Abstract
Introduction
Several publications described neurological manifestations caused by SARS-CoV-2. Immune-mediated neurological damages caused by COVID-19 are increasingly recognized.
Case report
A young male presented in March 2020 with a new-onset seizure. Later, he started to experience a severe headache. During the second admission in May, the MRI of the brain showed left frontal lesion. Nasal PCR for SARS-CoV-2 was negative, but the serology was positive, raising the suspicion of immune-mediated encephalitis. Elevated cerebrospinal fluid immunoglobulin G with two oligoclonal bands were also seen. The patient received IV immunoglobulin and showed improvement in headache. Follow-up MRIs of the brain revealed complete resolution of the lesion.
Discussion
Neurological complications from COVID-19 have been increasingly recognized. The proposed pathophysiology is either direct damage of neurological tissues, or indirectly through immune-mediated mechanisms. The timeline of the patient's presentation with seizure, as well as the lesion on the brain MRI with complete resolution after the IV immunoglobulin, strongly suggest that the patient had immune-mediated encephalitis after exposure to SARS-CoV-2.
Conclusions
Several cases of encephalitis caused by SARS-CoV-2 have been reported. Immune-mediated encephalitis as probable pathophysiology is described here.
Keywords: COVID-19, encephalitis, high-grade glioma, seizure
Introduction
Non-human coronaviruses were known to cause nervous tissue damage, even before the emergence of SARS-CoV-2.1 Since the pandemic by SARS-CoV-2 started, multiple publications discussed the neurological manifestations caused by the new human coronavirus.
SARS-CoV-2 has a peculiarity to produce strong immune response thereby causing damage to multiple organs and systems, including the nervous system. Although some research on COVID-19 related neurological complications proposed direct viral invasions and indirect damages from systemic hypoxia or thrombosis causing circulatory disruption as pathogenesis, the exact mechanism is largely unknown. Recently, immune-mediated neurological damages have increasingly been recognized.2,3 We report a case of a young male who presented with seizure and brain lesion on MRI with initial concern for neoplastic process. Workup revealed SARS-CoV-2 IgG antibody, raising the suspicion of immune-mediated encephalitis. The patient had clinical and radiological improvement after therapy with IV immunoglobulin.
Case report
A 20-year-old previously healthy man was hospitalized in early May of 2020 because of an intractable headache. In March, about 9 weeks prior to admission, he had travelled to Florida. Around the same time on March 9th, Florida was declared to be in state of emergency for COVID-19.4 While there, the patient had mild upper respiratory symptoms and subjective fever. He did not seek medical attention or get tested for SARS-CoV-2, but he isolated himself for the duration of his symptoms. Six weeks prior to the current admission, he was hospitalized for a new onset seizure. He was playing video games at home when he suddenly lost his consciousness. He woke up on the bathroom floor and noted a tongue bite. The seizure was unwitnessed. An EEG was performed at that time, which demonstrated interictal epileptiform abnormalities over the left frontotemporal head region. MRI of the brain during the same admission revealed hyperintensity involving the left frontal gyrus (Figure 1A). Oral levetiracetam 500 mg twice daily was started with plan to continue the medication as outpatient, and the patient was discharged. Two weeks prior to the current admission, he had had a breakthrough seizure. Around this time, he developed a throbbing headache located over the left frontal region. It was associated with nausea and occasional emesis. The patient’s neurologist was contacted on the day prior to admission due to persistence of headache, and a hospital admission was recommended for further work-up.
Figure 1. MRI of the brain with and without contrast. MRI of the brain after initial seizure (A), axial FLAIR showing area of increased signal within the left frontal gyrus (A.a), with no enhancement on post-contrast axial (A.b) and coronal (A.c). During the second admission (B), axial FLAIR showing increased signal within cortex and subcortical white matter (B.a), and area of enhancement (arrowhead) within the same region on post contrast axial (B.b), and coronal (B.c). On follow-up imaging (C), disappearance of hyperintense signal (C.a).
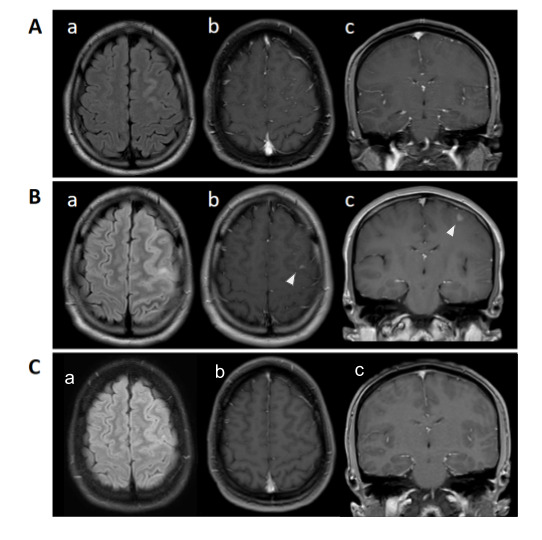
On admission, he was afebrile and hemodynamically stable. The physical examination was normal, including the neurological examination. The complete blood cell count, comprehensive metabolic panel, C-reactive protein and ferritin were within normal limits. CSF studies showed lymphocytic pleocytosis (WBC 153/mm3, 66% lymphocytes and 20% neutrophils). CSF proteins were elevated (52 mg/dL) and glucose was normal. CSF immunoglobulin G (IgG) was elevated and two oligoclonal bands were also seen in the CSF favored inflammatory process. CSF culture was sterile. The cranial MRI showed increased signal within the cortex and subcortical white matter of the left frontal lobe along with an area of post contrast enhancement. This abnormal area had increased in size compared to the previous MRI (Figure 1B). Magnetic resonance venography of the brain did not show venous thrombosis. Testing for cryptococcal antigen, cytomegalovirus, herpes, syphilis, and HIV was negative. Because of unusual MRI and CSF findings, and the COVID-19 pandemic, nasal swab for SARS-CoV-2 PCR, and serum SARS-CoV-2 IgG testing using the Abbott Architect i1000 SR was performed (Abbott Laboratories, USA). PCR was negative, but serum IgG was positive. PCR for SARS-CoV-2 in the CSF was not performed due to lack of a validated and FDA approved method. A CT scan of the chest, abdomen and pelvis were negative. The possibility for further testing was considered including brain biopsy, to check for autoimmune and paraneoplastic antibodies on the CSF sample. These were deferred due to positive COVID-19 serology in a setting of a pandemic, and lack of comorbidities in an otherwise healthy patient with no other symptoms on review of systems. Intravenous immunoglobulin (IVIG) 400 mg/kg was given for 5 days. The patient responded well to the initial therapy without major side effects and his headache completely resolved. At 2 months follow-up, he remained symptom free. CSF analysis was not repeated due to clinical improvement but follow-up cranial MRI was done (Figure 1C) and revealed complete resolution of the hyperintense signal (Figure 1C.a).
Discussion
COVID-19 is a multisystem disease, primarily targeting the respiratory system. Nervous system involvement is common. Reported neurological manifestations may result from direct invasion of the virus as seen in encephalitis,5-7 cerebrovascular accident,8 or rhabdomyolysis.9 Immune mediated late effects have been reported in cases of Guillain-Barré syndrome,10 transverse myelitis,11 or Miller Fisher syndrome.12 As regards encephalitis, one case report from Wuhan, China presented a patient with confusion and diagnosis of COVID-19.12 CT scan of the head was normal, and brain MRI was not performed. CSF was tested for anti-SARS-CoV-2 IgM and IgG (unpublished data), but the result was negative. Another case report5 described a young male with fever, seizure and abnormal cranial MRI. CSF PCR for SARS-CoV-2 was positive. A third publication9 reported a patient with a similar presentation to ours, with seizures and the brain MRI suspicious for high-grade glioma. The patient had a temporal lobectomy, and tested positive for SARS-CoV-2 after the surgery. In the first case,7 the confusion had appeared 12 days after the onset of respiratory symptoms, and it was speculated that SARS-CoV-2-induced immunologic response may cause inflammatory injury in the brain, but there was no clear inflammatory response seen in the CSF, or abnormal brain imaging studies.
Our patient had respiratory symptoms about 3 weeks prior to the seizure onset. He then had clinical and radiological progression, which along with the absence of nasal SARS-Cov-2 PCR, presence of positive serum serology and supporting CSF findings suggested post-infectious encephalitis. Multiple sclerosis was unlikely in view of lymphocytic pleocytosis and lack of characteristic MRI findings. The clinical picture was also atypical for a neoplastic process because of the relatively quick progression of MRI changes, CSF findings and response to IVIG.
Post-infectious encephalitis is an immune mediated response that typically occurs 2 to 4 weeks after a microbial infection. The most severe form is referred to as acute disseminated encephalomyelitis characterized by widespread, multifocal subcortical white matter lesions, and a case series presented 9 cases of COVID-19 with this inflammatory neurological syndrome.13 Milder forms with focal involvement have been described in cases of influenza,14 but for COVID-19, the MRI images showed either normal, or multiple lesions.13 The clinical presentation depends upon location and extent of CNS involvement. Headache, seizures, encephalopathy, and focal neurological deficits are commonly seen. The CSF typically shows a lymphocytic or mixed pleocytosis. Oligoclonal bands may be seen in a minority of patients. There is no standard treatment for post-infectious encephalitis. The main therapeutic options include high dose intravenous methylprednisolone, plasma exchange or IVIG. We avoided steroids in our patient so as not to alter the histopathology in case he required biopsy of the lesion. Post-infectious focal encephalitis has not been previously reported with COVID-19 infection. As the pandemic unfolds, more such cases may be seen in the future.
Conclusions
There is increasing awareness of probable immune-mediated sequelae from COVID-19.2,7,10,13 While most of the reported cases are based on speculations, temporal associations and clinical response to therapy make the suspicion highly likely. In our case, we believe the patient had enough evidence to suspect the novel coronavirus triggering the immune cascade and producing atypical presentation of focal encephalitis, namely the CSF findings suggestive of an immunological process, onset of seizure after respiratory symptoms in an area with high prevalence of COVID-19, and positive serology indicating prior exposure to the SARS-CoV-2.
It is highly likely that the treatment with immunoglobulin contributed to clinical improvement in this patient. Further research is required to define the optimal treatment.
Footnotes
Consent: The patient consented to the publication this case report and any accompanying images.
Authors’ contributions statement: EFAF obtained the history from the patient, performed literature search, wrote the manuscript, and edited the photograph of the MRI of the brain for the figure. AN gathered the information from the patient and reviewed the manuscript. AB gathered the information from the patient, wrote and reviewed the manuscript. SM provided expert opinion as a neurologist and wrote the manuscript. All authors read and approved the final version of the manuscript.
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
References
- 1.Perlman S, McIntosh K. Coronaviruses, including severe acute respiratory syndrome (SARS) and Middle Eastern Respiratory Syndrome (MERS) In: Bennett JE, Dolin R, Blaser M, editors. Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases. 9th ed. Piscataway, NJ: Elsevier; 2010. pp. 2187–94. [DOI] [Google Scholar]
- 2.Zhao H, Shen D, Zhou H, Liu J, Chen S. Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol. 2020;19:383–4. doi: 10.1016/S1474-4422(20)30109-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoo A, McLoughlin B, Cheema S, et al. Postinfectious brainstem encephalitis associated with SARS-CoV-2. J Neurol Neurosurg Psychiatry. 2020;91:1013–4. doi: 10.1136/jnnp-2020-323816. [DOI] [PubMed] [Google Scholar]
- 4.Florida Department of Health. Florida Department of Health announces new positive COVID-19 cases in Florida. 2020. [Accessed on: 02 April 2021]. Available at: http://www.floridahealth.gov/newsroom/2020/03/031020-florida-department-of-health-announces-new-positive-covid19-cases-florida.pr.html.
- 5.Moriguchi T, Harii N, Goto J, et al. A first case of meningitis/encephalitis associated with SARS-coronavirus-2. Int J Infect Dis. 2020;94:55–8. doi: 10.1016/j.ijid.2020.03.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Efe IE, Aydin OU, Alabulut A, Celik O, Aydin K. COVID-19-associated encephalitis mimicking glial tumor. World Neurosurg. 2020;140:46–8. doi: 10.1016/j.wneu.2020.05.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ye M, Ren Y, Lv T. Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. 2020;88:945–6. doi: 10.1016/j.bbi.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Modin D, Claggett B, Sindet-Pedersen C, et al. Acute COVID-19 and the incidence of ischemic stroke and acute myocardial infarction. Circulation. 2020;142:2080–2. doi: 10.1161/CIRCULATIONAHA.120.050809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chan KH, Farouji I, Abu Hanoud A, Slim J. Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19) Am J Emerg Med. 2020;38:1548.e1–3. doi: 10.1016/j.ajem.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Alberti P, Beretta S, Piatti M, et al. Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm. 2020;7:e741. doi: 10.1212/NXI.0000000000000741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Munz M, Wessendorf S, Koretsis G, et al. Acute transverse myelitis after COVID-19 pneumonia. J Neurol. 2020;267:2196–7. doi: 10.1007/s00415-020-09934-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gutiérrez-Ortiz C, Méndez-Guerrero A, Rodrigo-Rey S, et al. Miller Fisher syndrome and polyneuritis cranialis in COVID-19. Neurology. 2020;95:e601–5. doi: 10.1212/WNL.0000000000009619. [DOI] [PubMed] [Google Scholar]
- 13.Paterson RW, Brown RL, Benjamin L, et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143:3104–120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kimura S, Kobayashi T, Osaka H, Shimizu C, Uehara S, Ohtuki N. Serial magnetic resonance imaging in post-infectious focal encephalitis due to influenza virus. J Neurol Sci. 1995;131:74–7. doi: 10.1016/0022-510X(95)00041-Y. [DOI] [PubMed] [Google Scholar]


