Abstract
COVID-19-associated pulmonary aspergillosis (CAPA) is defined as invasive pulmonary aspergillosis occurring in COVID-19 patients. The purpose of this review was to discuss the incidence, characteristics, diagnostic criteria, biomarkers, and outcomes of hospitalized patients diagnosed with CAPA. A literature search was performed through Pubmed and Web of Science databases for articles published up to 20th March 2021. In 1421 COVID-19 patients, the overall CAPA incidence was 13.5% (range 2.5–35.0%). The majority required invasive mechanical ventilation (IMV). The time to CAPA diagnosis from illness onset varied between 8.0 and 16.0 days. However, the time to CAPA diagnosis from intensive care unit (ICU) admission and IMV initiation ranged between 4.0–15.0 days and 3.0–8.0 days. The most common diagnostic criteria were the modified AspICU–Dutch/Belgian Mycosis Study Group and IAPA-Verweij et al. A total of 77.6% of patients had positive lower respiratory tract cultures, other fungal biomarkers of bronchoalveolar lavage and serum galactomannan were positive in 45.3% and 18.2% of patients. The CAPA mortality rate was high at 48.4%, despite the widespread use of antifungals. Lengthy hospital and ICU stays ranging between 16.0–37.5 days and 10.5–37.0 days were observed. CAPA patients had prolonged IMV duration of 13.0–20.0 days. The true incidence of CAPA likely remains unknown as the diagnosis is limited by the lack of standardized diagnostic criteria that rely solely on microbiological data with direct or indirect detection of Aspergillus in respiratory specimens, particularly in clinical conditions with a low pretest probability. A well-designed, multi-centre study to determine the optimal diagnostic approach for CAPA is required.
Keywords: Severe acute respiratory syndrome coronavirus 2, Coronavirus disease 2019, COVID-19, COVID-19-Associated pulmonary aspergillosis, CAPA, Invasive pulmonary aspergillosis
Introduction
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) virus is known to cause coronavirus disease 2019 (COVID-19), resulting in the ongoing pandemic [1]. Secondary bacterial pulmonary infections have been increasingly recognized in hospitalized COVID-19 patients with an incidence as high as 15% and associated with poor outcomes of increased mechanical ventilation requirements, multi-organ dysfunction and mortality [2,3]. Therefore, surveillance cultures are being routinely obtained to exclude secondary bacterial infections from lower respiratory tract (LRT) specimens (sputum, endotracheal aspirate (ETA), bronchoalveolar lavage (BAL)), blood, and urinary source, especially in those who are critically ill. Despite the lack of systematic screening, invasive pulmonary aspergillosis (IPA) was reported in up to 4% of COVID-19 patients from China during the early period of the ongoing pandemic, but there were no details on its clinical significance or associated outcomes [4,5]. However, the clinical relevance of Aspergillus-positive cultures obtained on respiratory tract specimens is often difficult to distinguish actual infection from colonization as the more diagnostic procedures performed, the higher the chances of detecting Aspergillus microorganisms. Furthermore, it is impossible to differentiate IPA from COVID-19 pneumonia based on clinical signs and symptoms, and also chest imaging, where COVID-19 patients will display diffuse bilateral lung infiltrates that may obscure any diagnostic clues for IPA [6]. IPA has been described during or post severe viral-related pneumonia such as with influenza, SARS, Middle East Respiratory Syndrome (MERS), respiratory syncytial virus, and parainfluenza virus, especially in critically ill patients admitted to the intensive care unit (ICU) for respiratory failure [[7], [8], [9], [10], [11], [12]]. Whether COVID-19 predisposes the host to IPA as an independent host factor remains unknown, and the true incidence, outcomes, and diagnostic criteria have not been elucidated in this setting. The purpose of this systematic review was to examine and discuss the incidence of secondary IPA in COVID-19 patients defined as COVID-19-associated pulmonary aspergillosis (CAPA), clinical characteristics, diagnostic criteria, biomarkers, and associated outcomes based on the evidence available in the current literature.
Methods
This systematic review was conducted and presented in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Ethical approval and informed consent were not required for this study as it was a systematic review of previously published studies.
Search criteria
A literature search was performed through Pubmed and Web of Science databases for articles published, using the keywords of “coronavirus disease 2019 (COVID-19),” “severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2),” “COVID-19-associated pulmonary aspergillosis (CAPA),” “fungal infections,” “secondary infections,” “fungal pneumonia,” “mycosis,” “Aspergillosis,” “Aspergillus,” and “invasive pulmonary aspergillosis (IPA).” All specified keywords were combined using the “OR” operator and “AND” operator for searching the literature. Moreover, to detect additional studies, any cited references were reviewed to identify relevant literature that met our inclusion criteria.
Inclusion criteria
Articles that met the following criteria were included in our study: (1) observational studies that described the incidence, clinical characteristics, biomarkers, and outcomes of IPA in hospitalized adults with COVID-19 infections; (2) articles where the diagnosis of CAPA was made using several well-established diagnostic criteria (Table I ) that had been described in the current literature involving AspICU [13], CAPA-European Excellence Centre for Medical Mycology (ECMM) [14], Modified AspICU Gangneux et al. [15], Modified AspICU–Dutch/Belgian Mycosis Study Group [10], influenza-associated pulmonary aspergillosis (IAPA) criteria–Verweij et al. [16], or European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) [17]; (3) studies in which the diagnosis of COVID-19 was made by reverse transcriptase-polymerase chain reaction (RT-PCR) in all cases from respiratory tract specimens that included nasal and pharyngeal swabs, sputum, ETA and BAL; and (4) articles published between 1st January 2020 and 20th March 2021 in peer-reviewed journals.
Table I.
Diagnostic criteria for COVID-19-associated pulmonary aspergillosis (CAPA) according to various studies included in our review
| Diagnostic criteria | Clinical | Radiological | Mycological |
|---|---|---|---|
| EORTC/MSG [17] | One of the following host factors: (A) severe neutropenia, (B) allogeneic stem cell/solid organ transplant, (C) corticosteroid therapy (0.3 mg/kg per day for >3 months), (D) haematological malignancy, (E) congenital/inherited/acquired immunodeficiency, (F) treatment with T-cell/B-cell immunosuppressants | One of the following: (A) dense, well-circumscribed lesions with/without halo sign, (B) air-crescent sign, (C) cavity, (D) lobar or segmental consolidation | Proven: histopathological/microscopic evidence of septated hyphae with evidence of tissue damage or positive culture from sterile material Probable (all 3 criteria): (A) positive direct test (culture/microscopy on sputum, ETA, and BAL or 2 and more positive PCR on either BAL or serum), OR (B) positive indirect test (GM in serum or BAL) |
| AspICU [13] | One of the following: (A) refractory fever despite 3 days of antibiotic therapy, (B) recrudescent fever of at least 48 h despite antibiotic therapy, (C) pleuritic chest pain/rub, dyspnea, (D) haemoptysis, (E) worsening respiratory failure despite antibiotic therapy and ventilatory support | Abnormal imaging on chest radiography or chest CT | Proven: histopathological/microscopic evidence of septated hyphae with evidence of tissue damage or positive culture from sterile material Putative (all 3 criteria): (A) positive lower respiratory tract specimen in patient with either host risk factors (severe neutropenia, haematological/oncological malignancy treated with cytotoxic agents, corticosteroid therapy (prednisone equivalent, >20 mg/day), congenital/acquired immunodeficiency) OR (B) semiquantitative positive BAL culture in the absence of bacterial growth |
| CAPA-European Excellence Centre for Medical Mycology [14] | One of the following: (A) refractory fever despite 3 days of antibiotic therapy, (B) pleuritic chest pain/rub, dyspnea, (C) haemoptysis | Abnormal imaging on chest radiography or chest CT | Proven: histopathological/microscopic evidence of septated hyphae with evidence of tissue damage or positive culture from sterile material Probable (all 3 criteria): (A) positive lower respiratory tract specimen on BAL OR (B) BAL GM >1.0 ODI OR (C) serum GM >0.5 ODI OR (D) positive serum and BAL PCR, OR (E) positive serum PCR × 2 Possible (all 3 criteria): (A) positive non-BAL lower respiratory tract specimen OR (B) positive non-BAL GM >4.5 ODI OR (C) positive non-BAL GM >1.2 ODI × 2, OR (D) positive non-BAL GM >1.2 ODI with non-BAL PCR |
| Modified AspICU-Gangneux et al. [15] | One of the following: (A) refractory fever despite 3 days of antibiotic therapy, (B) recrudescent fever of at least 48 h despite antibiotic therapy, (C) pleuritic chest pain/rub, dyspnea, (D) haemoptysis, (E) worsening respiratory failure despite antibiotic therapy and ventilatory support | Abnormal imaging on chest radiography or chest CT | Proven: histopathological/microscopic evidence of septated hyphae with evidence of tissue damage or positive culture from sterile material Putative (all 3 criteria): (A) positive lower respiratory tract specimen in patient with either host risk factors (severe neutropenia, haematological/oncological malignancy treated with cytotoxic agents, corticosteroid therapy (prednisone equivalent, >20 mg/day), congenital/acquired immunodeficiency), OR (B) semiquantitative positive BAL culture/PCR in the absence of bacterial growth Probable: putative plus one positive serum biomarker |
| Modified AspICU–Dutch/Belgian Mycosis Study Group [10] | One of the following: (A) refractory fever despite 3 days of antibiotic therapy, (B) recrudescent fever of at least 48 h despite antibiotic therapy, (C) pleuritic chest pain/rub, dyspnea, (D) haemoptysis, (E) worsening respiratory failure despite antibiotic therapy and ventilatory support | Abnormal imaging on chest radiography or chest CT | Proven: histopathological/microscopic evidence of septated hyphae with evidence of tissue damage or positive culture from sterile material Putative (all 3 criteria): (A) positive BAL culture, OR (B) BAL GM >1.0 ODI, OR (C) serum GM >0.5 ODI |
| Influenza-Associated Pulmonary Aspergillosis (IAPA)–Verweij et al. [16] | Influenza-like illness between 7 days before and 4 days after ICU admission | Probable: (A) pulmonary infiltrate and at least one of the following mycological criteria, OR (B) cavitating infiltrate (not attributed to another aetiology) and at least one of the following mycological criteria | Positive influenza PCR/antigen test Proven: histopathological/microscopic evidence of septated hyphae with evidence of tissue damage or positive PCR in tissue Probable (all 3 criteria): (A) pulmonary infiltrate and at least one of the following mycological criteria (serum GM >0.5 ODI, BAL GM >1.0 ODI, positive BAL culture), OR (B) cavitating infiltrate (not attributed to another etiology) and at least one of the following mycological criteria (positive sputum/tracheal aspirate culture) |
BAL, bronchoalveolar lavage; CT, computed tomography; EORTC/MSG, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group; ETA, endotracheal aspirate; GM, galactomannan; IAPA, influenza-associated pulmonary aspergillosis (IAPA) criteria-Verweij et al.; ICU, intensive care unit; LRTC, lower respiratory tract cultures; NR, non recorded/negative; ODI, optimal density index; PCR, polymerase chain reaction; Spp., species.
Exclusion criteria
The exclusion criteria were specified as follows: (1) articles that did not meet or described specific diagnostic criteria for CAPA diagnosis (Table I) that could represent colonization or had coexisting bacterial and/or (non-Aspergillus) fungal micro-organisms simultaneously identified from the LRT specimens and/or blood cultures; (2) articles with fewer than 18 patients (defined as case series) and/or case reports; (3) articles involving COVID-19 patients of less than 18 years of age; (4) articles where pulmonary aspergillosis was concurrently diagnosed with other micro-organisms such as bacteria and/or viruses from similar respiratory tract cultures; and (5) articles describing aspergillosis obtained from non-respiratory tract cultures.
Data collection
Two researchers (W.C. and K.N.) independently screened the titles and abstracts, and reviewed the full texts of articles to identify studies that evaluated the incidence, clinical characteristics, diagnostic criteria, biomarkers and associated outcomes of hospitalized COVID-19 patients diagnosed with CAPA. Any disagreements were resolved by discussion. The extracted data from full texts of included studies were added into a standardized Excel (Microsoft Corporation) form.
The summary of the study qualities was shown in Table II . All included studies' characteristics and outcomes were analysed in Table III involving: study design (e.g., retrospective or prospective; cross-sectional, case–control, or cohort; single- or multi-centre); month/year; country; the number of patients; the age of the patient (e.g., mean ± standard deviation or median (interquartile range) years); incidence of CAPA; incidence of proven CAPA; time to diagnosis of CAPA; mortality; length of hospitalization; patients requiring IMV; and the median days of IMV. In Table IV , diagnostic evaluation and antifungal therapies were summarized comprising: positive lower respiratory tract cultures (LRTC); aspergillus species (spp.); CAPA diagnostic criteria; EORTC host risk factors; positivity of serum galactomannan (GM), BAL GM, and serum beta-D-glucan (BDG); therapeutic antifungals received.
Table II.
The results of Newcastle–Ottawa Scale (NOS) [18] performed for 19 cohort studies
| Author(s) | Cohort studies | Selection |
Comparability |
Outcome/exposure |
Total of 9 scores |
|||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | (∗∗) | a | b | c | |||
| Alanio et al. [21] | Prospective cohort | ∗ | ∗ | ∗ | ∗ | N/A | ∗ | ∗ | ∗ | 7 |
| Bartoletti et al. [22] | Prospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Chauvet et al. [26] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Delliere et al. [25] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Dupont et al. [43] | Prospective cohort | ∗ | ∗ | ∗ | ∗ | N/A | ∗ | ∗ | ∗ | 7 |
| Gangneux et al. [15] | Prospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Helleberg et al. [60] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | N/A | ∗ | ∗ | ∗ | 7 |
| Koehler et al. [28] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | N/A | ∗ | ∗ | ∗ | 7 |
| Lahmer et al. [29] | Prospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Lamoth et al. [42] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | N/A | ∗ | ∗ | ∗ | 7 |
| Machado et al. [24] | Prospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Meijer et al. [61] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Nasir et al. [19] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | N/A | ∗ | ∗ | ∗ | 7 |
| Roman-Montes et al. [27] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 |
| Rutsaert et al. [20] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | N/A | ∗ | ∗ | ∗ | 7 |
| Van Arkel et al. [62] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 |
| Van Biesen et al. [23] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
| Velez Pintado et al. [63] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | 8 |
| Versyck et al. [64] | Retrospective cohort | ∗ | ∗ | ∗ | ∗ | ∗∗ | ∗ | ∗ | ∗ | 9 |
1: Representatives of the exposed cohorts. 2: Selection of the non-exposed cohorts. 3: Ascertainment of exposure. 4: The outcome of interest was not present at the start of the study. a: Assessment of the outcome. b: Enough follow-up for the outcome. c: Adequacy of follow-up. N/A, non-available.
Table III.
Incidence, characteristics, and outcomes of COVID-19-associated pulmonary aspergillosis (CAPA) patients
| Observational studies | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Study design | Month, year | Country | Patients (N) | Age (years) mean ± SD | CAPA incidence (%) (proven/probable/putative) | Proven CAPA (%) | Time to CAPA (D) mean ± SD | Mortality (%) | IMV (%) | Median IMV duration (D) | Hospital LOS (D) mean ± SD |
| Alanio et al. [21] | Prospective cohort, single-centre | June 2020 | Italy | 27 | 63.0 ± 11.1 | 33.3 | NR | 3.0 (IMV) | 44.4 | 100 | NR | NR |
| Bartoletti et al. [22] | Prospective cohort, multi-centre | July 2020 | Italy | 108 | 63.0 ± 9.6 | 27.7 | NR | 4.0 ± 4.44 (ICU) | 44.0 | 100 | 13.0 ± 11.9 | 16.0 ± 13.3 (ICU) |
| Chauvet et al. [26] | Retrospective cohort, single-centre | November 2020 | France | 46 | 66.5 ± 6.8 | 13.0 | NR | 11.7 ± 9.7 (ICU) | 66.7 | 100 | NR | NR |
| Delliere et al. [25] | Retrospective cohort, multi-centre | December 2020 | France | 108 | 63.0 ± 8.5 | 19.4 | NR | 16.0 ± 8.9 (Symptoms), 6.0 ± 10.4 (ICU) | 71.4 | 100 | NR | 21.1 ± 17.6 |
| Dupont et al. [43] | Prospective cohort, multi-centre | August 2020 | France | 106 | 69.0 ± 8.1 | 17.9 | NR | 10.0 ± 5.9 (ICU) | 35.3 | 100 | NR | NR |
| Gangneux et al. [15] | Prospective cohort, single-centre | July 2020 | France | 45 | 70.0 ± 8.9 | 15.6 | NR | NR | 28.6 | 100 | 18.0 ± 13.3 | 27.0 ± 11.9 (ICU) |
| Helleberg et al. [60] | Retrospective cohort, single-centre | August 2020 | Denmark | 25 | 58.0 ± 0.0 | 8.0 | NR | 3.0 (IMV) | 100 | 100 | NR | 37.5 ± 0.0 |
| Koehler et al. [28] | Retrospective cohort, single-centre | June 2020 | Germany | 19 | 62.0 ± 13.2 | 26.3 | NR | NR | 60.0 | 100 | NR | NR |
| Lahmer et al. [29] | Prospective cohort, multi-centre | March 2021 | Germany | 32 | 69.5 ± 42.2 | 34.4 | NR | 4.0 ± 4.4 (ICU) | 36.4 | 100 | 20.0 ±14.8 | 21.0 ± 14.1 (ICU) |
| Lamoth et al. [42] | Retrospective cohort, single-centre | December 2020 | Switzerland | 118 | 65.0 ± 0.0 | 3.8 | NR | 8.0 (Symptoms), 7.0 (ICU), 6.0 (IMV) | 33.3 | 100 | NR | 22.3 ± 0.0 |
| Machado et al. [24] | Prospective cohort, single-centre | November 2020 | Spain | 239 | 64.5 ± 16.9 | 2.5 | NR | 15.0 (ICU) | 100 | 100 | NR | NR |
| Meijer et al. [61] | Retrospective cohort, single-centre | February 2021 | Netherlands | 66 | 67.3 ± 7.3 | 19.7 | NR | NR | 46.2 | 100 | NR | 31.8 ± 11.6 (ICU) |
| Nasir et al. [19] | Retrospective cohort, single-centre | August 2020 | Pakistan | 147 | 71.0 ± 25.2 | 3.4 | NR | 4.0 ± 5.6 (Admission) | 60.0 | 40.0 | NR | 16.0 ± 10.4 |
| Roman-Montes et al. [27] | Retrospective cohort, single-centre | November 2020 | Mexico | 144 | 48.3 ± 11.7 | 9.7 | NR | NR | 57.1 | 100 | NR | NR |
| Rutsaert et al. [20] | Retrospective cohort, single-centre | June 2020 | Belgium | 20 | 66.0 ± 15.6 | 35.0 | 57.1 | 8.0 ± 5.9 (IMV) | 57.1 | 100 | NR | 21.0 ± 11.9 |
| Van Arkel et al. [62] | Retrospective cohort, single-centre | May 2020 | Netherlands | 31 | 62.5 ± 29.6 | 19.4 | NR | 11.5 ± 25.2 (Symptoms), 5.0 ± 18.5 (ICU) | 66.7 | 100 | NR | 10.5 ± 31.9 (ICU) |
| Van Biesen et al. [23] | Retrospective cohort, single-centre | July 2020 | Netherlands | 42 | 68.0 ± 27.4 | 21.4 | NR | 3.0 ± 3.0 (Admission) | 22.2 | 100 | NR | 37.0 ± 15.6 (ICU) |
| Velez Pintado et al. [63] | Retrospective cohort, single-centre | March 2021 | Mexico | 83 | 64.0 ± 10.0 | 19.3 | NR | NR | 31.3 | 100 | NR | NR |
| Versyck et al. [64] | Retrospective cohort, single-centre | February 2021 | France | 56 | 63.5 ± 8.5 | 3.6 | NR | 11.0 ± 6.0 (Admission) | 100 | 100 | 18.0 ± 0.0 | 23.0 ± 5.0 |
D, days; ICU, intensive care unit; IMV, invasive mechanical ventilation; LOS, length of stay; NR, non reported/negative; SD, standard deviation; Y, years.
Table IV.
Diagnostic evaluation and antifungal therapies of COVID-19-associated pulmonary aspergillosis (CAPA) patients
| Cultures |
Biomarkers |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Author | Positive LRTC (%) | LRTC Source (%) | Positive LRT PCR (%) | Aspergillus Spp. (%) | CAPA diagnostic criteria (%) | EORTC host risk factors (%) | Serum GM >0.5 ODI (%) | BAL GM >1.0 ODI (%) | Serum Beta-D-Glucan >80 pg/mL (%) | Antifungals |
| Alanio et al. [21] | 77.7 | BAL (100) | BAL (44.4) | A. fumigatus (100) | Putative CAPA (Modified AspICU-DB) | 22.2 | 11.1 | 11.1 | 44.4 | Voriconazole (11.1), caspofungin (11.1) |
| Bartoletti et al. [22] | 63.0 | BAL (100) | BAL (67.0) | A. fumigatus (78.9), A. niger (15.8), A. flavus (5.3) | Probable CAPA (IAPA) | 27.0 | 3.0 | 100 | NR | Voriconazole (43) |
| Chauvet et al. [26] | 66.7 | BAL (50), ETA (50). | ETA (16.7) | A. fumigatus (100) | Putative CAPA (83.3) [Modified AspICU-DB], Possible CAPA (16.7) [EORTC/MSG] | 33.3 | NR | NR | NR | Voriconazole (33.3); amphotericin B (33.3); caspofungin (16.7). |
| Delliere et al. [25] | 100 | BAL (76), ETA (24) | BAL/ETA (71.4) | A. spp. | Probable CAPA (IAPA) | 23.8 | 23.8 | 14.3 | 52.4 | NR |
| Dupont et al. [43] | 94.7 | BAL (44.4), BAS (33.3), ETA (22.2) | NR | A. fumigatus (87.5), A. flavus (6.3), A. calidoustus (6.3) | Putative CAPA (Modified AspICU-DB) | NR | 5.3 | 42.1 | NR | Voriconazole (47.4) |
| Gangneux et al. [15] | 85.7 | BAL/ETA (100) | BAL/ETA (100) | A. fumigatus (100) | Probable (42.9), putative (57.1) CAPA [Modified AspICU-G] | NR | 28.6 | NR | NR | Voriconazole/ isavuconazole (100) |
| Helleberg et al. [60] | 100 | ETA (100), BAL (50) | NR | A. fumigatus (100) | Putative CAPA (Modified AspICU-DB) | NR | 50.0 | 50.0 | NR | Voriconazole (100) |
| Koehler et al. [28] | 60.0 | BAL (75), ETA (25) | BAL (60), ETA (20) | A. fumigatus (100) | Putative CAPA (Modified AspICU-DB) | NR | 40.0 | 60.0 | NR | Voriconazole (100), caspofungin (40), isavuconazole (20) |
| Lahmer et al. [29] | 81.8 | BAL (100) | NR | A. fumigatus (100) | Probable CAPA (IAPA) | NR | 36.4 | 100 | NR | Voriconazole (45.4); amphotericin B (45.4); isavuconazole (9.1). |
| Lamoth et al. [42] | 100 | BAS (100) | BAS (33.3) | A. fumigatus (100) | Putative CAPA (Modified AspICU-DB) | NR | 33.3 | NR | 33.3 | Voriconazole (100) |
| Machado et al. [24] | 100 | BAL (100) | NR | A. fumigatus (66.6), A. citrinoterreus (16.7), A. lentulus (16.7) | Putative CAPA (Modified AspICU-DB) | 50.0 | 66.7 | 33.3 | NR | Isavuconazole (50), voriconazole (25), amphotericin B (25) |
| Meijer et al. [61] | 100 | BAL (61.5), ETA (38.5). | BAL (38.5), ETA (15.4). | A. fumigatus (100) | Probable (61.5), Possible (38.5) CAPA [CAPA-ECMM] | NR | NR | 15.4 | 7.7 | Voriconazole (100); amphotericin B (38.5); caspofungin (38.5). |
| Nasir et al. [19] | 100 | Sputum/ ETA/BAL (100) | NR | A. flavus (60), A. fumigatus (20), A. terreus (20) | Putative CAPA (Modified AspICU-DB) | NR | NR | NR | 20.0 | Voriconazole (33.3), amphotericin B (22.2) |
| Roman-Montes et al. [27] | 78.6 | ETA (100) | NR | A. fumigatus (54.5), A. spp. (27.3), A. flavus (9.1), A. niger (9.1) | Putative CAPA (Modified AspICU-DB) | NR | 42.9 | 85.7 (ETA) | NR | Voriconazole (83.3), echinocandin (15.3) |
| Rutsaert et al. [20] | 85.7 | BAL (83.3), ETA (16.7) | NR | A. fumigatus (83.3), A. flavus (16.7) | Putative CAPA (AspICU) | 42.9 | 14.3 | 57.1 | NR | Voriconazole (85.7), isavuconazole (28.6) |
| Van Arkel et al. [62] | 83.3 | BAL (40), ETA (40), Sputum (20) | NR | A. fumigatus (100) | Probable CAPA (IAPA) | NR | NR | 50.0 | NR | Voriconazole/ anidulafungin (83.3), amphotericin B (16.7) |
| Van Biesen et al. [23] | 77.7 | BAL (100) | NR | A. fumigatus (71.4), A. flavus (14.3), A. terreus (14.3) | Probable CAPA (IAPA) | 11.1 | NR | 100 | NR | Voriconazole/ amphotericin B (100) |
| Velez Pintado et al. [63] | 12.5 | BAL (100) | NR | A. spp. | Probable (CAPA- ECMM) | NR | 43.8 | 56.3 | NR | NR |
| Versyck et al. [64] | 100 | BAL (50), ETA (50). | NR | A. fumigatus (100) | Putative CAPA (Modified AspICU-DB) | NR | 100 | 50.0 | 100 | Voriconazole (100) |
A., Aspergillus; BAL, bronchoalveolar lavage; BAS, bronchial aspirates; ,ECMM, European Excellence Centre for Medical Mycology; EORTC/MSG, European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group; ETA, endotracheal aspirate; GM, galactomannan; IAPA, influenza-associated pulmonary aspergillosis (IAPA) criteria–Verweij et al.; LRT, lower respiratory tract.
LRTC, lower respiratory tract cultures; Modified AspICU DB, Modified AspICU–Dutch/Belgian Mycosis Study Group; Modified AspICU-G, Modified AspICU–Gangneux et al.; NR, non-reported/negative; ODI, optimal density index; PCR, polymerase chain reaction; Spp., species.
Quality assessment
Two researchers performed quality assessments using the Newcastle–Ottawa Scale (NOS), containing nine items, for the cohort and case–control studies. In NOS, the total score ranged from 0 to 9 and was categorized into three groups: low quality ‘0–3’, moderate quality ‘4–6’, and high quality ‘7–9’ [18]. During the quality assessment of the included studies, any disagreements were resolved by discussion.
Results
Study selection
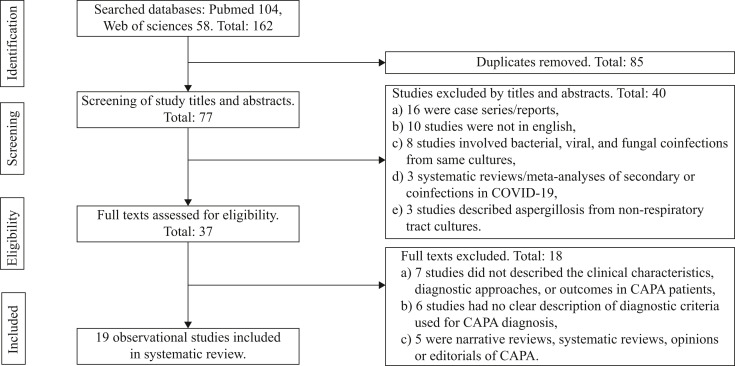
A total of 162 studies were identified in the initial search. After the removal of duplicates (N = 85) and those not meeting the inclusion criteria (by title, abstract, and full text: N = 114), 19 eligible observational studies were included in this review (Figure 1 ).
Figure 1.
Flowchart for observational studies.
Study characteristics
The study characteristics of the 19 observational studies included were described in Table III. All observational studies were cohort studies. Among the 19 cohorts, 68.4% (13/19) were retrospective, and 31.6% (6/19) were prospectively designed studies. 78.9% (15/19) were single-centre studies, and 21.1% (4/19) were multi-centre studies. Most of the studies were published from France (26.3% (5/19)), followed by the Netherlands (15.8% (3/19)) with the remainder from Belgium, Denmark, Germany, Italy, Mexico, Pakistan, Spain and Switzerland. The summary of the study qualities is shown in Table II. The majority of studies (47.4% (9/19)) had the maximum scores of 9, 15.8% (3/19) had scores of 8, and the remainder had scores of 7.
Epidemiology of CAPA
A total of 1421 hospitalized COVID-19 patients were included in our systematic review. The overall incidence of CAPA reported in COVID-19 pneumonia was 13.5% (192/1421) and ranging between 2.5 and 35.0% among the 19 observational studies included in our review (Table III). The majority of COVID-19 patients diagnosed with CAPA fell between the fifth and seventh decade of life, with a mean age of diagnosis ranging between 48.3 and 71.0 years. All of the hospitalized adult COVID-19 patients diagnosed with CAPA were critically ill and required ICU admission and IMV at the time of diagnosis, excluding observational studies by Nasir et al. [19] A small retrospective cohort study by Rutsaert et al. reported an incidence of CAPA at 35% among 20 critically ill COVID-19 patients and was the only study that described a proven CAPA diagnosis based on the biopsy results of four COVID-19 patients [20]. A total of 12.5% (24/192) of COVID-19 patients diagnosed with CAPA in 36.8% (7/19) of observational studies had pre-existing EORTC host risk factors (Table IV). The pre-existing EORTC host risk factors described were haematological malignancy, prolonged high-dose corticosteroid therapy, allogeneic stem cell/solid organ transplant, and inherited/acquired immunodeficiencies [[20], [21], [22], [23], [24], [25], [26]].
A multi-centre, retrospective cohort study by Delliere et al. described the incidence of CAPA at 19.4% among 108 COVID-19 patients requiring IMV in which respiratory tract cultures were obtained upon deterioration in clinical status [25]. A total of 23.8% of CAPA patients had EORTC host risk factors, and the diagnosis of probable CAPA was made according to IAPA criteria–Verweij et al. A multi-centre, prospective study by Bartoletti et al. reported the incidence of probable CAPA at 27.7% in 108 patients using similar diagnostic criteria of IAPA criteria–Verweij et al. and 27.0% had EORTC risk factors [22]. BAL was performed routinely on ICU admission, day 7 of IMV, and if there was a change in clinical status. A single-centre, retrospective study by Roman-Montes et al. revealed the incidence of putative CAPA at 9.7% in 144 patients using the modified AspICU–Dutch/Belgian Mycosis Study Group criteria [27]. Respiratory tract cultures were obtained if clinical status worsened during ICU admission. Finally, the largest study was a prospective single-centre cohort by Machado et al. with an incidence of putative CAPA at 2.5% in 239 patients using the modified AspICU–Dutch/Belgian Mycosis Study Group criteria [24].
Time to CAPA diagnosis
According to 14 observational studies (Table III), the overall time to CAPA diagnosis from the onset of COVID-19 symptoms ranged between 8.0 and 16.0 days. The time to CAPA diagnosis from ICU admission and after IMV initiation ranged between 4.0 and 15.0 days and between 3.0 and 8.0 days, respectively.
CAPA diagnostic criteria
The most common diagnostic criteria used (Table IV) to diagnose putative CAPA was modified AspICU–Dutch/Belgian Mycosis Study Group [10] in 52.6% (10/19) of observational studies reviewed. This was followed closely by IAPA diagnostic criteria–Verweij et al. [16] for probable CAPA diagnosis in 26.3% (5/19) of observational studies. The remainder of observational studies utilized AspICU [13], CAPA-ECMM [14], and modified AspICU–Gangneux et al. [15], to diagnose CAPA.
Cultures and biomarkers used in CAPA diagnosis
The CAPA diagnosis was made using LRTCs in 77.6 % (149/192) of CAPA patients, where the primary source of LRTCs was BAL. Around 30.7% (59/192) of CAPA patients had positive PCR from LRTCs observed in 42.1% (8/19) of studies reviewed. The Aspergillus species most frequently isolated were Aspergillus fumigatus in 84.2% (16/19) of studies.
In terms of fungal biomarkers used to assist with CAPA diagnosis, two different biomarkers were described involving GM and BDG. GM was obtained either from serum or BAL with a cut-off of 0.5 and 1.0 optimal density index (ODI) used, respectively (Table IV). Positive serum and BAL GM results were found in 73.7% (14/19) and 78.9% (15/19) of studies. Around 18.2% (35/192) of CAPA patients had positive serum GM, and 45.3% (87/192) of CAPA patients had positive BAL GM. Other serum biomarkers of BDG were rarely used in 31.6% (6/19) of observational studies and reportedly positive in only 10.4% (20/192) of patients diagnosed with CAPA.
Outcomes of CAPA
The hospital mortality observed among the 192 patients diagnosed with CAPA was highly variable, with an overall mortality rate of 48.4% (93/192) and ranging between 22.2% and 100% (Table III). According to 12 studies, the overall hospital and ICU length of stay (LOS) ranged between 16.0–37.5 days and 10.5–37.0 days. In four studies, the mean duration of IMV requirement ranged between 13.0 and 20.0 days. A total of 89.5% (17/19) of studies described the antifungal therapies received by CAPA patients that were predominantly voriconazole. Other antifungal therapies used were amphotericin B, anidulafungin, caspofungin, and isavuconazole (Table IV). No patients diagnosed with CAPA were on antifungal prophylaxis in all included studies.
Discussion
The overall incidence of CAPA was 13.5% and ranged between 2.5 and 35.0% among 1421 COVID-19 patients included. Generally, patients diagnosed with CAPA were critically ill and required IMV, although few had pre-existing EORTC risk factors. The mean age of CAPA patients ranged from 48.3 to 71.0 years. Many of these studies were limited by the lack of proven tissue diagnoses of CAPA. The time to CAPA diagnosis from onset of COVID-19 symptoms varied from 8.0 to 16.0 days. However, the time to CAPA diagnosis from ICU admission and after IMV initiation ranged from 4.0–15.0 days to 3.0–8.0 days. The most common diagnostic criteria used to diagnose putative/probable CAPA was the modified AspICU–Dutch/Belgian Mycosis Study Group, followed closely by IAPA–Verweij et al. Around 77.6 % of CAPA patients had positive LRTCs in which BAL was the most common method used for specimen sampling, and Aspergillus fumigatus was the most common Aspergillus species identified. Other fungal biomarkers used were BAL and serum GM in which BAL and serum GM returned positive in 45.3% and 18.2% of cases. Serum BDG was rarely used and returned positive in only 10.4% of cases. The mortality rate in CAPA patients was high at 48.4%, despite many patients receiving antifungal therapies. There was also a lengthy hospital and ICU LOS ranging between 16.0–37.5 days and 10.5–37.0 days. CAPA patients also had a prolonged duration of IMV ranging from 13.0 to 20.0 days.
Six observational studies reported an incidence of CAPA exceeding 20% among hospitalized COVID-19 patients, and five of these studies had a small sample size of fewer than 42 patients observed (Table III) [[20], [21], [22], [23],28]. Among these five studies described, Bartoletti et al. routinely performed serial samplings of BAL in a large sample size of 108 critically ill COVID-19 patients on the day of ICU admission, day seven after requiring IMV, and at the time of clinical deterioration, which likely contributed to the high observed CAPA incidence of 27.7% [22]. A prospective study by Alanio et al. reported a CAPA incidence of 33.3% among 27 COVID-19 patients requiring IMV in which bronchoscopy with BAL was performed routinely on day three post-intubation [21]. According to two observational studies by Lahmer et al. and Van Biesen et al., the high incidence of CAPA at 21.4% and 34.4% was due to a non-directed BAL approach used to minimize aerosolization and routinely performed within two days of ICU admission [23,29]. The non-directed BAL approach consists of advancing 12-French suction catheter via a closed-circuit until bronchial wedging is achieved, followed by lavage. Rutsaert et al. also described an elevated incidence of CAPA (35%) in their study in which routine bronchoscopy with BAL culture and GM testing was performed for indications of atelectasis and worsening clinical status among critically ill COVID-19 patients [20]. Furthermore, any suspicious tracheobronchial lesions concerning for Aspergillus tracheobronchitis were biopsied via bronchoscopy in that study. Therefore, the high CAPA incidence was at least partially explained by the frequent invasive BAL sampling approach across these five studies.
As the clinical course of COVID-19 demonstrates many features shared with severe influenza infection that include ARDS, lymphocytopenia, sepsis, and cytokine storm leading to multi-organ failure, it is reasonable to suspect that patients with severe COVID-19 may be similarly susceptible to IPA [30]. The clinical state of immunosuppression and underlying influenza-related acute respiratory failure is an independent risk factor for IPA [10,31]. When compared with critically ill adult patients being treated for influenza-related acute respiratory failure, several multi-centre retrospective studies reported an overall incidence of influenza-associated pulmonary aspergillosis (IAPA) amounting to 16–19% [10,31]. In influenza patients who are immunocompromised, the incidence of IAPA increased up to 32% from 14% in immunocompetent influenza patients. Critically ill patients with influenza-related respiratory failure have also been observed to have a 5.2-fold increased risk of contracting IAPA [10]. The evidence of IPA has also been found in 7.1–12.5% of autopsy series in SARS patients [[7], [8], [9]].
The diagnosis of CAPA from the onset of COVID-19 symptoms, ICU admission, and after initiation of IMV was highly variable. For the diagnosis of secondary bacterial pulmonary infections among COVID-19 patients, the time to diagnosis is 10 days (range 2–21 days) from hospital admission and nine days (range 4–18 days) after ICU admission but can occur as rapidly within five days after initiation of IMV [2,3,32]. Conversely, in critically ill adult patients with influenza-related acute respiratory failure, the median onset of IPA was three days after ICU admission [10,31]. The suspicion for secondary pulmonary infections typically arises when there is a sudden deterioration in the patient's clinical status or worsening chest imaging findings that cannot be explained by the underlying illness when managing critically ill COVID-19 patients. In the setting of this ongoing pandemic, clinicians' reluctance to perform BAL and rely on sputum and ETA specimens is not surprising and likely explained the delay in diagnosis of secondary bacterial and fungal pulmonary infections in COVID-19 patients compared with critically ill influenza patients.
There are multiple diagnostic criteria used for clinical decision-making in determining the probability of CAPA among the 19 observational studies included. These diagnostic criteria are a composite of host factors/clinical features, radiological findings, and mycological results described in Table I. Historically, EORTC/MSG criteria are used to classify patients who are immunocompromised into proven, probable, or possible aspergillosis while heavily reliant on characteristic radiological features of IPA that can be helpful to distinguish from COVID-19 pneumonia [17]. The diagnosis of IPA in critically ill patients can be challenging as the EORTC/MSG criteria are not necessarily applicable in the ICU setting or validated in immunocompetent patients, including COVID-19 patients, where many lack the typical host factors and often have less specific radiological features, especially in the presence of diffuse lung infiltrates from acute respiratory distress syndrome (ARDS) [17,22]. The EORTC/MSG diagnostic criteria were not used despite 36.8% of observational studies reporting COVID-19 patients with pre-existing EORTC host risk factors (Table IV), although this comprises 12.5% of patients diagnosed with CAPA. For patients with no underlying immunosuppressive comorbidities and in the ICU setting, the AspICU algorithm has emerged as the diagnostic criteria used to distinguish IPA into proven or putative from Aspergillus colonization in patients who are critically ill [13]. Putative AspICU diagnosis requires the presence of Aspergillus-positive LRT cultures (entry criterion) with compatible clinical, radiological and mycological findings described in Table I. More recently, several expert consensuses have proposed a modified case definition of CAPA from the AspICU algorithm, based on the addition of GM biomarker from serum or BAL specimens, irrespective of results of BAL cultures termed CAPA-ECMM [14], Modified AspICU–Dutch/Belgian Mycosis Study Group [10], and Influenza-associated Pulmonary Aspergillosis (IAPA)–Verweij et al. [16]. A BAL PCR testing for Aspergillus species has even been proposed as part of mycological criteria not only in EORTC/MSG but also in CAPA-ECMM and Modified AspICU–Gangneux et al. [14,15,17].
Furthermore, depending on the criteria, it is not unusual for a COVID-19 patient diagnosed with CAPA as per AspICU, CAPA-ECMM, and Modified AspICU-Gangneux et al. criteria to be initially misclassified as Aspergillus colonization in the absence of positive BAL culture or serum/BAL GM that may significantly impact management of COVID-19 patients and also their outcome [14,33,34]. A large, prospective study of 135 critically ill COVID-19 patients by White et al. described a huge variation in the incidence of CAPA at 5.9%, 14.8% and 14.1%, respectively, using the AspICU, modified AspICU–Dutch/Belgian Mycosis Study Group, and IAPA criteria [35]. It is likely that AspICU criteria significantly underestimate the incidence of CAPA compared with other CAPA criteria (Table I) with classification based on BAL culture in the absence of host risk factors that has slow turnover, lacks sensitivity, and is of limited utility in the ICU compared with the use of rapid serum/BAL biomarkers and PCR. Similar findings were noted in another large, prospective study by Machado et al. involving 239 critically ill COVID-19 patients where the incidence of CAPA increased from 1.3% using EORTC/MSG criteria to 2.5% using modified AspICU criteria–Dutch/Belgian Mycosis Study Group [24]. Therefore, the many CAPA definitions provided have enabled clinicians to utilize a strategic approach to identify and classify CAPA in critically ill COVID-19 patients. It also provides a framework for early diagnosis and possibly allows prompt treatment initiation, which may confer a survival benefit. In the prospective study by Alanio et al., the reported incidence of CAPA was 33% (9/27); however, five out of nine CAPA patients had positive BAL culture but negative BAL/serum GM suggesting a lack of tissue invasion [21]. Undeniably, 60% (3/5) of these COVID-19 patients were not treated with antifungals and survived. Several other diagnostic criteria have been suggested to allow early screening and diagnosis of CAPA, specifically in critically ill patients; yet, these criteria have not been validated in any studies [30,35,36]. Consequently, as the diagnostic criteria of CAPA continue to evolve during this current pandemic, large, prospective, multi-centre validation studies are required to determine which diagnostic CAPA criteria are most pragmatic or needed to be refined.
In the setting of this ongoing pandemic, the reluctance of clinicians to perform invasive diagnostic procedures such as BAL and over-reliance on sputum and ETA specimens is not unexpected. Elevated serum levels of procalcitonin and higher neutrophil to low lymphocyte ratio from dysregulated immune response have been suggested to predict secondary bacterial infection in critically ill COVID-19 patients [37]. However, these findings do not apply during the evaluation of suspected CAPA or invasive fungal disease [35]. Therefore, systematic screening using a combination of biomarkers such as serum and BAL GM is essential to assist with the diagnosis of CAPA and has since been included as part of the CAPA-ECMM [14], modified diagnostic CAPA criteria used involving Modified AspICU–Gangneux et al. [15], Modified AspICU–Dutch/Belgian Mycosis Study Group [10], and Influenza-associated Pulmonary Aspergillosis (IAPA)–Verweij et al. [16], given the poor specificity (20–50%) of a positive Aspergillus culture identified in sputum and ETA, that may represent colonization [38]. BAL GM, when performed, has an observed 90% sensitivity and 94% specificity for diagnosing proven or probable IPA according to the EORTC/MSG criteria [39]. However, in non-immunocompromised critically ill patients who do not have the typical host factors meeting EORTC/MSG criteria (Table I), BAL GM ODI of more than 0.5 cut-offs had 76% sensitivity and 81% specificity in diagnosing IPA [40]. Therefore, BAL PCR has been increasingly used to assist with the diagnosis of CAPA in 42.9% of observational studies included (Table III) with a reported sensitivity of 80% and specificity of 93% in non-neutropenic critically ill patients [41]. Using the Modified AspICU–Dutch/Belgian Mycosis Study Group criteria, a BAL GM will return positive in 92% cases using an index of more than 0.5 cut-offs for diagnosing IAPA [10]. The use of BAL PCR may allow early CAPA diagnosis; however, this was not observed in the study by Gangneux et al. using the Modified AspICU–Gangneux et al. criteria with a reported incidence of 15.6% [15]. It is important to note that Roman-Montes et al. used GM obtained from ETA as an equivalent for BAL GM, whereas studies by Dupont et al. and Lamoth et al. used bronchial aspirates (BAS) as an equivalent for BAL in assisting with diagnosis of putative CAPA due to concerns of exposure and transmission associated with bronchoscopy [27,42,43].
It remains open to what extent COVID-19 patients were co-infected, super-infected, or maybe sub-clinically colonized with Aspergillus. The distinction between colonization and angio-invasive disease can be difficult in CAPA because serum biomarkers are often negative, suggesting colonization. Serum GM is a relatively sensitive (82%) and specific (81%) diagnostic tool in neutropenic critically ill patients with proven IPA [[44], [45], [46]]. However, in non-neutropenic or IAPA patients, a considerably lower sensitivity of around 25–37% has been reported, which is similar to what we encountered in COVID-19 patients included in our review [10,16,45]. In contrast, BAL GM was 88–90% sensitive in both neutropenic and non-neutropenic groups. The low positivity rate of serum GM results encountered in non-neutropenic COVID-19 patients can be explained by multiple potential aetiologies. Hydroxychloroquine, which many hospitalized COVID-19 patients received during the early course of the pandemic and the use of empirical antifungals among critically ill patients may alter serum GM performance from drug inhibition in vitro activity and hyphal invasion by Aspergillus species [21,47,48]. This finding is supported by the lack of angio-invasion observed in multiple post mortem examinations of COVID-19 patients [49,50]. An autopsy of six critically ill COVID-19 patients by Flikweert et al. revealed no histopathological evidence of CAPA despite a high BAL GM index of 1.7–5.7 [50]. Few studies have described high serum GM levels supplementing histopathological evidence of Aspergillus angio-invasion on autopsies of COVID-19 patients [20,51,52]. A small retrospective study by Rutsaert et al. involving 20 critically ill COVID-19 patients had attempted to debunk this theory that angio-invasion by Aspergillus species was required for serum GM to return positive in which four patients with proven CAPA on tracheobronchial biopsies had undetectable serum GM level despite an elevated BAL GM level [20]. This finding further supports the notion that BAL GM is a more sensitive biomarker for IPA than serum GM, especially in those with non-angio-invasive CAPA. The specificity of serum BDG to distinguish between IPA and those with Aspergillus colonization has been shown to be as high as 86%, with two consecutive positive results [53]. Yet, the role of serum BDG in supporting the diagnosis of CAPA remains uncertain due to the scarcity of the tests being performed in the limited literature available. Lung biopsy remains the gold standard to confirm the diagnosis of proven CAPA, regardless of diagnostic criteria used (Table I), by revealing evidence of hyphae invasion and damage of lung tissue but generally avoided due to its associated risk, especially in ventilated COVID-19 patients. Again, a small retrospective study by Rutsaert et al. was the only study that performed routine bronchoscopy-guided biopsies of any suspicious tracheobronchial lesions, which likely explained the high incidence and proven CAPA diagnosis observed [20]. Moreover, it is not unusual for the diagnosis of proven CAPA to be confirmed during post mortem examination, which was seen in several studies [52,54]. The difficulties in CAPA diagnosis are further outlined in a study by Blaize et al., where both LRT culture and serum biomarkers were initially negative but repeat LRT cultures eventually returned positive after the patient's death [54]. In order to optimize microbiological and molecular diagnostics to better improve protection strategies for vulnerable patient groups, bronchoscopy, including tracheobronchial inspection and BAL sampling for culture, PCR and GM, should be part of the diagnostic gold standards whenever CAPA is suspected, providing local infection prevention and control guidance for aerosol-generating procedures can be adhered.
Critically ill ARDS patients with putative IPA diagnosis are shown to have significant mortality (OR 9.58; 95% CI 1.97–46.52; P=0.005) while Aspergillus colonizers are not [13,55]. The increased awareness of the high mortality rate observed in severe viral pneumonia such as SARS and IAPA has generated concerns relating to CAPA, especially in critically ill COVID-19 patients. One may even hypothesize that CAPA, which appears to be almost exclusively described in critically ill COVID-19 patients requiring IMV, is associated with high hospital mortality. Additionally, several of these observational studies have compared hospital mortality, LOS, and duration of IMV requirement. Initially, during the early course of the pandemic, two smaller single-centre studies by Gangneux et al. and Van Biesen et al. demonstrated similar ICU mortality rates (22.2–28.6% vs 13.3–15.1%; P>0.05) and IMV days (18 days vs 17 days; P=0.66) among CAPA and non-CAPA patients, although ICU LOS was significantly longer in CAPA patients (27–37 days vs 12–19 days; P<0.05) [15,23]. However, multiple recent large sample size studies refuted the findings of ICU LOS and duration of IMV requirement. The largest single-centre, prospective cohort study by Machado et al. revealed an increase in overall mortality (100% vs 40%; P=0.04) when comparing CAPA patients with those without CAPA [24]. A multi-centre study by Delliere et al. observed that many non-survivors of COVID-19 were those diagnosed with CAPA (71.4% vs 36.8%; OR 4.3; 95% CI 1.5–12.1), although no difference was noted for hospital LOS (21.1 days vs 25.1 days; P=0.31) [25]. Another multi-centre study by Bartoletti et al. reported similar findings with increased mortality among CAPA patients (44% vs 19%; P=0.02) but comparable ICU LOS (16 days vs 21 days; P=0.08) and days of IMV (13 days vs 16 days; P=0.09) [22]. Comparing critically ill influenza patients diagnosed with IAPA, ICU LOS (19 days vs 9 days; P<0.0001), IMV days (14 days vs 9 days; P=0.001), ICU (45% vs 20%; P<0.0001) and hospital mortality (49% vs 26%; P<0.0001) were significantly higher in those with IAPA than those without IAPA [10,31]. Therefore, critically ill CAPA patients have higher mortality than those without CAPA, although the length of hospitalization and duration of IMV requirements are similar.
Even after identifying the population at risk, the role of antifungal prophylaxis is unclear in CAPA. No COVID-19 patients received antifungal prophylaxis to prevent CAPA in our systematic review. Moreover, the choice, timing of administration, and total duration of therapeutic antifungals in CAPA patients remain understudied. The majority of CAPA patients received antifungal voriconazole, which is in line with ECMM expert guidelines that voriconazole or isavuconazole as recommended first-line therapy for suspected and confirmed CAPA [14]. Six to 12 weeks' duration of treatment is recommended with follow-up chest imaging being a valuable tool to determine treatment response and in whom longer treatment duration is required. Echinocandins are not recommended as monotherapy but in combination with an azole in areas with a high prevalence of azole resistance. Amphotericin B can be salvage therapy or even initial therapy if local azole resistance patterns are high while awaiting Aspergillus susceptibility results. The association between antifungals and mortality in CAPA patients remains unclear, despite the widespread use of antifungals in the included studies (Table IV). A study by Alanio et al. demonstrated low CAPA mortality despite minimal use of antifungals suggesting that patients are likely to be colonized despite meeting Modified AspICU-DB criteria that suggest actual infection [21]. Although antifungals are safe and effective, even as prophylaxis in immunocompromised patients, drug interactions, adverse effects, costs, and capability to measure drug levels are always of concern, especially in critically ill patients with healthcare systems already stretched to the limit. Moreover, countries with lower socioeconomic status are likely to have inadequate resources available to diagnose and treat patients with CAPA. This can very well explain the low incidence of CAPA (10% and less) observed in developing countries (e.g., Mexico and Pakistan), although mortality rates observed were similar [19,27]. Better studies are required to confirm this hypothesis.
Several possible limitations explain why the incidence of CAPA varied widely from 2.5% to 35% across studies included in our review (Table III). First, lack of clinical awareness and standardized diagnostic approach for evaluating CAPA and heterogeneity of diagnostic criteria/classifications used to define CAPA in COVID-19 patients. CAPA is challenging to diagnose and likely under-recognized and under-reported, particularly in the setting of ARDS in which clinical features and radiological findings in CAPA resemble those of severe COVID-19 pneumonia [17,22,56]. Some centres have implemented and performed routine bronchoscopy with BAL or serum GM testing regardless of clinical suspicion, leading to the high CAPA incidence reported [[20], [21], [22], [23],28]. Second, the reluctance to perform aerosol-generating procedures, such as autopsies and bronchoscopies in critically ill patients due to concerns of further transmission and risk of lung de-recruitment, leading to the variability of LRT samples, confined to sputum and ETA, and the lack of BAL GM available [56]. The use of bronchoscopy in COVID-19 patients has been recommended when current respiratory samples from sputum and endotracheal aspirates are negative, and an alternate diagnosis provided by BAL would significantly impact clinical management [57]. This recommendation is supported by several observational studies by Torrego et al. and Chang et al. [58,59]. Torrego et al. demonstrated that although BAL obtained from routine use of broncoscopy in COVID-19 patients resulted in a change of antibiotic prescribed in 83% of critically ill, mechanically ventilated COVID-19 patients, the bacterial micro-organisms identified from mechanically ventilated COVID-19 patients are similar to those identified in mechanically ventilated non-COVID-19 patients [58]. However, Chang et al. reported that in COVID-19 patients with negative ETA cultures, up to 35% of patients would have positive BAL cultures when routine bronchoscopy is performed without increased risk of peri-procedural complications such as hypoxia, arrhythmia, or pneumothorax [59]. ETAs are potentially safer alternative investigative options; however, their use for GM detection has not been validated. Third, the inconsistency in serum biomarkers of GM and BDG obtained in CAPA patients together with the low positivity rate, especially in non-neutropenic patients [56]. Fourth, the wide variability of in-hospital mortality observed in CAPA patients may underestimate the actual duration of hospitalization, ICU admission, and IMV requirement. Lastly, the lack of generalizability of our systematic review is limited by the limited number of studies published from developing countries. Therefore, we cannot determine the relationship between socioeconomic status with the risk of developing CAPA and associated outcomes among COVID-19 patients.
In conclusion, currently there is no agreed case definition of CAPA in which distinguishing from colonization remains highly variable depending on the classification used, which is well illustrated in our review; hence the difficulty in comparing different observational studies and the ongoing need to find a consensus on which of these criteria is most sensitive in defining and diagnosing CAPA. The complexity of CAPA presentation further increases this difficulty in critically ill COVID-19 patients. Therefore, taking into account host factors, clinical risk, and radiological findings are essential to help raise the pretest probability of CAPA. Understanding the clinical features of this novel disease requires a continued collaborative and systematic approach. This review highlights the ongoing need for a large, well-designed, multi-centre study to assess and determine whether there is an association between CAPA in critically ill patients with COVID-19, the optimal diagnostic approach, and if all critically ill COVID-19 patients require routine screening for secondary fungal pulmonary infections, especially in the presence of known risk factors.
Author contributions
All authors had access to the data and were involved in writing the manuscript.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Funding sources
None.
References
- 1.Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O., et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health — the latest 2019 novel coronavirus outbreak in Wuhan, China. International Journal of Infectious Diseases. 2020 Feb;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chong W.H., Saha B.K., Ramani Ananthakrishnan, Chopra A. State-of-the-art review of secondary pulmonary infections in patients with COVID-19 pneumonia. Infection. 2021;11:1–15. doi: 10.1007/s15010-021-01602-z. [Internet] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yang X., Yu Y., Xu J., Shu H., Xia J., Liu H., et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. 2020;8(5):475–481. doi: 10.1016/S2213-2600(20)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ye Z., Zhang Y., Wang Y., Huang Z., Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381–4389. doi: 10.1007/s00330-020-06801-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Franks T.J., Chong P.Y., Chui P., Galvin J.R., Lourens R.M., Reid A.H., et al. Lung pathology of severe acute respiratory syndrome (SARS): a study of 8 autopsy cases from Singapore. Hum Pathol. 2003;34:743–748. doi: 10.1016/S0046-8177(03)00367-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hwang D.M., Chamberlain D.W., Poutanen S.M., Low D.E., Asa S.L., Butany J. Pulmonary pathology of severe acute respiratory syndrome in Toronto. Mod Pathol. 2005;18(1):1–10. doi: 10.1038/modpathol.3800247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chong P.Y., Chui P., Ling A.E., Franks T.J., Tai D.Y.H., Leo Y.S., et al. Analysis of Deaths During the Severe Acute Respiratory Syndrome (SARS) Epidemic in Singapore. Arch Pathol Lab Med. 2004;128:10. doi: 10.5858/2004-128-195-AODDTS. [DOI] [PubMed] [Google Scholar]
- 10.Schauwvlieghe A.F.A.D., Rijnders B.J.A., Philips N., Verwijs R., Vanderbeke L., Van Tienen C., et al. Invasive aspergillosis in patients admitted to the intensive care unit with severe influenza: a retrospective cohort study. Lancet Respir Med. 2018;6(10):782–792. doi: 10.1016/S2213-2600(18)30274-1. [DOI] [PubMed] [Google Scholar]
- 11.Guberina H., Witzke O., Timm J., Dittmer U., Müller M.A., Drosten C., et al. A patient with severe respiratory failure caused by novel human coronavirus. Infection. 2014;42(1):203–206. doi: 10.1007/s15010-013-0509-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Magira E.E., Chemaly R.F., Jiang Y., Tarrand J., Kontoyiannis D.P. Outcomes in invasive pulmonary aspergillosis infections complicated by respiratory viral infections in patients with hematologic malignancies: a case–control study. Open Forum Infect Dis. 2019;6:ofz247. doi: 10.1093/ofid/ofz247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blot S.I., Taccone F.S., Van den Abeele A.-M., Bulpa P., Meersseman W., Brusselaers N., et al. A clinical algorithm to diagnose invasive pulmonary aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2012;186:56–64. doi: 10.1164/rccm.201111-1978OC. [DOI] [PubMed] [Google Scholar]
- 14.Koehler P., Bassetti M., Chakrabarti A., Chen S.C.A., Colombo A.L., Hoenigl M., et al. Defining and managing COVID-19-associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2020 Dec doi: 10.1016/S1473-3099(20)30847-1. S1473309920308471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gangneux J.-P., Reizine F., Guegan H., Pinceaux K., Le Balch P., Prat E., et al. Is the COVID-19 pandemic a good time to include Aspergillus molecular detection to categorize Aspergillosis in ICU patients? A monocentric experience. J Fungi (Basel) 2020;6(3):105. doi: 10.3390/jof6030105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Verweij P.E., Rijnders B.J.A., Brüggemann R.J.M., Azoulay E., Bassetti M., Blot S., et al. Review of influenza-associated pulmonary aspergillosis in ICU patients and proposal for a case definition: an expert opinion. Intensive Care Med. 2020;46(8):1524–1535. doi: 10.1007/s00134-020-06091-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Donnelly J.P., Chen S.C., Kauffman C.A., Steinbach W.J., Baddley J.W., Verweij P.E., et al. Revision and update of the consensus definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer and the Mycoses Study Group Education and Research Consortium. Clin Infect Dis. 2020;71:1367–1376. doi: 10.1093/cid/ciz1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stang A. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 19.Nasir N., Farooqi J., Mahmood S.F., Jabeen K. COVID-19-associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID-19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63:766–770. doi: 10.1111/myc.13135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rutsaert L., Steinfort N., Van Hunsel T., Bomans P., Naesens R., Mertes H., et al. COVID-19-associated invasive pulmonary aspergillosis. Ann Intensive Care. 2020;10:71. doi: 10.1186/s13613-020-00686-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alanio A., Dellière S., Fodil S., Bretagne S., Mégarbane B. Prevalence of putative invasive pulmonary aspergillosis in critically ill patients with COVID-19. Lancet Respir Med. 2020;8:e48–e49. doi: 10.1016/S2213-2600(20)30237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bartoletti M., Pascale R., Cricca M., Rinaldi M., Maccaro A., Bussini L., et al. Epidemiology of invasive pulmonary Aspergillosis among intubated patients with COVID-19: a prospective study. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1065. ciaa1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Van Biesen S., Kwa D., Bosman R.J., Juffermans N.P. Detection of Invasive Pulmonary Aspergillosis in COVID-19 with Nondirected BAL. Am J Respir Crit Care Med. 2020;202(8):1171–1173. doi: 10.1164/rccm.202005-2018LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Machado M., Valerio M., Álvarez-Uría A., Olmedo M., Veintimilla C., Padilla B., et al. Invasive pulmonary aspergillosis in the COVID-19 era: An expected new entity. Mycoses. 2020:13213. doi: 10.1111/myc.13213. myc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dellière S., Dudoignon E., Fodil S., Voicu S., Collet M., Oillic P.-A., et al. Risk factors associated with COVID-19-associated pulmonary aspergillosis in ICU patients: a French multicentric retrospective cohort. Clin Microbiol Infect. 2020 Dec doi: 10.1016/j.cmi.2020.12.005. S1198743X20307564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chauvet P., Mallat J., Arumadura C., Vangrunderbeek N., Dupre C., Pauquet P., et al. Risk factors for invasive pulmonary Aspergillosis in critically ill patients with coronavirus disease 2019-induced acute respiratory distress syndrome. Crit Care Explor. 2020;2 doi: 10.1097/CCE.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roman-Montes Authors C.M., Martinez-Gamboa A., Diaz-Lomelí P., Cervantes-Sanchez A., Rangel-Cordero A., Sifuentes-Osornio J., et al. Accuracy of galactomannan testing on tracheal aspirates in COVID-19-associated pulmonary aspergillosis. Mycoses. 2020:13216. doi: 10.1111/myc.13216. myc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koehler P., Cornely O.A., Böttiger B.W., Dusse F., Eichenauer D.A., Fuchs F., et al. COVID-19 associated pulmonary aspergillosis. Mycoses. 2020;63:528–534. doi: 10.1111/myc.13096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lahmer T., Kriescher S., Herner A., Rothe K., Spinner C.D., Schneider J., et al. Invasive pulmonary aspergillosis in critically ill patients with severe COVID-19 pneumonia: results from the prospective AspCOVID-19 study. PLoS One. 2021;16 doi: 10.1371/journal.pone.0238825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Armstrong-James D., Youngs J., Bicanic T., Abdolrasouli A., Denning D.W., Johnson E., et al. Confronting and mitigating the risk of COVID-19 associated pulmonary aspergillosis (CAPA) Eur Respir J. 2020:2002554. doi: 10.1183/13993003.02554-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van de Veerdonk F.L., Kolwijck E., Lestrade P.P.A., Hodiamont C.J., Rijnders B.J.A., van Paassen J., et al. Influenza-associated aspergillosis in critically ill patients. Am J Respir Crit Care Med. 2017;196:524–527. doi: 10.1164/rccm.201612-2540LE. [DOI] [PubMed] [Google Scholar]
- 32.Zhang H., Zhang Y., Wu J., Li Y., Zhou X., Li X., et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9:1958–1964. doi: 10.1080/22221751.2020.1812437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ghelfenstein-Ferreira T., Saade A., Alanio A., Bretagne S., Araujo de Castro R., Hamane S., et al. Recovery of a triazole-resistant Aspergillus fumigatus in respiratory specimen of COVID-19 patient in ICU – a case report. Med Mycol Case Rep. 2020 doi: 10.1016/j.mmcr.2020.06.006. S2211753920300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Abdalla S., Almaslamani M.A., Hashim S.M., Ibrahim A.S., Omrani A.S. Fatal coronavirus disease 2019-associated pulmonary aspergillosis; a report of two cases and review of the literature. IDCases. 2020;22 doi: 10.1016/j.idcr.2020.e00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.White P.L., Dhillon R., Cordey A., Hughes H., Faggian F., Soni S., et al. A national strategy to diagnose COVID-19 associated invasive fungal disease in the ICU. Clin Infect Dis. 2020 Aug 29:ciaa1298. doi: 10.1093/cid/ciaa1298. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mohamed A., Rogers T.R., Talento A.F. COVID-19 associated invasive pulmonary Aspergillosis: diagnostic and therapeutic challenges. J Fungi (Basel) 2020;6:115. doi: 10.3390/jof6030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Qin C., Zhou L., Hu Z., Zhang S., Yang S., Tao Y., et al. Dysregulation of immune response in patients with coronavirus 2019 (COVID-19) in Wuhan, China. Clin Infect Dis. 2020;71:762–768. doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yusuf E., Vonk A., van den Akker J.P.C., Bode L., Sips G.J., Rijnders B.J.A., et al. Frequency of positive Aspergillus tests in COVID-19 patients in comparison to other patients with pulmonary infections admitted to the ICU. J Clin Microbiol. 2021;59:20. doi: 10.1128/JCM.02278-20. e02278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guo Y.-L., Chen Y.-Q., Wang K., Qin S.-M., Wu C., Kong J.-L. Accuracy of BAL galactomannan in diagnosing invasive aspergillosis: a bivariate metaanalysis and systematic review. Chest. 2010;138(4):817–824. doi: 10.1378/chest.10-0488. [DOI] [PubMed] [Google Scholar]
- 40.Zhou W., Li H., Zhang Y., Huang M., He Q., Li P., et al. Diagnostic value of galactomannan antigen test in serum and bronchoalveolar lavage fluid samples from patients with nonneutropenic invasive pulmonary aspergillosis. J Clin Microbiol. 2017;55:2153–2161. doi: 10.1128/JCM.00345-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chong G.-L.M., van de Sande W.W.J., Dingemans G.J.H., Gaajetaan G.R., Vonk A.G., Hayette M.-P., et al. Validation of a new Aspergillus real-time PCR assay for direct detection of Aspergillus and azole resistance of Aspergillus fumigatus on bronchoalveolar lavage fluid. J Clin Microbiol. 2015;53:868–874. doi: 10.1128/JCM.03216-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lamoth F., Glampedakis E., Boillat-Blanco N., Oddo M., Pagani J.-L. Incidence of invasive pulmonary aspergillosis among critically ill COVID-19 patients. Clin Microbiol Infect. 2020;26:1706–1708. doi: 10.1016/j.cmi.2020.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dupont D., Menotti J., Turc J., Miossec C., Wallet F., Richard J.-C., et al. Pulmonary aspergillosis in critically ill patients with coronavirus disease 2019 (COVID-19) Med Mycol. 2020 Sep 10:myaa078. doi: 10.1093/mmy/myaa078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meersseman W., Lagrou K., Maertens J., Wilmer A., Hermans G., Vanderschueren S., et al. Galactomannan in bronchoalveolar lavage fluid: a tool for diagnosing Aspergillosis in intensive care unit patients. Am J Respir Crit Care Med. 2008;177:27–34. doi: 10.1164/rccm.200704-606OC. [DOI] [PubMed] [Google Scholar]
- 45.Meijer E.F.J., Dofferhoff A.S.M., Hoiting O., Buil J.B., Meis J.F. Azole-resistant COVID-19-associated pulmonary Aspergillosis in an immunocompetent host: a case report. J Fungi (Basel) 2020;6:79. doi: 10.3390/jof6020079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leeflang M.M.G., Debets-Ossenkopp Y.J., Wang J., Visser C.E., Scholten R.J.P.M., Hooft L., et al. Galactomannan detection for invasive aspergillosis in immunocompromised patients. Cochrane Database Syst Rev. 2015;12:CD007394. doi: 10.1002/14651858.CD007394.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Henriet S.S.V., Jans J., Simonetti E., Kwon-Chung K.J., Rijs A.J.M.M., Hermans P.W.M., et al. Chloroquine modulates the fungal immune response in phagocytic cells from patients with chronic granulomatous disease. J Infect Dis. 2013;207(12):1932–1939. doi: 10.1093/infdis/jit103. [DOI] [PubMed] [Google Scholar]
- 48.Saha B.K., Bonnier A., Chong W. Antimalarials as antivirals for COVID-19: believe it or not! Am J Med Sci. 2020 Aug doi: 10.1016/j.amjms.2020.08.019. S0002962920303712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.De Michele S., Sun Y., Yilmaz M.M., Katsyv I., Salvatore M., Dzierba A.L., et al. Forty postmortem examinations in COVID-19 patients. Am J Clin Pathol. 2020;154:748–760. doi: 10.1093/ajcp/aqaa156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Flikweert A.W., Grootenboers M.J.J.H., Yick D.C.Y., du Mée A.W.F., van der Meer N.J.M., Rettig T.C.D., et al. Late histopathologic characteristics of critically ill COVID-19 patients: different phenotypes without evidence of invasive aspergillosis, a case series. J Crit Care. 2020;59:149–155. doi: 10.1016/j.jcrc.2020.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Santana M.F., Pivoto G., Alexandre M.A.A., Baía-da-Silva D.C., Borba M.G. da S., Val F.A., et al. Confirmed Invasive Pulmonary Aspergillosis and COVID-19: the value of postmortem findings to support antemortem management. Rev Soc Bras Med Trop. 2020;53 doi: 10.1590/0037-8682-0401-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Antinori S., Rech R., Galimberti L., Castelli A., Angeli E., Fossali T., et al. Invasive pulmonary aspergillosis complicating SARS-CoV-2 pneumonia: a diagnostic challenge. Travel Med Infect Dis. 2020;38:101752. doi: 10.1016/j.tmaid.2020.101752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Talento A.F., Dunne K., Joyce E.A., Palmer M., Johnson E., White P.L., et al. A prospective study of fungal biomarkers to improve management of invasive fungal diseases in a mixed specialty critical care unit. J Crit Care. 2017;40:119–127. doi: 10.1016/j.jcrc.2017.03.025. [DOI] [PubMed] [Google Scholar]
- 54.Blaize M., Mayaux J., Nabet C., Lampros A., Marcelin A.-G., Thellier M., et al. Fatal Invasive Aspergillosis and Coronavirus Disease in an Immunocompetent Patient. Emerg Infect Dis. 2020;26:1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Contou D., Dorison M., Rosman J., Schlemmer F., Gibelin A., Foulet F., et al. Aspergillus-positive lower respiratory tract samples in patients with the acute respiratory distress syndrome: a 10-year retrospective study. Ann Intensive Care. 2016;6(1):52. doi: 10.1186/s13613-016-0156-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hoenigl M. Invasive fungal disease complicating coronavirus disease 2019: when it rains, it spores. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1342. ciaa1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wahidi M.M., Lamb C., Murgu S., Musani A., Shojaee S., Sachdeva A., et al. American Association for Bronchology and Interventional Pulmonology (AABIP) Statement on the Use of Bronchoscopy and Respiratory Specimen Collection in Patients with Suspected or Confirmed COVID-19 Infection. J Bronchology Interv Pulmonol. 2020;27:e52–e54. doi: 10.1097/LBR.0000000000000681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Torrego A., Pajares V., Fernández-Arias C., Vera P., Mancebo J. Bronchoscopy in COVID-19 patients with invasive mechanical ventilation: a center experience. Am J Respir Crit Care Med. 2020 May 15 doi: 10.1164/rccm.202004-0945LE. rccm.202004-0945LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chang S.H., Jiang J., Kon Z.N., Williams D.M., Geraci T.C., Smith D.E., et al. Safety and efficacy of bronchoscopy in critically ill patients with coronavirus disease 2019. Chest. 2020 Oct doi: 10.1016/j.chest.2020.09.263. S001236922034873X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Helleberg M., Steensen M., Arendrup M.C. Invasive aspergillosis in patients with severe COVID-19 pneumonia. Clin Microbiol Infect. 2021;27:147–148. doi: 10.1016/j.cmi.2020.07.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meijer E.F.J., Dofferhoff A.S.M., Hoiting O., Meis J.F. COVID-19–associated pulmonary aspergillosis: a prospective single-center dual case series. Mycoses. 2021;64:457–464. doi: 10.1111/myc.13254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Arkel A.L.E., Rijpstra T.A., Belderbos H.N.A., van Wijngaarden P., Verweij P.E., Bentvelsen R.G. COVID-19 associated pulmonary Aspergillosis. Am J Respir Crit Care Med. 2020 May 12:202004. doi: 10.1164/rccm.202004-1038LE. rccm. 1038LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vélez Pintado M., Camiro-Zúñiga A., Aguilar Soto M., Cuenca D., Mercado M., Crabtree-Ramirez B., et al. COVID-19-associated invasive pulmonary aspergillosis in a tertiary care center in Mexico City. Med Mycol. 2021 Mar 16 doi: 10.1093/mmy/myab009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Versyck M., Zarrougui W., Lambiotte F., Elbeki N., Saint-Leger P. Invasive pulmonary aspergillosis in COVID-19 critically ill patients: Results of a French monocentric cohort. J Med Mycol. 2021;31(2):101122. doi: 10.1016/j.mycmed.2021.101122. [DOI] [PMC free article] [PubMed] [Google Scholar]