Abstract
The COVID-19 pandemic constitutes an arduous global health challenge, and the increasing number of fatalities calls for the speedy pursuit of a remedy. This review emphasizes the changing aspects of the COVID-19 disease, featuring the cytokine storm’s pathological processes. Furthermore, we briefly reviewed potential therapeutic agents that may modulate and alleviate cytokine storms. The literature exploration was made using PubMed, Embase, MEDLINE, Google scholar, and China National Knowledge Infrastructure databases to retrieve the most recent literature on the etiology, diagnostic markers, and the possible prophylactic and therapeutic options for the management of cytokine storm in patients hospitalized with COVID-19 disease. The causative agent, severe acute respiratory coronavirus-2 (SARS-CoV-2), continually threatens the efficiency of the immune system of the infected individuals. As the first responder, the innate immune system provides primary protection against COVID-19, affecting the disease’s progression, clinical outcome, and prognosis. Evidence suggests that the fatalities associated with COVID-19 are primarily due to hyper-inflammation and an aberrant immune function. Accordingly, the magnitude of the release of pro-inflammatory cytokines such as interleukin (IL)-1, (IL-6), and tumor necrosis alpha (TNF-α) significantly differentiate between mild and severe cases of COVID-19. The early prediction of a cytokine storm is made possible by several serum chemistry and hematological markers. The prompt use of these markers for diagnosis and the aggressive prevention and management of a cytokine release syndrome is critical in determining the level of morbidity and fatality associated with COVID-19. The prophylaxis and the rapid treatment of cytokine storm by clinicians will significantly enhance the fight against the dreaded COVID-19 disease.
Keywords: COVID-19, SARS-CoV-2, cytokine storm, hyper-inflammation, fatality
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) is the causative agent of the coronavirus disease 2019 (COVID-19), which broke out in Wuhan, China, in December 2019 and spread around the entire globe.1 On the 11th March 2020, the World Health Organization (WHO) declared the disease a pandemic. Currently, 132 046 206cases and 2 867 242 fatalities have been documented across 233 nations and regions by April 7–2021, 09: 34 pm GMT+8.2 COVID-19 infection is characterized by complications of the lower respiratory system, including pneumonia, and clinical presentations of the disease range from asymptomatic, mild, moderate, to severe forms. Immunodeficiency, senility, and other disease conditions such as diabetes, coronary heart disease, hypertension, cerebral infarction, pneumonia, severe asthma, and chronic bronchitis complicate the COVID-19 illness.3,4
SARS-CoV-2 is a single-stranded RNA virus that is classified into the beta-coronavirus genus and the Coronaviridae family. The genome of the coronavirus encodes four predominant proteins, which are the Envelope (E), Membrane (M), Nucleocapsid (N), and Spike (S) proteins. The S protein is responsible for viral access into respiratory tissue through the Angiotensin-Converting Enzyme 2 (ACE-2) expressing epithelial cells.5,6 Being the first central point of contact with the host cell, the S protein is known to have strong immunogenic properties.5 There are three main clinical stages of COVID-19. Stage one is the viral response phase, which is the period of early infection. It lasts for about four days, and it is typically characterized by non-specific symptoms such as fever, cough, and diarrhea.6 Stage two is the pulmonary phase which usually lasts between days 5 to 13. At this phase, the pulmonary symptoms are first without hypoxia, and later hypoxia develops.6 Stage three is the systemic hyper-inflammation phase, which is usually from day 14.6 Most times, patients report to the hospital at the end of stage 1 or the beginning of stage 2. At this time, the innate immune system’s potential to combat the infection has been threatened.7
Evidence proposes that deaths associated with COVID-19 are principally owing to hyperinflammation and uncontrolled immune response. The COVID-19 infection triggers a cytokine storm characterized by potentially life-threatening pathologies such as hyper-inflammation, septic shock complications, coagulation dysfunction, and impairment of several vital organs.8–11 Hypercytokinemia in COVID-19 patients is characterized by the speedy propagation and hyperactivation of T-cells, macrophages, natural killer (NK) cells, and the excessive production of a host of pro-inflammatory cytokines (Figure 1) and chemical mediators discharged by immune or non-immune cells.8,12,13 The early prediction, rigorous prevention, and therapeutic intervention of a cytokine release syndrome are crucial in reducing the level of morbidity and fatality related to COVID-19. The development of hypercytokinemia is a strong indication of disease progression, and immune suppression is a key therapeutic strategy in combating this complication.14 Therefore, during the investigation and therapy of pneumonia caused by COVID-19 infection, it has become necessary to monitor cytokine levels and other markers to improve the rate of cure and, in turn, reduce the rate of human mortality from the burden of disease.15 Concerning the COVID-19 infection and the host cell, this research paper focuses on the changing pathophysiological aspects of the COVID-19 infection, accentuating the cytokine storm’s pathogenesis and markers. It also critically explores the current and potential therapeutic options that can be exploited to alleviate and control cytokine storms.
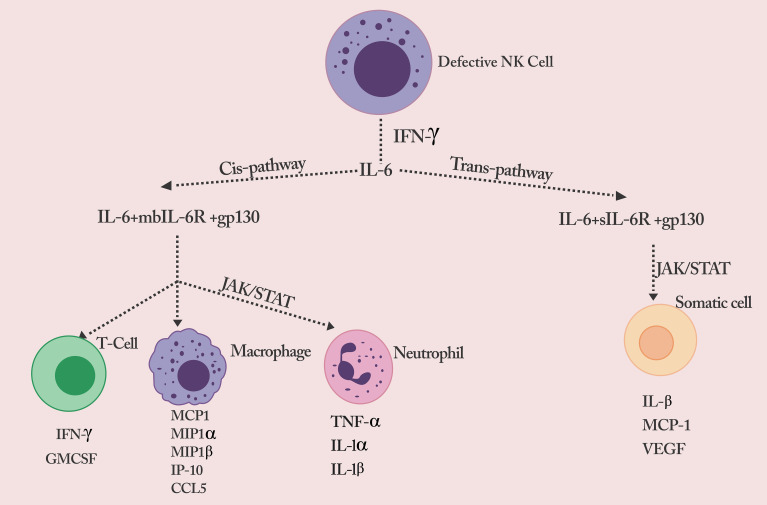
Figure 1.
Defective NK cell signals the production of a flurry of cytokines.
Notes: Reproduced from Rowaiye, A.; Okpalefe, O.; Onuh, O.; Ogidigo, J.; Oladipo, O.; Ogu, A.; Oli, A.; Olofinase, S.; Onyekwere, O. Preparing for the Storm: Mitigating the Effect of SARS-CoV-2 Induced Hypercytokinemia. Preprints 2020, 2020110604. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.
Search Strategy
This study is a narrative review. Relevant literature was retrieved from the databases (PubMed, Embase, MEDLINE, and China National Knowledge Infrastructure) and search engine (Google scholar). The search keywords included but were not limited to COVID-19 pathogenesis, SARS-CoV-2, Cytokine Storm, Hyperinflammation markers, and hypercytokinemia. Over 600 articles written only in the English language were retrieved. These articles were assessed for quality and eligibility based on key features such as straightforward protocol/study design, flawless methodology, complete data, and lack of selection or reporting bias. A total of 236 articles, vital information on the etiology, pathogenesis, diagnostic markers, prophylaxis, and possible cure of cytokine release syndrome in patients hospitalized for COVID-19 pneumonia, was obtained.
Pathogenesis of COVID-19-Triggered Cytokine Storm
The term cytokine release syndrome (CRS), also known as a cytokine storm, has been used to describe an abnormal discharge of soluble mediators and the associated immunopathological event that occurs after severe bacterial and viral infections.16 CRS is also referred to as hemophagocytic lymphohistiocytosis (HLH) or macrophage activation syndrome (MAS), depending on the underlining medical condition. CRS is associated with exaggerating pro-inflammatory-mediated response and an ineffective control mechanism by the anti-inflammatory system, leading to tissue damage.16,17 The human immune system plays an essential role in eradicating infectious agents such as influenza and coronaviruses through leucocyte recruitment and cytokines’ release. An unconstrained and well-harmonized stimulation of immune responses is usually the first mechanism of action the body presents to build a defense against any viral infection.18 Nevertheless, unregulated and aggravated immune responses may alter immunological function, leading to tissue damage and multiple organ failures.15,16 The production of various cytokines that cause cytokine storms in SARS-CoV-2 patients results in immunopathogenic injuries. Therefore, the effective lowering of pro-inflammatory cytokine levels in severe COVID-19 patients is crucial in preventing health deterioration in infected persons.19–21
The SARS-CoV-2 has been shown to infect the human respiratory epithelial cells, macrophages, and dendritic cells.22–24 It also induces the stimulation of pro-inflammatory cytokines and chemokines.25 The SARS-CoV-2 contains a receptor-binding domain (RBD) located in the S1 region that identifies a binding pocket in the Angiotensin-Converting Enzyme 2 (ACE2) and plays a role in the pathogenesis of COVID-19 infection.26 ACE2 is expressed by epithelial cells of the human respiratory tract, digestive tract, kidney, veins, terminal ileum, and colon.27 Due to genetic similarity, the ACE2 gene’s polymorphisms may affect the susceptibility to the SARS CoV infection and COVID-19 disease.26,28,29 As shown in a murine model, mutations might also alter the expression level of ACE2.30
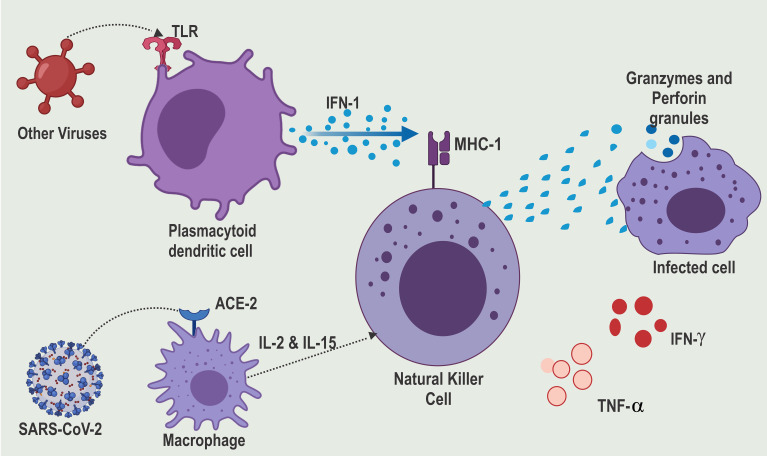
In the infected cells, the nucleic acids of the invading virus are recognized by Pattern Recognition Receptors (PPR) like the Toll-Like Receptors (TLR3, TLR7, TLR8, and TLR9).31–33 The single nucleotide polymorphisms in the gene coding for these TLR may lead to poor viral recognition and, subsequently, a susceptibility to cytokine storms.34 The activated TLR release cytokines such as type 1 Interferon (IFN-1) are key for antiviral immunity and are made up of IFN-α and IFN-β.35–38 IFN-1 is produced in large quantities by activated plasmacytoid dendritic cells. On binding with the IFN-1 receptor, IFN-1 triggers the phosphorylation of transcription factors such as signal transducer and activator of transcription 1 (STAT1) in a downstream signaling pathway that triggers hundreds of IFN-stimulated genes in the nucleus.16,39 IFN-1 stimulates the NK cells through dependent or independent mechanisms on class I major histocompatibility complex (MHC) protein.40 Polymorphisms of class 1 MHC may also lead to increased susceptibility to COVID-19 infection.7 Unfortunately, the SARS-CoV-2 evades the TLR8 and TLR9 receptors (Figure 2). This is achieved through an intricate capping mechanism of the mRNA by the methyltransferase.41 This suppresses IFN-I production and the downregulation of interferon-stimulated genes in COVID-19 patients.42 However, independent of IFN-1, the NK cells can be triggered through other cytokines such as IL-2 and IL-15. These compensatory signaling mechanisms are essential in the innate antiviral defense of most COVID-19 survivors.43
Figure 2.
SARS-CoV-2 evades TLR recognition and thereby suppressing IFN-1 production.
Notes: Reproduced from Rowaiye, A.; Okpalefe, O.; Onuh, O.; Ogidigo, J.; Oladipo, O.; Ogu, A.; Oli, A.; Olofinase, S.; Onyekwere, O. Preparing for the Storm: Mitigating the Effect of SARS-CoV-2 Induced Hypercytokinemia. Preprints 2020, 2020110604. Creative Commons license and disclaimer available from: http://creativecommons.org/licenses/by/4.0/legalcode.
The NK cells are responsible for the immune surveillance of tumor cells and cells infected by viruses.44 To effectively carry out this role, the NK cells are furnished with a set of pro-inflammatory cytokines (IFN-γ and TNF-α) and cytotoxic molecules (perforin and granzymes).45 Primarily due to the presence of inhibitory receptors and regulatory cytokines like IL-10, IL-3, and Granulocyte-macrophage colony-stimulating factor (GM-CSF), NK cells also play an immunomodulatory role. They can control exaggerated immune responses, as seen in auto-inflammatory conditions and cytokine storms.46 However, reduced activity, decreased number, and genetic deficiency of NK cells have been associated with cytokine storm progression.46,47 Mutations in the genes of specific proteins expressed by NK cells affect cytotoxic activities. For example, mutations in the PRF1 and GZMB genes lead to defective perforin and granzyme B expression, respectively.47–50 Perforinopathy and other cytotoxic protein expression deficiencies could induce intense inflammatory response signals seen in cytokine storm syndrome.51,52 Empirical evidence suggests that the failure of Granzyme/Perforin-induced apoptosis of target cells causes severe immune dysregulation. The prolonged survival of target cells causes IFN-γ secreted by the NK cells to trigger naive macrophages, consequently overproducing proinflammatory cytokines.47 Therefore, though there is an absence of the death of virally-infected cells due to a decrease in NK cell cytotoxic activity, a flurry of cytokines is released by macrophages resulting in the IL-6 cascade.52
Upstream of the IL-6 signaling pathway, IL-1β, and TNF alpha are two important pro-inflammatory cytokines involved in the pathogenesis of virus-induced cytokine storms.53 The TNF-α and IL-1β trigger the Nuclear Factor kappa light chain enhancer of activated B cells (NF-κB) signaling pathway. On activation, the NF-κB, a DNA binding protein, activates the transcription of various genes and thereby regulates inflammation.53
The induction of NF-κB further triggers IL-6, other pro-inflammatory cytokines, chemokines, enzymes, and adhesion molecules. Additionally, the NF-κB regulates the proliferation, morphogenesis, and apoptosis of inflammatory cells.53–55 IL-6 is a pleiotropic cytokine produced from multiple cell types, including macrophages, dendritic cells, fibroblasts, keratinocytes, mesangial cells, vascular endothelial cells, B and T cells.56 It does not only have significant pro-inflammatory properties but can also trigger dual signaling pathways (cis or trans) based on their cellular distribution.57 In the trans-signaling pathway, IL-6 binds to the soluble form of the IL-6 receptor (SIL-6R) found on all somatic cells.57 The IL-6/sIL-6R composite binds to gp130 and triggers the JAK-STAT3 signaling gateway. The CRS is aggravated through the release of monocyte chemo-attractant protein–1 (MCP-1), IL-8, vascular endothelial growth factor (VEGF), and monocyte chemo-attractant protein–1 (MCP-1), which are associated with tissue damage and inflammation. The vascular permeability and leakage associated with the hypotension and pulmonary dysfunction in ARDS are due to reduced E-cadherin and increased VEGF expression.58–61
In the cis signaling pathway, IL-6 binds with the membrane-bound IL-6 receptor (mbIL-6R), expressed on neutrophils, naive T cells, and monocytes/macrophages, which cover both the innate and acquired immune systems.57 The IL-6/mbIL-6R complex binds with gp130 and triggers the Janus kinases (JAKs) and signal transmitter and inducer of transcription 3 (STAT3) proteins.57 This signal cascade causes multiple effects on the cells involved and culminate in a cytokine storm. Cytokines are usually elevated according to the level of inflammation. Activated T cells secrete cytokines such as IFN-γ and GM-CSF. The activated monocytes/macrophages secrete cytokines and chemokines such as Monocyte Chemoattractant Protein-1 (MCP1), Macrophage Inflammatory Protein 1-alpha (MIP1α), Macrophage Inflammatory Protein 1-beta (MIP1 β), monokine induced by gamma interferon (MIG), IFN-γ-induced protein 10 (IP-1O), and CCL-5. The triggered neutrophils also secrete pro-inflammatory cytokines such as TNF-α, IL-1-α, and IL-1-β.58–63
The invasion of SARS-CoV-2 induces an alteration in T-cell responses leading to the elevated serum level of cytokines and chemokines in severe cases compared with mild or moderate cases. For example, IL-6 was reported to be 76% higher than normal in severe cases compared with 30% in mild conditions.3, 64–69 As the cytokines and chemokines level increase, they tend to recruit many other inflammatory cells, including neutrophils and monocytes, which results in the permeation of the lung tissue, causing acute respiratory distress syndrome (ARDS). The ARDS is characterized by apoptosis of the pulmonary epithelial and endothelial cells, damages of the lung micro-vascular and alveolar epithelial cell barrier, vascular leakage, alveolar edema, hypoxia, and pulmonary fibrosis.8,70
In addition to the direct attack of SARS-CoV-2 on CD4 lymphocytes, IL-6 has also been shown to inhibit lymphopoiesis through lymphocyte trafficking and the direct suppression of progenitor cells.71,72 The uncommitted hematopoietic stem cells, which contain precursors for both lymphoid and myeloid fates, express the IL-6 receptor-α chain. These cells respond to IL-6 activation through the JAK/STAT3 pathway, which causes the expression of the Id1 transcription factor. Consequently, lymphopoiesis is inhibited, and myelopoiesis is elevated in a mitogen-activated protein kinase (MAPK)-dependent manner.73,74
The overall immunopathology of COVID-19 infection suggests remarkable lymphocytopenia, thrombocytopenia, basopenia, eosinopenia, monocytopenia, hyper-gamma-immunoglobulinemia, neutrophilia, T-cell activation, lymphocyte dysfunction, and increased pro-inflammatory cytokine production.75 Regarding specific clinical symptoms, IFN-γ causes fever, headaches, chills, fatigue, malaise, cardiomyopathy, vascular leakage, lung injury, and acute-phase protein production. Also, TNF-α causes flu-like symptoms.75 IL-6 induces cardiomyopathy, vascular leakage, activation of complement and the coagulation cascade, and diffuse intravascular coagulation.76–78 The excessive production of cytokines also leads to the generation of tissue factors that culminate in the blood’s over-coagulation. Tissue hypoxia and ischemia result from thrombosis and viscera embolization.66
Markers of COVID-19-Induced Cytokine Storm
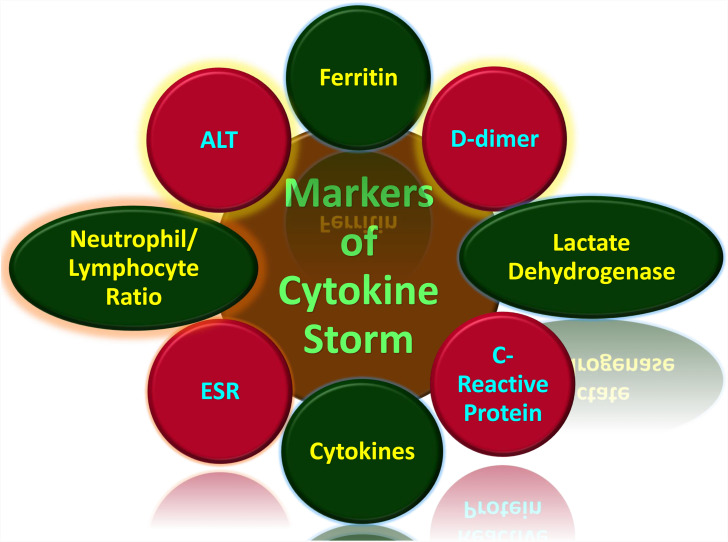
Generally, cytokine storms are diagnosed in the form of the underlying medical condition. Therefore, a viral infection-induced cytokine storm is different from a macrophage activation syndrome induced by autoimmune defects such as systemic juvenile idiopathic arthritis (JIA) and in lupus or familial hemophagocytic lymphohistiocytosis (HLH), which is induced by certain genetic syndromes.79,80 The symptoms of cytokine storms include fatigue, fever, chills, nausea, emesis, headache, cough, seizures, tremor, dyspnea, lethargy, and rash.81 Other symptoms include increased blood clotting, low blood pressure, multiple organ failure, and death75,82 The quick and early prediction of hypercytokinemia through specific biological markers would prevent many deaths. This use of these markers would allow for closer clinical monitoring and aggressive supportive treatment to avoid a poor prognosis (Figure 3).83 The clinical markers (Table 1) that can diagnose tissue damage in COVID-19 infection include serum chemistry and hematological parameters.84
Figure 3.
Markers of cytokine storm associated with COVID-19.75,79–86,90–132
Table 1.
Markers of Cytokine Storm
| Marker | Source | Association with Cytokine Storm | Normal Range | COVID-19 Marker Cut-Off | References |
|---|---|---|---|---|---|
| Ferritin | Serum Chemistry | Cellular ferritin leaks from damaged cells | 18–350 ng/mL | ≥ 400 μg/L* | [85] |
| D-dimer | Serum Chemistry | Defective plasma coagulation | 250–500 ng/mL | ≥ 1000 ng/mL* | [86] |
| Lactate dehydrogenase | Serum Chemistry | Lactic acidosis | 140 −280 U/L | ≥ 450U/L * | [83] |
| C-Reactive Protein | Serum Chemistry | Associated to IL-6 stimulation | 0.3 to 1.0 mg/dL | ≥ 20 mg/dL* | [87] |
| ALT | Serum Chemistry | Damage of hepatocytes | 19 to 33 IU/L | 67 (47–100) IU/L* | [88] |
| Cytokines | Serum Chemistry | ||||
| IL-1beta | Pro-inflammatory activity | 0.10 pg/mL | 0.67 pg/mL | [89] | |
| IL-6 | Pro-inflammatory activity | 0.5 to 5 pg/mL | <25 pg/mL* | [90,91] | |
| TNF-α | Pro-inflammatory activity | 0 to 8.1pg/mL | < 35pg/mL* | [92] | |
| Neutrophil/Lymphocyte Ratio | Hematological | Increased neutrophil counts and lymphopenia. | 1.0–3.0 | 6.6 (2.1–11.1)* | [7,93] |
| Erythrocyte Sedimentation Rate | Hematological | Platelet destruction | 0 to 29 mm/hr | >100 mm/hr* | [94] |
Notes: *Marker levels suggest a remarkable increase from the normal range. They are not to be taken as fixed as they do vary as the disease progresses due to several other factors.
Ferritin
The function of serum ferritin as a marker of inflammation and immune dysregulation is well established. While Ferritin is considered an early phase reactant, its synthesis does not occur within the serum but intracellular iron storage protein.85 Iron and inflammations are inescapably linked. An increase in cellular ferritin production allows for greater binding of intracellular iron. This is a host defense mechanism to inhibit free radical production.95 Serum ferritin originates from the leakage from damaged cells and therefore serves as an important disease marker. As ferritin exits the cells into the serum, it leaves an unbound Iron and lacks most of the Iron it contains intracellularly. Though serum ferritin is benign, the unbound Iron further causes cellular damage through lipid oxidation.85,95 Unliganded Iron catalyzes hydroxyl radical formation and increases the production of markers such as malondialdehyde, 27-hydroxycholesterol, 8-hydroxydeoxyguanosine, 4-hydroxynonenal, and isoprostanes.85,95 Since the red blood cells contain the highest ferritin volume, and unbound Iron distorts their structure and function. Unbound Iron also causes pathological changes in fibrin morphology, consequently making it trap deformed erythrocytes.85,95 Clinical data suggest that increased ferritin level is linked to the severity of COVID19 disease, the normal serum ferritin range being 18–350 ng/mL.96 In severe cases, ferritin levels are >400 μg/mL and could be between 1.5 and 5.3 times higher than moderate cases.97 Notably, in the absence of liver disease or the need for blood transfusion for anemia, a disproportionately high and continuously increasing serum ferritin level suggests the presence of a cytokine storm.98
D-Dimer (DD)
Soluble fibrin is generated during plasma coagulation. D-dimer is released as a product of degeneration of cross-linked fibrin. Hence, it is a sensitive biomarker to rule out venous thromboembolism. However, increased DD level is an indicator of the activation of coagulation.86 There is a correlation between D-dimer and the progression of severe COVID-19 infection. D-dimer is directly connected with the activation of the proinflammatory cytokine cascade. On the contrary, it is not associated with anti-inflammatory cytokines (IL-10).99
A defective coagulation system in severe COVID-19 patients and DD can identify patients at increased risk of the cytokine storm.86 Beyond inflammation and coagulopathy is strongly involved in the pathogenesis of severe COVID-19 illness. Thrombosis, especially venous thrombosis, is a serious complication in COVID-19 patients, and it leads to multiple infarcts and ischemia of the extremities.99 D-dimer is an important marker of coagulopathy, and elevated level is a predictor of COVID-19 progression.99 The normal plasma level of D-dimer is below 250ng/mL for D-dimer units or below 500 ng/mL for fibrin equivalent units.100 D-dimer levels higher than 1000 ng/mL indicate poor clinical prognosis and the need for anticoagulant therapy.101,102
Lactate Dehydrogenase (LDH)
This is an enzyme found in nearly all living cells. It is an essential indicator of cytokine storm in COVID-19 infection because it is associated with metabolic acidosis.103 In viral infections, tissue hypoxia conditions prevail when pyruvate is converted to lactic acid and accumulates. Lactic acidosis induces the monocytes and macrophages to produce IL-1β and triggers the inflammasome to activate inflammatory responses.104,105 In homeostatic response to the acidosis, the LDH level is increased. Therefore, LDH is a marker of tissue damage caused by viremia and dysregulated immune response because as tissue deterioration progresses, the LDH increases. It reflects the degree of various pathophysiological processes, and therefore it can predict the progression or regression of disease. There is a significant difference in LDH levels between mild and severe groups of COVID-19 pneumonia patients.106 Normal LDH levels range from 140 −280 units per liter (U/L), and the upper limit of 450U/L is used as a threshold for severe COVID 19 infection.81
C-Reactive Protein (CRP)
In response to IL-6 stimulation, CRP is released from the liver cells. CRP is an acute-phase reactant, which is markedly elevated in COVID-19 patients. CRP is a useful indicator to determine the severity of disease and a reliable predictor of cytokine release syndrome. Extremely high CRP levels are directly linked to poor disease prognosis. Based on the relationship between CRP levels and IL-6, it can be used as a surrogate marker. Daily monitoring is important to distinguish between those whose fever would resolve and those whose symptoms would gravitate to a cytokine storm. The normal CRP range is 0.3 to 1.0 mg/dL but values greater than 20 mg/dL suggests hyper-inflammation.87 A patient whose CRP exceeds this threshold is at particularly high risk of the cytokine storm.107 The LDH and CRP may also be associated with respiratory function; may also be used to predict respiratory failure in COVID-19 patients.87
Alanine Aminotransferase (ALT)
Alanine aminotransferase is an enzyme found inside the hepatocytes and is released excessively into the bloodstream during liver damage. Liver damage is a clinical sign characteristic of COVID-19 infection, and it has been reported in at least one-half of patients suffering from the disease.108,109 Impaired liver function is characterized by ALT elevations in multiples factors of the upper reference limit.88 During SARS-CoV-2, the ACE-2 receptor acts as a target for entrance into the cells. This receptor plays a crucial role in propagating the virus as it is expressed on the bile duct and liver’s endothelial cells, therefore exposing the organ to possible infection.110 A direct viral attack on the hepatocytes and the cholangiocytes elevates ALT levels. Other causes of liver damage in COVID-19 patients include drug-induced toxicity.109
Blood Urea Nitrogen (BUN) to Creatinine Ratio
The glomerulus filters blood Urea, Nitrogen, and Creatinine (Cr). While the creatinine’s tubular reabsorption is minimal, that of BUN can either be increased or decreased. The normal BUN range is 7–20 mg/dL, and that of creatinine is 0.7–1.2 mg/dL.111 When the BUN to Cr ratio exceeds 20, this is predictive of acute kidney injury, making BUN reabsorption increased due to the kidneys’ hypoperfusion. BUN is generally increased during COVID-19 infection due to acute kidney damage and other factors.112 Gastrointestinal bleeding has been observed in patients with severe COVID-19 disease. The gastrointestinal tract’s epithelial cells, which positively express the ACE2 protein, are attacked by SARS-CoV-2.113 Consequently, histological degeneration, necrosis, and varying degrees of mucosal spillage are detected in a deceased COVID-19 patient’s gastrointestinal mucosa.113 Gastrointestinal bleeding may also be due to an existing coagulopathy developed from multiple organ failure or the use of corticosteroids with heparin or salicylic acid in intravascular thrombosis treatment.114 Gastrointestinal bleeding can cause an elevation in the BUN to Cr ratio due to amino acid digestion. Hemoglobin is metabolized into amino acids by the enzymes in the upper intestinal tract. The reabsorbed amino acids are broken down into urea, and this increases BUN level.115,116 High BUN level is associated with mortality in COVID-19 patients.117
Creatinine Kinase-Muscle/Brain Activity (CK-MB)
Cardiac damage possibly occurs during SARS-CoV-2 infection, and this is due to the activity of proinflammatory cytokines such as TNF-α, IL-1, and IL-6. These cytokines may play an important role in the development and progression of heart failure.118 CK-MB is a marker for myocarditis. Meta-analysis reveals that elevated CK-MB levels suggest cardiac damage and are significantly associated with disease severity and an increased risk of mortality in COVID-19 patients.119,120
Cytokines
Though expensive to test, cytokines can be used to predict the cytokine storm. The rise in the levels of pro-inflammatory cytokines such as IL-1beta, IL-6, and TNF alpha are predictors of disease progression and an impending cytokine storm.121,122 These cytokines can also be used as prognostic markers to determine the clinical outcome of therapeutic agents administered to combat the SARS-CoV-2 disease. The normal range of IL-6 for healthy individuals is 0.5–5 pg/mL, and it could exceed 25pg/mL during infection.90,91 The normal range for IL-1beta is 0.10pg/mL, and it could exceed 0.67pg/mL during infection.89 The normal range for TNF-alpha is 0.0–8.1 pg/mL, and it could exceed 35pg/mL during infection.92,121 Cytokine cut-offs are difficult to establish and may vary depending on population genetics, pleiotropic nature, and patient’s health status. Therefore, it is ideal for comparing cytokine signatures with their baseline values to establish a diagnostic or prognostic decision.123
The Neutrophil/Lymphocyte Ratio (NLR)
The Neutrophil/Lymphocyte Ratio is potentially a predictive prognostic biomarker in patients infected with the SARS-CoV-2.93 Severe COVID-19 is characterized by high levels of circulating pro-inflammatory cytokines, increased neutrophil counts, and lymphopenia.124 Lymphopenia as a reliable and effective predictor for the COVID-19 disease state.125 While the neutrophilia is due to prolonged neutrophil activation, and the lymphocytopenia could result from a straight attack of SARS-CoV-2 on lymphocytes, sequestration of the lymphocyte in the lung, suppression of hematopoietic stem cells, or the cytokine-mediated disruption of lymphocyte trafficking.126
Erythrocyte Sedimentation Rate (ESR)
The Erythrocyte Sedimentation Rate measures the rate at which red blood cells (RBC) settle in anti-coagulated whole blood. During inflammation, the ESR is faster than usual because a high proportion of fibrinogen in the blood causes the RBC to stick together and increases its density.94 In SARS-CoV-2 infection, thrombocytopenia is due to a direct attack of the virus on the platelets and megakaryocyte progenitor cells in the bone marrow.127,128 Platelets express specialized PRR and can activate thrombo-inflammatory responses against viruses. Functional interaction between the platelets and the innate immune system promotes thrombo-inflammation and tissue damage.129 Platelet activation potentiates the recruitment, activation, and transmigration of innate immune cells. The progression and resolution of the inflammation of platelets are promoted in a disease‐specific manner. Also, thrombo-inflammation is differentially regulated by platelet-leukocyte interactions.130 As the severe COVID-19 disease progresses, the platelet counts decrease, and consequently, the erythrocyte sedimentation rate is lowered. The normal ESR ranges for men and women are usually is very high during severe inflammation due to infection.131,132
Mitigation and Therapeutic Intervention of COVID-19-Induced Cytokine Storm
To effectively prevent or mitigate a cytokine storm, several pathological factors must be considered. The earliest possible time pharmacological interventions (Figure 4) have a higher possibility of success in managing severe COVID-19 infected cases.133,134 These include viremia, cellular oxidation, immuno-senescence, and comorbidities.7 The administration of immunomodulators would further strengthen the therapeutic strategy (Table 2). While WHO is yet to approve any cure for COVID-19, many antiviral agents have been proposed. Some of these are natural compounds that can also serve as antioxidants to combat oxidative stress caused by SARS-CoV-2 infection.7 Immuno-senescence is an age-related decline in immune function. This is one of the most important reasons for the increased morbidity and mortality in elderly patients hospitalized with COVID-19. Immunosenescence is characterized by the diminished activity of hematopoietic stem cells due to continuous oxidative damage to DNA and the decreased number or activity of circulating phagocytes, dendritic cells, NK cells, and B-cells.135 Patients from 65 years and above stand a high risk of COVID-19 fatality.135 Immunosenescence can be combated through bone marrow transplantation and genetic reprogramming, which is not logistically or economically feasible in treating COVID-19.135 Comorbidities constitute a significant risk factor for cytokine storm syndrome in COVID-19. Immunity in patients with SARS-CoV-2 infection is further weakened by other diseases like hypertension, diabetes, cardiovascular diseases, and lung diseases.136 These conditions reduce the resistance to the invading virus and, therefore, enhance the disease’s progression. The clinician should take care of these comorbidities to prevent a cytokine storm.136
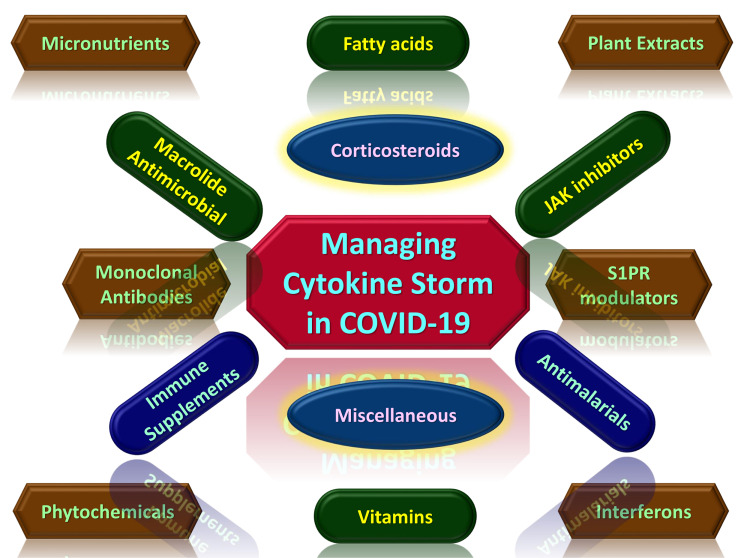
Figure 4.
Potential therapeutic agents for managing cytokine storm associated with COVID-19.7,30,44,76,121,133–137,139–249
Table 2.
Potential Therapeutic Agents for Mitigating and Managing COVID-19 Cytokine Storms
| Class | Therapeutic Agent | Mechanism of Action | Stage of Development | References |
|---|---|---|---|---|
| Micronutrients | Zinc | Decreased gene expression of pro-inflammatory cytokines, Regulates several enzymes within the apoptotic cascade, Recruitment of Lck to the TCR complex | Nutraceutical | [138,139] |
| Fatty acids | Omega-3 Fatty acid (PUFA) | Induce apoptosis in leucocytes | Nutraceutical | [140] |
| Butyrate | Suppress IL-12 expression, Enhance IL-10 expression | Nutraceutical | [141] | |
| Vitamins | Vit. C | Inhibit mRNA expression of pro-inflammatory cytokines, increased production of type 1 interferons | Nutraceutical | [142] |
| Vit. D | Suppress IL-17 production | Nutraceutical | [143] | |
| Phytochemicals | Resveratrol | Increased expression of IL-10 | Nutraceutical | [144] |
| Kaempferol | Decreased expression of IL-6 and TNF-alpha | Nutraceutical | [144] | |
| Apigenin | Decreased expression of IL-6 and TNF-alpha | Nutraceutical | [144] | |
| Quercetin | Decreased expression of IL-6 and TNF-alpha | Nutraceutical | [144] | |
| Plant Extracts | Echinacea purpurea | Inhibit the release of TNF-α | Nutraceutical | [145] |
| Camellia sinensis | Promote γδ T lymphocyte functions | Nutraceutical | [146] | |
| Echium amoenum | Decrease expression of IL-1β, IL-6, TNF-α, iNOS and COX2 | Herbal Tea | [147] | |
| Abelmoschus esculentus | Activation of NK cells | Nutraceutical | [148] | |
| Andrographis paniculata | Activation of NK cells | Nutraceutical | [44] | |
| Interferons | IFN-λ | Suppression of neutrophil infiltration and IL-1β production. | Clinical trial | [149,150] |
| Monoclonal Antibodies | Tocilizumab | Blocks IL-6 receptors | Approved | [151,152] |
| Anakinra | Interleukin-1 receptor antagonist | Approved | [153,154] | |
| S1PR modulators | Fingolimod | Agonist of S1P1R, S1P3R, S1P4R, and S1P5R receptors. | Approved | [155,156] |
| JAK inhibitors | Baricitinib | JAK1, JAK2, JAK 3, and Tyrosine Kinase 2 inhibition | Approved | [157,158] |
| Pacritinib | JAK2 and IRAK1 inhibition | Under investigation | [159] | |
| Antimalarials | Hydroxychloroquine | Inhibits antigen processing | Approved | [160,161] |
| Macrolides (Antibiotics) | Azithromycin | Inhibits bacterial protein synthesis and neutrophil activity | Approved | [162] |
| Clarithromycin | Inhibits bacterial protein synthesis and neutrophil activity | Approved | [163] | |
| Immune Supplements | Transfer Factor | Activate NK cells, Reduce IL-6, and Increase IL-10 | Nutraceutical | [164,165] |
| Corticosteroids | Dexamethasone | Promote anti-inflammatory activities | Approved | [166] |
| Methylprednisolone | Promote anti-inflammatory activities | Approved | [167] | |
| Others | Ulinastatin | Increases IL-10 level, reduces the levels of TNF-α, IL-6, and IFN-γ | Approved | [168] |
| Eritoran | TLR4 antagonist | [167] |
Immunomodulators are therapeutic agents that can be used in regulating immune responses. In the case of COVID-19, the suppression of hypercytokinemia may be able to resolve ARDS fully. Immunomodulators are of different categories, and some are currently being developed or repurposed for use based on compassionate recommendations for the management of COVID-19 [Figure 5]. 137 They are usually administered in combination with other drugs. They can be used as prophylaxis to prevent cytokine storms by promoting an effective innate immune response cascade through specific TLR to bring about viral clearance.30 Therapeutically, immunomodulators can also be used to activate an adaptive immune response.137 Immunomodulators range from synthetic drugs, natural compounds to recombinant antibodies, many of which are still undergoing clinical trials.
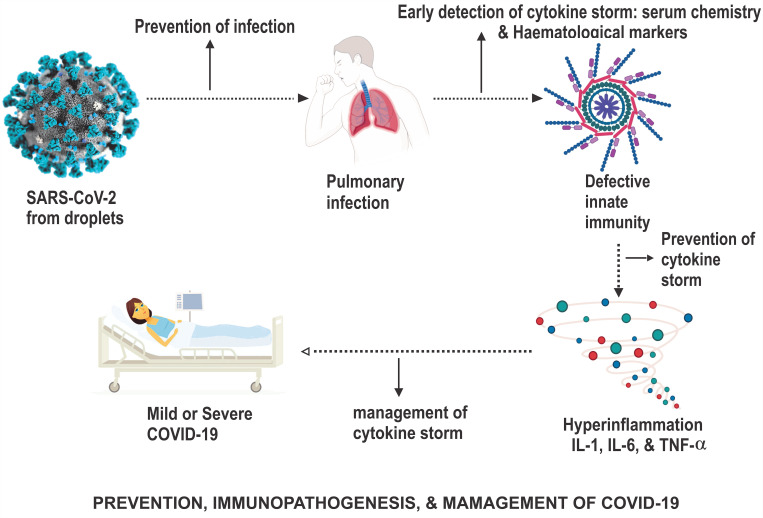
Figure 5.
The prevention, immunopathogenesis and management of COVID-19.
Micronutrients
These include trace elements, polyunsaturated fatty acids (PUFA), and vitamins.
Trace Elements
Zinc is an indispensable trace element involved in gene expression, protein folding, enzymatic reactions, and physiological processes.169–173 Indeed, zinc’s multifunctional effects are cell-specific, and many proteins bind to zinc through their zinc finger motifs.174 In combating SARS-CoV-2 viremia and its associated cytokine storm, zinc is vital in decreasing oxidative stress and optimizing immune function.175 Zinc modulates the activities of immune cells. As a signaling molecule, it triggers several cascades.175 It is involved in regulating lipopolysaccharide (LPS) signaling through the TLR-4 receptor in macrophages/monocytes and dendritic cells (DCs) of the innate immune system. In the adaptive immune system, zinc is involved in T Cell Receptor (TCR) signaling by recruiting lymphocyte-explicit protein tyrosine kinase (Lck) to the TCR complex. Zinc is also a regulator of the activity of enzymes of apoptotic signal transduction of B cell function.138,175 Studies have shown that zinc modulates the response B-cell response (antibody production), monocytes, NK cells, neutrophils, and lymphocyte development, including mediating the killing of viruses.139 Zinc deficiency triggers the release of cytokines such as IL-1β, IL-2, IL-6, and TNF-α, which triggers the expression of cellular zinc transporters.175 Conversely, zinc supplementation alters plasma cytokines in a dose-dependent manner.176 It causes the downregulation of pro-inflammatory cytokines due to the decreased gene expression of TNF-α, IL-1β, and IL-8.177
Interestingly, significantly lower levels of Zinc have been found in COVID-19 patients than in uninfected persons. This low zinc level has also increased the risk of complications by 5-fold.178 Also, in the treatment of COVID-19, zinc supplementation has proved to be essential due to the micronutrient’s natural antiviral and immunomodulatory properties. It is useful for the majority of the population, particularly those having suboptimal zinc levels.176 A case report on four COVID-19 patients revealed that administering a high dose of oral zinc salts had a beneficial effect on COVID-19 progression.178 On a larger scale, there are 12 different RCTs (as of August 2020) associated with Zinc Supplementation in the prophylaxis and therapy of COVID-19.179 Other trace elements play an essential role in the management of cytokine storms include copper, iron, magnesium, and selenium.180–182
Polyunsaturated Fatty Acids (PUFA) and Butyrate
Polyunsaturated fatty acids, including Omega-3 fatty acid, can be found in fish, seafood, and plant oils such as soybean and canola oils. Omega-3 fatty acids are responsible for modulating monocyte and lymphocyte functions and modify host immunity during inflammatory disease.183,184 Omega-3 fatty acids are made up of eicosapentaenoic and docosahexaenoic acids, which extensively enhance an immune response to viral infections.140,180 The conversion of Omega-3 by lipoxygenase to lipids mediators such as lipoxins, resolvins, and protectins facilitates a dual role of promoting pro-resolution of inflammation and anti-inflammation.183 The programmed resolution of acute inflammation reduces neutrophils’ infiltration, increases the recruitment of monocytes, and activates the macrophages to consume dead neutrophils.183,185 Consequently, a high dose of Omega-3 supplementation causes decreased plasma concentration of IL-1, IL-6, and TNF-α. This anti-inflammatory property makes PUFA useful in the prevention and treatment of COVID-19 cytokine storms.186 There is an ongoing RCT on the efficacy of using Omega-3 Polyunsaturated Fatty Acids to combat cytokine storms in COVID-19 patients. It is a single-blinded, randomized, placebo-controlled study involving 40 participants.187
Butyrate is a short-chain fatty acid synthesized by intestinal microbiota as a lactic acid fermentation product, and it serves as a source of energy for the intestinal epithelial cells. It also enhances the protection of the epithelium barrier through its anti-inflammatory activities.188 By the inhibition of NFκB activation and the degradation of I-kappa B alpha (IκBα) protein, butyrate suppresses the production of 1L-12. It also suppresses the discharge of other pro-inflammatory cytokines like IL-2 and IFN-γ in anti-CD3-stimulated peripheral blood mononuclear cells.141,188,189 Butyrate also enhances the production of anti-inflammatory cytokines, IL-10 and IL-4.189 There are several ongoing RCTs on the efficacy of probiotics species such as Bifidobacterium and Lactobacillus in treating COVID-19 patients.190
Vitamins
These are known to have immunomodulatory, anti-viral, and antioxidant properties.142 Vitamin C is an essential micronutrient that modulates both innate and adaptive immune functions. By stimulating p38 mitogen-activated protein kinase, vitamin C inhibits TNF-mediated activation of NFκB by the phosphorylation of IκBα.191 Dietary supplementation of vitamin C significantly decreases the mRNA expression of pro-inflammatory cytokines and the 70 kilodalton heat shock protein (HSP70).192 Also, the intravenous administration of vitamin C in high doses can ameliorate the neutrophil-related cytokine storm in COVID-19 patients.191,192 Vitamin C also causes increased production of IFN-1, which causes the suppression of SARS-CoV-2 replication.7,192 IFN-1 modulates the NK cell via both direct and indirect pathways.193,194 There is an ongoing study on the efficacy of Vitamin C in treating Acute Lung Injury in COVID-19 patients. The protocol is 50 mg/kg of Vitamin C administered intravenously every 6 hours for 96 hours.195
Vitamin D has also been shown to suppress cytokine production in COVID-19 patients.143 Vitamin D binds with the vitamin D receptor (VDR) on Th17 cells and suppresses the production of the IL-17 by inducing C/EBP homologous protein (CHOP) expression. Consequently, the recruitment of neutrophils is impaired.196 Data from observational studies suggest that vitamin D supplementation can reduce the risk of developing respiratory infections, especially in vitamin D-deficient people.197
Phytochemicals and Plant Extracts
The extracts of many plants such as fruits, herbs, and spices can produce anti-inflammatory activities. For instance, the extract of Echinacea purpurea has been proven to stimulate phagocytosis in macrophages and inhibit the release of TNF-α. It also acts as an agonist against cannabinoid B2 receptor, trigger NK cells, and increase leucocyte circulation.145 Camellia sinensis (green tea) extract reportedly reduced flu infection’s clinical symptoms in a double-blind study and promoted γδ T lymphocyte functions.146 Echium amoenum (Boraginaceae) extract to act on the macrophages to reduce iNOS and COX2 enzymes and TNF-α, IL-1β, and IL-6 cytokine levels.147 The extracts of plants such as Abelmoschus esculentus and Andrographis paniculata have been predicted to be stimulators of the KIR2DS2 and KIR2DS4 receptors of NK cells, respectively.144,148 The stimulation of these activating receptors, which engage the DAP12-ZAP 70/Syk ITAM-dependent signaling pathway, would prevent cytokine storm by reducing the viral load.44 Thereby, multiple studies reported that assessment of cytokinin levels with differentiation was a potentially beneficial correlation with the prognosis of severe COVID-19 infected cases.121,198–200
Natural compounds such as resveratrol, kaempferol, diosmetin, naringenin, capsaicin, apigenin, chrysin, quercetin, and luteolin can reduce the expression of TNF-alpha, IL-6, inducible nitric oxide synthase (iNOS), or cyclooxygenase-2 (COX2). They can also increase the expression of anti-inflammatory cytokines such as IL-10.201 Dietary polyphenolic compounds such as flavonoids have been shown to have beneficial effects on host defense reactions and inhibit the transcription factor, NF-kB, from producing pro-inflammatory cytokines.202,203 544
Clinical trials using only medicinal plants for the prevention and treatment of COVID-19 are scarce. However, numerous ongoing RCTs use these natural products as the adjuvant component. For example, in China, diazotized glycyrrhizinate, extracted from licorice root, has been tried to control COVID-19 alongside Vitamin C and a standard antiviral agent. In Iran, clinical studies have been carried out to investigate the anti-COVID-19 efficacy of the combination of several medicinal plants such as licorice, mallow, turmeric, echinacea, ginger, sage, fennel, and St. John’s wort.204
Interferons
Interferon lambda (IFN-λ) is a Type III interferon with established antiviral activity in the respiratory tract’s epithelial cells.149 In nature, Type III interferons act as the first line of the weapon in fighting viruses. IFN-λ is induced because of pathogen identification through pattern recognition receptors (PRR).150 The receptors of Type III interferon are expressed on all epithelial cells. Hence, they are found prominently in the respiratory, gastrointestinal, and reproductive tracts.205,206 IFN-λ binds to IFN-λ receptor 1 (IFNLR1) via strong affinity and recruits its subunit, IL10Rb.207 The complex formed transduces signals through the JAK-STAT pathway, culminating in the expression of hundreds of IFN-stimulated genes (ISG) such as NF-kB.150 Consequently, IFN-λ relieves inflammation by the suppression of neutrophil infiltration into the epithelial cells. IFN-λ also suppresses IL-1β production and reduces the activity of IFN-αβ, thereby downplaying immune-response in inflammation.208 With these anti-inflammatory properties, IFN-λ has the therapeutic potential to combat neutrophil-related cytokine storms.209
The early administration of IFN-λ as an immunomodulator during the low viral titer stage of coronavirus infection recorded considerable clinical therapy success. Similarly, some research work has shown considerable success in IFN-λ in combating the COVID 19 infection. The WHO has approved IFN-λ to manage COVID-19 patients due to the promise it has shown.149 A multicentre, double-blind, placebo-controlled RCT investigated the safety and efficacy of peginterferon lambda in treating mild-to-moderate cases of COVID-19. The study titled Interferon Lambda for Immediate Antiviral Therapy at Diagnosis in COVID-19 (ILIAD) involved 60 participants. The study revealed that Peginterferon lambda accelerated viral decline compared with the placebo indicating its usefulness in preventing clinical deterioration and reducing the time the virus is shed.210 Similarly, the safety and efficacy of interferon (IFN) β-1 in treating severe COVID-19 cases have been evaluated by an open-label RCT. It was observed that IFN β-1b significantly reduced mortality, decreased the need for invasive mechanical ventilation, and shortened the recovery time of patients.211
Interleukin 6 (IL-6) Inhibitors
IL-6 Inhibitors block the pro-inflammatory activity of IL-6, which is critical in developing cytokine storms in severe COVID-19 infection. Sarilumab and Tocilizumab are human monoclonal antibodies that inhibit IL-6 signaling by binding to the IL-6 receptor, responsible for processes such as activation of T-cells, stimulation of immunoglobulin secretion, the proliferation of the hematopoietic cell, and differentiation stimulation.76 Tocilizumab interacts with transmembrane and soluble forms of IL-6 receptors, thereby blocking the transduction of signals that trigger an immune response.76 Tocilizumab has been recommended for severe COVID-19 patients showing extensive bilateral pulmonary injury and high IL-6 levels.151,212 A retrospective examination of 20 COVID-19 cases revealed that Tocilizumab decreased fever and lung lesion opacity and improved the number of lymphocytes in peripheral blood.152 However, at 15 days, RCT shows that in severe cases of COVID-19, tocilizumab plus standard care did not perform better than standard care alone in improving clinical outcome.213 Furthermore, there are documented reports of tocilizumab linked with increased chances of developing opportunistic infections such as fungal, tuberculosis, or other viral infections due to immune suppression.153
Interleukin 1 (IL-1) Receptor Antagonists
The pro-inflammatory properties of IL-1 pre-dominate the innate immune system and are closely linked to damaging inflammation. A study revealed that the primary inflammatory cells found in the broncho-alveolar fluids and lung tissues of COVID-19 patients are the highly inflammatory pro-fibrosing monocyte-derived macrophages.154 This shows that IL-1α and IL-1β, two major juxtacrine and paracrine stimulatory cytokines of macrophagic-monocyte cells, are good targets for combating hypercytokinemia.
The management of cytokine storms resulting from infection has been meticulously studied by testing the agonists’ activities of IL-1 cytokines produced during infection. Inhibition of IL-1β, a cytokine of the IL-1 family, produced alongside IL-33 and IL-18 during infection by a drug named Anakinra, has considerably reduced cytokine storm.154 Anakinra is a human recombinant monoclonal antibody antagonist that connects to the IL-1R receptor and has shown efficacy in treating COVID-19 hypercytokinemia.154 Investigation on some individuals suffering from either moderate or severe COVID-19 pneumonia treated with Anakinra showed good clinical and biological outcomes.214 However, on a full-scale multicentre, open-label, Bayesian RCT, Anakinra did not improve clinical outcome in mild-to-moderate cases of COVID-19 pneumonia.215
Tumor Necrosis Factor (TNF) Blocker
Tumor necrosis factor blockers are agents that inhibit the TNF signaling pathway and consequently suppress hyperinflammation. Anti-TNF therapies have been proven to suppress inflammation in inflammatory ailments like rheumatoid arthritis and inflammatory bowel disease.216 Empirical evidence reports that TNF-inhibitors could treat the cytokine storm in COVID-19 patients.216 Several natural compounds have also been shown to inhibit TNF production. They include curcumin from turmeric and catechins from green tea.217 One major advantage TNF inhibitors have over cytokine blockers is that they down-regulate the expression of several pro-inflammatory intermediaries such as IL-1, IL-6, and Granulocyte-macrophage colony-stimulating factor (GM-CSF) as well as down-regulate clotting markers such as DD and pro-thrombin fragments.218
Multiple sourced, observational data generated from the management of COVID-19 patients on anti-TNF therapy show improved clinical outcomes and reduced death rate. In the face of this body of compelling evidence, there is a compelling need for a full-scale evaluation of TNF inhibitors through an RCT.219 However, there are safety concerns about secondary bacterial infection following the administration of TNF inhibitors.220
Sphingosine-1-Phosphate Receptor (S1PR) Agonists
Sphingosine-1-phosphate receptors are a group of G-protein-coupled receptors (GPCRs) to which the biologically active lipid called sphingosine 1-phosphate (S1P) has a strong affinity. They are sub-divided into S1PR1, S1PR2, S1PR3, S1PR4, and S1PR5.155 SIP acts as a lipid signaling molecule that targets and up-regulates these receptors as a potential strategy in combating hyper-inflammation associated with SARS-CoV-2 infection.156,221 Specifically, S1PR1 abundantly found on endothelial cells plays two critical roles: (a) modulation of immune responses by regulating the maturation, migration, and trafficking of lymphocytes.222,223 (b) Protection of the vascular endothelium against infection by regulating the vascular maturation, migration, cytoskeletal structure, and capillary-like network formation of endothelial cells.224,225
The use of S1PR1 signaling can significantly reduce the immunopathology associated with cytokine storms. This is because the treatment with S1PR1 ligands has a profound anti-inflammatory effect. Activated by phosphorylation, S1PR1 ligands bind to the S1P1 receptor inhibiting the release of specific lymphoid cells, suppress excessive cytokine production, and consequently reduce immune-mediated pulmonary injury.226,227
Fingolimod is an approved activator of S1PR1, S1PR3, S1PR4, and S1PR5 receptors. The oral administration of Fingolimod in COVID-19 patients stabilized the pulmonary endothelial barrier, decreased the inflammatory infiltrate, and reduced histopathology associated with ARDS.156,228 Widely used in the treatment of multiple sclerosis, Fingolimod is yet to undergo RCT for its efficacy in the treatment of COVID-19.229
Also, CYM5442, S1PR1-specific agonist, significantly reduced hyper-inflammation in H1N1-induced mice and improved survival. It also alleviated H1N1-induced acute lung injury (ALI).230 It has been suggested that it should be used as adjunctive therapy in battling COVID-19 disease.208
Janus Kinase (JAK) Inhibitors
Interleukin (IL)-1 and IL-6 signaling are critical to cytokine syndrome in COVID-19 patients. Janus kinase inhibitors have dual therapeutic potential.157 They exhibit their antiviral effect by blocking viral endocytosis and blocking multiple pro-inflammatory cytokines activation.157 A known example is baricitinib, a typical numb-associated kinase (NAK) antagonist (a JAK1/2 antagonist). It also inhibits other enzymes such as tyrosine kinase 2 and JAK3.158 Baricitinib has a high affinity for a clathrin-mediated viral endocytosis regulator, AP2-linked protein kinase - 1 (AAK1), found in pulmonary AT2 alveolar epithelial cells.231 The binding of baricitinib to AAK1 prevent SARS-CoV-2 penetration into the AT2 alveolar epithelial cells.232
In an open-labeled investigation, patients administered baricitinib and existing therapies for two weeks significantly showed improvement in fever, oxygen saturation, the partial pressure of arterial oxygen/percentage of inspired oxygen ratio, C-reactive protein, and early warning scores.232 1033 COVID-19 patients were subjected to another randomized, double-blind, placebo-controlled trial and the results revealed that baricitinib administer with remdesivir showed better clinical outcomes than remdesivir alone by accelerating improvement and reducing recovery time.233
Another example of JAK inhibitors is pacritinib, which is under investigation. Pacritinib is Interleukin-1 receptor-associated kinase 1 (IRAK1) and JAK2 inhibitor that could mitigate IL-1 and IL-6 expression and macrophage activation syndrome.159 Due to its anti-inflammatory potential, pacritinib has recently undergone a multicenter Phase 3, randomized, double-blind placebo-controlled study. The study called PRE-VENT evaluates the ability of pacritinib to prevent the disease progression into ARDS in hospitalized patients with severe COVID-19 with or without cancer.234
Hydroxychloroquine (HCQ)
Hydroxychloroquine is employed in the management of rheumatoid arthritis, malaria, and the autoimmune disease, Lupus.160 Quinoline, a component of HCQ, is a potential immunomodulator that can reduce the production of proinflammatory cytokines such as TNF-α, IL-1, IL-6, and IFN-α implicated in hypercytokinemia. This can be achieved by inhibiting MHC II expression, antigen processing, immune activation through TLR signaling, and the cyclic GMP–AMP synthase (cGAS) stimulation of interferon genes T-cells.160 HCQ increases endosomal pH inhibiting intracellular viral activity and its presentation on the MHC 1 protein.7 HCQ shows several potential antiviral and anti-inflammatory effects against COVID-19 infection during the early stages, and its prompt administration is key to the prevention of disease progression and severity.161,235 Additionally, it has been reported that administration of HCQ had a positive association with serious psychiatric conditions including suicide, and, between these patients, some of them committed suicide among COVID-19 cases236 and rheumatoid arthritis patent.237
Macrolides
Macrolides are antibiotics with immunomodulatory properties such as neutrophil activation and clearance through apoptosis, prevention of pro-inflammatory cytokine released by immune cells, modulation of macrophage differentiation, and inhibition of mucus, etc.238 Macrolides have been suggested for use in controlling hypercytokinemia in influenza infections.239 Chronic inflammation of the lung parenchyma is characterized by an elevation of alveolar macrophages, Th1 and Th17 T-cells, neutrophils, and innate lymphoid cells. There is also an elevation of the blood levels of pro-inflammatory cytokines such as IL-1β, IL-4, IL-8, and TNF-α.239 Azithromycin, erythromycin, and Clarithromycin are macrolides that have been used frequently in combination with other therapies for the treatment of COVID-19. Beyond treating bacterial infections, these macrolides have been shown to significantly decrease neutrophil count, inhibit neutrophil chemotaxis, and reduce the concentration of neutrophil elastase concentration, IL-4, IL-8, TNF-α, IFN-γ, C- reactive protein, calprotectin, myeloperoxidase (MPO), and serum amyloid A in the blood, through various immunological mechanisms.162,240
In an intervention study involving 90 participants, the oral administration of 500 mg clarithromycin twice daily for seven days significantly increased anti-inflammatory activity and improved clinical outcome in early moderate COVID-19 infection.163 The proposed mechanism of action of clarithromycin is the modulation of the Th1/Th2 mononuclear responses and the suppression of SARS-CoV-2 viral load.163
In a randomized clinical trial conducted among patients with chronic obstructive pulmonary disease (COPD), azithromycin showed decreased platelet and white blood cell counts; and decreased concentrations of IL-8, C-reactive protein, E-selectin, and lactoferrin in the plasma.163 Interestingly, the University of Oxford reported that the Platform Randomised Trial of Interventions against COVID-19 in older people (PRINCIPLE) trial showed that azithromycin is not effective the treatment of COVID-19 in older people. 500mg of azithromycin was administered three times daily for 14 days to 526 subjects. These were compared with 862 subjects receiving usual care. No beneficial effect of azithromycin was observed in subjects aged over 50 years treated in the early stages of COVID-19.241 Also, clinical outcomes were not improved when azithromycin was added to standard of care treatment (which included hydroxychloroquine) in severe COVID-19 patients. This was observed in an open-label RCT that took place in 37 locations across Brazil.242
Transfer Factors (TF)
Transfer factors are low molecular weight polypeptides fractions obtained from natural sources such as colostrum, egg yolks, or porcine spleen.164 Specifically, it is derived from T-lymphocytes, and the immunomodulatory properties of TF have been well documented. It has been used to combat viral infections successfully.164 Transfer factors increase innate defense by the activation of NK cells against viruses. TF can decrease IL-6 production and trigger the release of IL-10. Thus, TF can potentially combat immune hyper-responsiveness and hyper-inflammatory associated with COVID-19.165 Though yet to undergo RCT, TF’s use has been proposed as a therapeutic option against COVID-19.243 However, a 7-week double-blinded study evaluates the effect of TF supplementation in healthy adults against Upper Respiratory Tract Infections caused by Influenza or Rhinovirus. There are no results posted for this study.244
Corticosteroids and Other Agents
Corticosteroids such as methylprednisolone and dexamethasone have significantly decreased the death toll in COVID-19 patients with ARDS.245 Methylprednisolone has been found to decrease death risk in ARDS patients in China’s clinical study.245 Another study revealed that a single-dose of 40–500 mg of methylprednisolone stopped the hyper-inflammation without a negative effect of virus removal and specific IgG production. However, continuous administration of methylprednisolone slowed down a viral clearance and suppressed the immune system.246 Prolonged use of methylprednisolone can also cause infections and other adverse effects, which include musculoskeletal effects such as osteoporosis, myopathy, and osteonecrosis; endocrine effects such as Cushing syndrome and growth impairment; cardiovascular effects such as hypertension and arrhythmias; dermatologic effects such as ecchymosis, mild hirsutism, and facial erythema; ophthalmologic effects such as cataract and glaucoma; gastrointestinal effects such as gastritis, gastric ulcer, and fatty liver; and neuropsychiatric effects such as psychosis, sleep disturbances, and akathisia.247
According to the findings by the UK Recovery Group headed by Horby P, dexamethasone emerges as the most reliable remedy for lung inflammation due to COVID-19 infection to date. It significantly suppressed cytokine storm, reduced mortality, and the duration of hospital stay among 6425 patients with severe COVID-19 infection.166 Also, dexamethasone has been reported to reduce the risk of death linked to hypercytokinemia in 33% of patients with severe COVID-19 disease who received mechanical ventilation.248 Dexamethasone is beneficial in hospitalized women who are COVID-19 patients and require mechanical ventilation or supplemental oxygen support. Though the same is beneficial in pediatric COVID-19 patients who also require mechanical ventilation or any other form of supplemental oxygen support, this should be considered on a case-by-case basis.249
Glucocorticosteroids have been used to manage lung inflammation during SARS and MERS epidemics but produced harmful effects such as prolonged viral presence (due to immunosuppression) and induced diabetes.248 The WHO has recommended the administration of systemic corticosteroids in severe COVID-19 patients; however, it discouraged its use in treating COVID-19 patients with mild infection.167 This is to prevent worsening of a problem with complications such as asthma and cardiogenic shock. The WHO advised that the risk and benefit analysis should be done for individual patients before administering corticosteroids.167
Unlike corticosteroids, ulinastatin, a non-immunosuppressant drug, is a widely used clinical drug to combat inflammation. It increases IL-10 level (anti-inflammatory factor) and reduces TNF-α, IL-6, and IFN-γ (proinflammatory factors). Therefore, ulinastatin may have a good prospect in treating COVID-19 infection, although further RCT is needed to substantiate this claim.168
In infections with pathogenic human coronaviruses, there is an accumulation of oxidized phospholipids (OxPL) in the pulmonary tissues.250 The OxPL stimulates the Toll-like receptor 4 (TLR4), which increases the production of cytokines/chemokines in the macrophages of the lungs. Eritoran is a TLR4 antagonist, and therefore, it decreases the secretion of OxPL, pro-inflammatory cytokines, and chemokines. Along with other OxPL inhibitors, Eritoran is a potential therapeutic candidate for COVID-19 disease.250
Conclusion
The COVID-19-induced cytokine storm is characterized by immune dysregulation and hyperinflammation. It is also associated with tissue damage, multi-organ failure, and death. The early diagnosis and aggressive mitigation of cytokine storms are essential to reduce COVID-19 morbidities and mortalities effectively. Clinical data suggests that specific hyper-inflammation markers have proven to be accurate and reliable in distinguishing between mild and severe cases of COVID-19 infection. Laboratory tests using these markers on the first day of hospitalization and a few days after would be useful in the clinical evaluation of disease status and progression. For would-be severe cases, a quick and aggressive administration of the potential therapeutic agents discussed would prevent or attenuate the effect of the advancing cytokine storm.
Recommendation
Several diagnostic markers for cytokine storms indicate that they could be easily identified and promptly arrested. Rapid prophylaxis and treatment of cytokine storm by clinicians will significantly enhance success in the fight against the dreaded COVID-19 pandemic. Policymakers should consider the recommendation for the off-label use of medications with potential activity against cytokine storm. Original studies on the genetic and other remote causes of the defective innate immune system are needed.
Key Messages
The cytokine storm is a major cause of mortality and morbidity in COVID-19 infection.
COVID-19-induced cytokine storm is caused by a defective innate immune response and an exaggerated adaptive immune response.
The magnitude of the release of pro-inflammatory cytokines such as IL-1, IL-6, and TNF-α significantly differentiates between mild and severe cases of COVID-19.
Through the effective use of serum chemistry and hematological markers, a cytokine storm may be promptly diagnosed and controlled.
Several biologicals, synthetic drugs, and herbal products have shown good promise in combating COVID-19-induced cytokine storms, but there is a need to validate their efficacy and safety in randomized clinical trials.
Reports from clinical trials by the UK RECOVERY Group reveal that dexamethasone is the most promising drug in combating cytokine storms and consequently preventing and reducing mortality caused by the COVID-19.
Acknowledgment
The images were created using the BioRender Webserver. We also appreciate the Department of Pharmaceutical Microbiology and Biotechnology, Faculty of Pharmaceutical Sciences, Nnamdi Azikiwe University, Anambra State, Nigeria.
Consent for Publication
Every author had reviewed and approved the final copy of the manuscript and has agreed to be responsible for all aspects of the work, including any issues related to accuracy or integrity.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they do not have any financial involvement or affiliations with any organization, association, or entity directly or indirectly with the subject matter or materials presented in this article. This also includes honoraria, expert testimony, employment, ownership of stocks or options, patents or grants received or pending, or royalties.
References
- 1.Zhu N, Zhang D, Wang W, et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N Engl J Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization Fact Sheet. WHO Health Emergency Dashboard. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019. Accessed April 8, 2021.
- 3.Chen N, Zhou M, Dong X, et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395(10223):507–513. doi: 10.1016/S0140-6736(20)30211-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nile SH, Nile A, Qiu J, Li L, Jia X, Kai G. COVID-19: pathogenesis, cytokine storm, and therapeutic potential of interferons. Cytokine Growth Factor Rev. 2020;53:66–70. doi: 10.1016/j.cytogfr.2020.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Li L, Feng Z, et al. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020;6:11. doi: 10.1038/s41421-020-0147-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu R, Zhao X, Li J, et al. Genomic characterization and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395(10224):565–574. doi: 10.1016/S0140-6736(20)30251-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rowaiye AB, Onuh OA, Oli AN, Okpalefe OA, Oni S, Nwankwo EJ. The pandemic COVID-19: a tale of viremia, cellular oxidation, and immune dysfunction. Pan Afr Med J. 2020;36:188. doi: 10.11604/pamj.2020.36.188.23476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun X, Wang T, Cai D, et al. Cytokine storm intervention in the early stages of COVID-19 pneumonia. Cytokine Growth Factor Rev. 2020;53:38–42. doi: 10.1016/j.cytogfr.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ragab D, Salah Eldin H, Taeimah M, Khattab R, Salem R. The COVID-19 Cytokine Storm; What We Know So Far. Front Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bhaskar S, Sinha A, Banach M, et al. Cytokine Storm in COVID-19-Immunopathological Mechanisms, Clinical Considerations, and Therapeutic Approaches: the REPROGRAM Consortium Position Paper. Front Immunol. 2020;11:1648. doi: 10.3389/fimmu.2020.01648 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim JS, Lee JY, Yang JW, et al. Immunopathogenesis and treatment of cytokine storm in COVID-19. Theranostics. 2021;11(1):316–329. doi: 10.7150/thno.49713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eccleston-Turner M, Phelan A, Katz R. Preparing for the Next Pandemic - The WHO’s Global Influenza Strategy. N Engl J Med. 2019;381(23):2192–2194. doi: 10.1056/NEJMp1905224 [DOI] [PubMed] [Google Scholar]
- 13.Teijaro JR, Walsh KB, Rice S, Rosen H, Oldstone MB. Mapping the innate signaling cascade essential for cytokine storm during influenza virus infection. Proc Natl Acad Sci USA. 2014;111(10):3799–3804. doi: 10.1073/pnas.1400593111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin YH, Cai L, Cheng ZS, et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil Med Res. 2020;7(1):4. doi: 10.1186/s40779-020-0233-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76(1):16–32. doi: 10.1128/MMBR.05015-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciavarella C, Motta I, Valente S, Pasquinelli G. Pharmacological (or Synthetic) and Nutritional Agonists of PPAR-γ as Candidates for Cytokine Storm Modulation in COVID-19 Disease. Molecules. 2020;25(9):2076. doi: 10.3390/molecules25092076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye Q, Wang B, Mao J. The pathogenesis and treatment of the `Cytokine Storm’ in COVID-19. J Infect. 2020;80(6):607–613. doi: 10.1016/j.jinf.2020.03.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Channappanavar R, Fehr AR, Vijay R, et al. Dysregulated Type I Interferon and Inflammatory Monocyte-Macrophage Responses Cause Lethal Pneumonia in SARS-CoV-Infected Mice. Cell Host Microbe. 2016;19(2):181–193. doi: 10.1016/j.chom.2016.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Davidson S, Maini MK, Wack A. Disease-promoting effects of type I interferons in viral, bacterial, and coinfections. J Interferon Cytokine Res. 2015;35(4):252–264. doi: 10.1089/jir.2014.0227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shaw AC, Goldstein DR, Montgomery RR. Age-dependent dysregulation of innate immunity. Nat Rev Immunol. 2013;13(12):875–887. doi: 10.1038/nri3547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li S, Zhang Y, Guan Z, et al. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct Target Ther. 2020;5(1):235. doi: 10.1038/s41392-020-00334-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J, Jiang M, Chen X, Montaner LJ. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol. 2020;108(1):17–41. doi: 10.1002/JLB.3COVR0520-272R [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yang D, Chu H, Hou Y, et al. Attenuated Interferon and Proinflammatory Response in SARS-CoV-2-Infected Human Dendritic Cells Is Associated with Viral Antagonism of STAT1 Phosphorylation. J Infect Dis. 2020;222(5):734–745. doi: 10.1093/infdis/jiaa356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim ES, Choe PG, Park WB, et al. Clinical Progression and Cytokine Profiles of Middle East Respiratory Syndrome Coronavirus Infection. J Korean Med Sci. 2016;31(11):1717–1725. doi: 10.3346/jkms.2016.31.11.1717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hoffmann M, Kleine-Weber H, Schroeder S, et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell. 2020;181(2):271–280.e8. doi: 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harmer D, Gilbert M, Borman R, Clark KL. Quantitative mRNA expression profiling of ACE 2, a novel homolog of angiotensin-converting enzyme. FEBS Lett. 2002;532(1–2):107–110. doi: 10.1016/s0014-5793(02)03640-2 [DOI] [PubMed] [Google Scholar]
- 28.Niu W, Qi Y, Hou S, Zhou W, Qiu C. Correlation of angiotensin-converting enzyme two gene polymorphisms with stage 2 hypertension in Han Chinese. Transl Res. 2007;150(6):374–380. doi: 10.1016/j.trsl.2007.06.002 [DOI] [PubMed] [Google Scholar]
- 29.Chen YY, Liu D, Zhang P, et al. Impact of ACE2 gene polymorphism on antihypertensive efficacy of ACE inhibitors. J Hum Hypertens. 2016;30(12):766–771. doi: 10.1038/jhh.2016.24 [DOI] [PubMed] [Google Scholar]
- 30.Wysocki J, Ye M, Soler MJ, et al. ACE and ACE2 activity in diabetic mice. Diabetes. 2006;55(7):2132–2139. doi: 10.2337/db06-0033 [DOI] [PubMed] [Google Scholar]
- 31.IIwasaki A, Pillai PS. Innate immunity to influenza virus infection. Nat Rev Immunol. 2014;14(5):315–328. doi: 10.1038/nri3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yang L, Liu S, Liu J, et al. COVID-19: immunopathogenesis and Immunotherapeutics. Signal Transduct Target Ther. 2020;5(1):128. doi: 10.1038/s41392-020-00243-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xagorari A, Chlichlia K. Toll-like receptors and viruses: induction of innate antiviral immune responses. Open Microbiol J. 2008;2(1):49–59. doi: 10.2174/1874285800802010049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wurfel MM, Gordon AC, Holden TD, et al. Toll-like receptor 1 polymorphisms affect innate immune responses and outcomes in sepsis. Am J Respir Crit Care Med. 2008;178(7):710–720. doi: 10.1164/rccm.200803-462OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bastard P, Rosen LB, Zhang Q, et al. Autoantibodies against type I IFNs in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4585. doi: 10.1126/science [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Q, Bastard P, Liu Z, et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370(6515):eabd4570. doi: 10.1126/science [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider WM, Chevillotte MD, Rice CM. Interferon-stimulated genes: a complex web of host defenses. Annu Rev Immunol. 2014;32(1):513–545. doi: 10.1146/annurev-immunol-032713-120231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kawai T, Akira S. Antiviral signaling through pattern recognition receptors. J Biochem. 2007;141(2):137–145. doi: 10.1093/jb/mvm032 [DOI] [PubMed] [Google Scholar]
- 39.Sallard E, Lescure FX, Yazdanpanah Y, Mentre F, Peiffer-Smadja N. Type 1 interferons as a potential treatment against COVID-19. Antiviral Res. 2020;178:104791. doi: 10.1016/j.antiviral.2020.104791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reiter Z. Interferon–a major regulator of natural killer cell-mediated cytotoxicity. J Interferon Res. 1993;13(4):247–257. doi: 10.1089/jir.1993.13.247 [DOI] [PubMed] [Google Scholar]
- 41.Shibabaw T, Molla MD, Teferi B, Ayelign B. Role of IFN and Complements System: innate Immunity in SARS-CoV-2. J Inflamm Res. 2020;13:507–518. doi: 10.2147/JIR.S267280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hadjadj J, Yatim N, Barnabei L, et al. Impaired type I interferon activity and inflammatory responses in severe COVID-19 patients. Science. 2020;369(6504):718–724. doi: 10.1126/science.abc6027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Becknell B, Caligiuri MA. Interleukin-2, interleukin-15, and their roles in human natural killer cells. Adv Immunol. 2005;86:209–239. doi: 10.1016/S0065-2776(04)86006-1 [DOI] [PubMed] [Google Scholar]
- 44.Rowaiye AB, Asala T, Oli AN, Uzochukwu IC, Akpa A, Esimone CO. The Activating Receptors of Natural Killer Cells and Their Inter-Switching Potentials. Curr Drug Targets. 2020;21(16):1733–1751. doi: 10.2174/1389450121666200910160929 [DOI] [PubMed] [Google Scholar]
- 45.Soe WM, Lim JHJ, Williams DL, et al. Using Expanded Natural Killer Cells as Therapy for Invasive Aspergillosis. J Fungi. 2020;6(4):231. doi: 10.3390/jof6040231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandenhaute J, Wouters CH, Matthys P. Natural Killer Cells in Systemic Autoinflammatory Diseases: a Focus on Systemic Juvenile Idiopathic Arthritis and Macrophage Activation Syndrome. Front Immunol. 2020;10:3089. doi: 10.3389/fimmu.2019.03089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jenkins MR, Rudd-Schmidt JA, Lopez JA, et al. Failed CTL/NK cell killing and cytokine hypersecretion are directly linked through prolonged synapse time. J Exp Med. 2015;212(3):307–317. doi: 10.1084/jem.20140964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brisse E, Wouters CH, Matthys P. Advances in the pathogenesis of primary and secondary haemophagocytic lymphohistiocytosis: differences and similarities. Br J Haematol. 2016;174(2):203–217. doi: 10.1111/bjh.14147 [DOI] [PubMed] [Google Scholar]
- 49.Sepulveda FE, Debeurme F, Ménasché G, et al. Distinct severity of HLH in both human and murine mutants with complete loss of cytotoxic effector PRF1, RAB27A, and STX11. Blood. 2013;121(4):595–603. doi: 10.1182/blood-2012-07-440339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Trapani JA, Voskoboinik I, Jenkins MR. Perforin-dependent cytotoxicity: ‘Kiss of death’ or prolonged embrace with darker elocation-idnseque11es? Oncoimmunology. 2015;4(9):e1036215. doi: 10.1080/2162402X.2015.1036215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Canna SW, Behrens EM. Making sense of the cytokine storm: a conceptual framework for understanding, diagnosing, and treating hemophagocytic syndromes. Pediatr Clin North Am. 2012;59(2):329–344. doi: 10.1016/j.pcl.2012.03.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Voskoboinik I, Trapani JA. Perforinopathy: a spectrum of human immune disease caused by defective perforin delivery or function. Front Immunol. 2013;4:441. doi: 10.3389/fimmu.2013.00441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rex J, Lutz A, Faletti LE, et al. IL-1β and TNFα Differentially Influence NF-κB Activity and FasL-Induced Apoptosis in Primary Murine Hepatocytes During LPS-Induced Inflammation. Front Physiol. 2019;10:117. doi: 10.3389/fphys.2019.00117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hoesel B, Schmid JA. The complexity of NF-κB signaling in inflammation and cancer. Mol Cancer. 2013;12(1):86. doi: 10.1186/1476-4598-12-86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu T, Zhang L, Joo D, Sun SC. NF-κB signaling in inflammation. Sig Transduct Target Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Velazquez-Salinas L, Verdugo-Rodriguez A, Rodriguez LL, Borca MV. The Role of Interleukin 6 During Viral Infections. Front Microbiol. 2019;10:1057. doi: 10.3389/fmicb.2019.01057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lacroix M, Rousseau F, Guilhot F, et al. Novel Insights into Interleukin 6 (IL-6) Cis- and Trans-signaling Pathways by Differentially Manipulating the Assembly of the IL-6 Signaling Complex. J Biol Chem. 2015;290(45):26943–26953. doi: 10.1074/jbc.M115.682138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tamura K, Kanazawa T, Tsukada S, Kobayashi T, Kawamura M, Morikawa A. Increased serum monocyte chemoattractant protein-1, macrophage inflammatory protein-1beta, and interleukin-8 concentrations in hemophagocytic lymphohistiocytosis. Pediatr Blood Cancer. 2008;51(5):662–668. doi: 10.1002/pbc.21660 [DOI] [PubMed] [Google Scholar]
- 59.Takada H, Takahata Y, Nomura A, Ohga S, Mizuno Y, Hara T. Increased serum levels of interferon-gamma-inducible protein 10 and monokine induced by gamma interferon in patients with haemophagocytic lymphohistiocytosis. Clin Exp Immunol. 2003;133(3):448–453. doi: 10.1046/j.1365-2249.2003.02237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Osugi Y, Hara J, Tagawa S, et al. Cytokine production regulating Th1 and Th2 cytokines in hemophagocytic lymphohistiocytosis. Blood. 1997;89(11):4100–4103. doi: 10.1182/blood.V89.11.4100 [DOI] [PubMed] [Google Scholar]
- 61.Briso EM, Dienz O, Rincon M. Cutting edge: soluble IL-6R is produced by IL-6R ectodomain shedding in activated CD4 T cells. J Immunol. 2008;180(11):7102–7106. doi: 10.4049/jimmunol.180.11.7102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Calabrese LH, Rose-John S. IL-6 biology: implications for clinical targeting in rheumatic disease. Nat Rev Rheumatol. 2014;10(12):720–727. doi: 10.1038/nrrheum.2014.127 [DOI] [PubMed] [Google Scholar]
- 63.Ott LW, Resing KA, Sizemore AW, et al. Tumor Necrosis Factor-alpha- and interleukin-1-induced cellular responses: coupling proteomic and genomic information. J Proteome Res. 2007;6(6):2176–2185. doi: 10.1021/pr060665l [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tsang K, Zhong NS. SARS: pharmacotherapy. Respirology. 2003;8(Suppl):S25–S30. doi: 10.1046/j.1440-1843.2003.00525 [DOI] [PubMed] [Google Scholar]
- 65.Chien JY, Hsueh PR, Cheng WC, Yu CJ, Yang PC. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology. 2006;11(6):715–722. doi: 10.1111/j.1440-1843.2006.00942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi: 10.1016/j.cyto.2020.155151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhou Y, Fu B, Zheng X, et al. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. National Sci Rev. 2020;7(6):998–1002. doi: 10.1093/nsr/nwaa041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang Y, Shen C, Li J, et al. Plasma IP-10 and MCP-3 levels are highly associated with disease severity and predict the progression of COVID-19. J Allergy Clin Immunol. 2020;146(1):119–127.e4. doi: 10.1016/j.jaci.2020.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Herrero R, Sanchez G, Lorente JA. New insights into the mechanisms of pulmonary edema in acute lung injury. Ann Transl Med. 2018;6(2):32. doi: 10.21037/atm.2017.12.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wee JL, Greenwood DL, Han X, Scheerlinck JP. Inflammatory cytokines IL-6 and TNF-α regulate lymphocyte trafficking through the local lymph node. Vet Immunol Immunopathol. 2011;144(1–2):95–103. doi: 10.1016/j.vetimm.2011.07.007 [DOI] [PubMed] [Google Scholar]
- 72.Li H, Liu L, Zhang D, et al. SARS-CoV-2 and viral sepsis: observations and hypotheses. Lancet. 2020;395(10235):1517–1520. doi: 10.1016/S0140-6736(20)30920-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Maeda K, Baba Y, Nagai Y, et al. IL-6 blocks a discrete early step in lymphopoiesis. Blood. 2005;106(3):879–885. doi: 10.1182/blood-2005-02-0456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Maeda K, Malykhin A, Teague-Weber BN, Sun XH, Farris AD, Coggeshall KM. Interleukin-6 aborts lymphopoiesis and elevates production of myeloid cells in systemic lupus erythematosus-prone B6. Sle1.Yaa animals. Blood. 2009;113(19):4534–4540. doi: 10.1182/blood-2008-12-192559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shimabukuro-Vornhagen A, Gödel P, Subklewe M, et al. Cytokine release syndrome. J Immunother Cancer. 2018;6(1):56. doi: 10.1186/s40425-018-0343-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanaka T, Narazaki M, Kishimoto T. Immunotherapeutic implications of IL-6 blockade for cytokine storm. Immunotherapy. 2016;8(8):959–970. doi: 10.2217/imt-2016-0020 [DOI] [PubMed] [Google Scholar]
- 77.Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16(5):448–457. doi: 10.1038/ni.3153 [DOI] [PubMed] [Google Scholar]
- 78.Pathan N, Hemingway CA, Alizadeh AA, et al. Role of interleukin 6 in myocardial dysfunction of meningococcal septic shock. Lancet. 2004;363(9404):203–209. doi: 10.1016/S0140-6736(03)15326-3 [DOI] [PubMed] [Google Scholar]
- 79.Schulert GS, Grom AA. Pathogenesis of macrophage activation syndrome and potential for cytokine-directed therapies. Annu Rev Med. 2015;66:145–159. doi: 10.1146/annurev-med-061813-012806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sieni E, Cetica V, Hackmann Y, et al. Familial hemophagocytic lymphohistiocytosis: when rare diseases shed light on immune system functioning. Front Immunol. 2014;5:167. doi: 10.3389/fimmu.2014.00167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124(2):188–195. doi: 10.1182/blood-2014-05-552729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Khadka RH, Sakemura R, Kenderian SS, Johnson AJ. Management of cytokine release syndrome: an update on emerging antigen-specific T cell engaging immunotherapies. Immunotherapy. 2019;11(10):851–857. doi: 10.2217/imt-2019-0074 [DOI] [PubMed] [Google Scholar]
- 83.Poggiali E, Zaino D, Immovilli P, et al. Lactate dehydrogenase and C-reactive protein as predictors of respiratory failure in CoVID-19 patients. Clin Chim Acta. 2020;509:135–138. doi: 10.1016/j.cca.2020.06.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Potempa LA, Rajab IM, Hart PC, Bordon J, Fernandez-Botran R. Insights into the Use of C-Reactive Protein as a Diagnostic Index of Disease Severity in COVID-19 Infections. Am J Trop Med Hyg. 2020;103(2):561–563. doi: 10.4269/ajtmh.20-0473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kell DB, Pretorius E. Serum ferritin is an important inflammatory disease marker, as it is mainly a leakage product from damaged cells. Metallomics. 2014;6(4):748–773. doi: 10.1039/c3mt00347g [DOI] [PubMed] [Google Scholar]
- 86.Shorr AF, Thomas SJ, Alkins SA, Fitzpatrick TM, Ling GS. D-dimer correlates with proinflammatory cytokine levels and outcomes in critically ill patients. Chest. 2002;121(4):1262–1268. doi: 10.1378/chest.121.4.1262 [DOI] [PubMed] [Google Scholar]
- 87.Nehring SM, Goyal A, Bansal P, et al. C Reactive Protein. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2020. Available from https://www.ncbi.nlm.nih.gov/books/NBK441843/. Accessed November4th, 2020. [Google Scholar]
- 88.Bertolini A, van de Peppel IP, Bodewes FAJA, et al. Abnormal Liver Function Tests in Patients with COVID-19: relevance and Potential Pathogenesis. Hepatology. 2020. doi: 10.1002/hep.31480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Mandel M, Harari G, Gurevich M, Achiron A. Cytokine prediction of mortality in COVID19 patients. Cytokine. 2020;134:155190. doi: 10.1016/j.cyto.2020.155190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.O’Neill CM, Lu C, Corbin KL, et al. Nunemaker CS. Circulating levels of IL-1B+IL-6 cause ER stress and dysfunction in islets from prediabetic male mice. Endocrinology. 2013;154(9):3077–3088. doi: 10.1210/en.2012-2138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Grifoni E, Valoriani A, Cei F, et al. Interleukin-6 as prognosticator in patients with COVID-19. J Infect. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Li G, Wu W, Zhang X, et al. Serum levels of tumor necrosis factor-alpha in patients with IgA nephropathy are closely associated with disease severity. BMC Nephrol. 2018;19(1):326. doi: 10.1186/s12882-018-1069-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kong M, Zhang H, Cao X, Mao X, Lu Z. Higher level of neutrophil-to-lymphocyte is associated with severe COVID-19. Epidemiol Infect. 2020;148:e139. doi: 10.1017/S0950268820001557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu P, Zhou Q, Xu J. Mechanism of thrombocytopenia in COVID-19 patients. Ann Hematol. 2020;99(6):1205–1208. doi: 10.1007/s00277-020-04019-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. 2017;29(9):401–409. doi: 10.1093/intimm/dxx031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vargas-Vargas M, Cortés-Rojo C. Ferritin levels and COVID-19. Rev Panam Salud Publica. 2020;44:e72. doi: 10.26633/RPSP.2020.72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Gómez-Pastora J, Weigand M, Kim J, et al. Hyperferritinemia in critically ill COVID-19 patients - Is ferritin the product of inflammation or a pathogenic mediator? Clin Chim Acta. 2020;509:249–251. doi: 10.1016/j.cca.2020.06.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Shaw TY, Schivo M. Weathering a Cytokine Storm: a Case of EBV-Induced Hemophagocytic Lymphohistiocytosis. J Investig Med High Impact Case Rep. 2016;4(2):2324709616647409. doi: 10.1177/2324709616647409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Magro G. Cytokine Storm: is it the only major death factor in COVID-19 patients? Coagulation role. Med Hypotheses. 2020;142:109829. doi: 10.1016/j.mehy.2020.109829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pagana KD, Pagana TJ, Pagana TN. Mosby’s Diagnostic and Laboratory Test Reference. 14th ed. St Louis, MO: Elsevier; 2019. [Google Scholar]
- 101.Zhang L, Yan X, Fan Q, et al. D-dimer levels on admission to predict in-hospital mortality in patients with Covid-19. J Thromb Haemost. 2020;18(6):1324–1329. doi: 10.1111/jth.14859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yu B, Li X, Chen J, et al. Evaluation of variation in D-dimer levels among COVID-19 and bacterial pneumonia: a retrospective analysis. J Thromb Thrombolysis. 2020;50(3):548–557. doi: 10.1007/s11239-020-02171-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chhetri S, Khamis F, Pandak N, Al Khalili H, Said E, Petersen E. A fatal case of COVID-19 due to metabolic acidosis following dysregulate inflammatory response (cytokine storm). IDCases. 2020;21:e00829. doi: 10.1016/j.idcr [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kelley N, Jeltema D, Duan Y, He Y. The NLRP3 Inflammasome: an Overview of Mechanisms of Activation and Regulation. Int J Mol Sci. 2019;20(13):3328. doi: 10.3390/ijms20133328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Erra Díaz F, Dantas E, Geffner J. Unravelling the Interplay between Extracellular Acidosis and Immune Cells. Mediators Inflamm. 2018;2018:1218297. doi: 10.1155/2018/1218297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wu MY, Yao L, Wang Y, et al. Clinical evaluation of potential usefulness of serum lactate dehydrogenase (LDH) in 2019 novel coronavirus (COVID-19) pneumonia. Respir Res. 2020;21(1):171. doi: 10.1186/s12931-020-01427-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Wang Z, Han W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark Res. 2018;6(1):4. doi: 10.1186/s40364-018-0116-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fan Z, Chen L, Li J, et al. Clinical Features of COVID-19-Related Liver Functional Abnormality. Clin Gastroenterol Hepatol. 2020;18(7):1561–1566. doi: 10.1016/j.cgh.2020.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Alqahtani SA, Schattenberg JM. Liver injury in COVID-19: the current evidence. United European Gastroenterol J. 2020;8(5):509–519. doi: 10.1177/2050640620924157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Xu L, Liu J, Lu M, Yang D, Zheng X. Liver injury during highly pathogenic human coronavirus infections. Liver Int. 2020;40(5):998–1004. doi: 10.1111/liv.14435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Cai Q, Huang D, Yu H, et al. COVID-19: abnormal liver function tests. J Hepatol. 2020;73(3):566–574. doi: 10.1016/j.jhep.2020.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Morgan DB, Carver ME, Payne RB. Plasma creatinine and urea: creatinine ratio in patients with raised plasma urea. Br Med J. 1977;2(6092):929–932. doi: 10.1136/bmj.2.6092.929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zhang C, Shi L, Wang FS. Liver injury in COVID-19: management and challenges. Lancet Gastroenterol Hepatol. 2020;5(5):428–430. doi: 10.1016/S2468-1253(20)30057-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Gulen M, Satar S. Uncommon presentation of COVID-19: gastrointestinal bleeding. Clin Res Hepatol Gastroenterol. 2020;44(4):e72–e76. doi: 10.1016/j.clinre.2020.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tian Y, Rong L, Nian W, He Y. Review article: gastrointestinal features in COVID-19 and the possibility of fecal transmission. Aliment Pharmacol Ther. 2020;51(9):843–851. doi: 10.1111/apt.15731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yang X, Yu Y, Xu J, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cheng A, Hu L, Wang Y, et al. Diagnostic performance of initial blood urea nitrogen combined with D-dimer levels for predicting in-hospital mortality in COVID-19 patients. Int J Antimicrob Agents. 2020;56(3):106110. doi: 10.1016/j.ijantimicag.2020.106110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wrigley BJ, Lip GY, Shantsila E. The role of monocytes and inflammation in the pathophysiology of heart failure. Eur J Heart Fail. 2011;13(11):1161–1171. doi: 10.1093/eurjhf/hfr122 [DOI] [PubMed] [Google Scholar]
- 119.Yang J, Liao X, Yin W, et al. Elevated cardiac biomarkers may be effective prognostic predictors for patients with COVID-19: a multicenter, observational study. Am J Emerg Med. 2021;39:34–41. doi: 10.1016/j.ajem.2020.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Shi L, Wang Y, Wang Y, Duan G, Yang H. Meta-Analysis of Relation of Creatine kinase-MB to Risk of Mortality in Coronavirus Disease 2019 Patients. Am J Cardiol. 2020;130:163–165. doi: 10.1016/j.amjcard.2020.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26(10):1636–1643. doi: 10.1038/s41591-020-1051-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Han H, Ma Q, Li C, et al. Profiling serum cytokines in COVID-19 patients reveals IL-6 and IL-10 are disease severity predictors. Emerg Microbes Infect. 2020;9(1):1123–1130. doi: 10.1080/22221751.2020.1770129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wu D, Dinh TL, Bausk BP, Walt DR. Long-Term Measurements of Human Inflammatory Cytokines Reveal Complex Baseline Variations between Individuals. Am J Pathol. 2017;187(12):2620–2626. doi: 10.1016/j.ajpath.2017.08.007 [DOI] [PubMed] [Google Scholar]
- 124.Liu W, Tao ZW, Wang L, et al. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Tan A, Sullenbarger B, Prakash R, McDaniel JC. Supplementation with eicosapentaenoic acid and docosahexaenoic acid reduces high levels of circulating proinflammatory cytokines in aging adults: a randomized, controlled study. Prostaglandins Leukot Essent Fatty Acids. 2018;132:23–29. doi: 10.1016/j.plefa.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.He Z, Zhao C, Dong Q, et al. Effects of severe acute respiratory syndrome (SARS) coronavirus infection on peripheral blood lymphocytes and their subsets. Int J Infect Dis. 2005;9(6):323–330. doi: 10.1016/j.ijid.2004.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Hottz ED, Bozza FA, Bozza PT. Platelets in Immune Response to Virus and Immunopathology of Viral Infections. Front Med. 2018;5:121. doi: 10.3389/fmed.2018.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wool GD, Miller JL. The Impact of COVID-19 Disease on Platelets and Coagulation. Pathobiology. 2021;88(1):15–27. doi: 10.1159/000512007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Rayes J, Bourne JH, Brill A, Watson SP. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res Pract Thromb Haemost. 2019;4(1):23–35. doi: 10.1002/rth2.12266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ito K, Hirao A, Arai F, et al. Regulation of oxidative stress by ATM is required for self-renewal of hematopoietic stem cells. Nature. 2004;431(7011):997–1002. doi: 10.1038/nature02989 [DOI] [PubMed] [Google Scholar]
- 131.Tishkowski K, Gupta V. Erythrocyte Sedimentation Rate. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2021. Available from https://www.ncbi.nlm.nih.gov/books/NBK557485/. Accessed February27, 2021. [PubMed] [Google Scholar]
- 132.Al Musawi MS, Jaafar MS, Al-Gailani B, Ahmed NM, Suhaimi FM, Bakhsh M. Erythrocyte sedimentation rate of human blood exposed to low-level laser. Lasers Med Sci. 2016;31(6):1195–1201. doi: 10.1007/s10103-016-1972-1 [DOI] [PubMed] [Google Scholar]
- 133.Kim PS, Read SW, Fauci AS. Therapy for Early COVID-19: a Critical Need. JAMA. 2020;324(21):2149–2150. doi: 10.1001/jama.2020.22813 [DOI] [PubMed] [Google Scholar]
- 134.Sahu KK, Kumar R. Preventive and treatment strategies of COVID-19: from community to clinical trials. J Family Med Prim Care. 2020;9(5):2149–2157. doi: 10.4103/jfmpc.jfmpc_728_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Stahl EC, Brown BN. Cell Therapy Strategies to Combat Immunosenescence. Organogenesis. 2015;11(4):159–172. doi: 10.1080/15476278.2015.1120046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Das S, Anu KR, Birangal SR, et al. Role of comorbidities like diabetes on severe acute respiratory syndrome coronavirus-2: a review. Life Sci. 2020;258:118202. doi: 10.1016/j.lfs.2020.118202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Burrage DR, Koushesh S, Sofat N. Immunomodulatory Drugs in the Management of SARS-CoV-2. Front Immunol. 2020;11:1844. doi: 10.3389/fimmu.2020.01844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Hojyo S, Fukada T. Roles of Zinc Signaling in the Immune System. J Immunol Res. 2016;2016:6762343. doi: 10.1155/2016/6762343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Prasad AS. Zinc in human health: effect of zinc on immune cells. Mol Med. 2008;14(5–6):353–357. doi: 10.2119/2008-00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Szabó Z, Marosvölgyi T, Szabó É, Bai P, Figler M, Verzár Z. The Potential Beneficial Effect of EPA and DHA Supplementation Managing Cytokine Storm in Coronavirus Disease. Front Physiol. 2020;11:752. doi: 10.3389/fphys.2020.00752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Baud D, Dimopoulou Agri V, Gibson GR, Reid G, Giannoni E. Using Probiotics to Flatten the Curve of Coronavirus Disease COVID-2019 Pandemic. Front Public Health. 2020;8:186. doi: 10.3389/fpubh.2020.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Boretti A, Banik BK. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. Pharma Nutrition. 2020;12:100190. doi: 10.1016/j.phanu.2020.100190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Grant WB, Lahore H, McDonnell SL, et al. Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients. 2020;12(4):988. doi: 10.3390/nu12040988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sheeja K, Kuttan G. Modulation of natural killer cell activity, antibody-dependent cellular cytotoxicity, and antibody-dependent complement-mediated cytotoxicity by andrographolide in normal and Ehrlich ascites carcinoma-bearing mice. Integr Cancer Ther. 2007;6(1):66–73. doi: 10.1177/1534735406298975 [DOI] [PubMed] [Google Scholar]
- 145.Chicca A, Raduner S, Pellati F, et al. Synergistic immunomopharmacological effects of N-alkylamides in Echinacea purpurea herbal extracts. Int Immunopharmacol. 2009;9(7–8):850–858. doi: 10.1016/j.intimp.2009.03.006 [DOI] [PubMed] [Google Scholar]
- 146.Rowe CA, Nantz MP, Bukowski JF, Percival SS. Specific formulation of Camellia sinensis prevents cold and flu symptoms and enhances gamma, delta T cell function: a randomized, double-blind, placebo-controlled study. J Am Coll Nutr. 2007;26(5):445–452. doi: 10.1080/07315724.2007.10719634 [DOI] [PubMed] [Google Scholar]
- 147.Naseri N, Kalantar K, Amirghofran Z. Anti-inflammatory activity of Echium amoenum extract on macrophages mediated by inhibition of inflammatory mediators and cytokines expression. Res Pharm Sci. 2018;13(1):73–81. doi: 10.4103/1735-5362.220970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rowaiye AB, Oni SO, Uzochukwu IC, Akpa A, Esimone CO. The Structure-Based Virtual Screening for Natural Compounds that Bind with the Activating Receptors of Natural Killer Cells. Trop J Nat Prod Res. 2021;5(1):145–164. doi: 10.26538/tjnpr/v5i1.21 [DOI] [Google Scholar]
- 149.Broggi A, Ghosh S, Sposito B, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020;369(6504):706–712. doi: 10.1126/science.abc3545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Syedbasha M, Egli A. Interferon Lambda: modulating Immunity in Infectious Diseases. Front Immunol. 2017;8:119. doi: 10.3389/fimmu.2017.00119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Alattar R, Ibrahim TBH, Shaar SH, et al. Tocilizumab for the treatment of severe coronavirus disease 2019. J Med Virol. 2020;92(10):2042–2049. doi: 10.1002/jmv.25964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Xu X, Han M, Li T, et al. Effective treatment of severe COVID-19 patients with tocilizumab. Proc Natl Acad Sci USA. 2020;117(20):10970–10975. doi: 10.1073/pnas.2005615117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rutherford AI, Subesinghe S, Hyrich KL, Galloway JB. Serious infection across biologic-treated patients with rheumatoid arthritis: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Ann Rheum Dis. 2018;77(6):905–910. doi: 10.1136/annrheumdis-2017-2128 [DOI] [PubMed] [Google Scholar]
- 154.Aouba A, Baldolli A, Geffray L, et al. Targeting the inflammatory cascade with anakinra in moderate to severe COVID-19 pneumonia: case series. Ann Rheum Dis. 2020;79(10):1381–1382. doi: 10.1136/annrheumdis-2020-217706 [DOI] [PubMed] [Google Scholar]
- 155.Kunkel GT, Maceyka M, Milstien S, Spiegel S. Targeting the sphingosine-1-phosphate axis in cancer, inflammation and beyond. Nat Rev Drug Discov. 2013;12(9):688–702. doi: 10.1038/nrd4099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Naz F, Arish M. Battling COVID-19 Pandemic: sphingosine-1-Phosphate Analogs as an Adjunctive Therapy? Front Immunol. 2020;11:1102. doi: 10.3389/fimmu.2020.01102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Mehta P, Ciurtin C, Scully M, Levi M, Chambers RC. JAK inhibitors in COVID-19: the need for vigilance regarding increased inherent thrombotic risk. Eur Respir J. 2020;56(3):2001919. doi: 10.1183/13993003.01919-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.La Rosée F, Bremer HC, Gehrke I, et al. The Janus kinase 1/2 inhibitor ruxolitinib in COVID-19 with severe systemic hyperinflammation. Leukemia. 2020;34(7):1805–1815. doi: 10.1038/s41375-020-0891-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.CTI Biopharma. CTI Biopharma Announces Initiation of Phase 3 PRE-VENT Study Evaluating Pacritinib in Hospitalized Patients with Severe COVID-19; 2020. Available from:https://cbc.gcs-web.com/news-releases/news-release-details/cti-biopharma-announces-initiation-phase-3-pre-vent-study. Accessed November4th, 2020.
- 160.Schrezenmeier E, Dörner T. Mechanisms of action of hydroxychloroquine and chloroquine: implications for rheumatology. Nat Rev Rheumatol. 2020;16(3):155–166. doi: 10.1038/s41584-020-0372-x [DOI] [PubMed] [Google Scholar]
- 161.Li X, Wang Y, Agostinis P, et al. Is hydroxychloroquine beneficial for COVID-19 patients? Cell Death Dis. 2020;11(7):512. doi: 10.1038/s41419-020-2721-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Parnham MJ, Culić O, Eraković V, et al. Modulation of neutrophil and inflammation markers in chronic obstructive pulmonary disease by short-term azithromycin treatment. Eur J Pharmacol. 2005;517(1–2):132–143. doi: 10.1016/j.ejphar.2005.05.023 [DOI] [PubMed] [Google Scholar]
- 163.ClinicalTrails.gov. Anti-inflammatory Clarithromycin for Improving COVID-19 Infection Early (ACHIEVE). ClinicalTrials.gov Identifier NCT04398004. US National Library of Medicine; Available from: https://clinicaltrials.gov/ct2/show/NCT04398004. Accessed March1, 2021. [Google Scholar]
- 164.Krishnaveni M. A review on transfer factor an immune modulator. Drug Invent Today. 2013;5:153–156. doi: 10.1016/j.dit.2013.04.002 [DOI] [Google Scholar]
- 165.Castrejón Vázquez MI, Reséndiz-Albor AA, Ynga-Durand MA, et al. Dialyzable Leukocyte Extract (Transferon™) Administration in Sepsis: experience from a Single Referral Pediatric Intensive Care Unit. Biomed Res Int. 2019;2019:8980506. doi: 10.1155/2019/8980506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Horby P, Lim WS, Emberson JR, et al.; RECOVERY Collaborative Group. Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med. 2021;384(8):693–704. doi: 10.1056/NEJMoa2021436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Siemieniuk R, Rochwerg B, Agoritsas T, et al. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379 [DOI] [PubMed] [Google Scholar]
- 168.Meng WT, Qing L, Li CZ, et al. Ulinastatin: a Potential Alternative to Glucocorticoid in the Treatment of Severe Decompression Sickness. Front Physiol. 2020;11:273. doi: 10.3389/fphys.2020.00273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Chasapis CT, Loutsidou AC, Spiliopoulou CA, Stefanidou ME. Zinc and human health: an update. Arch Toxicol. 2012;86(4):521–534. doi: 10.1007/s00204-011-0775-1 [DOI] [PubMed] [Google Scholar]
- 170.Samad N, Sodunke TE, Abubakar AR, et al. The Implications of Zinc Therapy in Combating the COVID-19 Global Pandemic. J Inflamm Res. 2021;14:527–550. doi: 10.2147/JIR.S295377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Razzaque MS. COVID-19 Pandemic: can Maintaining Optimal Zinc Balance Enhance Host Resistance? Tohoku J Exp Med. 2020;251(3):175–181. doi: 10.1620/tjem.251.175 [DOI] [PubMed] [Google Scholar]
- 172.Razzaque MS. COVID-19 pandemic: can boosting immune responses by maintaining adequate nutritional balance reduce viral insults? Adv Hum Biol. 2020;10(3):99–102. doi: 10.4103/AIHB.AIHB_75_20 [DOI] [Google Scholar]
- 173.Razzaque MS. COVID-19 pandemic: can zinc supplementation provide an additional shield against the infection. Comput Struct Biotech J. 2021;19:1371–1378. doi: 10.1016/j.csbj.2021.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Cassandri M, Smirnov A, Novelli F, et al. Zinc-finger proteins in health and disease. Cell Death Discov. 2017;3(1):17071. doi: 10.1038/cddiscovery.2017.71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Haase H, Rink L. Functional significance of zinc-related signaling pathways in immune cells. Annu Rev Nutr. 2009;29(1):133–152. doi: 10.1146/annurev-nutr-080508-141119 [DOI] [PubMed] [Google Scholar]
- 176.Wessels I, Rolles B, Rink L. The Potential Impact of Zinc Supplementation on COVID-19 Pathogenesis. Front Immunol. 2020;11:1712. doi: 10.3389/fimmu.2020.01712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Foster M, Samman S. Zinc and regulation of inflammatory cytokines: implications for cardiometabolic disease. Nutrients. 2012;4(7):676–694. doi: 10.3390/nu4070676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Finzi E Treatment of SARS-CoV-2 with high dose oral zinc salts: a report on four patients. Int J Infect Dis. 2020; 99:307–309. doi: 10.1016/j.ijid.2020.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Joachimiak MP. Zinc against COVID-19? Symptom surveillance and deficiency risk groups. PLoS Negl Trop Dis. 2021;15(1):e0008895. doi: 10.1371/journal.pntd.0008895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Maggini S, Wintergerst ES, Beveridge S, Hornig DH. Selected vitamins and trace elements support immune function by strengthening epithelial barriers and cellular and humoral immune responses. Br J Nutr. 2007;98(Suppl 1):S29–S35. doi: 10.1017/S0007114507832971 [DOI] [PubMed] [Google Scholar]
- 181.Otieno CA. Coronavirus (COVID-19)-Kenyan case: a Review on prioritizing immunonutrition in Prevention and Management. World J Res Rev. 2020;11(2):1–13. doi: 10.31871/WJRR.11.2.4 [DOI] [Google Scholar]
- 182.Gasmi A, Tippairote T, Mujawdiya PK, et al. Micronutrients as immunomodulatory tools for COVID-19 management. Clin Immunol. 2020;220:108545. doi: 10.1016/j.clim.2020.108545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Serhan CN, Chiang N, Van Dyke TE. Resolving inflammation: dual anti-inflammatory and pro-resolution lipid mediators. Nat Rev Immunol. 2008;8(5):349–361. doi: 10.1038/nri2294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Weitzel LR, Mayles WJ, Sandoval PA, Wischmeyer PE. Effects of pharmaconutrients on cellular dysfunction and the microcirculation in critical illness. Curr Opin Anaesthesiol. 2009;22(2):177–183. doi: 10.1097/ACO.0b013e328328d32f [DOI] [PubMed] [Google Scholar]
- 185.Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–1197. doi: 10.1038/ni1276 [DOI] [PubMed] [Google Scholar]
- 186.Kiecolt-Glaser JK, Belury MA, Andridge R, Malarkey WB, Glaser R. Omega-3 supplementation lowers inflammation and anxiety in medical students: a randomized controlled trial. Brain Behav Immun. 2011;25(8):1725–1734. doi: 10.1016/j.bbi.2011.07.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.ClinicalTrails.gov. The Effect of Omega-3 on Selected Cytokines Involved in Cytokine Storm (NIH, 2020, Omega-3). ClinicalTrials.gov Identifier: NCT04483271. US National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04483271. Accessed February27, 2021. [Google Scholar]
- 188.Säemann MD, Böhmig GA, Osterreicher CH, et al. Anti-inflammatory effects of sodium butyrate on human monocytes: potent inhibition of IL-12 and up-regulation of IL-10 production. FASEB J. 2000;14(15):2380–2382. doi: 10.1096/fj.00-0359fje [DOI] [PubMed] [Google Scholar]
- 189.Segain JP, Raingeard de la Blétière D, Bourreille A, et al. Butyrate inhibits inflammatory responses through NFkappaB inhibition: implications for Crohn’s disease. Gut. 2000;47(3):397–403. doi: 10.1136/gut.47.3.397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Olaimat AN, Aolymat I, Al-Holy M, et al. The potential application of probiotics and prebiotics for the prevention and treatment of COVID-19. NPJ Sci Food. 2020;4:17. doi: 10.1038/s41538-020-00078-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Bowie AG, O’Neill LA. Vitamin C inhibits NF-kappa B activation by TNF via the activation of p38 mitogen-activated protein kinase. J Immunol. 2000;165(12):7180–7188. doi: 10.4049/jimmunol.165.12.7180 [DOI] [PubMed] [Google Scholar]
- 192.Jang IS, Ko YH, Moon YS, Sohn SH. Effects of Vitamin C or E on the Pro-Inflammatory Cytokines, Heat Shock Protein 70 and Antioxidant Status in Broiler Chicks under Summer Conditions. Asian Australas J Anim Sci. 2014;27(5):749–756. doi: 10.5713/ajas.2013.13852 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Stackaruk ML, Lee AJ, Ashkar AA. Type I interferon regulation of natural killer cell function in primary and secondary infections. Expert Rev Vaccines. 2013;12(8):875–884. doi: 10.1586/14760584.2013.814871 [DOI] [PubMed] [Google Scholar]
- 194.Lee AJ, Mian F, Poznanski SM, et al. Type I Interferon Receptor on NK Cells Negatively Regulates Interferon-γ Production. Front Immunol. 2019;10:1261. doi: 10.3389/fimmu.2019.01261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.ClinicalTrails.gov. SAFEty Study of Early Infusion of Vitamin C for Treatment of Novel Coronavirus Acute Lung Injury (SAFE EVICT CORONA-ALI). ClinicalTrials.gov Identifier: NCT04344184. US National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04344184. Accessed March1, 2021. [Google Scholar]
- 196.Chang SH, Chung Y, Dong C. Vitamin D suppresses Th17 cytokine production by inducing C/EBP homologous protein (CHOP) expression. J Biol Chem. 2010;285(50):38751–38755. doi: 10.1074/jbc.C110.185777 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Diabetes TL. The Lancet Diabetes Endocrinology. Vitamin D and COVID-19: why the controversy? Lancet Diabetes Endocrinol. 2021;9(2):53. doi: 10.1016/S2213-8587(21)00003-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Liuzzo G, Patrono C. COVID 19: in the eye of the cytokine storm. Eur Heart J. 2021;42(2):150–151. doi: 10.1093/eurheartj/ehaa1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Jesenak M, Brndiarova M, Urbancikova I, et al. Immune Parameters and COVID-19 Infection - Associations with Clinical Severity and Disease Prognosis. Front Cell Infect Microbiol. 2020;10:364. doi: 10.3389/fcimb.2020.00364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.McElvaney OJ, McEvoy NL, McElvaney OF, et al. Characterization of the Inflammatory Response to Severe COVID-19 Illness. Am J Respir Crit Care Med. 2020;202(6):812–821. doi: 10.1164/rccm.202005-1583OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Aravindaram K, Yang NS. Anti-inflammatory plant natural products for cancer therapy. Planta Med. 2010;76(11):1103–1117. doi: 10.1055/s-0030-1249859 [DOI] [PubMed] [Google Scholar]
- 202.Shapiro H, Lev S, Cohen J, Singer P. Polyphenols in the prevention and treatment of sepsis syndromes: rationale and pre-clinical evidence. Nutrition. 2009;25(10):981–997. doi: 10.1016/j.nut.2009.02.010 [DOI] [PubMed] [Google Scholar]
- 203.Lewicka A, Szymański Ł, Rusiecka K, et al. Supplementation of Plants with Immunomodulatory Properties during Pregnancy and Lactation-Maternal and Offspring Health Effects. Nutrients. 2019;11(8):1958. doi: 10.3390/nu11081958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Nugraha RV, Ridwansyah H, Ghozali M, Khairani AF, Atik N. Traditional Herbal Medicine Candidates as Complementary Treatments for COVID-19: a Review of Their Mechanisms, Pros, and Cons. Evid Based Complement Alternat Med. 2020;2020:2560645. doi: 10.1155/2020/2560645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Kotenko SV, Durbin JE. Contribution of type III interferons to antiviral immunity: location, location, location. J Biol Chem. 2017;292(18):7295–7303. doi: 10.1074/jbc.R117.777102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lazear HM, Nice TJ, Diamond MS. Interferon-λ: immune Functions at Barrier Surfaces and Beyond. Immunity. 2015;43(1):15–28. doi: 10.1016/j.immuni.2015.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lazear HM, Schoggins JW, Diamond MS. Shared and Distinct Functions of Type I and Type III Interferons. Immunity. 2019;50(4):907–923. doi: 10.1016/j.immuni.2019.03.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Blazek K, Eames HL, Weiss M, et al. IFN-λ resolves inflammation via suppression of neutrophil infiltration and IL-1β production. J Exp Med. 2015;212(6):845–853. doi: 10.1084/jem.20140995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Rivera A. Interferon Lambda’s New Role as Regulator of Neutrophil Function. J Interferon Cytokine Res. 2019;39(10):609–617. doi: 10.1089/jir.2019.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Feld JJ, Kandel C, Biondi MJ, et al. Peginterferon lambda for the treatment of outpatients with COVID-19: a Phase 2, placebo-controlled randomized trial. Lancet Respir Med. 2021. doi: 10.1016/S2213-2600(20)30566-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Rahmani H, Davoudi-Monfared E, Nourian A, et al. Interferon β-1b in treatment of severe COVID-19: a randomized clinical trial. Int Immunopharmacol. 2020;88:106903. doi: 10.1016/j.intimp.2020.106903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Released by National Health Commission & National Administration of Traditional Chinese Medicine on March 3, 2020. Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin Med J. 2020;133(9):1087–1095. doi: 10.1097/CM9.0000000000000819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Veiga VC, Prats JAGG, Farias DLC, et al. Coalition covid-19 Brazil VI Investigators. Effect of tocilizumab on clinical outcomes at 15 days in patients with severe or critical coronavirus disease 2019: randomized controlled trial. BMJ. 2021;372:n84. doi: 10.1136/bmj.n84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.King A, Vail A, O’Leary C, et al. Anakinra in COVID-19: important considerations for clinical trials. Lancet Rheumatol. 2020;2(7):e379–e381. doi: 10.1016/S2665-9913(20)30160-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.CORIMUNO-19 Collaborative group. Effect of anakinra versus usual care in adults in hospital with COVID-19 and mild-to-moderate pneumonia (CORIMUNO-ANA-1): a randomized controlled trial. Lancet Respir Med. 2021. doi: 10.1016/S2213-2600(20)30556-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Robinson PC, Richards D, Tanner HL, Feldmann M. Accumulating evidence suggests anti-TNF therapy needs to be given trial priority in COVID-19 treatment. Lancet Rheumatol. 2020;2(11):E653–E655. doi: 10.1016/S2665-9913(20)30309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Starzonek J, Roscher K, Blüher M, et al. Effects of a blend of green tea and curcuma extract supplementation on lipopolysaccharide-induced inflammation in horses and ponies. Peer J. 2019;7:e8053. doi: 10.7717/peerj.8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Kany S, Vollrath JT, Relja B. Cytokines in Inflammatory Disease. Int J Mol Sci. 2019;20(23):6008. doi: 10.3390/ijms20236008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Robinson PC, Liew DFL, Liew JW, et al. The Potential for Repurposing Anti-TNF as a Therapy for the Treatment of COVID-19. Med. 2020;1(1):90–102. doi: 10.1016/j.medj.2020.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Baddley JW, Cantini F, Goletti D, et al. ESCMID Study Group for Infections in Compromised Hosts (ESGICH) Consensus Document on the safety of targeted and biological therapies: an infectious diseases perspective (Soluble immune effector molecules [I]: anti-tumor necrosis factor-α agents). Clin Microbiol Infect. 2018;24(Suppl 2):S10–S20. doi: 10.1016/j.cmi.2017.12.025 [DOI] [PubMed] [Google Scholar]
- 221.McGowan EM, Haddadi N, Nassif NT, Lin Y. Targeting the SphK-S1P-SIPR Pathway as a Potential Therapeutic Approach for COVID-19. Int J Mol Sci. 2020;21(19):7189. doi: 10.3390/ijms21197189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Allende ML, Dreier JL, Mandala S, Proia RL. Expression of the sphingosine 1-phosphate receptor, S1P1, on T-cells controls thymic emigration. J Biol Chem. 2004;279(15):15396–15401. doi: 10.1074/jbc.M314291200 [DOI] [PubMed] [Google Scholar]
- 223.Du X, Zeng H, Liu S, et al. Mevalonate metabolism-dependent protein geranylgeranylation regulates thymocyte egress. J Exp Med. 2020;217(2):e20190969. doi: 10.1084/jem.20190969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Lee MJ, Van Brocklyn JR, Thangada S, et al. Sphingosine-1-phosphate as a ligand for the G protein-coupled receptor EDG-1. Science. 1998;279(5356):1552–1555. doi: 10.1126/science.279.5356.1552 [DOI] [PubMed] [Google Scholar]
- 225.Liu CH, Thangada S, Lee MJ, Van Brocklyn JR, Spiegel S, Hla T. Ligand-induced trafficking of the sphingosine-1-phosphate receptor EDG-1. Mol Biol Cell. 1999;10(4):1179–1190. doi: 10.1091/mbc.10.4.1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Iwasaki A, Medzhitov R. A new shield for a cytokine storm. Cell. 2011;146(6):861–862. doi: 10.1016/j.cell.2011.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Subei AM, Cohen JA. Sphingosine 1-phosphate receptor modulators in multiple sclerosis. CNS Drugs. 2015;29(7):565–575. doi: 10.1007/s40263-015-0261-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Foerch C, Friedauer L, Bauer B, Wolf T, Adam EH. Severe COVID-19 infection in a patient with multiple sclerosis treated with fingolimod. Mult Scler Relat Disord. 2020;42:102180. doi: 10.1016/j.msard.2020.102180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.ClinicalTrails.gov. Fingolimod in COVID-19. ClinicalTrials.gov Identifier: NCT04280588. US National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04280588. Accessed February27th, 2021. [Google Scholar]
- 230.Zhao J, Zhu M, Jiang H, Shen S, Su X, Shi Y. Combination of sphingosine-1-phosphate receptor 1 (S1PR1) agonist and antiviral drug: a potential therapy against pathogenic influenza virus. Sci Rep. 2019;9(1):5272. doi: 10.1038/s41598-019-41760-7 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 231.Stebbing J, Phelan A, Griffin I, et al. COVID-19: combining antiviral and anti-inflammatory treatments. Lancet Infect Dis. 2020;20(4):400–402. doi: 10.1016/S1473-3099(20)30132-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Cantini F, Niccoli L, Matarrese D, Nicastri E, Stobbione P, Goletti D. Baricitinib therapy in COVID-19: a pilot study on safety and clinical impact. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kalil AC, Patterson TF, Mehta AK, et al. Baricitinib plus Remdesivir for Hospitalized Adults with Covid-19. N Engl J Med. 2020. doi: 10.1056/NEJMoa2031994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.ClinicalTrails.gov. PRE-VENT Study in Hospitalized Patients with Severe COVID-19 With or Without Cancer. ClinicalTrials.gov Identifier: NCT04404361. US National Library of Medicine. Available from: https://clinicaltrials.gov/ct2/show/NCT04404361. Accessed March1, 2021. [Google Scholar]
- 235.Abubakar AR, Sani IH, Godman B, et al. Systematic Review on the Therapeutic Options for COVID-19: clinical Evidence of Drug Efficacy and Implications. Infect Drug Resist. 2020;13:4673–4695. doi: 10.2147/IDR.S289037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Garcia P, Revet A, Yrondi A, Rousseau V, Degboe Y, Montastruc F. Psychiatric Disorders and Hydroxychloroquine for Coronavirus Disease 2019 (COVID-19): a VigiBase Study. Drug Saf. 2020;43(12):1315–1322. doi: 10.1007/s40264-020-01013-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Lane JCE, Weaver J, Kostka K, et al. Risk of depression, suicide, and psychosis with hydroxychloroquine treatment for rheumatoid arthritis: a multinational network cohort study. Rheumatology. 2020. doi: 10.1093/rheumatology/keaa771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bermejo-Martin JF, Kelvin DJ, Eiros JM, Castrodeza J, Ortiz de Lejarazu R. Macrolides for the treatment of severe respiratory illness caused by novel H1N1 swine influenza viral strains. J Infect Dev Ctries. 2009;3(3):159–161. doi: 10.3855/jidc.18 [DOI] [PubMed] [Google Scholar]
- 239.Zimmermann P, Ziesenitz VC, Curtis N, Ritz N. The Immunomodulatory Effects of Macrolides-A Systematic Review of the Underlying Mechanisms. Front Immunol. 2018;9:302. doi: 10.3389/fimmu.2018.00302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.He ZY, Ou LM, Zhang JQ, et al. Effect of 6 months of erythromycin treatment on inflammatory cells in induced sputum and exacerbations in chronic obstructive pulmonary disease. Respiration. 2010;80(6):445–452. doi: 10.1159/000321374 [DOI] [PubMed] [Google Scholar]
- 241.Clinical Trial Arena. Study finds azithromycin and doxycycline ineffective for Covid-19; 2021. Available from: https://www.clinicaltrialsarena.com/news/uk-study-azithromycin-doxycycline/#:~:text=The%20University%20of%20Oxford%20ineffective%20treatments%20for%20Covid%2D19. Accessed March2, 2021.
- 242.Furtado RHM, Berwanger O, Fonseca HA, et al. Azithromycin in addition to standard of care versus standard of care alone in the treatment of patients admitted to the hospital with severe COVID-19 in Brazil (COALITION II): a randomized clinical trial. Lancet. 2020;396(10256):959–967. doi: 10.1016/S0140-6736(20)31862-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Viza D, Pizza G, De Vinci C, Brandi G, Ablashi D. Transfer Factor as an Option for Managing the COVID-19 Pandemic. Folia Biol. 2020;66(3):86–90. [PubMed] [Google Scholar]
- 244.ClinicalTrails.gov. Efficacy of Transfer Factor to Prevent Upper Respiratory Tract Infections in Healthy Adults. ClinicalTrials.gov Identifier: NCT01106183. US National Library of Medicine. Available from: https://www.clinicaltrials.gov/ct2/show/NCT01106183. Accessed March1, 2021. [Google Scholar]
- 245.Mahase E. Covid-19: low dose steroid cuts death in ventilated patients by one-third, trial finds. BMJ. 2020;369:m2422. doi: 10.1136/bmj.m2422 [DOI] [PubMed] [Google Scholar]
- 246.Liu J, Zheng X, Huang Y, Shan H, Huang J. Successful use of methylprednisolone for treating severe COVID-19. J Allergy Clin Immunol. 2020;146(2):325–327. doi: 10.1016/j.jaci.2020.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Kapugi M, Cunningham K. Corticosteroids. Orthop Nurs. 2019;38(5):336–339. doi: 10.1097/NOR.0000000000000595 [DOI] [PubMed] [Google Scholar]
- 248.Soy M, Keser G, Atagündüz P, Tabak F, Atagündüz I, Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin Rheumatol. 2020;39(7):2085–2094. doi: 10.1007/s10067-020-05190-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pau AK, Aberg J, Baker J, et al. Convalescent Plasma for the Treatment of COVID-19: perspectives of the National Institutes of Health COVID-19 Treatment Guidelines Panel. Ann Intern Med. 2021;174(1):93–95. doi: 10.7326/M20-6448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Imai Y, Kuba K, Neely GG, et al. Identification of oxidative stress and Toll-like receptor 4 signaling as a key pathway of acute lung injury. Cell. 2008;133(2):235–249. doi: 10.1016/j.cell.2008.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]