [Google Scholar]
- 39.Shi L, Li Y. One case of severe pneumonia caused by Chlamydia psittaci. Chin J Infect Chemother. 2019;19(3):309–311. [Google Scholar]
- 40.He X, Wang Y, Tan Y. A case report of primary Sjogren’s syndrome infected with Chlamydia psittaci (in chinese). J Clin Pulm Med. 2020;25(03):483–484. [Google Scholar]
- 41.Katsura D, Tsuji S, Kimura F, Tanaka T, Eguchi Y, Murakami T. Gestational psittacosis: a case report and literature review. J Obstet Gynaecol Res. 2020;46(5):673–677. doi: 10.1111/jog.14217 [DOI] [PubMed] [Google Scholar]
- 42.Zhang G, Zhu L, Zhou J, et al. Treatment and nursing of a severe case of chlamydia psittaci pneumonia (in chinese). Chin J Clin Infect Dis. 2020;13(2):134–137. [Google Scholar]
- 43.Zhang H, Zhan D, Chen D, et al. Next-generation sequencing diagnosis of severe pneumonia from fulminant psittacosis with multiple organ failure: a case report and literature review. Ann Transl Med. 2020;8(6):401. doi: 10.21037/atm.2020.03.17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fernández P, Iborra MA, Simón M, Segovia M. Outbreak of Chlamydia psittaci pneumonia in the Region of Murcia. Enfermedades infecciosas y microbiologia clinica. 2020;38(6):300–301. [DOI] [PubMed] [Google Scholar]
- 45.Yu L, Wen-ting J, Yu-yan M, Qing M, Jue P, Bi-jie H. Diagnosis of 5 cases of Chlamydia psittaci pneumonia and clinical characteristics. China Acad J Electron Publ House. 2020;30(22):3394–3398. [Google Scholar]