Abstract
Melanoma affects about 6000 patients a year in Spain. A group of medical oncologists from Spanish Society of Medical Oncology (SEOM) and Spanish Multidisciplinary Melanoma Group (GEM) has designed these guidelines to homogenize the management of these patients. The diagnosis must be histological and determination of BRAF status has to be performed in patients with stage ≥ III. Stage I–III resectable melanomas will be treated surgically. In patients with stage III melanoma, adjuvant treatment with immunotherapy or targeted therapy is also recommended. Patients with unresectable or metastatic melanoma will receive treatment with immunotherapy or targeted therapy, the optimal sequence of these treatments remains unclear. Brain metastases require a separate consideration, since, in addition to systemic treatment, they may require local treatment. Patients must be followed up closely to receive or change treatment as soon as their previous clinical condition changes, since multiple therapeutic options are available.
Keywords: Melanoma, Adjuvant treatment metastatic treatment, Staging
Methodology
The authors have reviewed the published clinical guidelines, as well as clinical trials from which the aspects referred to in these guidelines can be concluded. Each author has been responsible for reviewing a part of the guideline that has been shared and discussed among all the authors to reach a consensus. Finally, the degrees of evidence and recommendation have been established based on the recommendations for the development of guidelines [1, 2].
Incidence and epidemiology
The annual incidence of melanoma in Europe is < 10–25/100.000, in the US is 20–30/100.000 and in Australia 50–60/100.000 [3]. It is expected an increase in the next years, and therefore, there is a need to improve prevention and early diagnosis measures [4]. In Spain, an increase in the melanoma mortality rate was observed in the last decades of the twentieth century; however, later, stabilization was observed in women and a decrease in middle age and young men [5, 6]. In 2020, the estimated new cases of melanoma in Spain are 6179 [7].
Ultraviolet radiation is the most important risk factor, especially if intermittent sun exposure, particularly early in life [8]. Individuals with skin types I and II have the highest risk of developing melanoma, as well as individuals with a high numbers of typical nevi, large congenital nevi, and atypical nevi [9, 10]. About 10% of melanomas occur in patients with familial history of melanoma and can present a germline mutation; CDKN2A is the main gene involved [11]. Physical protection is the best prevention of melanoma, and regular use of sunscreen reduces the incidence of cutaneous melanoma (level of evidence 1, grade of recommendation A) [12].
Diagnosis and pathology
Clinical analysis of suspicious lesions includes three aspects: (1) ABCD rule (Asymmetry, Border irregularities, Color heterogeneity, and Dynamics or evolution in the color, size, or elevation), (2) the ugly duckling sign (the lesion is different from the rest in the same patient), and (3) chronological analysis of changes: assessment of rapid growth in a previous lesion [13]. Dermatoscopy by an experienced physician is recommended for the diagnosis of pigmented lesions (Level of evidence 1b, grade of recommendation A) [14]. Whole-body photography and digital dermatoscopy are useful in people at high risk of melanoma, especially in early diagnosis (Level of evidence 2b, grade of recommendation B) [15]. Reflectance confocal microscopy can be helpful in lesions difficult to diagnose by visual inspection and dermatoscopy (Level of evidence 2b, grade of recommendation B) [16]. All suspicious lesions must be confirmed histologically by excisional biopsy following the eighth edition of the American Joint Committee on Cancer (AJCC 8th edition) [17].
Molecular testing
Determination of BRAF V600 status is mandatory in patients with resectable or unresectable stage III or IV melanoma (Level of evidence 1, grade of recommendation A). Determination of C-KIT and NRAS status is recommended in BRAF wild-type patients (Level of evidence 2, grade of recommendation C) [18, 19]. A new melanoma subtype with NF1 mutation has recently been defined, but its clinical implications are unknown [20]. Programmed death ligand 1 (PD-L1) expression can be tested in resectable or unresectable stage III and IV, although its determination is not mandatory, since negative cases can respond to anti-PD-1 treatments.
Staging (Table 1)
Table 1.
Melanoma staging AJCC 8th edition
| T category | Thickness | Ulceration status |
|---|---|---|
| TX: Primary tumor thickness cannot be assessed (e.g., diagnosis by curettage) | Not applicable | Not applicable |
| T0: No evidence of primary tumor (e.g., unknown primary or completely regressed melanoma) | Not applicable | Not applicable |
| Tis (melanoma in situ) | Not applicable | Not applicable |
| T1 | ≤ 1.0 mm | Unknown or unspecified |
| T1a | < 0.8 mm | Without ulceration |
| T1b | < 0.8 mm | With ulceration |
| 0.8–1.0 mm | With or without ulceration | |
| T2 | > 1.0–2.0 mm | Unknown or unspecified |
| T2a | > 1.0–2.0 mm | Without ulceration |
| T2b | > 1.0–2.0 mm | With ulceration |
| T3 | > 2.0–4.0 mm | Unknown or unspecified |
| T3a | > 2.0–4.0 mm | Without ulceration |
| T3b | > 2.0–4.0 mm | With ulceration |
| T4 | > 4.0 mm | Unknown or unspecified |
| T4a | > 4.0 mm | Without ulceration |
| T4b | > 4.0 mm | With ulceration |
| N category | No. of tumor-involved regional lymph nodes | Presence of in-transit, satellite, and/or microsatellite metastases |
|---|---|---|
| NX | Regional nodes not assessed (e.g., sentinel lymph-node biopsy not performed, regional nodes previously removed for another reason) | No |
| Exception: pathological N category is not required for T1 melanomas, use clinical N information | ||
| N0 | No regional metastases detected | No |
| N1 | One tumor-involved node or any number of in-transit, satellite, and/or microsatellite metastases with no tumor-involved nodes | |
| N1a | One clinically occult (i.e., detected by SLN biopsy) | No |
| N1b | One clinically detected | No |
| N1c | No regional lymph-node disease | Yes |
| N2 | Two or three tumor-involved nodes or any number of in-transit, satellite, and/or microsatellite metastases with one tumor-involved node | |
| N2a | Two or three clinically occult (i.e., detected by SLN biopsy) | No |
| N2b | Two or three, at least one of which was clinically detected | No |
| N2c | One clinically occult or clinically detected | Yes |
| N3 | Four or more tumor-involved nodes or any number of in-transit, satellite, and/or microsatellite metastases with two or more tumor-involved nodes, or any number of matted nodes without or with in-transit, satellite, and/or microsatellite metastases | |
| N3a | Four or more clinically occult (i.e., detected by SLN biopsy) | No |
| N3b | Four or more, at least one of which was clinically detected, or the presence of any number of matted nodes | No |
| N3c | Two or more clinically occult or clinically detected and/or presence of any number of matted nodes | Yes |
| M category | Anatomic site | LDH level |
|---|---|---|
| M0 | No evidence of distant metastasis | Not applicable |
| M1 | Evidence of distant metastasis | See below |
| M1a | Distant metastasis to skin, soft tissue including muscle, and/or nonregional lymph node | Not recorded or unspecified |
| M1a(0) | Not elevated | |
| M1a (1) | Elevated | |
| M1b | Distant metastasis to lung with or without M1a sites of disease | Not recorded or unspecified |
| M1b(0) | Not elevated | |
| M1b (1) | Elevated | |
| M1c | Distant metastasis to non-CNS visceral sites with or without M1a or M1b sites of disease | Not recorded or unspecified |
| M1c(0) | Not elevated | |
| M1c (1) | Elevated | |
| M1d | Distant metastasis to CNS with or without M1a, M1b, or M1c sites of disease | Not recorded or unspecified |
| M1d(0) | Not elevated | |
| M1d (1) | Elevated |
| T | N | M | Stage group |
|---|---|---|---|
| Tis | N0 | M0 | 0 |
| T1a | N0 | M0 | IA |
| T1b–T2a | N0 | M0 | IB |
| T2b–T3a | N0 | M0 | IIA |
| T3b–T4a | N0 | M0 | IIB |
| T4b | N0 | M0 | IIC |
| Any T, Tis | ≥N1 | M0 | III |
| Any T | Any N | M1 | IV |
TNM staging in melanoma includes physical examination of the entire body. In pT1b–pT4b melanoma, ultrasound (US) for locoregional lymph-node metastasis, and/or computed tomography (CT) or positron emission tomography (PET) scans and brain magnetic resonance imaging (MRI), is recommended for proper tumor assessment (Level of evidence 3, grade of recommendation A) [17].
Treatment of localized disease
Treatment of primary tumors
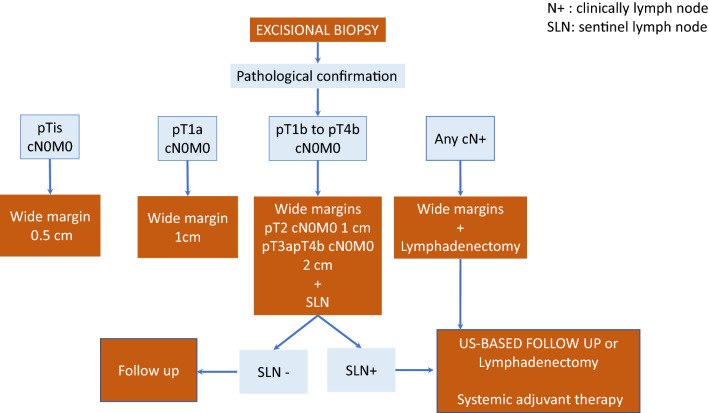
Excisional biopsy with a 2 mm lateral margin and deep subcutaneous margin is indicated for any suspicious lesion (Level of evidence 1a, grade of recommendation A). Upon pathological confirmation of the diagnosis, definitive surgery with wide margins is performed. The deep margin should extend to the fascia, whereas lateral margins will depend on Breslow thickness: 0.5 cm for in situ melanomas, 1 cm for tumors with thickness of up to 2 mm, and 2 cm for > 2 mm (Level of evidence 1b, grade of recommendation A) [21]. Figure 1 shows the treatment algorithm for primary tumors.
Fig. 1.
Treatment algorithm for primary tumors and regional lymph notes
Sentinel lymph-node biopsy is recommended for melanomas with Breslow > 0.8 mm of thickness or < 0.8 mm with ulceration, i.e., melanomas with stage ≥ IB of the AJCC 8th edition classification (Level of evidence 2a, grade of recommendation A) [22–24].
Complete lymph-node dissection in patients with positive sentinel lymph nodes carries severe morbidity and has shown no impact on survival compared with US-based follow-up (Level of evidence 1b, grade of recommendation A) [25, 26]. For this reason, complete dissection has been abandoned as routine practice. However, the procedure is recommended in the case of clinically detected regional lymph-node metastases (Level of evidence 4, grade of recommendation C) [27].
Resection of satellite or in-transit metastases is associated with a high risk of local and regional progression. With the advent of effective systemic therapies, this strategy could only be considered in highly selected cases (Level of evidence 4, grade of recommendation C).
Adjuvant therapy
Adjuvant radiotherapy
Adjuvant radiotherapy in the local tumor can be considered in cases of inadequate resection margins of lentigo maligna when further resection is not advisable (Level of evidence 3b, grade of recommendation B. The role of radiotherapy for in-transit metastasis has not been established (Level of evidence 5, grade of recommendation D). Nodal adjuvant radiotherapy reduces the risk of regional recurrence after resection of palpable regional lymph nodes or extracapsular extension [28]. However, it increases the risk of regional toxicity—particularly lymphedema—with no impact on overall survival, so it is not longer routinely recommended (Level of evidence 2b, grade of recommendation B) [29]. Radiotherapy may be discussed in resected head and neck melanoma with palpable lymph nodes, where local control is critical and has a lower risk of lymphedema.
Adjuvant systemic therapy
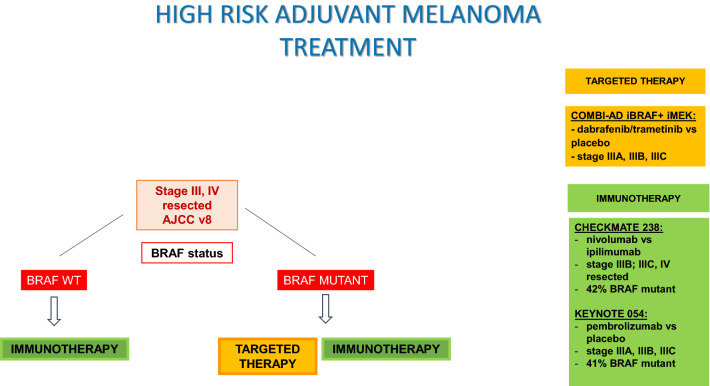
For patients with complete resection of a cutaneous melanoma, to recommend adjuvant systemic therapy depends upon the risk of disease recurrence, based on the stage at diagnosis, along with a consideration of patient age, comorbidity, and personal preferences. For patients with stage III melanoma, adjuvant immunotherapy with nivolumab or pembrolizumab is indicated; and dabrafenib and trametinib are an alternative for patients with BRAF V600 mutation. In patients with completely resected stage IV, adjuvant nivolumab is also indicated.
Nivolumab for 1 year prolonged RFS at 4-year follow-up compared with ipilimumab (52% vs. 41%, HR: 0.71, p < 0.0001, respectively) in patients with completely resected stage IIIB, IIIC, or IV. Efficacy was observed both in BRAF mutant and wild-type patients. In the first report of overall survival, with fewer events than expected, OS rates were similar in both treatment groups (78% Nivolumab and 77% ipilimumab), taking into account that more patients treated with ipilimumab received subsequent therapy, including immunotherapy (57% with ipilimumab and 49% with nivolumab) [30, 31].
Pembrolizumab for 1 year has also shown prolonged RFS compared with placebo in patients with completely resected stage III melanoma at 3.5-year follow-up (59′8% vs. 41′4%, HR 0,59, p < 0.0001, respectively). Efficacy was observed both in BRAF mutant and wild-type patients. Pembrolizumab also decreased the 3.5-year incidence of distant metastases as first recurrence and locoregional recurrence only. The S1404 phase III trial compares pembrolizumab with high-dose interferon or high-dose ipilimumab in patients with completely resected high-risk stage III–IVA disease; accrual is complete, and results are pending [32, 33].
Dabrafenib plus trametinib for 1 year showed a longer RFS compared with placebo in completely resected stage III BRAF V600 mutant melanoma at 5 year follow-up (52% vs. 36%, HR 0.51, 95% CI 0.42–0.61), irrespective of baseline factors. Overall survival, at a median follow-up of 2.8 years, was prolonged with the targeted therapy, but remains immature [34–37].
In summary, in patients with completely resected stage III or IV melanoma, adjuvant immunotherapy and targeted therapies have shown to improve RFS (Level of evidence 1, grade of recommendation A). Although in all these pivotal clinical trials, lymphadenectomy was required as an inclusion criteria, the impact of avoiding a lymphadenectomy on the results of adjuvant treatment is unknown, leaving the decision whether or not performing it before in the context of a discussion with the patient (Level of evidence 3, grade of recommendation C). Finally, stage IIIA AJCC v7 patients were included in COMBI-AD and Keynote 054 trials with the inclusion criteria of sentinel lymph nodes > 1 mm; regulatory approvals of FDA, EMA, and AEMPS admit to extend the use of adjuvant systemic treatment to with anti-PD-1 antibodies all stage III, including stage IIIA, but there is no clear evidence of its use in IIIA with < 1 mm (Level of evidence 3, grade of recommendation B–C). Selection of a specific agent depends largely on BRAF mutation status and toxicity profiles (Level of evidence 3–4, grade of recommendation B). Figure 2 shows adjuvant treatment algorithm in high-risk melanoma.
Fig. 2.
Adjuvant treatment algorithm in high-risk melanoma
For patients who relapse with metastatic disease after initial adjuvant therapy, options include treatment with an alternative active systemic therapy or inclusion in a clinical trial (Level of evidence 3, grade of recommendation C) [38].
Treatment of advanced disease
Oligometastatic disease
Some stage IV patients present with a resectable, oligometastatic disease and surgical excision or stereotactic radiosurgery (SRS) of solitary metastases should be considered whenever feasible. One-third of patients with resected metastasis may become long-term survivors (Level of evidence 2b, grade of recommendation B) [39]. Surgery may be the preferred option for selected patients if feasible, preferentially combined with adjuvant systemic therapies as previously commented.
Systemic treatment of unresectable advanced/m1 disease
Treatment options in first line
Immunotherapy
Immunotherapy based on immune checkpoint inhibitors has demonstrated its superiority over chemotherapy in terms of response, PFS and OS in first-line treatment (Level of evidence 1, grade of recommendation A). This option, unlike that occurs with targeted therapy against BRAF, is not based on any biomarker such as the expression of the programmed death-ligand 1 (PD-L1) (Level of evidence I, grade of recommendation B) or tumor mutational burden (TMB) (Level of evidence IV, grade of recommendation C). Ipilimumab, an anti-CTLA-4 (cytotoxic T-lymphocyte-associated protein 4) antibody, was the first treatment to show an improvement in overall survival in patients with metastatic melanoma [40, 41]. This option has been exchanged in favor to PD-1 inhibitors such as nivolumab [42, 43], pembrolizumab [44, 45], or the combination of ipilimumab plus nivolumab [46, 47]. Table 2 summarizes the main characteristics of the pivotal trials of immunotherapy in advanced melanoma.
Table 2.
Main characteristics of the pivotal trials of immunotherapy in advanced melanoma
| Treatment | Considerations | PFS (median months 95% IC) | OS (median months, 95% IC) | Update overall survival (%) |
|---|---|---|---|---|
|
CheckMate 066 |
BRAF WT patients Control arm: DTIC Protocol amendment: cross over nivolumab |
5.1 (3.5–10.8) | NR (survival rate at 1 year 72.9% (65.5–78.9) | 51.2% at 3 years |
|
KEYNOTE-006 |
Control arm: ipilimumab 34% second-line treatment 36% BRAF mutant |
4.1 (2.9 a 6.9)* | NR (survival rate at 1 year 68.4%) | 32.7 (24.5–41.6) at 5 years** |
|
CheckMate 067 |
Control arm: ipilimumab 31.5% BRAF mutant |
11.5 (8.9–16.7) | 64% survival rate at 2 years | 58% at 3 years; 52% at 5 years |
NR not reached, NC unknown
*At dose 10 mg/kg every 3 weeks
**Combined pembrolizumab groups
The clinical criteria that can help us to choose the best first-line treatment option with immune checkpoint inhibitors (monotherapy or combination) or the duration of treatment are not yet established. Different studies have proposed several clinical markers such as LDH (lactate dehydrogenase), lymphocytes, leukocytes, or eosinophils count that could predict the response to ICI, but data are inconclusive (Level of evidence IV, grade of recommendation C) [48].
On the other hand, there is other type of immunotherapy such as Talimogene Laherparepvec, an intralesional virotherapy that has shown an improvement in durable response rate, overall survival, and loco-regional control respect to GM-CSF in patients with injectable lesions and unresectable stage IIIB–C or IV melanoma, specially when used as first-line therapy (Level of evidence 1, grade of recommendation B) [49].
Targeted therapy
Currently, there are three different combinations of BRAF and MEK inhibitors based in four randomized phase III clinical trials that improve both progression-free survival and overall survival in comparison with BRAF inhibitors alone (Table 3) [50, 51]. The combination of BRAF and MEK inhibitors should be the therapy of choice when targeted therapy is considered, unless absolute contraindication for MEK inhibitors is present (Level of evidence 1, grade of recommendation A).
Table 3.
Randomized trials with of BRAF and MEK inhibitors in advanced BRAF mutant melanoma
| Combination | Progression-free survival (median months, 95% IC) | Overall survival (median months, 95% IC) | Patients alive at 3, 4, and 5 years (%) | References |
|---|---|---|---|---|
| Dabrafenib + trametinib | 11.1 (9.5–12.8) | 25.9 (22.6–31.5) | 44,37,34 | [1] |
| Vemurafenib + cobimetinib | 12.6 (9.5–14.7) | 22.5 (20.3–28.8) | 38.5, 34.7, NA | [2] |
| Encorafenib + binimetinib | 14.9 (11.0–20.2) | 33.6 (24.4–39.2) | 47, 39, NA | [3] |
There are certain subgroups that of poor prognosis, being the most important factors an elevated LDH, ECOG > 1 and high tumor burden (expressed in number of organs involved) [50, 52]. However, a combination of BRAF and MEK inhibitor would be the first option over monotherapy (Level of evidence 2, grade of recommendation B).
Direct comparison among the three different combos does not exist, and similar efficacy with small differences in the toxicity profile has been described. When selecting the combination, patient preferences, drug availability, and efficiency criteria should be taken into account (Level of evidence 4, grade of recommendation C).
First-line selection in BRAFm
The best treatment sequence for BRAF mutant patients is unknown, since no direct comparison exists. First-line decision between targeted therapies or immunotherapy is currently being studied in prospective trials (SECOMBIT, NCT02631447) to define the best sequencing combination treatment in terms of OS, the primary efficacy variable. Meanwhile, the decision should be based on patient’s profile (comorbidities, ECOG, symptoms, and life expectancy) and on melanoma characteristics (tumor burden, site of metastasis, and level of LDH) [52, 53] (Level of evidence 2, grade of recommendation B).
Most studies have demonstrated a higher number of events during the first 12 months with immunotherapy than with targeted therapy and, by contrast, patients with immunotherapy have better survival beyond the first year [50, 54]. With all the aforementioned, it can be advised that in those patients where the first months of treatment can be administered safely (for example, those who will not progress quickly, or where immediate response is not required due to involvement of an organ or its function) would be good candidates for immunotherapy, reserving targeted therapy for later lines (Level of evidence 2, grade of recommendation B).
Treatment options in second line
Treatment options in second line depend on the treatment administered in the first line, as well as, on the mutational status. For the BRAF wild-type patients, options for a second-line treatment are limited, and inclusion in clinical trials is a priority. When the first line was an anti-PD-1 antibody, ipilimumab (Level of evidence 2, grade of recommendation B) and the combination of nivolumab and ipilimumab (Level of evidence 4, grade of recommendation B) are two valid options [55]. Finally, chemotherapy (Dacarbazine and Temozolomide) can be considered for patients who exhausted other options (Level of evidence 2, grade of recommendation D).
In the BRAF mutant population treated with an anti-PD-1 antibody in first line, the combination of BRAF and MEK inhibitors is the preferred option. Although their activity has not been prospectively studied after progression to anti-PD-1, it seems to be similar to the first line of treatment in terms of response (Level of evidence 2, grade of recommendation A) [56]. Data from the Columbus Study, where BRAF mutant patients may have previously received immunotherapy, showed that these patients also benefited from the combination (Level of evidence 2, grade of recommendation A) [57].
In the BRAF mutant population treated with the combination of BRAF and MEK inhibitors in first line, anti-PD-1 antibody and the combination of nivolumab and ipilimumab are valid options.
Treatment beyond progression
Treatment beyond progression might be an option in selected patients both on targeted as well as on immunotherapy based on retrospective data [58]. No randomized data are available at this time. Treatment beyond progression is generally not recommended unless there is a suspicion of pseudo-progression, bearing in mind that the analysis of these data is subject to many biases related to patient status.
Management of brain metastasis
It is estimated that up to 50–60% of metastatic melanoma patients will develop brain metastasis. First, a stepwise and multidisciplinary approach is highly recommended to design an individualized plan for every patient (Level of evidence 4, grade of recommendation A) [59]. In an effort to classify patients into similar prognostic groups, some key clinical factors such as Karnofsky Performance Score (KPS), number of brain metastases, extracranial metastases, age, and BRAF status constitute the basis of the specific graded prognostic assessment (GPA) index for melanoma (Melanoma-mol GPA) [60] and could be extremely helpful to guide clinical decision-making (Level of evidence 3, grade of recommendation B).
Among locoregional strategies, surgery must be considered to treat symptomatic and large brain metastasis, especially in the case of solitary brain metastasis and when a pathological and/or molecular diagnosis is needed (Level of evidence 4, grade of recommendation B) [61]. SRS is generally recommended in patients with 1–4 brain metastases with less than 3–4 cm (Level of evidence 3, grade of recommendation A), although the role of SRS has been tested for up to 15 brain metastasis [61]. Adjuvant SRS yields the same overall survival but with fewer declines in cognitive function, so SRS on the surgical cavity and not WBRT is recommended after excision of brain metastases (Level of evidence 1b, grade of recommendation A) [62]. Whole brain radiation therapy (WBRT) is discouraged except in the palliative setting when other options are not feasible (Level of evidence 4, grade of recommendation C) [59].
When locoregional strategies are discarded, systemic therapies must be considered. At this point, two main clinical situations emerge. In asymptomatic, previously untreated brain metastasis patients, combination immunotherapy with nivolumab and ipilimumab has demonstrated outstanding results with intracranial response rates ranging from 46 to 57% and median PFS and DOR not reached with a median follow-up of 20.6 months [63]. Pembrolizumab, nivolumab, and ipilimumab as single therapies also show some degree of activity (ORR around 20%) [64]. In BRAF mutant patients, BRAF and MEK inhibitors obtain high intracranial responses, which rises up to 58% with the combination of dabrafenib and trametinib [65]. However, these responses seem to be shorter than those obtained in extracranial sites. Aforementioned results make reasonable advising nivolumab plus ipilimumab as the preferred first-line treatment for patients with asymptomatic brain metastasis not amenable to surgery or SRS, irrespective of BRAF status, whenever possible (Level of evidence 3, grade of recommendation A). In BRAF-mutated patients, combination of BRAF and MEK inhibitors remains a good alternative option in first and second lines (Level of evidence 3, grade of recommendation B).
Unfortunately, symptomatic and/or previously treated patients obtain poorer results, especially with immunotherapy, and new approaches are eagerly needed in this setting [59].
Follow-up
On average, 20–30% of early stage melanoma patients will develop a recurrence within 5 years [66]. For stage I–II melanomas, recurrences will be in 50% of cases at regional lymph nodes, 30% as distant metastasis and 20% will be local relapses or in-transit metastasis. For stage III melanoma, up to 95% occur during the first 3 years of follow-up [66], and up to 50% will be distant recurrences, 25% regional and 25% local relapses. About 2–10% will have a second primary melanoma, most of them during the first year after initial diagnosis [67].
Self-examination is an essential component of the follow-up and can lead to early recognition of recurrences and new melanomas [68]. Patients should receive instructions on self-examination (Level of evidence 3, grade of recommendation B). Over the last few years, several skin cancer detection applications for smartphones have been developed through analysis of artificial intelligence algorithms. Further studies are needed before implementing these techniques in the general population (Level of evidence 4, grade of recommendation C).
Physical examination has proven to be an effective procedure for early recurrence detection [68] and should be performed in all melanoma patients during follow-up (Level of evidence 2, grade of recommendation A). Physical exam must include skin and nodes exam. Total body photography, sequential digital dermatoscopy imaging, and reflectance confocal microscopy, must be helpful in patients with a high number of moles or presence of clinical atypical nevi (Level of evidence 3, grade of recommendation A). For stage I–IIA melanomas, the frequency of physical exams should be, at least, annually, life-long, but it depends on the risk factors of each patient (Level of evidence 3, grade of recommendation A). For patients with stage IIB–IV melanomas, physical examinations should be performed every 3–6 months during the first 2 years, and then every 3–6 months for 3 years, and after 5 years, they could be done annually (Level of evidence 3, grade of recommendation A).
Routine blood testing is optional, as few recurrences are detected by increased LDH and S-100 levels [68] (Level of evidence 4, grade of recommendation C). Liquid biopsy for melanoma screening using the determination of BRAF mutation in cfDNA from blood or other body fluids is a promising technique, but it is not routinely indicated yet (Level of evidence 4, grade of recommendation C).
Lymph-node sonography has proven to be the most sensitive and most specific procedure for the detection of locoregional lymph-node metastases [69]. In patients with stage IIC–III melanomas, lymph-node sonography of regional areas must be performed regularly, and it should be performed every 4 months during the first 2 years, and every 6 months during the next 3 years, especially in patients with positive sentinel lymph nodes without lymph-node dissection (Grade of recommendation A; level of evidence 1).
General recommendation about imaging procedures is not possible, since no prospective studies have assessed if early recurrence detection impacts in the overall survival. Some studies in IIC–III melanoma patients have demonstrated that an extensive follow-up including CT body scan and brain MRI detects almost 50%, and 8% of recurrences [70, 71], so imaging follow up every 3 months is recommended (Grade of recommendation B; level of evidence 3). Routine follow-up with PET–CT is not recommended, although some studies have demonstrated a higher sensitivity for detecting distant metastases in the extremities (Grade of recommendation C; level of evidence 4) [72]. For earlier stages I–IIA where the risk of relapse is lower, radiological follow-up with body CT scan and brain MRI is optional (Grade of recommendation C; level of evidence 4).
Summary of recommendations and evidence
| Level of evidence | Grade of recommendation | |
|---|---|---|
| Physical protection and regular use of sunscreen reduce the incidence of cutaneous melanoma | 1 | A |
| Diagnosis and pathology | 1b | A |
| Dermatoscopy by an experienced physician is recommended for the diagnosis of pigmented lesions | ||
| Whole-body photography and digital dermatoscopy are useful in people at high risk of melanoma, especially in early diagnosis | 2b | B |
| Determination of BRAF V600 status is mandatory in patients with resectable or unresectable stage III or IV melanoma | 1 | A |
| Staging | ||
| In pT1b–pT4b melanoma, US for locoregional LN metastasis, CT or PET-TC and brain MRI, is recommended | 3 | A |
| Treatment of primary tumor | ||
| Excisional biopsy is recommended on all suspicious lesions | 1a | A |
| Safety surgical margins should be Breslow-adapted | 1b | A |
| SLN is indicated if Breslow > 0.8 mm or < 0.8 mm with ulceration | 2a | A |
| Routine CLND is not recommended for SLN + patients | 1b | A |
| CLND is recommended for clinically detected regional lymph nodes | 4 | C |
| Adjuvant therapy | ||
| Adjuvant radiotherapy is not recommended, but it may be considered for selected cases | 2b | B |
| Adjuvant anti-PD-1 treatment with nivolumab and pembrolizumab or targeted therapies with dabrafenib and trametinib are recommended in resected stage III–IV melanoma | 1 | A |
| Oligometastatic disease | ||
| Surgical excision or SRS of solitary metastases should be considered | 2b | B |
| Management of advanced disease | ||
| First line advanced/M1 disease | ||
| Anti-PD-1 treatment (nivolumab or pembrolizumab) or ipilimumab and nivolumab are a standard of care for all patients with advanced melanoma | 1 | A |
| Intralesional T-VEC is also an option for unresectable stage IIIB–C or IV | 1 | C |
| In BRAF V600 melanoma, BRAF and MEK inhibitors (dabrafenib–trametinib, vemurafenib–cobimetinib, encorafenib–binimetinib) are additional first-line options | 1 | A |
| Second line and beyond advanced/M1 disease | ||
| In BRAF wild-type melanoma, ipilimumab and the combination of nivolumab and ipilimumab are two valid options after an anti-PD-1 treatment | 2, 4 | B |
| In BRAFV600 melanoma, BRAF and MEK inhibitors are the preferred option after an anti-PD-1 treatment | 2 | A |
| In BRAFV600 melanoma treated with BRAF and MEK inhibitors in first line, anti-PD-1 treatment (nivolumab or pembrolizumab) or ipilimumab and nivolumab are valid options | 2 | A |
| Chemotherapy can be considered for patients who exhausted other options | 2 | D |
| Brain metastases | ||
| Multidisciplinary approach is highly recommended | 4 | A |
| Melanoma-mol GPA index could help to guide clinical decision | 3 | B |
| Surgery must be considered in symptomatic, large BM specially in solitary BM | 4 | B |
| SRS is recommended in 1–4 BM metastases with less than 3–4 cm | 3 | A |
| Follow-up | ||
| Self-examination is recommended in all patients | 3 | B |
| Physical examinations is recommended in all patients | 2 | A |
| Routine blood testing is optional | 4 | C |
| Follow-up with CT body scan and brain MRI in stages IIB–IV is recommended | 3 | B |
| Follow-up with CT body scan and brain MRI in stages I–IIA is optional | 4 | C |
| Lymph-node US is recommended in SLN-positive patients | 1 | A |
SLN sentinel lymph node, CLND complete lymph-node resection, SRS stereotactic radiosurgery, BM brain metastasis
Compliance with ethical standards
Conflict of interest
MMT reports grants and personal fees from BMS; personal fees from Astra Zeneca, Novartis, Roche, MSD, Boehringer Ingelheim, Takeda, Pierre-Fabre and Bayer; non-financial support from Astra Zeneca, MSD, Boehringer Ingelheim outside the submitted work. JLM reports personal fees from Novartis, Roche, Pierre-Fabre, BMS, MSD and grants from; Merck, Roche, Abbvie, Astra Zeneca outside the submitted work. I.M.-R. has received grants as advisory board member and travel and congress accommodation from BMS, MSD, Roche, Novartis, Amgen, Pierre Fabre, Merck Serono, Sanofi, Regeneron, Incyte, AstraZeneca, and Bioncotech. LCM reports grants and personal fees from BMS, Roche, MSD-Merck, and Amgen, personal fees from Pierre Fabre, Novartis, and grants from Celgene, outside the submitted work. EE reports personal fees from BMS, MSD, Novartis, Pierre Fabre, and Roche. MGC reports personal fees from NOVARTIS, BMS, and Roche. AB reports personal fees and non-financial support from BMS, MSD, Roche, Novartis, Sanofi, and Merck-Pfizer, and personal fees from Pierre Fabre and Incyte, outside the submitted work. KM, EPR, and EMC have nothing to disclose.
Ethical approval
The current study has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Informed consent
Not applicable.
Footnotes
All authors are members of the Spanish Society of Medical Oncology (SEOM) and Spanish Multidisciplinary Melanoma Group (GEM).
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All authors have contributed equally to the present manuscript.
Contributor Information
M. Majem, Email: mmajem@santpau.cat
J. L. Manzano, Email: jmanzano@iconcologia.net
I. Marquez-Rodas, Email: ivanpantic@hotmail.com
K. Mujika, Email: kmujika@onkologikoa.org
E. Muñoz-Couselo, Email: emunoz@vhio.net
E. Pérez-Ruiz, Email: eliperu@gmail.com
L. de la Cruz-Merino, Email: lucme12@yahoo.es
E. Espinosa, Email: eespinosa00@hotmail.com
M. Gonzalez-Cao, Email: mgonzalezcao@oncorosell.com
A. Berrocal, Email: berrocal.alf@gmail.com
References
- 1.Shekelle PG, Woolf SH, Eccles M, Grimshaw J. Developing clinical guidelines. West J Med. 1999;170(6):348–351. [PMC free article] [PubMed] [Google Scholar]
- 2.Dykewicz CA. Guidelines for preventing opportunistic infections among hematopoietic stem cell transplant recipients: focus on community respiratory virus infections. Biol Blood Marrow Transpl. 2001;7(Suppl):19S–22S. doi: 10.1053/bbmt.2001.v7.pm11777100. [DOI] [PubMed] [Google Scholar]
- 3.Garbe C, Amaral T, Peris K, Hauschild A, Arenberger P, Bastholt L, et al. European consensus-based interdisciplinary guideline for melanoma. Part 1: diagnostics—update 2019. Eur J Cancer (Oxford, England: 1990) 2020;126:141–158. doi: 10.1016/j.ejca.2019.11.014. [DOI] [PubMed] [Google Scholar]
- 4.Whiteman DC, Green AC, Olsen CM. The Growing Burden of Invasive Melanoma: projections of incidence rates and numbers of new cases in six susceptible populations through 2031. J Invest Dermatol. 2016;136(6):1161–1171. doi: 10.1016/j.jid.2016.01.035. [DOI] [PubMed] [Google Scholar]
- 5.Gutierrez-Gonzalez E, Lopez-Abente G, Aragones N, Pollan M, Pastor-Barriuso R, Sanchez MJ, et al. Trends in mortality from cutaneous malignant melanoma in Spain (1982–2016): sex-specific age-cohort-period effects. J Eur Acad Dermatol Venereol. 2019;33(8):1522–1528. doi: 10.1111/jdv.15565. [DOI] [PubMed] [Google Scholar]
- 6.Rubio-Casadevall J, Puig-Vives M, Puigdemont M, Vilardell L, Carbo-Bague A, Marcos-Gragera R, et al. Patterns of increased incidence and survival of cutaneous melanoma in Girona (Spain) 1994–2013: a population-based study. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mexico. 2018;20(12):1617–1625. doi: 10.1007/s12094-018-1900-x. [DOI] [PubMed] [Google Scholar]
- 7.Cifras del cancer en España. 2020. https://seom.org/dmcancer/cifras-del-cancer/.
- 8.Elwood JM, Jopson J. Melanoma and sun exposure: an overview of published studies. Int J Cancer. 1997;73(2):198–203. doi: 10.1002/(sici)1097-0215(19971009)73:2<198::aid-ijc6>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 9.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Zanetti R, Masini C, et al. Meta-analysis of risk factors for cutaneous melanoma: III. Family history, actinic damage and phenotypic factors. Eur J Cancer (Oxford, England: 1990) 2005;41(14):2040–2059. doi: 10.1016/j.ejca.2005.03.034. [DOI] [PubMed] [Google Scholar]
- 10.Gandini S, Sera F, Cattaruzza MS, Pasquini P, Abeni D, Boyle P, et al. Meta-analysis of risk factors for cutaneous melanoma: Common and atypical naevi. Eur J Cancer (Oxford, England: 1990) 2005;41(1):28–44. doi: 10.1016/j.ejca.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 11.Toussi A, Mans N, Welborn J, Kiuru M. Germline mutations predisposing to melanoma. J Cutan Pathol. 2020;47(7):606–616. doi: 10.1111/cup.13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Green AC, Williams GM, Logan V, Strutton GM. Reduced melanoma after regular sunscreen use: randomized trial follow-up. J Clin Oncol. 2011;29(3):257–263. doi: 10.1200/JCO.2010.28.7078. [DOI] [PubMed] [Google Scholar]
- 13.Grob JJ, Bonerandi JJ. The ‘ugly duckling’ sign: identification of the common characteristics of nevi in an individual as a basis for melanoma screening. Arch Dermatol. 1998;134(1):103–104. doi: 10.1001/archderm.134.1.103-a. [DOI] [PubMed] [Google Scholar]
- 14.Kittler H, Pehamberger H, Wolff K, Binder M. Diagnostic accuracy of dermoscopy. Lancet Oncol. 2002;3(3):159–165. doi: 10.1016/s1470-2045(02)00679-4. [DOI] [PubMed] [Google Scholar]
- 15.Lallas A, Apalla Z, Kyrgidis A, Papageorgiou C, Boukovinas I, Bobos M, et al. Second primary melanomas in a cohort of 977 melanoma patients within the first 5 years of monitoring. J Am Acad Dermatol. 2020;82(2):398–406. doi: 10.1016/j.jaad.2019.08.074. [DOI] [PubMed] [Google Scholar]
- 16.Dinnes J, Deeks JJ, Saleh D, Chuchu N, Bayliss SE, Patel L, et al. Reflectance confocal microscopy for diagnosing cutaneous melanoma in adults. Cochrane Database Syst Rev. 2018;12:CD013190. doi: 10.1002/14651858.CD013190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gershenwald JE, Scolyer RA, Hess KR, Sondak VK, Long GV, Ross MI, et al. Melanoma staging: Evidence-based changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67(6):472–492. doi: 10.3322/caac.21409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yao Z, Yaeger R, Rodrik-Outmezguine VS, Tao A, Torres NM, Chang MT, et al. Tumours with class 3 BRAF mutants are sensitive to the inhibition of activated RAS. Nature. 2017;548(7666):234–238. doi: 10.1038/nature23291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Meng D, Carvajal RD. KIT as an oncogenic driver in melanoma: an update on clinical development. Am J Clin Dermatol. 2019;20(3):315–323. doi: 10.1007/s40257-018-0414-1. [DOI] [PubMed] [Google Scholar]
- 20.Cirenajwis H, Lauss M, Ekedahl H, Torngren T, Kvist A, Saal LH, et al. NF1-mutated melanoma tumors harbor distinct clinical and biological characteristics. Mol Oncol. 2017;11(4):438–451. doi: 10.1002/1878-0261.12050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hayes AJ, Maynard L, Coombes G, Newton-Bishop J, Timmons M, Cook M, et al. Wide versus narrow excision margins for high-risk, primary cutaneous melanomas: long-term follow-up of survival in a randomised trial. Lancet Oncol. 2016;17(2):184–192. doi: 10.1016/S1470-2045(15)00482-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Balch CM, Gershenwald JE, Soong SJ, Thompson JF, Ding S, Byrd DR, et al. Multivariate analysis of prognostic factors among 2,313 patients with stage III melanoma: comparison of nodal micrometastases versus macrometastases. J Clin Oncol. 2010;28(14):2452–2459. doi: 10.1200/JCO.2009.27.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nguyen CL, McClay EF, Cole DJ, O’Brien PH, Gillanders WE, Metcalf JS, et al. Melanoma thickness and histology predict sentinel lymph node status. Am J Surg. 2001;181(1):8–11. doi: 10.1016/s0002-9610(00)00533-x. [DOI] [PubMed] [Google Scholar]
- 24.Han D, Zager JS, Shyr Y, Chen H, Berry LD, Iyengar S, et al. Clinicopathologic predictors of sentinel lymph node metastasis in thin melanoma. J Clin Oncol. 2013;31(35):4387–4393. doi: 10.1200/JCO.2013.50.1114. [DOI] [PubMed] [Google Scholar]
- 25.Leiter U, Stadler R, Mauch C, Hohenberger W, Brockmeyer N, Berking C, et al. Complete lymph node dissection versus no dissection in patients with sentinel lymph node biopsy positive melanoma (DeCOG-SLT): a multicentre, randomised, phase 3 trial. Lancet Oncol. 2016;17(6):757–767. doi: 10.1016/S1470-2045(16)00141-8. [DOI] [PubMed] [Google Scholar]
- 26.Faries MB, Thompson JF, Cochran AJ, Andtbacka RH, Mozzillo N, Zager JS, et al. Completion dissection or observation for sentinel-node metastasis in melanoma. N Engl J Med. 2017;376(23):2211–2222. doi: 10.1056/NEJMoa1613210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morton DL, Wanek L, Nizze JA, Elashoff RM, Wong JH. Improved long-term survival after lymphadenectomy of melanoma metastatic to regional nodes. Analysis of prognostic factors in 1134 patients from the John Wayne Cancer Clinic. Ann Surg. 1991;214(4):491–499. doi: 10.1097/00000658-199110000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strom T, Torres-Roca JF, Parekh A, Naghavi AO, Caudell JJ, Oliver DE, et al. Regional radiation therapy impacts outcome for node-positive cutaneous melanoma. J Natl Compr Canc Netw. 2017;15(4):473–482. doi: 10.6004/jnccn.2017.0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Henderson MA, Burmeister BH, Ainslie J, Fisher R, Di Iulio J, Smithers BM, et al. Adjuvant lymph-node field radiotherapy versus observation only in patients with melanoma at high risk of further lymph-node field relapse after lymphadenectomy (ANZMTG 01.02/TROG 02.01): 6-year follow-up of a phase 3, randomised controlled trial. Lancet Oncol. 2015;16(9):1049–1060. doi: 10.1016/S1470-2045(15)00187-4. [DOI] [PubMed] [Google Scholar]
- 30.Weber J, Mandala M, Del Vecchio M, Gogas HJ, Arance AM, Cowey CL, et al. Adjuvant Nivolumab versus Ipilimumab in resected stage III or IV Melanoma. N Engl J Med. 2017;377(19):1824–1835. doi: 10.1056/NEJMoa1709030. [DOI] [PubMed] [Google Scholar]
- 31.Ascierto PA, Del Vecchio M, Mandala M, Gogas H, Arance AM, Dalle S, et al. Adjuvant nivolumab versus ipilimumab in resected stage IIIB-C and stage IV melanoma (CheckMate 238): 4-year results from a multicentre, double-blind, randomised, controlled, phase 3 trial. Lancet Oncol. 2020;21(11):1465–1477. doi: 10.1016/S1470-2045(20)30494-0. [DOI] [PubMed] [Google Scholar]
- 32.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson VG, Dalle S, et al. Longer follow-up confirms recurrence-free survival benefit of adjuvant pembrolizumab in high-risk stage III Melanoma: Updated results from the EORTC 1325-MG/KEYNOTE-054 trial. J Clin Oncol. 2020;38(33):3925–3936. doi: 10.1200/JCO.20.02110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Eggermont AMM, Blank CU, Mandala M, Long GV, Atkinson V, Dalle S, et al. Adjuvant Pembrolizumab versus placebo in resected stage III Melanoma. N Engl J Med. 2018;378(19):1789–1801. doi: 10.1056/NEJMoa1802357. [DOI] [PubMed] [Google Scholar]
- 34.Long GV, Hauschild A, Santinami M, Atkinson V, Mandala M, Chiarion-Sileni V, et al. Adjuvant dabrafenib plus trametinib in stage III BRAF-mutated Melanoma. N Engl J Med. 2017;377(19):1813–1823. doi: 10.1056/NEJMoa1708539. [DOI] [PubMed] [Google Scholar]
- 35.Hauschild A, Dummer R, Schadendorf D, Santinami M, Atkinson V, Mandala M, et al. Longer follow-up confirms relapse-free survival benefit with adjuvant dabrafenib plus trametinib in patients with resected BRAF V600-Mutant stage III Melanoma. J Clin Oncol. 2018;36(35):3441–3449. doi: 10.1200/JCO.18.01219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dummer R, Brase JC, Garrett J, Campbell CD, Gasal E, Squires M, et al. Adjuvant dabrafenib plus trametinib versus placebo in patients with resected, BRAF(V600)-mutant, stage III melanoma (COMBI-AD): exploratory biomarker analyses from a randomised, phase 3 trial. Lancet Oncol. 2020;21(3):358–372. doi: 10.1016/S1470-2045(20)30062-0. [DOI] [PubMed] [Google Scholar]
- 37.Dummer R, Hauschild A, Santinami M, Atkinson V, Mandala M, Kirkwood JM, et al. Five-year analysis of adjuvant Dabrafenib plus Trametinib in stage III Melanoma. N Engl J Med. 2020;383(12):1139–1148. doi: 10.1056/NEJMoa2005493. [DOI] [PubMed] [Google Scholar]
- 38.Owen CN, Shoushtari AN, Chauhan D, Palmieri DJ, Lee B, Rohaan MW, et al. Management of early melanoma recurrence despite adjuvant anti-PD-1 antibody therapy() Ann Oncol Off J Eur Soc Med Oncol. 2020;31(8):1075–1082. doi: 10.1016/j.annonc.2020.04.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sosman JA, Moon J, Tuthill RJ, Warneke JA, Vetto JT, Redman BG, et al. A phase 2 trial of complete resection for stage IV melanoma: results of Southwest Oncology Group Clinical Trial S9430. Cancer. 2011;117(20):4740–4746. doi: 10.1002/cncr.26111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Robert C, Thomas L, Bondarenko I, O’Day S, Weber J, Garbe C, et al. Ipilimumab plus dacarbazine for previously untreated metastatic melanoma. N Engl J Med. 2011;364(26):2517–2526. doi: 10.1056/NEJMoa1104621. [DOI] [PubMed] [Google Scholar]
- 41.Ascierto PA, Del Vecchio M, Robert C, Mackiewicz A, Chiarion-Sileni V, Arance A, et al. Ipilimumab 10 mg/kg versus ipilimumab 3 mg/kg in patients with unresectable or metastatic melanoma: a randomised, double-blind, multicentre, phase 3 trial. Lancet Oncol. 2017;18(5):611–622. doi: 10.1016/S1470-2045(17)30231-0. [DOI] [PubMed] [Google Scholar]
- 42.Ascierto PA, Long GV, Robert C, Brady B, Dutriaux C, Di Giacomo AM, et al. Survival outcomes in patients with previously untreated BRAF wild-type advanced melanoma treated with nivolumab therapy: three-year follow-up of a randomized phase 3 trial. JAMA Oncol. 2019;5(2):187–194. doi: 10.1001/jamaoncol.2018.4514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Robert C, Long GV, Brady B, Dutriaux C, Maio M, Mortier L, et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med. 2015;372(4):320–330. doi: 10.1056/NEJMoa1412082. [DOI] [PubMed] [Google Scholar]
- 44.Schachter J, Ribas A, Long GV, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab for advanced melanoma: final overall survival results of a multicentre, randomised, open-label phase 3 study (KEYNOTE-006) Lancet. 2017;390(10105):1853–1862. doi: 10.1016/S0140-6736(17)31601-X. [DOI] [PubMed] [Google Scholar]
- 45.Robert C, Ribas A, Schachter J, Arance A, Grob JJ, Mortier L, et al. Pembrolizumab versus ipilimumab in advanced melanoma (KEYNOTE-006): post hoc 5-year results from an open-label, multicentre, randomised, controlled, phase 3 study. Lancet Oncol. 2019;20(9):1239–1251. doi: 10.1016/S1470-2045(19)30388-2. [DOI] [PubMed] [Google Scholar]
- 46.Larkin J, Hodi FS, Wolchok JD. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N Engl J Med. 2015;373(13):1270–1271. doi: 10.1056/NEJMc1509660. [DOI] [PubMed] [Google Scholar]
- 47.Wolchok JD, Chiarion-Sileni V, Gonzalez R, Rutkowski P, Grob JJ, Cowey CL, et al. Overall survival with combined nivolumab and ipilimumab in advanced Melanoma. N Engl J Med. 2017;377(14):1345–1356. doi: 10.1056/NEJMoa1709684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Espinosa E, Marquez-Rodas I, Soria A, Berrocal A, Manzano JL, Gonzalez-Cao M, et al. Predictive factors of response to immunotherapy-a review from the Spanish Melanoma Group (GEM) Ann Transl Med. 2017;5(19):389. doi: 10.21037/atm.2017.08.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Andtbacka RH, Kaufman HL, Collichio F, Amatruda T, Senzer N, Chesney J, et al. Talimogene laherparepvec improves durable response rate in patients with advanced melanoma. J Clin Oncol. 2015;33(25):2780–2788. doi: 10.1200/JCO.2014.58.3377. [DOI] [PubMed] [Google Scholar]
- 50.Liszkay G, Gogas H, Mandalà M, Fernandez AMA, Garbe C, Schadendorf D, et al. Update on overall survival in COLUMBUS: a randomized phase III trial of encorafenib (ENCO) plus binimetinib (BINI) versus vemurafenib (VEM) or ENCO in patients with BRAF V600–mutant melanoma. J Clin Oncol. 2019;37(15_suppl):9512. [Google Scholar]
- 51.Dreno B, Ascierto PA, McArthur GA, Atkinson V, Liszkay G, Giacomo AMD, et al. Efficacy and safety of cobimetinib (C) combined with vemurafenib (V) in patients (pts) with BRAFV600 mutation–positive metastatic melanoma: analysis from the 4-year extended follow-up of the phase 3 coBRIM study. J Clin Oncol. 2018;36(15):9522. [Google Scholar]
- 52.Long GV, Grob JJ, Nathan P, Ribas A, Robert C, Schadendorf D, et al. Factors predictive of response, disease progression, and overall survival after dabrafenib and trametinib combination treatment: a pooled analysis of individual patient data from randomised trials. Lancet Oncol. 2016;17(12):1743–1754. doi: 10.1016/S1470-2045(16)30578-2. [DOI] [PubMed] [Google Scholar]
- 53.Hauschild A, Larkin J, Ribas A, Dréno B, Flaherty KT, Ascierto PA, et al. Modeled prognostic subgroups for survival and treatment outcomes in BRAF V600-mutated metastatic melanoma: pooled analysis of 4 randomized clinical trials. JAMA Oncol. 2018;4(10):1382–1388. doi: 10.1001/jamaoncol.2018.2668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ugurel S, Röhmel J, Ascierto PA, Flaherty KT, Grob JJ, Hauschild A, et al. Survival of patients with advanced metastatic melanoma: the impact of novel therapies-update 2017. Eur J Cancer (Oxford, England : 1990) 2017;83:247–257. doi: 10.1016/j.ejca.2017.06.028. [DOI] [PubMed] [Google Scholar]
- 55.Zimmer L, Apuri S, Eroglu Z, Kottschade LA, Forschner A, Gutzmer R, et al. Ipilimumab alone or in combination with nivolumab after progression on anti-PD-l therapy in advanced melanoma. Eur J Cancer (Oxford, England: 1990) 2017;75:47–55. doi: 10.1016/j.ejca.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Aya F, Fernandez-Martinez A, Gaba L, Victoria I, Tosca M, Pineda E, et al. Sequential treatment with immunotherapy and BRAF inhibitors in BRAF-mutant advanced melanoma. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mexico. 2017;19(1):119–124. doi: 10.1007/s12094-016-1514-0. [DOI] [PubMed] [Google Scholar]
- 57.Dummer R, Ascierto PA, Gogas HJ, Arance A, Mandala M, Liszkay G, et al. Encorafenib plus binimetinib versus vemurafenib or encorafenib in patients with BRAF-mutant melanoma (COLUMBUS): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2018;19(5):603–615. doi: 10.1016/S1470-2045(18)30142-6. [DOI] [PubMed] [Google Scholar]
- 58.Beaver JA, Hazarika M, Mulkey F, Mushti S, Chen H, He K, et al. Patients with melanoma treated with an anti-PD-1 antibody beyond RECIST progression: a US Food and Drug Administration pooled analysis. Lancet Oncol. 2018;19(2):229–239. doi: 10.1016/S1470-2045(17)30846-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Michielin O, van Akkooi ACJ, Ascierto PA, Dummer R, Keilholz U. Cutaneous melanoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(12):1884–1901. doi: 10.1093/annonc/mdz411. [DOI] [PubMed] [Google Scholar]
- 60.Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, et al. Estimating survival in melanoma patients with brain metastases: an update of the graded prognostic assessment for melanoma using molecular markers (Melanoma-molGPA) Int J Radiat Oncol Biol Phys. 2017;99(4):812–816. doi: 10.1016/j.ijrobp.2017.06.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wasif N, Bagaria SP, Ray P, Morton DL. Does metastasectomy improve survival in patients with Stage IV melanoma? A cancer registry analysis of outcomes. J Surg Oncol. 2011;104(2):111–115. doi: 10.1002/jso.21903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown PD, Ballman KV, Cerhan JH, Anderson SK, Carrero XW, Whitton AC, et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC.3): a multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18(8):1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N Engl J Med. 2018;379(8):722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Rishi A, Yu HM. Current treatment of melanoma brain metastasis. Curr Treat Opt Oncol. 2020;21(6):45. doi: 10.1007/s11864-020-00733-z. [DOI] [PubMed] [Google Scholar]
- 65.Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, et al. Dabrafenib plus trametinib in patients with BRAF(V600)-mutant melanoma brain metastases (COMBI-MB): a multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol. 2017;18(7):863–873. doi: 10.1016/S1470-2045(17)30429-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rockberg J, Amelio JM, Taylor A, Jorgensen L, Ragnhammar P, Hansson J. Epidemiology of cutaneous melanoma in Sweden-Stage-specific survival and rate of recurrence. Int J Cancer. 2016;139(12):2722–2729. doi: 10.1002/ijc.30407. [DOI] [PubMed] [Google Scholar]
- 67.Dicker TJ, Kavanagh GM, Herd RM, Ahmad T, McLaren KM, Chetty U, et al. A rational approach to melanoma follow-up in patients with primary cutaneous melanoma, Scottish Melanoma Group. Br J Dermatol. 1999;140(2):249–254. doi: 10.1046/j.1365-2133.1999.02657.x. [DOI] [PubMed] [Google Scholar]
- 68.Garbe C, Paul A, Kohler-Spath H, Ellwanger U, Stroebel W, Schwarz M, et al. Prospective evaluation of a follow-up schedule in cutaneous melanoma patients: recommendations for an effective follow-up strategy. J Clin Oncol. 2003;21(3):520–529. doi: 10.1200/JCO.2003.01.091. [DOI] [PubMed] [Google Scholar]
- 69.Xing Y, Bronstein Y, Ross MI, Askew RL, Lee JE, Gershenwald JE, et al. Contemporary diagnostic imaging modalities for the staging and surveillance of melanoma patients: a meta-analysis. J Natl Cancer Inst. 2011;103(2):129–142. doi: 10.1093/jnci/djq455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Podlipnik S, Carrera C, Sanchez M, Arguis P, Olondo ML, Vilana R, et al. Performance of diagnostic tests in an intensive follow-up protocol for patients with American Joint Committee on Cancer (AJCC) stage IIB, IIC, and III localized primary melanoma: a prospective cohort study. J Am Acad Dermatol. 2016;75(3):516–524. doi: 10.1016/j.jaad.2016.02.1229. [DOI] [PubMed] [Google Scholar]
- 71.Podlipnik S, Moreno-Ramirez D, Carrera C, Barreiro A, Manubens E, Ferrandiz-Pulido L, et al. Cost-effectiveness analysis of imaging strategy for an intensive follow-up of patients with American Joint Committee on Cancer stage IIB, IIC and III malignant melanoma. Br J Dermatol. 2019;180(5):1190–1197. doi: 10.1111/bjd.16833. [DOI] [PubMed] [Google Scholar]
- 72.Schroer-Gunther MA, Wolff RF, Westwood ME, Scheibler FJ, Schurmann C, Baumert BG, et al. F-18-fluoro-2-deoxyglucose positron emission tomography (PET) and PET/computed tomography imaging in primary staging of patients with malignant melanoma: a systematic review. Syst Rev. 2012;1:62. doi: 10.1186/2046-4053-1-62. [DOI] [PMC free article] [PubMed] [Google Scholar]