Abstract
Background and purpose
Studies have demonstrated the potential of online adaptive radiotherapy (oART). However, routine use has been limited due to resource demanding solutions. This study reports on experiences with oART in the pelvic region using a novel cone-beam computed tomography (CBCT)-based, artificial intelligence (AI)-driven solution.
Material and methods
Automated pre-treatment planning for thirty-nine pelvic cases (bladder, rectum, anal, and prostate), and one hundred oART simulations were conducted in a pre-clinical release of Ethos (Varian Medical Systems, Palo Alto, CA). Plan quality, AI-segmentation accuracy, oART feasibility and an integrated calculation-based quality assurance solution were evaluated. Experiences from the first five clinical oART patients (three bladder, one rectum and one sarcoma) are reported.
Results
Auto-generated pre-treatment plans demonstrated similar planning target volume (PTV) coverage and organs at risk doses, compared to institution reference. More than 75% of AI-segmentations during simulated oART required none or minor editing and the adapted plan was superior in 88% of cases. Limitations in AI-segmentation correlated to cases where AI model training was lacking. The five first treated patients complied well with the median adaptive procedure duration of 17.6 min (from CBCT acceptance to treatment delivery start). The treated bladder patients demonstrated a 42% median primary PTV reduction, indicating a 24%-30% reduction in V45Gy to the bowel cavity, compared to non-ART.
Conclusions
A novel commercial oART solution was demonstrated feasible for various pelvic sites. Clinically acceptable AI-segmentation and auto-planning enabled adaptation within reasonable timeslots. Possibilities for reduced PTVs observed for bladder cancer indicated potential for toxicity reductions.
Keywords: Online Adaptive Radiotherapy (oART), CBCT image-guided radiotherapy, Artificial Intelligence, Automated treatment planning, Bladder cancer, Workflow
1. Introduction
While offline adaptive radiotherapy (ART) is sufficient for a range of disease sites, inter-fractional anatomical variations in the pelvic area are not always predictable, indicating the need for online ART (oART) [1], [2]. Several oART approaches have previously been investigated, including selection from a library of plans based on cone-beam computed tomography (CBCT) scans [3], [4], [5], [6], [7], [8]. Such strategies all support the sparing of organs at risk (OAR) compared to non-ART strategies. Conducting full re-optimization on the anatomy of the day has furthermore been demonstrated superior to plan selection [9], [10]. However, the clinical use of online re-optimization has been limited due to the cumbersome re-delineation and treatment planning required within a short time frame. Therefore, improvements in deformable image registration, auto-segmentation, auto-planning, and quality assurance (QA) procedures are required. Commercially available oART solutions with full re-optimization, previously demonstrated to be feasible within a clinical setting, have mainly consisted of magnetic resonance (MR) guided oART approaches [11], [12], [13]. These systems have benefits of superior soft tissue contrast and real-time image guidance, but also typically require additional time and resources. Therefore, a fast CBCT-based approach would be interesting for routine practice, especially in anatomical sites with expected inter- and intra-fractional variations and with targets visible on CBCT (e.g. bladder).
One major contributor to the rapid development of oART has been the increased use of artificial intelligence (AI) [14]. Applications of AI in this field include automated organ segmentation [15], [16], [17], the use of synthetic image data [18], [19] as well as automated treatment planning [20], [21], [22]. The purpose of this project was to describe the clinical implementation of a commercial solution for CBCT-guided and AI-driven oART. The aim was to evaluate the feasibility, time-efficiency, and potential clinical impact of the solution for a range of common disease sites in the pelvic area, and to report on the first five patients treated with oART using this system.
2. Material and methods
The Ethos therapy solution for AI-driven CBCT-based daily oART (Varian Medical Systems, Palo Alto, CA) was installed in our department in August 2019. This system applies an AI-algorithm based on convolutional neural networks for the detection of daily anatomy. A technical description of the system has been provided elsewhere [23] and an additional brief explanation is provided in Appendix A. Supplementary material. Prior to clinical implementation, staff was trained on a pre-clinical release, equipped with an emulator for simulated oART. Training was focused on patients with targets in the pelvic and abdominal region. Pre-treatment planning procedures, online plan re-optimization and the treatment workflow were evaluated and optimized. Furthermore, an integrated solution for calculation-based plan QA was assessed. Experiences from the first five patients treated after clinical implementation were reported on. All patients provided informed written consent and treatments were delivered under institutional approval.
2.1. Pre-treatment plan generation
Retrospective pre-treatment data was collected for thirty-nine pelvic patients with included pelvic lymph nodes (eight bladder, eight prostate, nine rectum, and fourteen anus cancer), selected from the most recent clinical cases at our institution between January 2018 and July 2019. All patients had CT simulations using a 2 mm slice thickness (16 slice Philips Brilliance CT Big Bore scanner, Philips Medical Systems, Cleveland, OH) with contouring and treatment planning in accordance with local clinical protocols (Appendix A. Table S 1) in Eclipse (v.15.6, Varian Medial systems) treatment planning system (TPS), using the Anisotropic-Analytical-Algorithm (AAA) for dose calculation. Automated pre-treatment planning for non-ART was retrospectively conducted in a pre-clinical release of the Ethos TPS. For each patient, three intensity-modulated radiotherapy (IMRT) plans (seven, nine, and twelve fields at system-defined beam angles), two volumetric modulated arc therapy (VMAT) plans (two and three arcs), as well as one re-calculated and one re-optimized plan, using the beam configuration of the original clinical plan, were generated. Dose-to-medium was calculated using the AcurosXB algorithm with 2.5 mm resolution and an angular resolution determined from control points (2 degrees for a full arc). Thus, eight plans per patient and a total of 312 plans were generated for comparison. Plans were normalized to achieve a mean dose of the high-dose planning target volume (PTV) equal to the prescribed dose. Plan evaluation was based on fulfillment of standard constraints on clinical target volume (CTV) and PTV coverage, and dose to OAR (Appendix A. Table S 1). Furthermore, an overall modulation factor (MU/Gy), the high-dose PTV conformity index (CI) and homogeneity index (HI) were evaluated. The CI was derived as the ratio between the volume receiving at least 98% (V98%) of the prescribed dose and the total high-dose PTV (VPTV), according to CI = V98%/VPTV [24]. The HI was derived as HI=(D2%-D98%)/D50%, where D2%, D98% and D50%, were the doses received by 2%, 98% and 50% of the high-dose PTV, respectively [25].
2.2. Simulated online adaptive radiotherapy
Twenty-six patients (eight bladder, eight prostate, six rectum, four anus) were selected for simulated oART, from top-to-bottom in the list of patients included in the pre-treatment plan evaluation. The number of patients for each disease site were decided based on training needs before clinical implementation. A total of one hundred (forty-seven bladder, thirty-six prostate, thirteen rectum, four anus) adaptive sessions were simulated, using an emulator of the oART module. Reduced margins were applied in nineteen simulated oART sessions for bladder cancer patients, while standard non-ART margins were used for the remaining fractions and disease sites (Appendix A. Table S 1). The simulated oART sessions were based on retrospectively collected CBCT images (from the first fraction and, for patients with more than one simulation, every fifth clinical treatment fraction). The AI-segmented influencers (system-defined organs adjacent to and with high impact on the deformation of CTVs and OAR from the CT to the CBCT geometry) were reviewed and the amount of editing required for clinically acceptable delineations was qualitatively scored. Edits were classified as minor if adjustments were required on few CT slices (approximately < 10% of slices with organ present), intermediate if many slices required adjustments or if few slices required larger edits, and major if many slices required larger edits or if the structure needed to be cleared and manually delineated. Similarly, the propagated CTVs were reviewed and scored. Finally, the adapted plan (re-optimization of the pre-treatment plan on the anatomy of the day), was evaluated and compared to the scheduled plan (re-calculation of the pre-treatment plan on the anatomy of the day with isocenter optimization based on maximized PTV coverage). Plan comparisons were carried out by a single medical physicist (LMA) and was based on the fulfillment of clinical goals (CTV/PTV coverage and dose to OAR) and plan complexity (MU/Gy).
2.3. Patient-specific QA
Thirty-two pre-treatment generated plans (nine and twelve-field IMRT, two and three-arc VMAT) for the first four bladder and four rectum patients from the pre-treatment plan generation investigation, were evaluated using an integrated independent dose calculation QA tool (M3D) (Mobius3D version 2.2, Varian Medical Systems). Corresponding plans were measured using the Delta4+ (D4) phantom (ScandiDos AB, Uppsala, Sweden) as well as portal dosimetry (PD). Resulting local gamma passing rates were compared using institutional standard gamma criteria (M3D: 3%/3 mm, 10% threshold; D4: 3%/2 mm, 10% threshold; PD: 3%/2 mm, 20% threshold).
2.4. First clinical adaptive treatments
The first five patients treated on the AI-driven and CBCT-based oART system consisted of three patients with bladder cancer, one with rectum cancer, and one with a gastro-intestinal stromal tumor (GIST) located between rectum and vagina. The twenty oART sessions conducted for these patients were evaluated in terms of treatment duration, structure editing, primary PTV volume changes, and fulfillment of dose constraints.
2.5. Statistical analysis
The extracted data parameters were evaluated in Matlab (R2019b, The MathWorks, Inc.). A median value together with the interquartile range (IQR) or a mean value together with one standard deviation (SD) were extracted for data samples not belonging to or belonging to a normal distribution (Lilliefors test), respectively. Wilcoxon rank sum tests were used to test whether two samples came from distributions with equal medians, at a statistical significance level of 5%.
3. Results
3.1. Pre-treatment plan generation
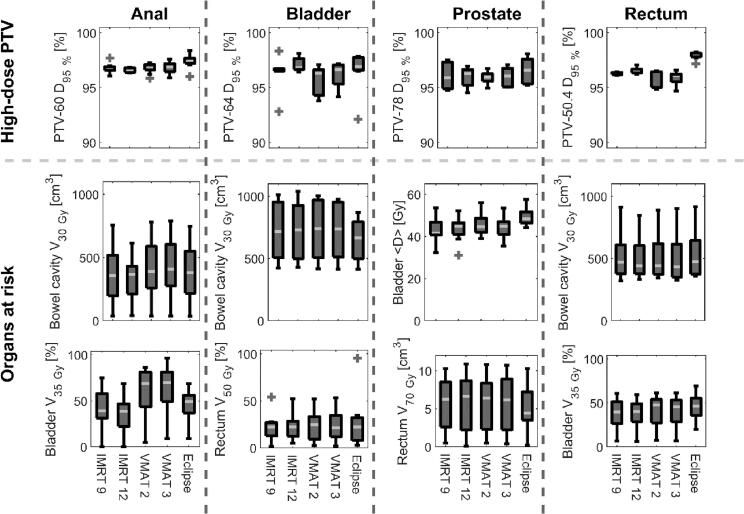
The automated TPS achieved PTV coverage, and doses to OAR, similar to the clinical plans (greater than95% VMAT) (Fig. 1). The median doses to 95% of the high-dose PTV (D95%) achieved with the automated TPS (all IMRT and VMAT plans) and the previous clinical standard TPS were 96.5% and 97.5%, respectively (p < 0.001). The plan quality indices were comparable between the two TPS, with the exceptions of an inferior HI for auto-generated VMAT plans and a superior CI for manually generated VMAT plans (Table 1). The higher MU/Gy for the auto-generated compared to the manually generated VMAT plans was an additional indication on the plan generation differences. For all cases in this study, the auto-generated IMRT plans (either nine or twelve fields) fulfilled more clinical goals than corresponding auto-generated VMAT plans and were therefore preferred as adaptive reference plans. The disease specific constraints on PTV coverage and doses to OAR were for these IMRT plans comparable to corresponding manually generated clinical VMAT plans (Table 2).
Fig. 1.
Comparison of clinically relevant dose parameters for automatically generated treatments plans (9 and 12 field IMRT as well as 2 and 3 arc VMAT) and plans manually generated in the previous clinical TPS for anal cancer (n = 14), rectum cancer (n = 9), prostate cancer (n = 8), and bladder cancer (n = 7) patients. The inner line denotes the median value, the box the interquartile range, the whiskers the 9th and 91st percentile, with the outliers presented as single markers.
Table 1.
Comparison of homogeneity index (HI), conformity index (CI), and the modulation factor (MU/Gy) of IMRT and VMAT plans generated in automated TPS and the previous clinical standard TPS. Results presented as average values and corresponding standard deviation (k = 1).
|
Automated TPS |
Standard TPS |
|||
|---|---|---|---|---|
| IMRT | VMAT | IMRT | VMAT | |
| HI | 0.08 ± 0.01 | 0.10 ± 0.05 | 0.06 ± 0.01 | 0.07 ± 0.03 |
| CI | 0.83 ± 0.06 | 0.82 ± 0.04 | 0.83 ± 0.05 | 0.90 ± 0.03 |
| MU/Gy | 1054 ± 320 | 365 ± 31 | 1068 ± 135 | 276 ± 23 |
Table 2.
Disease site specific relevant dose parameters for high-dose PTV coverage and doses to OAR, comparing the selected best auto-generated plan with corresponding manually created clinical reference plan. Results are presented as the average value together with one standard deviation.
| Disease site | Target / OAR | Constraint | Best auto-TPS plan | Clinical reference plan |
|---|---|---|---|---|
| Bladder | PTV | D99% [Gy] | 61.2 ± 0.4 | 61.0 ± 2.1 |
| Rectum | V50 Gy [%] | 22.6 ± 14.3 | 28.4 ± 28.5 | |
| Bowel cavity | V30 Gy [cm3] | 731.0 ± 14.9 | 644.5 ± 22.0 | |
| Prostate | PTV | D95% [Gy] | 74.9 ± 0.9 | 75.3 ± 0.8 |
| Bladder | Dmean [Gy] | 43.0 ± 5.8 | 48.9 ± 4.4 | |
| Rectum | V70 Gy [%] | 5.7 ± 3.3 | 5.8 ± 3.6 | |
| Rectum | PTV | V95% [%] | 98.6 ± 3.3 | 99.9 ± 0.1 |
| Bladder | V35 Gy [%] | 37.3 ± 17.5 | 43.7 ± 14.5 | |
| Bowel cavity | V30 Gy [cm3] | 513.7 ± 180.0 | 531.2 ± 176.0 | |
| Anal | PTV | V95% [%] | 99.7 ± 0.1 | 99.3 ± 0.6 |
| Bladder | V35 Gy [%] | 36.9 ± 19.7 | 43.0 ± 17.0 | |
| Bowel cavity | V30 Gy [cm3] | 344.9 ± 165.6 | 394.1 ± 207.7 |
The template-based automated treatment planning required one iteration of plan generation for cases with a single dose level to achieve clinically acceptable dose distributions with fulfillment of clinical goals, i.e. no additional revisions by adding or changing priorities of clinical goals followed by a new round of optimization were needed. However, cases with two dose levels delivered as simultaneously integrated boost required one to two additional revisions and the most complex cases, e.g. large anal cancer targets with two or more dose levels, required two to four revisions.
3.2. Simulated online adaptive radiotherapy
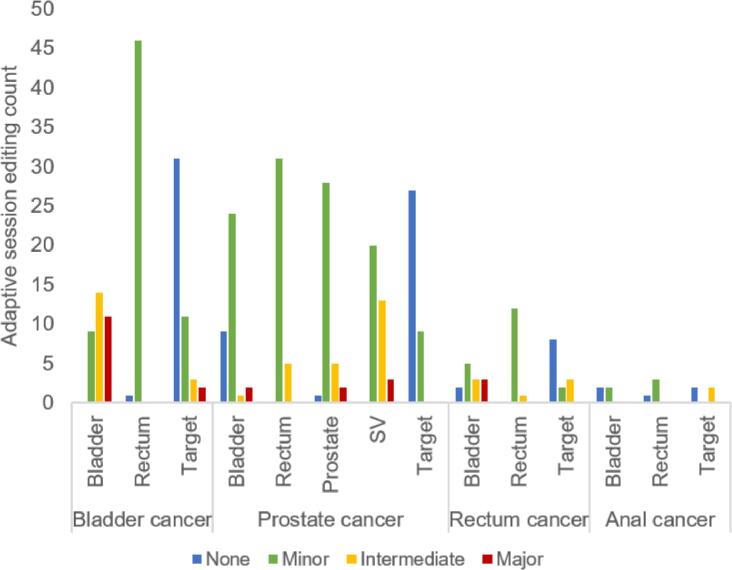
The AI-segmented influencers required none or minor editing in 76% out of the 259 influencers, while the corresponding value for the 100 propagated CTVs (primary and elective combined) was 90% (Fig. 2). Limitations in AI-driven auto-segmentation were correlated to cases where the applied version of the system was not yet trained, e.g. bladder patients with urinary catheter. Spending additional time on ensuring optimal influencer delineations increased the clinical acceptability of all propagated CTVs and OAR (including but not limited to those already defined as influencers) (Fig. 2).
Fig. 2.
Results of the qualitative assessment of AI-generated influencers (SV denotes the seminal vesicles) and subsequentially propagated clinical target volumes (Target) for 47, 36, 13 and 4 simulated adaptive sessions on 8 bladder, 8 prostate, 6 rectum and 4 anal cancer patients, respectively. Presented as a histogram over the extent of editing required to reach a clinically acceptable structure definition.
Re-optimizing the treatment plan to the anatomy of the day resulted in the adapted plan being selected in 88% of the simulated treatment sessions (98%, 83%, 67% and 100% for bladder, prostate, rectum and anal cases, respectively). The main deciding factor for most cases was CTV and PTV coverage (60%) or a combination of target coverage and OAR sparing (30%), while solely OAR sparing was the driving factor in only a few cases (3%). The remaining decisions were based on plan complexity, with e.g. one case with two times greater MU/Gy factor in the adapted compared to the scheduled plan.
3.3. Patient-specific QA
Overall, both calculation- and measurement-based QA demonstrated good agreements with dose calculations by the automated TPS, with all plans acceptable for treatment delivery independent of QA method. The M3D dose calculations resulted in local gamma passing rates above 97% for all cases, with a median of 99.2% (IQR = 1.2%). These results compared well with corresponding median passing rates of 98.4% (IQR = 1.4%) and 99.9% (IQR = 0.3%) for D4 and PD, respectively (Table 3).
Table 3.
Average gamma passing rates (±1 standard deviation) for plan-specific QA of auto-generated treatment plans (n = 12 per plan type) all evaluated using both Delta4 phantom (D4), portal dosimetry (PD), and Mobius3D (M3D).
| Bladder |
Rectum |
|||||
|---|---|---|---|---|---|---|
| D4 | PD | M3D | D4 | PD | M3D | |
| 9 field IMRT | 98.3 ± 0.9 | 99.9 ± 0.0 | 99.5 ± 0.3 | 96.9 ± 0.7 | 100.0 ± 0.0 | 98.8 ± 0.6 |
| 12 field IMRT | 97.3 ± 1.2 | 99.9 ± 0.1 | 98.6 ± 1.0 | 97.4 ± 1.0 | 100.0 ± 0.0 | 98.5 ± 0.8 |
| 2 arc VMAT | 98.7 ± 0.4 | 99.7 ± 0.1 | 98.7 ± 0.9 | 98.5 ± 0.5 | 99.6 ± 0.2 | 100.0 ± 0.0 |
| 3 arc VMAT | 98.9 ± 0.6 | 99.9 ± 0.1 | 97.8 ± 1.4 | 98.7 ± 0.3 | 99.7 ± 0.2 | 100.0 ± 0.0 |
| ALL | 98.3 ± 1.0 | 99.9 ± 0.1 | 98.6 ± 1.1 | 97.9 ± 1.0 | 99.8 ± 0.2 | 99.3 ± 0.8 |
3.4. First clinical adaptive treatments
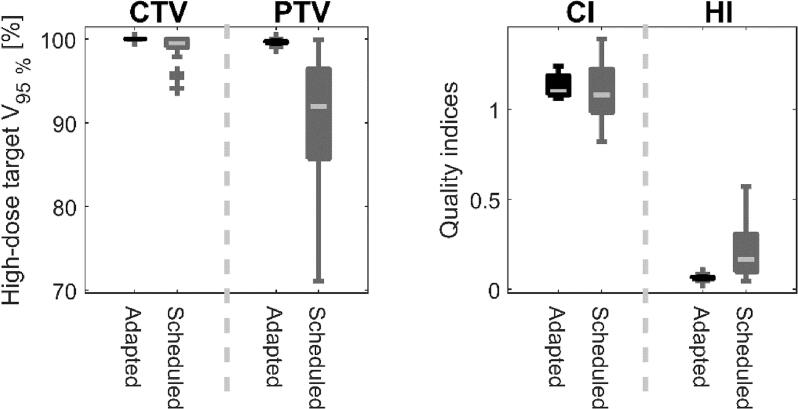
All patients complied well with the online adaptive procedure, which took a median of 17.6 min (IQR = 4.0 min) (from acceptance of acquired CBCT to start of treatment delivery) for the twenty first delivered fractions. The AI-driven influencer segmentation required approximately 3–6 min and review of propagated CTVs was carried out within an additional 1–3 min. The remaining time was used for plan generation and review, plan QA, and occasional extra verifying CBCT acquisitions. The median ratio between the propagated high-dose CTV based on the anatomy of the day and the corresponding reference volume was 1.0 (IQR = 0.3). For the bladder cases, margin reductions resulted in a statistically significant median reduction of the high-dose PTV by 42% (IQR = 19%) when moving from a non-ART to the studied oART approach (p < 0.001). The high-dose PTV V95% dose coverage was reduced to a median of 88.2% (IQR = 9.7%) for the scheduled plans, while regained at a median of 99.6% (IQR = 0.1) for the adapted plans (Fig. 3). The scheduled plans had a corresponding CTV V95% < 98% in 4 out of 15 bladder treatments (minimum 94.1%), while kept at 100% for all adapted plans. The CI and HI provided additional indications of the superiority of the adapted plans (Fig. 3). In terms of potential clinical impact, high-dose PTV volume reductions for bladder cases e.g. indicated a 24% − 30% reduction in V45 Gy to the bowel cavity, compared to non-ART. For the rectum and the GIST patients, the margins were not reduced and no significant changes in PTV volumes or OAR doses were observed. The adapted plans were selected in favor of the scheduled for 15 out of 15, 1 out of 2, and 0 out of 3 oART sessions for the bladder, rectum, and GIST patients, respectively.
Fig. 3.
The boxplots to the left present the high-dose CTV and PTV coverage as the volume receiving more than 95% of the prescribed dose (V95%) achieved by the adapted and scheduled plan during all 20 online treatment sessions for the five patients included in this study. Boxplots to the right presents corresponding quality indices as CI and HI values for adapted and scheduled plans. The inner line denotes the median value, the box the interquartile range, the whiskers the 9th and 91st percentile, with the outliers presented as single markers.
4. Discussion
This study aimed at describing the first clinical implementation of a novel commercial solution for AI-driven and CBCT-based oART in the pelvic region. Data from the use of a pre-clinical version demonstrated feasibility of re-optimization to the anatomy of the day, with deliverable plans of acceptable quality. The early clinical experiences treating the five first patients indicated potential for reduction of margins, and possibly toxicity related dose parameters, with patients complying well with the added time spent on the adaptive procedure.
Automated treatment planning is important for fast oART with full re-optimization to the anatomy of the day [14]. A range of pelvic disease sites were in this study included in a pre-clinical evaluation of the automated TPS, with emphasis on benchmarking the treatment plan quality to our previous standard practice. The system generated IMRT plans of clinically acceptable quality, comparable to our standard best practice for manual plan generation. The automated plan generation potentially minimizes inter-planner variability and reduce time spent on treatment planning for non-ART [20]. However, the current version of the automated TPS is limited by a fixed set of beam configurations and inferior VMAT plan quality. Adding user defined beam configurations would increase flexibility (e.g. to better avoid prothesis or to better optimize beam angles for unilateral treatments) and improved VMAT plan quality would enable faster treatment delivery. A stochastic behavior in the generation of plans was observed, with minor variations in dose distributions for identical settings and prioritization between clinical goals. For complex cases needing one or more revisions with repeated plan optimization, approximately ten to forty minutes was added to the offline pre-treatment plan generation process for each revision.
CBCT-based oART has been demonstrated feasible within reasonable session durations and with clinically acceptable deformation of structures to the anatomy of the day for a range of pelvic cases. Clinical oART sessions from acceptance of acquired CBCT to start of treatment delivery were achievable within a median of 17.6 min, which is competitive to other solutions [26], [27] and also corresponds well with recent findings for simulated use of the present oART system for head and neck cancer patients [28]. With the novel CBCT-guided approach there is a potential to enable oART also for standard-fractionated treatments that so far has not been extensively investigated [12], [29], [30]. However, oART still require solutions for logistical challenges, e.g. minimizing the required presence of physicist and radiation oncologists. Training of RTTs to manage influencer editing is thus crucial and has so far been successful at our institution. Further training of RTTs to manage also CTV and plan review is pending.
Auto-generated organs were observed to require none or only minor editing in more than three out of four cases, with subsequent propagation of CTVs and OAR to a clinically acceptable accuracy. The version of the studied oART system had limitations defining influencers in situations where the AI models had not yet been trained, e.g. patients with urinary catheter or male patients after prostatectomy. The adaptive process for cases requiring manual re-delineation was still considered feasible within reasonable session durations. Despite high quality CBCT imaging, organ definition can be challenging due to the limited soft-tissue contrast, e.g. in the most caudal part of the bladder and prostate. However, meticulous influencer definition minimizes the need to spend time on manual adjustments of propagated CTV. This corresponds well with observations in a recent study on a similar approach for head and neck cancer patients [28]. Editing propagated targets will break the automated treatment planning chain and increase the time spent on the adaptive procedure. This would imply a risk of larger intra-fractional variations invalidating the adaptive margins applied and adding the need for increased margins or additional verifying CBCT imaging prior to treatment delivery. The lack of more continuous intra-fraction monitoring, available with e.g. MR-guided oART systems, is currently a limitation of this CBCT-based approach, especially relevant also for e.g. hypo-fractionated prostate treatments.
As observed in this study, online adaptation and full re-optimization to the anatomy of the day result in selecting the adapted plan over the scheduled in most cases. That is to a large extent due to daily adaptation enabling reduction of margins otherwise necessary to account for inter-fractional variations. For the bladder cases treated with oART in this study, high-dose PTV volumes were reduced with a median of 42% compared to non-ART, correlating well with previous observations for bladder cancer patients [5], [10]. This study indicates that this will impact critical OAR dose parameters and could potentially reduce toxicity.
In conclusion, a novel commercial oART solution was demonstrated feasible for use in the pelvic region. CBCT-based AI-segmentation and automated treatment planning of clinically acceptable quality enabled online adaptation within reasonable session durations. Possibilities for reduced treatment volumes were observed for bladder cancer patients, indicating a potential for toxicity reductions.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Sune K. Buhl is acknowledged for technical support in setting up both the pre-clinical and clinical solution of the oART system. Geoffrey Hugo and Jeremy Booth, as representatives of the international Adaptive Intelligence Consortium, are acknowledged for their support during commissioning and contributions to scientific discussions.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2020.12.004.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Collins S.D., Leech M.M. A review of plan library approaches in adaptive radiotherapy of bladder cancer. Acta Oncol. 2018;57(5):566–573. doi: 10.1080/0284186X.2017.1420908. [DOI] [PubMed] [Google Scholar]
- 2.Green O.L., Henke L.E., Hugo G.D. Practical Clinical Workflows for Online and Offline Adaptive Radiation Therapy. Semin Radiat Oncol. 2019;29(3):219–227. doi: 10.1016/j.semradonc.2019.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ahmad R., Bondar L., Voet P., Mens J.-W., Quint S., Dhawtal G. A margin-of-the-day online adaptive intensity-modulated radiotherapy strategy for cervical cancer provides superior treatment accuracy compared to clinically recommended margins: A dosimetric evaluation. Acta Oncol. 2013;52(7):1430–1436. doi: 10.3109/0284186X.2013.813640. [DOI] [PubMed] [Google Scholar]
- 4.Burridge N., Amer A., Marchant T., Sykes J., Stratford J., Henry A. Online adaptive radiotherapy of the bladder: Small bowel irradiated-volume reduction. Int J Radiat Oncol Biol Phys. 2006;66(3):892–897. doi: 10.1016/j.ijrobp.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Vestergaard A., Muren L.P., Lindberg H., Jakobsen K.L., Petersen J.B.B., Elstrøm U.V. Normal tissue sparing in a phase II trial on daily adaptive plan selection in radiotherapy for urinary bladder cancer. Acta Oncol. 2014;53(8):997–1004. doi: 10.3109/0284186X.2014.928419. [DOI] [PubMed] [Google Scholar]
- 6.Bondar M.L., Hoogeman M.S., Mens J.W., Quint S., Ahmad R., Dhawtal G. Individualized Nonadaptive and Online-Adaptive Intensity-Modulated Radiotherapy Treatment Strategies for Cervical Cancer Patients Based on Pretreatment Acquired Variable Bladder Filling Computed Tomography Scans. Int J Radiat Oncol Biol Phys. 2012;83(5):1617–1623. doi: 10.1016/j.ijrobp.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Foroudi F., Wong J., Kron T., Rolfo A., Haworth A., Roxby P. Online Adaptive Radiotherapy for Muscle-Invasive Bladder Cancer: Results of a Pilot Study. Int J Radiat Oncol Biol Phys. 2011;81(3):765–771. doi: 10.1016/j.ijrobp.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 8.Foroudi F., Pham D., Rolfo A., Bressel M., Tang C.I., Tan A. The outcome of a multi-centre feasibility study of online adaptive radiotherapy for muscle-invasive bladder cancer TROG 10.01 BOLART. Radiother Oncol. 2014;111(2):316–320. doi: 10.1016/j.radonc.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 9.Ahunbay E.E., Peng C., Holmes S., Godley A., Lawton C., Li X.A. Online Adaptive Replanning Method for Prostate Radiotherapy. Int J Radiat Oncol Biol Phys. 2010;77(5):1561–1572. doi: 10.1016/j.ijrobp.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 10.Vestergaard A., Muren L.P., Søndergaard J., Elstrøm U.V., Høyer M., Petersen J.B. Adaptive plan selection vs. re-optimisation in radiotherapy for bladder cancer: A dose accumulation comparison. Radiother Oncol. 2013;109(3):457–462. doi: 10.1016/j.radonc.2013.08.045. [DOI] [PubMed] [Google Scholar]
- 11.Acharya S., Fischer-Valuck B.W., Kashani R., Parikh P., Yang D., Zhao T. Online Magnetic Resonance Image Guided Adaptive Radiation Therapy: First Clinical Applications. Int J Radiat Oncol Biol Phys. 2016;94(2):394–403. doi: 10.1016/j.ijrobp.2015.10.015. [DOI] [PubMed] [Google Scholar]
- 12.Henke L., Kashani R., Yang D., Zhao T., Green O., Olsen L. Simulated Online Adaptive Magnetic Resonance–Guided Stereotactic Body Radiation Therapy for the Treatment of Oligometastatic Disease of the Abdomen and Central Thorax: Characterization of Potential Advantages. Int J Radiat Oncol Biol Phys. 2016;96(5):1078–1086. doi: 10.1016/j.ijrobp.2016.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Henke L., Kashani R., Robinson C., Curcuru A., DeWees T., Bradley J. Phase I trial of stereotactic MR-guided online adaptive radiation therapy (SMART) for the treatment of oligometastatic or unresectable primary malignancies of the abdomen. Radiother Oncol. 2018;126(3):519–526. doi: 10.1016/j.radonc.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 14.Brock K.K. Adaptive Radiotherapy: Moving Into the Future. Sem Radiat Oncol. 2019;29(3):181–184. doi: 10.1016/j.semradonc.2019.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang S., Tang F., Huang X., Yang K., Zhong T., Hu R. Deep-learning-based detection and segmentation of organs at risk in nasopharyngeal carcinoma computed tomographic images for radiotherapy planning. Eur Radiol. 2019;29(4):1961–1967. doi: 10.1007/s00330-018-5748-9. [DOI] [PubMed] [Google Scholar]
- 16.Fu Y., Lei Y., Wang T., Tian S., Patel P., Jani A.B. Pelvic multi‐organ segmentation on cone‐beam CT for prostate adaptive radiotherapy. Med Phys. 2020;47(8):3415–3422. doi: 10.1002/mp.14196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liang Y., Schott D., Zhang Y., Wang Z., Nasief H., Paulson E. Auto-segmentation of pancreatic tumor in multi-parametric MRI using deep convolutional neural networks. Radiother Oncol. 2020;145:193–200. doi: 10.1016/j.radonc.2020.01.021. [DOI] [PubMed] [Google Scholar]
- 18.Lei Y, Wang T, Tian S, Dong X, Jani AB, Schuster D, et al. Male pelvic multi-organ segmentation aided by CBCT-based synthetic MRI. Phys Med Biol 2020;65. https://doi.org/10.1088/1361-6560/ab63bb. [DOI] [PMC free article] [PubMed]
- 19.Liu Y., Lei Y., Wang T., Fu Y., Tang X., Curran W.J. CBCT‐based synthetic CT generation using deep‐attention cycleGAN for pancreatic adaptive radiotherapy. Med Phys. 2020;47(6):2472–2483. doi: 10.1002/mp.14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McIntosh C, Welch M, McNiven A, Jaffray DA, Purdie TG. Fully automated treatment planning for head and neck radiotherapy using a voxel-based dose prediction and dose mimicking method. Phys Med Biol 2017;62:5926–44. Doi:10.1088/1361-6560/aa71f8. [DOI] [PubMed]
- 21.Kearney V., Chan J.W., Valdes G., Solberg T.D., Yom S.S. The application of artificial intelligence in the IMRT planning process for head and neck cancer. Oral Oncol. 2018;87:111–116. doi: 10.1016/j.oraloncology.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 22.Fan J., Wang J., Chen Z., Hu C., Zhang Z., Hu W. Automatic treatment planning based on three-dimensional dose distribution predicted from deep learning technique. Med Phys. 2019;46(1):370–381. doi: 10.1002/mp.13271. [DOI] [PubMed] [Google Scholar]
- 23.Archambault Y., Boylan C., Bullock D., Morgas T., Peltola J., Ruokokoski E. Making on-Line Adaptive Radiotherapy Possible Using Artificial Intelligence and Machine Learning for Efficient Daily Re-Planning. Med Phys Int J. 2020;8:77–86. [Google Scholar]
- 24.Shaw E., Kline R., Gillin M., Souhami L., Hirschfeld A., Dinapoli R. Radiation therapy oncology group: Radiosurgery quality assurance guidelines. Int J Radiat Oncol Biol Phys. 1993;27(5):1231–1239. doi: 10.1016/0360-3016(93)90548-A. [DOI] [PubMed] [Google Scholar]
- 25.The international commission on radiation units and measurements. Prescribing, Recording, and Reporting Photon-Beam Intensity-Modulated Radiation Therapy (IMRT). J ICRU 2010;10:1–106. Doi: 10.1093/jicru/ndq001.
- 26.Tetar S.U., Bruynzeel A.M.E., Lagerwaard F.J., Slotman B.J., Bohoudi O., Palacios M.A. Clinical implementation of magnetic resonance imaging guided adaptive radiotherapy for localized prostate cancer. Phys Imag Radiat Oncol. 2019;9:69–76. doi: 10.1016/j.phro.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bertelsen A.S., Schytte T., Møller P.K., Mahmood F., Riis H.L., Gottlieb K.L. First clinical experiences with a high field 1.5 T MR linac. Acta Oncol. 2019;58(10):1352–1357. doi: 10.1080/0284186X.2019.1627417. [DOI] [PubMed] [Google Scholar]
- 28.Yoon SW, Lin H, Alonso-Basanta M, Anderson N, Apinorasethkul O, Cooper K, et al. Initial Evaluation of a Novel Cone-Beam CT-Based Semi-Automated Online Adaptive Radiotherapy System for Head and Neck Cancer Treatment – A Timing and Automation Quality Study. Cureus 2020;12. https://doi.org/10.7759/cureus.9660. [DOI] [PMC free article] [PubMed]
- 29.Fischer-Valuck B.W., Henke L., Green O., Kashani R., Acharya S., Bradley J.D. Two-and-a-half-year clinical experience with the world's first magnetic resonance image guided radiation therapy system. Adv Radiat Oncol. 2017;2(3):485–493. doi: 10.1016/j.adro.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Henke L.E., Olsen J.R., Contreras J.A., Curcuru A., DeWees T.A., Green O.L. Stereotactic MR-Guided Online Adaptive Radiation Therapy (SMART) for Ultracentral Thorax Malignancies: Results of a Phase 1 Trial. Adv Radiat Oncol. 2019;4(1):201–209. doi: 10.1016/j.adro.2018.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.