Abstract
Recent advances in integrating 1.5 Tesla magnetic resonance (MR) imaging with a linear accelerator (MR-Linac) allow MR-guided stereotactic body radiotherapy (SBRT) for prostate cancer. Choosing an optimal strategy for daily online plan adaptation is particularly important for MR-guided radiotherapy. We analyzed deformable dose accumulation on scans from four patients and found that daily anatomy changes had little impact on the delivered dose, with the dose to the prostate within 0.5% and dose to the rectum/bladder mostly less than 0.5 Gy. These findings could help in the choice of an optimal strategy for online plan adaptation for MR-guided prostate SBRT.
Keywords: MR-Linac, MRgRT, Prostate, SBRT
1. Introduction
Magnetic resonance imaging (MRI) is increasingly recognized as the primary modality for staging prostate cancer and has been used for contouring targets and organs at risk (OARs) in radiotherapy [1], [2], [3]. Recent advances in the integration of MRI with a linear accelerator (MR-Linac) have enabled MR-guided radiotherapy (MRgRT) [4], [5], [6]. Integrated MR-Linacs allow simultaneous MRI with radiation delivery, thereby providing superior visualization of the soft tissues during treatment and enabling real-time motion monitoring [7], [8]. The 1.5 Tesla MR-Linac system also permits online adaptation of treatment plans to account for daily soft-tissue deformation [4]. Several groups have reported using online plan adaptation with a 1.5 Tesla MR-Linac system for various treatment sites [9], [10], [11]. Recently Alongi et al. published a study describing MR-Linac for prostate stereotactic body radiotherapy (SBRT), focusing on clinical aspects such as tolerability, quality of life, and patient-reported outcomes [12]. However, no protocols have been established for prostate SBRT that include daily online plan adaptation with the 1.5 Tesla MR-Linac system, particularly for selecting an appropriate plan-adaptation strategy. Dose accumulation in daily adaptive plans for MR-Linac treatments has been studied [13], [14], but these daily adaptive plans have yet to be used to select an optimal plan-adaptation strategy. In this short communication, we analyzed the deformable accumulated dose from daily adaptive plans to help in choosing an optimal online plan-adaptation strategy for MR-guided prostate SBRT.
2. Methods and materials
2.1. Patient selection
Four patients with the following characteristics were identified for treatment with an Elekta Unity MR-Linac system (Elekta, Stockholm, Sweden): (1) intermediate-risk prostate cancer (stage T2c or less and Gleason 7 disease with prostate-specific antigen level < 10.0 ng/mL); (2) prostate volume < 50 cm3; (3) minimal obstructive urinary symptoms (an American Urological Association symptom score of 15 or less); and (4) no history of transurethral resection of the prostate or urethral surgery. Suitability for MR-Linac treatment was considered first by the attending physician, and was subsequently evaluated by a multidisciplinary team of physicists, dosimetrists, therapists, and physicians. The evaluation focused on MR safety issues, fit feasibility within the MR-Linac body coil, and the patient’s ability to maintain a consistent position for extended treatment times. Once a patient was deemed to be a good candidate for treatment on the MR-Linac, that patient was counseled on the logistics and side effects of the treatment. This study was approved by the institutional review board of MD Anderson Cancer Center.
2.2. Simulation
Before the simulation, a rectal spacer was placed between the rectum and the prostate of each patient to separate these two structures and thereby reduce the radiation dose to the rectum during treatment. Three gold fiducials were also placed during that procedure. Just before the treatment simulation, the patient was instructed to have a comfortably full bladder and an empty rectum (ensured by use of an enema). The simulation involved obtaining a CT scan on a Philips Brilliance 16-slice CT scanner, followed by an MR scan on the MR-Linac. The MR scan included the acquisition of 3D T1 and T2 weighted sequences via the same scanning protocol as that used for daily MR image acquisition.
2.3. Treatment planning
Treatment planning was done with the CT images by using the Monaco treatment planning system (Elekta, Inc. Maryland Heights, MO), according to institutional guidelines established by the Prostate Radiation Oncology Service that address planning constraints for targets and normal tissue structures. The clinical target volume (CTV) included the prostate and intraprostatic portion of the seminal vesicles (SVs). The planning target volume (PTV) was created by expanding the CTV by 5 mm from the prostate in the lateral and superior-inferior directions and by 3 mm in the posterior direction. All CTVs and PTVs were drawn by a single physician. The dose was prescribed to cover at least 95% of the PTV, with the dose prescribed being 36.25 Gy for three patients (our standard clinical practice) and 40 Gy (for one patient participating in a clinical trial to evaluate dose escalation), delivered in 5 fractions every other day. The OAR dose constraints are listed in Supplementary Table S1.
Pretreatment quality assurance (QA) included a secondary verification of monitor units (MU) using RadCalc (Version 6.3, Lifeline Software Inc., Austin TX) and an IMRT QA measurement using ArcCheck MR (Sun Nuclear Corporation, Melbourne FL). We also performed an offline plan adaptation test using the MR images acquired during MR simulation. This test was used to check the end-to-end plan-adaptation workflow and determine the MR sequence for plan adaptation.
2.4. Online plan adaptation and treatment delivery
Patient setup on the MR-Linac couch was based on an index value recorded at simulation to determine the patient’s longitudinal position. No external lasers were present inside the MR-Linac room; rather, therapists relied on an internal sagittal laser and leveling marks on the patients’ skin to determine the lateral position and minimize body rotation. A T2-weighted MR image was acquired for plan adaptation. This MR image was then rigidly registered to the reference CT to determine the shift of the treatment isocenter. Then, the isocenter and all contours on the reference CT were shifted to a new location on the CT scan based on the registration, and the original treatment plan was re-optimized to meet the dosimetric criteria set forth in the reference plan. This plan adaptation approach, known as “Adapt to Position” (ATP), was used for all fractions delivered to all four patients.
All adaptive plans had an independent MU check using RadCalc before beam delivery. While the beam was on, motion was monitored in real-time by using three orthogonal cine MR images centered at the prostate. After treatment, an IMRT QA measurement of the adaptive plan was obtained for a further quality check.
2.5. Dose accumulation and evaluation
After treatment was finished for each patient, we evaluated the accumulated dose of the adaptive plans as follows. Because the ATP plan dose was calculated on the reference CT, the fraction dose of each ATP plan was added together to create a summed ATP plan, which represented the planned delivered dose. In parallel evaluations, we shifted each ATP plan to the corresponding MR image, and deformed the fraction dose back to the reference CT image for accumulation by using an in-house deformable registration tool [15], [16]. The dose deformation was based on the deformable registration between the daily MR scan and the simulation MR scan (Fig. 1). The accumulated deformed dose approximated the actual delivered dose by accounting for daily anatomic variations and was compared with the summed ATP dose to evaluate the accuracy of the ATP approach.
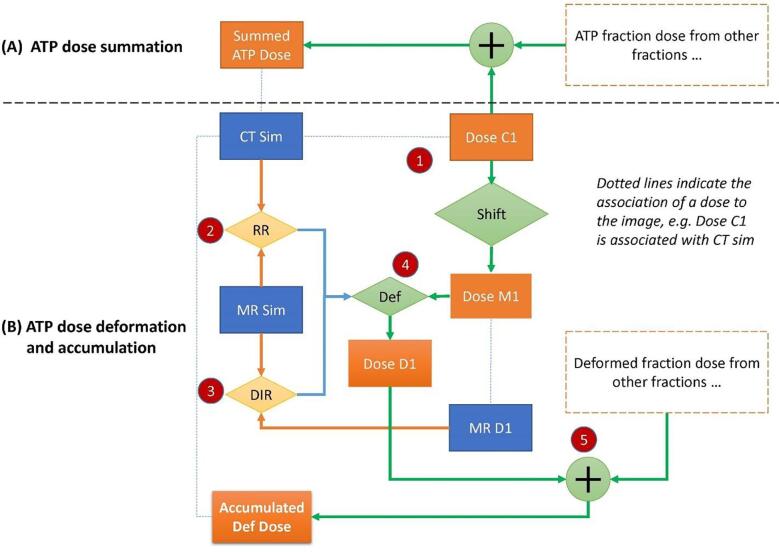
Fig. 1.
Illustration of dose accumulation in adapt-to-position (ATP) plans. “Dose C1” is the fraction dose of one ATP plan. (A) Direct summation of fraction doses of all ATP plans creates the summed ATP dose, representing the planned delivered dose. (B) To generate the accumulated deformed dose, the “Dose C1” is first shifted to the corresponding daily MR space “MR D1” by using the isoshift obtained during treatment. Then rigid registration (RR) between “CT sim” and “MR sim” and deformable image registration (DIR) between “MR D1” and “MR sim” are combined to deform “Dose M1” to “CT sim” space for accumulation, creating the accumulated deformed dose.
3. Results
3.1. Adaptive plan and treatment delivery
All MR-Linac treatments were successfully delivered. The average treatment time per fraction was 38.3 ± 8.6 min (range 26–65 min) and included patient setup, MR acquisition, online plan adaptation, and beam delivery (Supplementary Table S2). The adaptive plans achieved all of the dosimetric goals set in the reference plans and were consistent with reference plan. Fig. 2 shows one example of the adaptive plan dose overlaid on the daily MR image and compared with the reference plan dose. We observed that the location of the rectal spacer and the patient anatomy could change slightly from fraction to fraction. Although real-time motion monitoring visualized the motion of the prostate and the extent of bladder filling during treatment, the prostate was in all fractions within the prescribed isodose line, while the bladder filling increased the bladder volume towards the opposite side of the prostate.
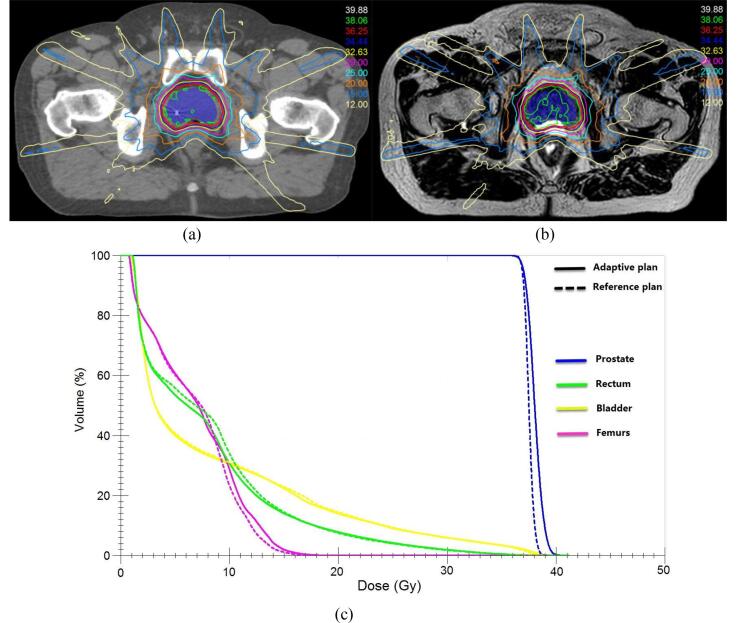
Fig. 2.
Illustration of an online adaptive plan created during patient treatment. (a) The reference plan overlaid on the CT image. (b) The adaptive plan for the 5th fraction of the treatment overlaid on the daily MR image. (c) The dose-volume histogram (DVH) comparison between the reference plan (dashed lines) and the adaptive plan (solid lines).
3.2. Dose accumulation
The accumulated deformed dose was consistent with the summed ATP dose in comparisons of their dose-volume histograms (Supplementary Fig. S1). Using the mean dose for evaluation (Supplementary Table S3), the difference in prostate dose was within 0.5% for all four patients. The rectum and bladder mean doses were low and the absolute dose difference was small (<0.5 Gy), with the exception of one patient who received a higher rectum dose (actual 15.1 Gy vs. planned 11.2 Gy) and another who received a higher bladder dose (actual 10.3 Gy vs. planned 8.5 Gy). The rectal spacer effectively pushed the rectum away from the high-dose region so that the rectum did not receive a substantial dose, and daily anatomic variations of the rectum had little dosimetric effect. The dose to the SVs exhibited the largest differences between fractions (Supplementary Table S3). The accumulated deformed dose met the clinical criteria for the target and all OARs.
4. Discussion
We presented our clinical experience with prostate SBRT delivered with a 1.5 Tesla MR-Linac. The major advantage of this type of treatment is the excellent soft-tissue visualization enabled by MRI during treatment. Use of a rectal spacer in combination with the MR images greatly enhanced our confidence in simultaneously delivering high doses to the target and sparing the rectum. Also, the use of motion monitoring helped to understand the intrafraction changes in bladder volume and assured us that these changes had minimal dosimetric effects.
All four patients tolerated the treatment well, without any grade 2 or higher gastrointestinal (GI) or genitourinary (GU) toxicity (per the modified Radiation Therapy Oncology Group assessment system) during treatment. At a median follow-up interval of 5 months, all four patients were doing well, with no GI or GU toxicity and no evidence of recurrence. We do acknowledge that this follow-up interval has been very short and additional follow-up will be needed to assess the long-term effects of the treatment.
The overall time spent for each fraction delivered with a 1.5 Tesla MR-Linac was comparable to that for prostate SBRT on regular linacs [17]. However, interruptions to the integrated workflow of the MR-Linac can considerably extend the time needed for delivery of a fraction. Moreover, a 1.5 Tesla MR-Linac also provides diffusion and perfusion MRI sequences, which are not available on a 0.35 Tesla MR-Linac [18]. Although a 0.35 Tesla MR-Linac provides gated dose delivery for motion management, which is not currently available in the 1.5 Tesla MR-Linac system, prostate SBRT does not benefit from this functionality because intra-fraction prostate motion may not occur frequently.
Dose accumulation calculated from daily adaptive plans has been recognized as an important tool for evaluating treatment effects with the MR-Linac, particularly with regard to the overall dosimetric consequences to OARs [12], [13], [14]. However, this tool has not yet been used to inform the choice of a plan adaptation strategy, either prospectively and retrospectively. In addition to the ATP workflow, the 1.5 Tesla MR-Linac system also offers an “Adapt to Shape” (ATS) workflow, which includes contour deformation from the reference CT to the daily MR image, with plan reoptimization based on the updated anatomy from the MR image. The advantage of ATS is in the use of daily assessments of anatomic changes for adaptive planning, which could create more accurate plans. However, the ATS approach requires additional time for contouring verification, which often is substantial. To evaluate whether the ATS approach is needed, we compared accumulated deformed dose with the summed ATP dose to evaluate the dosimetric impact from anatomic variations, thereby avoiding the need to recontour targets and OARs for the evaluation [19]. Our accumulated dose study showed that the ATP plan dose was close to the estimated actual delivered dose, implying that daily changes in anatomy have little effect on the treatment.
Notably, one of the challenges in this study is the accuracy of dose deformation, which relies on the accuracy of the deformable registration algorithm. Significant anatomic changes can affect the accuracy of deformable registration, as has been seen in contour propagation during online plan adaptation [14], [20]. In this study, we used an in-house deformable registration tool [16] that has been validated for MR image registration, and the registration results were visually checked for accuracy.
In conclusion, we found that ATP is feasible for prostate SBRT in terms of the need to balance treatment time and dose accuracy. However, we also found that the SVs could move between fractions, which would mean that the ATP approach may not be good enough for prostate SBRT if the SVs are included as a treatment target.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
This paper is part of a special issue that contains contributions originally submitted to the scientific meeting MR in RT, which was planned to take place 05/2020, organized by the German Research Center (DKFZ) in Heidelberg. We acknowledge funding by DKFZ for the publication costs of this special issue. The authors would like to thank Christine F Wogan from Department of Radiation Oncology for reviewing this manuscript.
Funding
This work was support in part by the National Institutes of Health through Cancer Center Support Grant P30CA016672.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2020.12.001.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Mottet N., Bellmunt J., Bolla M., Briers E., Cumberbatch M.G., De Santis M. EAU-ESTRO-SIOG guidelines on prostate cancer. Part 1: screening, diagnosis, and local treatment with curative intent. Eur Urol. 2017;71:618–629. doi: 10.1016/j.eururo.2016.08.003. [DOI] [PubMed] [Google Scholar]
- 2.Pathmanathan A.U., Schmidt M.A., Brand D.H., Kousi E., van As N.J., Tree A.C. Improving fiducial and prostate capsule visualization for radiotherapy planning using MRI. J Appl Clin Med Phys. 2019;20:27–36. doi: 10.1002/acm2.12529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Murray J., Tree A.C. Prostate cancer – advantages and disadvantages of MR-guided RT. Clin Transl Radiat Oncol. 2019;18:68–73. doi: 10.1016/j.ctro.2019.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Winkel D., Bol G.H., Kroon P.S., van Asselen B., Hackett S.S., Werensteijn-Honingh A.M. Adaptive radiotherapy: the Elekta Unity MR-linac concept. Clin Transl Radiat Oncol. 2019;18:54–59. doi: 10.1016/j.ctro.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Raaymakers B.W., Lagendijk J.J.W., Overweg J., Kok J.G.M., Raaijmakers A.J.E., Kerkhof E.M. Integrating a 1.5 T MRI scanner with a 6 MV accelerator: proof of concept. Phys Med Biol. 2009;54:N229–N237. doi: 10.1088/0031-9155/54/12/N01. [DOI] [PubMed] [Google Scholar]
- 6.Tetar S.U., Bruynzeel A.M.E., Lagerwaard F.J., Slotman B.J., Bohoudi O., Palacios M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imaging Radiat Oncol. 2019;9:69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Corradini S., Alongi F., Andratschke N., Belka C., Boldrini L., Cellini F. MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiat Oncol. 2019;14:92. doi: 10.1186/s13014-019-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pathmanathan A.U., van As N.J., Kerkmeijer L.G.W., Christodouleas J., Lawton C.A.F., Vesprini D. Magnetic resonance imaging-guided adaptive radiation therapy: a “Game Changer” for prostate treatment? Int J Radiat Oncol Biol Phys. 2018;100:361–373. doi: 10.1016/j.ijrobp.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 9.Werensteijn-Honingh A.M., Kroon P.S., Winkel D., Aalbers E.M., van Asselen B., Bol G.H. Feasibility of stereotactic radiotherapy using a 1.5 T MR-linac: multi-fraction treatment of pelvic lymph node oligometastases. Radiother Oncol. 2019;134:50–54. doi: 10.1016/j.radonc.2019.01.024. [DOI] [PubMed] [Google Scholar]
- 10.Winkel D., Bol G.H., Werensteijn-Honingh A.M., Kiekebosch I.H., van Asselen B., Intven M.P.W. Evaluation of plan adaptation strategies for stereotactic radiotherapy of lymph node oligometastases using online magnetic resonance image guidance. Phys Imaging Radiat Oncol. 2019;9:58–64. doi: 10.1016/j.phro.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bertelsen A.S., Schytte T., Møller P.K., Mahmood F., Riis H.L., Gottlieb K.L. First clinical experiences with a high field 1.5 T MR linac. Acta Oncol. 2019;58:1352–1357. doi: 10.1080/0284186X.2019.1627417. [DOI] [PubMed] [Google Scholar]
- 12.Alongi F., Rigo M., Figlia V., Cuccia F., Giaj-Levra N., Nicosia L. 1.5 T MR-guided and daily adapted SBRT for prostate cancer: feasibility, preliminary clinical tolerability, quality of life and patient-reported outcomes during treatment. Radiat Oncol. 2020;15:69. doi: 10.1186/s13014-020-01510-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dunlop A., Mitchell A., Tree A., Barnes H., Bower L., Chick J. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Transl Radiat Oncol. 2020;23:35–42. doi: 10.1016/j.ctro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mannerberg A., Persson E., Jonsson J., Gustafsson C.J., Gunnlaugsson A., Olsson L.E. Dosimetric effects of adaptive prostate cancer radiotherapy in an MR-linac workflow. Radiat Oncol. 2020;15:168. doi: 10.1186/s13014-020-01604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang H., Dong L., Lii M.F., Lee A.L., de Crevoisier R., Mohan R. Implementation and validation of a three-dimensional deformable registration algorithm for targeted prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2005;61:725–735. doi: 10.1016/j.ijrobp.2004.07.677. [DOI] [PubMed] [Google Scholar]
- 16.Ger R.B., Yang J., Ding Y., Jacobsen M.C., Fuller C.D., Howell R.M. Accuracy of deformable image registration on magnetic resonance images in digital and physical phantoms. Med Phys. 2017;44:5153–5161. doi: 10.1002/mp.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miralbell R. Stereotactic body radiotherapy for prostate cancer: treatment approaches and clinical outcomes. J Radiosurg SBRT. 2011;1:147–154. [PMC free article] [PubMed] [Google Scholar]
- 18.Bruynzeel A.M.E., Tetar S.U., Oei S.S., Senan S., Haasbeek C.J.A., Spoelstra F.O.B. A Prospective single-arm phase 2 study of stereotactic magnetic resonance guided adaptive radiation therapy for prostate cancer: early toxicity results. Int J Radiat Oncol Biol Phys. 2019;105:1086–1094. doi: 10.1016/j.ijrobp.2019.08.007. [DOI] [PubMed] [Google Scholar]
- 19.Winkel D., Werensteijn-Honingh A.M., Kroon P.S., Eppinga W.S.C., Bol G.H., Intven M.P.W. Individual lymph nodes: “See it and Zap it”. Clin Transl Radiat Oncol. 2019;18:46–53. doi: 10.1016/j.ctro.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Christiansen R.L., Dysager L., Bertelsen A.S., Hansen O., Brink C., Bernchou U. Accuracy of automatic deformable structure propagation for high-field MRI guided prostate radiotherapy. Radiat Oncol. 2020;15:32. doi: 10.1186/s13014-020-1482-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.