Highlights
-
•
DCA in most cases is superior to VMAT for multi metastases single isocenter SRS.
-
•
Normal brain V12Gy was significantly reduced with DCA, predicting for lower S-NEC.
-
•
Maximum doses to critical organs-at-risk were significantly lower with DCA.
-
•
Conformity was comparable between VMAT and DCA.
Keywords: Multiple brain metastases, Single isocenter SRS planning techniques, SRS, Radiosurgery
Abstract
Background and purpose
Whole brain radiation therapy use has decreased in favor of stereotactic radiosurgery (SRS) for the treatment of multiple brain metastases due to reduced neurotoxicity. Here we compare two single isocenter radiosurgery planning techniques, volumetric modulated arc therapy (VMAT) and dynamic conformal arcs (DCA) in terms of their dosimetric and delivery performance.
Materials and methods
Sixteen patients with 2– 18 brain metastases (total 103; median 4) previously treated with single fraction SRS were replanned for multiple lesion single isocenter treatments using VMAT and DCA using different treatment planning systems for each and three different plan geometries for DCA. Plans were evaluated using the Paddick conformity index, normal tissue V12Gy, the probability for symptomatic brain necrosis (S-NEC), maximum organ-at-risk (OAR) point doses, and total number of monitor units (MU).
Results
Conformity was not significantly different between VMAT and DCA plans. VMAT plans showed a trend towards higher MU with a median difference between 18% and 24% (p ≤ 0.09). Median V12Gy differences were 7.0 cm3–8.6 cm3 favoring DCA plans (p < 0.01). VMAT plans had median excess absolute and relative S-NEC risks compared to DCA plans of 8%–10% and 25%–31%, respectively (p < 0.01). Moreover for VMAT compared to DCA, maximum OAR doses were significantly higher for the brainstem (1.9 Gy; p < 0.01), chiasm (0.5 Gy; p ≤ 0.02), and optic nerves (0.5 Gy; p ≤ 0.04).
Conclusions
In most cases DCA plans were found to be dosimetrically superior to VMAT plans with reduced V12Gy and associated risk for S-NEC. Maximum doses to important OARs showed significant improvement, increasing the ability for subsequent salvage treatments involving radiation.
1. Introduction
The management of secondary malignancies of the brain, which occur in 20%–40% of patients with cancer, has traditionally involved whole-brain radiation therapy (WBRT) with the addition of radical local therapy like surgical resection and stereotactic radiosurgery (SRS) [1], [2]. WBRT, however, has been associated with neurological and neurocognitive decline in patients [3], [4] increasing the interest in partial brain irradiation techniques including SRS to multiple metastases. Furthermore, SRS can be repeated to treat lesions as they occur on imaging or become symptomatic, before hippocampal avoidant WBRT is used as a salvage treatment [5], [6].
The treatment of multiple metastases using a single isocenter for each increases the treatment time almost linearly with the number of lesions. Multi leaf collimator (MLC) equipped medical linear accelerators (linacs) however, offer the possibility to irradiate multiple lesions at the same time using either volumetric modulated arc therapy (VMAT) [7] or dynamic conformal arcs (DCA) [8].
Over the last years, SRS has been increasingly used as the upfront treatment for patients presenting with multiple metastases. VMAT has been used to treat multiple lesions using a single treatment isocenter to achieve high conformality and reduce overall treatment time compared to multiple isocenter DCA plans [7]. While VMAT can achieve superior conformality single isocenter DCA can achieve otherwise similar plan quality with increased dose gradients and reduced low-to-intermediate doses [8] which have been associated with symptomatic brain necrosis (S-NEC) [9]. Recently, a dedicated multi-target single isocenter DCA treatment planning system (TPS) was commissioned for clinical use at our institution. From a user-defined set of couch positions and gantry angle ranges this TPS optimizes collimator rotations, the number of gantry passes at each couch angle, and treatment apertures to minimize irradiation of normal tissue and maximize conformity of prescription dose to targets.
The performance of this single isocenter DCA technique was compared by Gevaert et al. to multiple isocenter DCA yielding comparable plans [10]. Compared to single isocenter VMAT, Gevaert et al. and Narayanasamy et al. found overall plan quality to be similar, while low to intermediate doses to normal tissue were reduced using DCA [10], [11]. Gevaert et al. included ten patients with one to eight metastases (median three), while Narayanasamy et al. included eight patients with three to seven lesions (median five) each. Vergalasova et al. compared single isocenter DCA with manual VMAT techniques for 16 patients, finding no significant difference in overall plan quality [12]. Another study by Hofmaier et al. [13] compared single isocenter DCA with VMAT finding improved healthy brain sparing but less conformity for irregular targets with DCA. Liu et al. [14] compared single isocenter DCA and VMAT for 30 patients with four to ten metastases (median 7.5) from two institutions and found that DCA performed more favorably at low (V5Gy, mean brain dose) and, in contrast to Gevaert et al., Narayanasamy et al., and Hofmaier et al. [13] that VMAT performed better for intermediate doses (V8Gy, V12Gy). Better conformality was achieved using VMAT compared to DCA plans [12], [14].
The aim of this study was to compare the performance of single isocenter DCA with VMAT in a larger patient cohort and for more lesions per case than were included in previous studies. Specifically, we aimed to investigate the difference in normal tissue sparing at intermediate doses and evaluate it in terms of the associated risk of symptomatic brain necrosis.
2. Materials and methods
Sixteen patients with 2–18 brain metastases (total 103; median 4) who were previously treated with single fraction SRS using a TrueBeam STx (Varian Medical Systems, Palo Alto, CA) equipped with a HD120 MLC at our institution were selected for this institutional review board exempt study. Treated plans used single or multiple isocenters employing DCA or VMAT or a combination thereof using a flattened 6 MV beam and Brainlab ExacTrac for initial patient setup and intra-fraction verification. These plans were originally created using the Brainlab iPlan and Varian Eclipse treatment planning systems (TPS). For this study, all treatments were replanned by the same physicist (more than one year of SRS experience) for single isocenter DCA and VMAT using Brainlab Elements Multiple Brain Mets SRS (MME) version 2 and Eclipse version 15.6, respectively. Plans were optimized and dose calculated on a planning computed tomography scan with 0.82 mm/px resolution in the axial plane and 1.25 mm slice thickness. Dose calculation algorithms were pencil beam convolution in MME and AAA in Eclipse with a dose grid size of 1 mm in both TPS.
Planning target volumes (PTV) were created from magnetic resonance imaging-defined gross tumor volumes by applying a 1 mm radial margin. Prescribed doses were 15 Gy to lesions having a largest diameter ≤4 cm and ≥3 cm, 18 Gy to lesions with a largest diameter <3 cm and ≥2 cm, and 21 Gy or 24 Gy to lesions having a largest diameter <2 cm. Coverage, the relative volume of the PTV receiving at least the prescription dose (V100%), was at least 99%, but ideally 99.5%.
2.1. Dynamic conformal arc planning technique
MME automatically placed the isocenter at the geometric center of all selected targets and mirrored left/right-sided templates to minimize the cumulative radiological path length. During optimization collimator angles were automatically chosen to prevent island blocking at each gantry pass and to minimize the effective field sizes. If all targets could not be treated by a single arc at the same time, another arc was added at the same couch angle for a maximum of two arcs per couch angle. Subsequently, the arc weights were automatically optimized to yield optimal conformity.
A subset of cases was used to evaluate different beam configurations (setups) – number of couch angles and gantry angle ranges – to create pre-defined beam setups (cf. Suppl. Fig. S1). From these three general purpose setups were created consisting of an asymmetric 4 angle setup, an asymmetric 5 angle setup, and a symmetric 6 angle setup. The 4-angle setup (4A) used 0°, 320°, 290°, and 60° couch angles with a 130° arc spread while, the 5-angle setup (5A) used 0°, 325°, 290°, 65°, and 30° couch angles with an arc spread of 160°. These two asymmetric templates were intended to be used for one to three lesions or lesion clusters away from midline. For cases that require more degrees of freedom and lesion clusters more uniformly distributed throughout the brain a symmetric 6-angle setup (6A) was created at 5°, 40°, 75°, 285°, 320°, 355°. The arc spread was again set to 130°.
In this study all plan setups were used to create DCA plans without making individual modifications to either couch or gantry angles. Plans were created using “SRS Prescription” mode, adding a D5% objective to each target (controlled inhomogeneity) whose dose value was calculated based on the isodose line prescription percentage with a default of 80%. The variance of monitor units (MU) between arcs was not penalized, by setting the MU spread to high. During preliminary testing it was found that using a high MU spread setting leads to the lowest total number of monitor units. No additional optimization parameters were used; while MME allows for the use of organ-at-risk (OAR) dose constraints their use was found to have little effect on OAR doses if the targets are sufficiently far from other structures.
2.2. Volumetric modulated arc therapy planning technique
VMAT plans used a single isocenter placed at the center of the union of all planning target volumes (PTVs) with one to four couch angles, chosen based on the number and distribution of lesions. Coplanar and moderately non-coplanar setups with couch angles ±10° from zero utilized full arcs spanning at least 300° with complementary collimator settings. Non-coplanar setups with three to four couch angles also used a full arc at 0° couch angle. Three-angle setups used couch angles of 0°, 40°–60°, and 320°–300°, while four-angle setups used partial arcs with couch angles spaced 40° apart starting at zero following the DCA setup for single lesions outlined above. Collimator angles were manually optimized to minimize island blocking, which was found to increase the MLC leaf pattern complexity.
These setups were chosen based on prior clinical experience with VMAT for multi-lesion single isocenter treatments: cases with large number of lesions with a more uniform distribution throughout the cranium benefitted least from non-coplanar setups, due to the usually large aperture sizes and the resulting complex MLC patterns. It was further found that increasing the number of arcs, corresponding to a larger cumulative arc length, did not improve plan quality, but severely increased the total number of monitor units and subjectively increased plan complexity. Thus, multiple arcs at the same couch angle were not allowed, unless they were treating different areas of the cranium.
Optimization ring structures were created having distances of 1, 5, and 10 mm from the edge of the PTVs to drive conformality of high and intermediate doses by setting the maximum dose constraints in those structures to 95%, 70%–75%, and 40%–60% of prescription dose, respectively. Separate ring structures were created per prescription dose level. Additionally, the normal tissue objective was enabled. To avoid overly modulated plans a MU objective was used with a maximum limit chosen based on experience with DCA plans; its priority was at least 50 (possible range 0–100). The volume of normal brain receiving 12 Gy or more (V12Gy) was controlled by adjusting a V10Gy constraint as the optimization progresses. Additional dose-volume constraints were added as needed. Maximum dose constraints were added for OARs only in cases with maximum doses exceeding several gray.
2.3. Dose and delivery metrics
Dosimetric indices analyzed included the inverse Paddick conformity index (CI) [15] the volume of normal brain receiving 12 Gy or more (V12Gy), as well as maximum point doses received by the brainstem, chiasm, optic nerves, and eyes. The inverse Paddick conformity index was calculated by
where PIV denotes the prescription isodose volume, TV the target volume, and is the target volume covered by the prescription isodose. The CI was calculated for each lesion separately and subsequently volume averaged or, where not possible due to close proximity of individual targets, for the aggregate target volume. Using the normal brain V12Gy the probability for symptomatic brain necrosis (S-NEC) was estimated using a published logistic regression model [9]
with literature reported parameters, , [7]. S-NEC differences between plans for the same patient were compared in terms of excess absolute and relative risk.
Indices used in this evaluation were TPS-reported using the respective implementation of dose-volume histogram (DVH) calculation and internal dose grid. DVHs can vary between different treatment planning systems depending on the implementation, especially concerning frequency and location of dose sampling [16], [17]. While the use of third-party software for DVH metric calculation could have overcome these differences, this would not have reflected the clinical use case. Treatment plans generated in different planning systems, here Elements and Eclipse, were optimized and ultimately reviewed by the treating physician in their respective TPS. Similarly, the dose metrics reported on the treatment plan printouts were calculated using each system’s DVH calculation implementation and internal dose grid, which can differ from the exported dose file in DICOM format.
The total number of monitor units delivered indicated how efficiently the radiation beam is used to deliver the same prescription dose. Lower MUs corresponded to less complex MLC motions reducing the possibility for dosimetric errors and reducing overall dose calculation uncertainty since larger average beam apertures are used.
Median metric values and interquartile ranges (IQR) were reported for VMAT and MME plans. All dosimetric and delivery metrics were compared using a paired two-sided Wilcoxon signed-rank test with a 0.05 level of significance.
3. Results
All plans created in this study were clinically acceptable and achieved the minimum target prescription dose coverage goal of 99%. The median cumulative tumor volume was 4.0 cm3 (IQR 7.2 cm3). Median conformity indices for different MME setups were between 1.45 and 1.51 with IQRs of 0.25–0.34 and 1.38 (0.29) for VMAT. Median normal brain V12Gy was 17.9 cm3 (58.7 cm3) in VMAT and 7.6 cm3–9.7 cm3 (26.2 cm3–33.3 cm3) in MME plans. Median values and interquartile ranges (IQR) for all investigated metrics are given in Table 1 for VMAT and MME plans.
Table 1.
Median values and interquartile ranges for investigated metrics for VMAT and three different MME setups. The median cumulative tumor volume was 4.0 cm3 (IQR 7.2 cm3).
| VMAT |
MME 4A |
MME 5A |
MME 6A |
|||||
|---|---|---|---|---|---|---|---|---|
| MED | (IQR) | MED | (IQR) | MED | (IQR) | MED | (IQR) | |
| CI | 1.38 | (0.29) | 1.51 | (0.34) | 1.45 | (0.34) | 1.48 | (0.25) |
| V12Gy (cm3) | 17.9 | (58.7) | 9.7 | (33.3) | 7.6 | (27.2) | 8.3 | (26.2) |
| S-NEC | 36% | (71%) | 23% | (46%) | 23% | (41%) | 24% | (40%) |
| Brainstem (Gy) | 6.95 | (6.69) | 4.09 | (7.01) | 4.02 | (3.65) | 4.09 | (3.84) |
| Chiasm (Gy) | 2.66 | (3.17) | 1.85 | (2.49) | 1.36 | (1.63) | 1.47 | (2.05) |
| Eye, Lt (Gy) | 1.23 | (1.47) | 1.27 | (1.53) | 1.10 | (0.99) | 1.07 | (1.14) |
| Eye, Rt (Gy) | 1.25 | (1.54) | 0.82 | (1.02) | 1.05 | (1.09) | 0.90 | (1.17) |
| Optic Nerve, Lt (Gy) | 1.75 | (3.05) | 1.11 | (1.70) | 1.04 | (1.45) | 1.02 | (2.01) |
| Optic Nerve, Rt (Gy) | 1.99 | (1.86) | 1.21 | (2.47) | 1.55 | (1.74) | 0.96 | (2.19) |
| MU | 10,910 | (7412) | 8972 | (5346) | 9049 | (4910) | 8868 | (5173) |
OAR maximum point doses were significantly higher in VMAT plans for the brainstem (p < 0.01), chiasm (p ≤ 0.02), optic nerves (p ≤ 0.04), and left eye with average median differences of 1.9 Gy (−1.2 to 6.6 Gy), 0.5 Gy (−1.7 to 4.0 Gy), 0.5 Gy (−1.2 to 2.1 Gy), and 0.4 (−0.5 to 2.0). Median differences in maximum doses to the chiasm, optic nerves, and eyes were only significant for some MME setups (cf. Table 2). Median, minimum, and maximum differences between VMAT and different MME setups are reported in Table 2 for dose metrics for which significant differences have been found. Despite not reaching significance (p ≥ 0.05), we have chosen to report CI and monitor unit differences.
Table 2.
Metric differences between VMAT and DCA plans for each MME setup template. Ranges are given as minimum and maximum values. Significant p-values are shown in bold. Differences for other dose metrics were not significant.
| Metric | Setup | Median | Range | p | ||
|---|---|---|---|---|---|---|
| Inverse Paddick CI | 4A | −0.07 | −0.60 | 0.25 | 0.08 | |
| 5A | −0.06 | −0.66 | 0.37 | 0.41 | ||
| 6A | −0.07 | −0.57 | 0.33 | 0.26 | ||
| Normal brain, V12Gy (cm3) | 4A | 7.5 | −10.5 | 77.6 | <0.01 | |
| 5A | 7.0 | 0.3 | 81.0 | <0.01 | ||
| 6A | 8.6 | 1.3 | 82.0 | <0.01 | ||
| Brainstem, Dmax (Gy) | 4A | 1.4 | −1.2 | 6.4 | <0.01 | |
| 5A | 2.2 | 0.5 | 5.6 | <0.01 | ||
| 6A | 2.2 | 0.1 | 6.6 | <0.01 | ||
| Chiasm, Dmax (Gy) | 4A | 0.6 | −1.6 | 3.4 | 0.12 | |
| 5A | 0.5 | −0.5 | 4.0 | <0.01 | ||
| 6A | 0.5 | −1.7 | 2.9 | 0.08 | ||
| Optic Nerves, Dmax (Gy) | 4A | 0.5 | −1.2 | 2.1 | 0.04 | |
| 5A | 0.6 | −0.6 | 1.9 | 0.08 | ||
| 6A | 0.5 | −0.6 | 2.0 | 0.03 | ||
| Eye, Lt, Dmax (Gy) | 4A | 0.2 | −1.2 | 2.0 | 0.30 | |
| 5A | 0.5 | −0.6 | 1.9 | 0.05 | ||
| 6A | 0.4 | −0.5 | 2.0 | 0.01 | ||
| Monitor Units | 4A | 18% | −44% | 91% | 0.09 | |
| 5A | 18% | −43% | 118% | 0.07 | ||
| 6A | 24% | −40% | 105% | 0.07 | ||
Based on the logistic regression model for S-NEC, VMAT plans had a significant (p < 0.01) median excess absolute risk (EAR) of 8%–10% (range −4% to 43%) and excess relative risk (ERR) of 25% to 31% (range −9% to 76%) compared to MME plans. EAR and ERR between different MME plans for the same patient were similar with a median excess risk of less than 1%. Differences for the other metrics were not significant between either VMAT and MME plans or between the different MME plans.
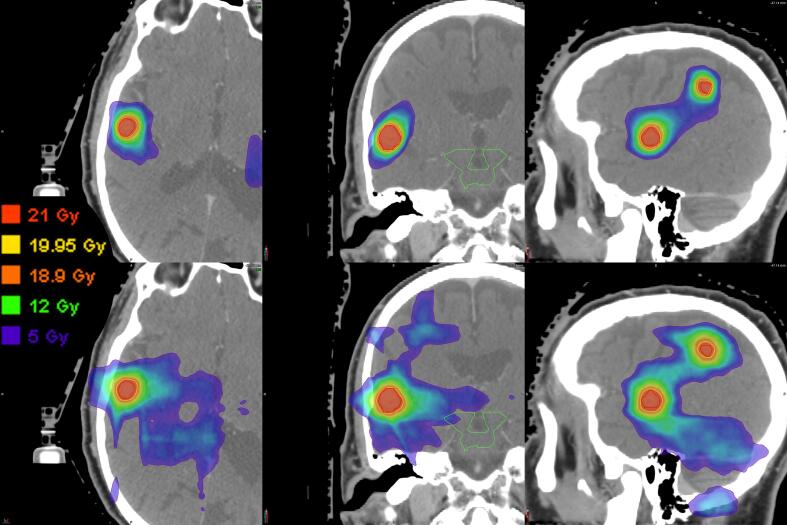
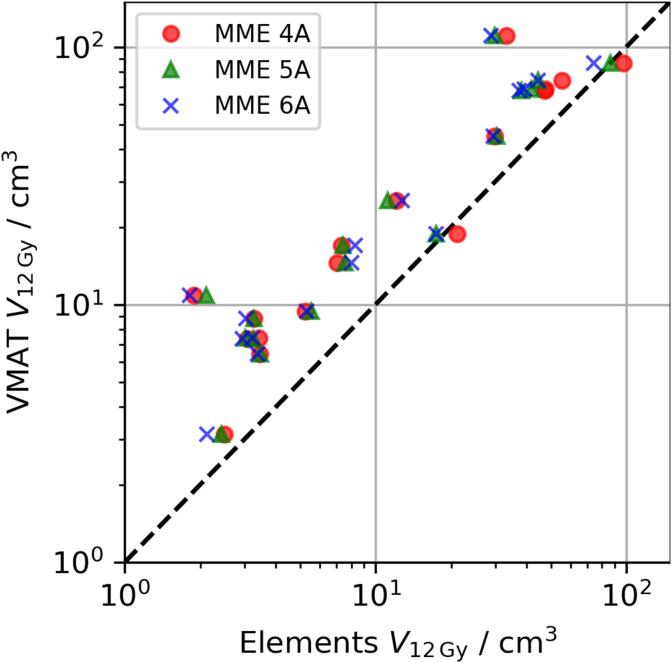
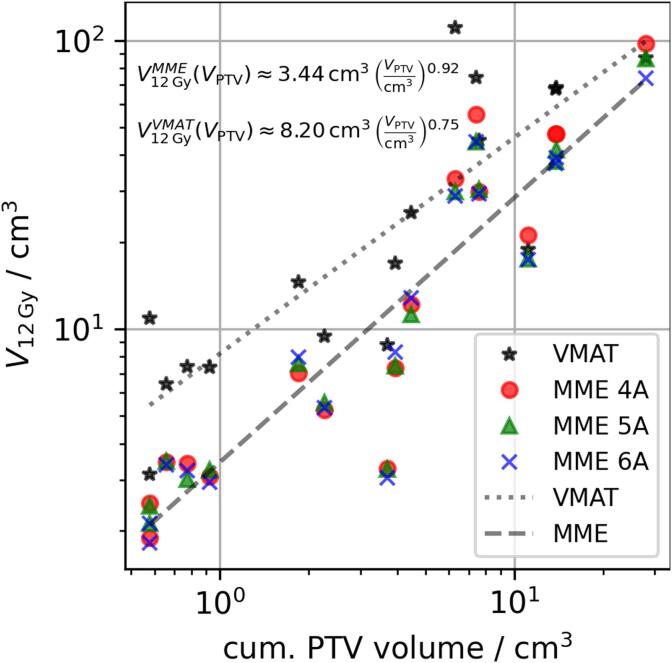
Fig. 1 shows the difference in low to intermediate dose performance between MME and VMAT plans for one example case. Qualitatively, the volume of 12 Gy (green) and 5 Gy (purple) doses was larger for VMAT leading to large dose bridges between targets. This is supported by comparing the normal brain V12Gy between MME and VMAT plans (cf. Table 2 and Fig. 2). For MME plans V12Gy was lower or comparable to that of VMAT plans, with little difference between the different MME setups at low to intermediate volumes. For larger V12Gy, setups with larger total arc length performed better. Increasing treatment volume also increased normal brain V12Gy and the relationships between V12Gy and cumualtive PTV volume were found using linear regression (cf. Fig. 3).
Fig. 1.
Dose distributions for a five-lesion case planned with four DCA (top row) and three arc VMAT (bottom row). The dose levels shown are 21 Gy (red), 19.95 Gy (95%, yellow), 18.9 Gy (90%, orange), 12 Gy (green), and 5 Gy (purple). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Fig. 2.
Comparison of normal brain V12Gy in MME (abscissa) plans and VMAT (ordinate). Markers below the dashed line correspond to higher V12Gy in VMAT plans.
Fig. 3.
Normal brain V12Gy depending on cumulative PTV volumes for MME and VMAT plans. Log-log regressions are shown for MME (dashed, averaged for all setups) and VMAT (dotted).
Again, MME performed better than VMAT with the difference between them narrowing for larger treatment volumes. The normal brain V12Gy ratio between both treatment planning systems, , is approximately 2.4, 1.6, and 1.2 for total target volumes of 1, 10, and 50 cm3, respectively. In two cases VMAT reduced the normal brain V12Gy compared to the MME four angle setup: from 21.1 to 18.8 cm3 treating two lesions and from 97.3 to 86.7 cm3 treating nine lesions.
4. Discussion
In this study we have found significant dosimetric differences between multi lesion single isocenter plans favoring DCA over VMAT plans. Most significantly, the volume of normal brain receiving 12 Gy or more was reduced in all five and six angle DCA setups compared to VMAT. Only in two cases VMAT performed better than the four angle DCA plans in reducing V12Gy. This highlights that in most cases even the use of a simple DCA (four angle) setup could produce dosimetrically superior plans over VMAT. The results of our study are consistent with those obtained in previous studies [10], [11], [12], [14] with overall similar plan quality in the high dose regime. This confirms the results from Gevaert et al. [10] and Narayanasamy et al. [11] in a larger cohort that also includes cases with more lesions. However, contrary to Liu et al. we found that DCA outperformed VMAT at intermediate doses (V12Gy). The distribution of low and intermediate doses in VMAT can depend strongly on the choice of table, gantry, and collimator rotations. In most cases we followed a similar approach as described by Liu et al. [14] and Clark et al. [18]. Compared to Liu et al. [14] we used more distinct DCA plan setups and evaluated plans created with each setup with each other and with VMAT. Furthermore, our planning approach for VMAT plans was more dynamic and less prescriptive to follow the same approach and trade-offs we would apply in a clinical situation. We found that regardless of beam setup the inverse optimization engine in Eclipse creates highly complex MLC leaf motions, which also increases the total number of monitor units. In complicated plans this effect increases with the total number of control points available during optimization and thus, with the cumulative gantry angle span. This could also lead to an increase in dosimetric uncertainty to the larger number of small MLC apertures. To reduce this uncertainty, we forced a monitor unit reduction by using maximum MU objectives, which leads to less MLC complexity, but possibly also increases the volume of normal tissue receiving intermediate doses. To decrease the MLC leaf motion complexity in VMAT one could increase the number of arcs or limit the lesions treated per arc to reduce island blocking and heavy modulation. This, however, would come at a significant increase in planning and treatment time, especially for larger numbers of lesions.
For DCA plans normal brain V12Gy increased almost linearly with the cumulative target volume, with an exponent of 0.92 from log-log regression. This was expected since DCA plans optimize the collimator rotations and MLC apertures to prevent island blocking and only expose one target in the direction of leaf travel. The volume receiving 12 Gy or more in DCA plans depends primarily on the prescription level and MLC aperture size. As the volume projected by the MLC aperture increases linearly with target volume, the V12Gy can also be expected to follow the same dependency. VMAT, however, generates complex leaf motions, distributing small MLC apertures over the entire treated volume. Together with island blocking this inadvertently leads to more MLC openings (minimum leaf gap) over normal tissue as MLC leaves move between different targets within one gantry pass. This explains the higher V12Gy in VMAT especially at smaller cumulative target volumes as MLC leaves move between these smaller targets, exposing a relatively large volume of normal tissue. For large cumulative target volumes, the ratio of normal tissue to target volume becomes smaller leading to more leaf openings over targets, corresponding to the smaller observed exponent of 0.75.
While prescription dose conformity was somewhat better but not statistically significant for VMAT, corresponding to lower volumes irradiated to high doses, all CIs were within a commonly clinically acceptable range, (≤3 acceptable). This range of CIs prevents unnecessarily excessive irradiation of normal brain but also allows for a dosimetric safety margin against small targeting uncertainties in addition to the PTV margin. Despite this, CI values closer to one are usually an indicator of increased plan quality.
In all investigated cases doses to the brainstem, chiasm, optic nerves, or eyes were not heavily constrained as no lesions were abutting these OARs. Due to the high dose gradient in both DCA and VMAT plans these OARs receive doses well below their tolerances by default. However, significant differences in maximum point doses were observed for all OARs between VMAT and DCA plans with DCA plans consistently achieving lower maximum OAR doses. Differences between DCA setups were not significant. The dose reduction to the brainstem showed the largest effect size with median differences of 1.4–2.2 Gy, followed by the chiasm and optic nerves with median differences of 0.5–0.6 Gy. While these differences are negligible in a radiation naïve patient they can accumulate to a substantial cumulative dose if SRS is used as salvage treatment after WBRT or if the patient will likely undergo multiple courses of SRS. Maximum point doses to the left eye were also found to be significantly lower in six angle DCA plans compared to VMAT. However, dose differences to the eyes are most likely due to isocenter location and gantry angle ranges, which were not specifically optimized for each case, which can explain why there was only a significant difference for the left eye. Similarly, maximum point doses to OARs that are sufficiently far away from the target volumes stem primarily from internal scatter and exit dose which is dictated by the choice of beam arrangement; in our experience, using optimization constraints one is usually not able to substantially reduce these point doses.
Monitor units were higher in VMAT plans compared to DCA plans. This is because the number of MU depends strongly on the distribution, size, and possible island blocking of lesions for VMAT, which can achieve efficient plans for cases with few lesions. Additionally, MME always uses at least as many arcs as there are couch angles, which might not be necessary for cases with few lesions. The average difference between the median monitor units given in Table 1 was approximately 1947 MU, corresponding to 3.25 min at 600 MU/min dose rate. This is comparable to the result from Narayanasamy et al. [11] who observed a median difference of 2356 MU favoring DCA.
We acknowledge that the dynamic nature of our VMAT plan creation, despite being planned by the same physicist, may limit the comparability with DCA plans. However, the objective of this study was to dosimetrically compare DCA plans with clinically acceptable and realistic VMAT plans. Additionally, the impact of Varian HyperArc, a workflow dedicated to the treatment of intracranial targets using VMAT could not be assessed in this study. Furthermore, while these results may have some dependence on the make and model of the linear accelerator, most notably the MLC and its minimum leaf width at isocenter, we believe that this will primarily have an impact on conformity, but not significantly change the performance at intermediate doses. A study by Hofmaier et al. [13] compared DCA plans generated with the same TPS with VMAT plans generated in a different TPS for delivery using a different linear accelerator from the one used in this study and found performance differences favoring DCA plans similar to our study. These findings provide additional evidence that the observed differences persist between different VMAT optimization algorithms and treatment planning systems. By extension this may also hold true for different DCA optimization algorithms.
This study demonstrated that multi lesion single isocenter SRS treatments using DCA can consistently produce dosimetrically superior plans compared to VMAT. The volume of normal brain receiving 12 Gy or more, which had been correlated to the probability of symptomatic brain necrosis, as well as maximum point doses to important OARs showed significant improvement.
Funding
None.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.phro.2021.01.002.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Arc geometries used in Brainlab Elements with four (4A), five (5A), and six (6A) couch positions. The 4A and 5A setups are asymmetric, while 6A is symmetric.
References
- 1.Smith M.L., Lee J.Y.K. Stereotactic radiosurgery in the management of brain metastasis. Neurosurg Focus. 2007;22:1–8. doi: 10.3171/foc.2007.22.3.6. [DOI] [PubMed] [Google Scholar]
- 2.Videtic G.M.M., Gaspar L.E., Aref A.M., Germano I.M., Goldsmith B.J., Imperato J.P. American college of radiology appropriateness criteria on multiple brain metastases. Int J Radiat Oncol Biol Phys. 2009;75:961–965. doi: 10.1016/j.ijrobp.2009.07.1720. [DOI] [PubMed] [Google Scholar]
- 3.McDuff S.G.R., Taich Z.J., Lawson J.D., Sanghvi P., Wong E.T., Barker F.G. Neurocognitive assessment following whole brain radiation therapy and radiosurgery for patients with cerebral metastases. J Neurol Neurosurg Psychiatry. 2013;84:1384–1391. doi: 10.1136/jnnp-2013-305166. [DOI] [PubMed] [Google Scholar]
- 4.Manapov F., Käsmann L., Roengvoraphoj O., Dantes M., Schmidt-Hegemann N.-S., Belka C. Prophylactic cranial irradiation in small-cell lung cancer: update on patient selection, efficacy and outcomes. Lung Cancer Targets Ther. 2018;9:49–55. doi: 10.2147/LCTT.S137577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gondi V., Pugh S.L., Tomé W.A., Caine C., Corn B., Kanner A. Preservation of memory with conformal avoidance of the hippocampal neural stem-cell compartment during whole-brain radiotherapy for brain metastases (RTOG 0933): a phase II multi-institutional trial. J Clin Oncol. 2014;32:3810–3816. doi: 10.1200/JCO.2014.57.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown P.D., Gondi V., Pugh S., Tomé W.A., Wefel J.S., Armstrong T.S. Hippocampal avoidance during whole-brain radiotherapy plus memantine for patients with brain metastases: phase III trial NRG oncology CC001. J Clin Oncol. 2020;38:1019–1029. doi: 10.1200/JCO.19.02767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hardcastle N., Tomé W.A. On a single isocenter volumetric modulated arc therapy SRS planning technique for multiple brain metastases. J Radiosurg SBRT. 2012;2:1–9. [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Y., Chin K., Robbins J.R., Kim J., Li H., Amro H. Radiosurgery of multiple brain metastases with single-isocenter dynamic conformal arcs (SIDCA) Radiother Oncol. 2014;112:128–132. doi: 10.1016/j.radonc.2014.05.009. [DOI] [PubMed] [Google Scholar]
- 9.Korytko T., Radivoyevitch T., Colussi V., Wessels B.W., Pillai K., Maciunas R.J. 12 Gy gamma knife radiosurgical volume is a predictor for radiation necrosis in non-AVM intracranial tumors. Int J Radiat Oncol Biol Phys. 2006;64:419–424. doi: 10.1016/j.ijrobp.2005.07.980. [DOI] [PubMed] [Google Scholar]
- 10.Gevaert T., Steenbeke F., Pellegri L., Engels B., Christian N., Hoornaert M.-T. Evaluation of a dedicated brain metastases treatment planning optimization for radiosurgery: a new treatment paradigm? Radiat Oncol. 2016;11:13. doi: 10.1186/s13014-016-0593-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Narayanasamy G., Stathakis S., Gutierrez A.N., Pappas E., Crownover R., Floyd J.R., et al. A systematic analysis of 2 monoisocentric techniques for the treatment of multiple brain metastases. Technol Cancer Res Treat. 2017;16:639–644. doi: 10.1177/1533034616666998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vergalasova I., Liu H., Alonso-Basanta M., Dong L., Li J., Nie K. Multi-institutional dosimetric evaluation of modern day stereotactic radiosurgery (SRS) treatment options for multiple brain metastases. Front Oncol. 2019;9:1–12. doi: 10.3389/fonc.2019.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hofmaier J., Bodensohn R., Garny S., Hadi I., Fleischmann D.F., Eder M. Single isocenter stereotactic radiosurgery for patients with multiple brain metastases: dosimetric comparison of VMAT and a dedicated DCAT planning tool. Radiat Oncol. 2019;14:4–11. doi: 10.1186/s13014-019-1315-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu H., Thomas E.M., Li J., Yu Y., Andrews D., Markert J.M. Interinstitutional plan quality assessment of 2 linac-based, single-isocenter, multiple metastasis radiosurgery techniques. Adv Radiat Oncol. 2020;5:1051–1060. doi: 10.1016/j.adro.2019.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paddick I. A simple scoring ratio to index the conformity of radiosurgical treatment plans. J Neurosurg. 2000;93:219–222. doi: 10.3171/jns.2000.93.supplement_3.0219. [DOI] [PubMed] [Google Scholar]
- 16.Ebert M.A., Haworth A., Kearvell R., Hooton B., Hug B., Spry N.A. Comparison of DVH data from multiple radiotherapy treatment planning systems. Phys Med Biol. 2010;55:N337–N346. doi: 10.1088/0031-9155/55/11/N04. [DOI] [PubMed] [Google Scholar]
- 17.Kennedy A.M., Lane J., Ebert M.A. An investigation of the impact of variations of DVH calculation algorithms on DVH dependant radiation therapy plan evaluation metrics. J Phys Conf Ser. 2014;489:012093. doi: 10.1088/1742-6596/489/1/012093. [DOI] [Google Scholar]
- 18.Clark G.M., Popple R.A., Prendergast B.M., Spencer S.A., Thomas E.M., Stewart J.G. Plan quality and treatment planning technique for single isocenter cranial radiosurgery with volumetric modulated arc therapy. Pract Radiat Oncol. 2012;2:306–313. doi: 10.1016/j.prro.2011.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Arc geometries used in Brainlab Elements with four (4A), five (5A), and six (6A) couch positions. The 4A and 5A setups are asymmetric, while 6A is symmetric.