Abstract
Background & Aims
Two SARS-CoV-2 mRNA vaccines were approved to prevent COVID-19 infection, with reported vaccine efficacy of 95%. Liver transplant (LT) recipients are at risk of lower vaccine immunogenicity and were not included in the registration trials. We assessed vaccine immunogenicity and safety in this special population.
Methods
LT recipients followed at the Tel-Aviv Sourasky Medical Center and healthy volunteers were tested for SARS-CoV-2 IgG antibodies directed against the Spike-protein (S) and Nucleocapsid-protein (N) 10–20 days after receiving the second Pfizer-BioNTech BNT162b2 SARS-CoV-2 vaccine dose. Information regarding vaccine side effects and clinical data was collected from patients and medical records.
Results
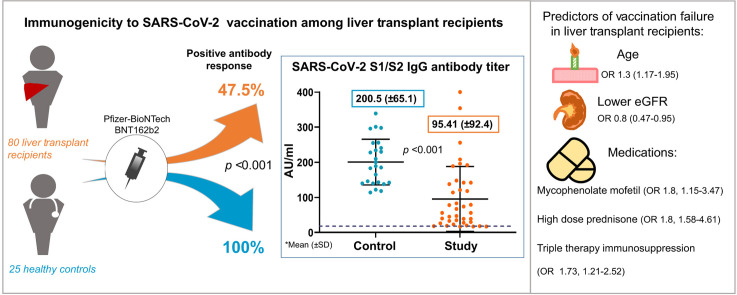
Eighty LT recipients were enrolled. Mean age was 60 years and 30% were female. Twenty-five healthy volunteer controls were younger (mean age 52.7 years, p = 0.013) and mostly female (68%, p = 0.002). All participants were negative for IgG N-protein serology, indicating immunity did not result from prior COVID-19 infection. All controls were positive for IgG S-protein serology. Immunogenicity among LT recipients was significantly lower with positive serology in only 47.5% (p <0.001). Antibody titer was also significantly lower in this group (mean 95.41 AU/ml vs. 200.5 AU/ml in controls, p <0.001). Predictors for negative response among LT recipients were older age, lower estimated glomerular filtration rate, and treatment with high dose steroids and mycophenolate mofetil. No serious adverse events were reported in either group.
Conclusion
LT recipients developed substantially lower immunological response to the Pfizer-BioNTech SARS-CoV-2 mRNA-based vaccine. Factors influencing serological antibody responses include age, renal function and immunosuppressive medications. The findings require re-evaluation of vaccine regimens in this population.
Lay summary
The Pfizer-BioNTech BNT162b2 SARS-CoV-2 vaccine elicited substantially inferior immunity in liver transplant recipients. Less than half of the patients developed sufficient levels of antibodies against the virus, and in those who were positive, average antibody levels were 2x less compared to healthy controls. Factors predicting non-response were older age, renal function and immunosuppressive medications.
Keywords: Liver transplantation, vaccination, COVID-19, Pfizer-BioNTech BNT162b2, SARS-CoV-2 vaccine
Graphical abstract
See Editorial, pages 265–266
Introduction
Two novel mRNA technology-based SARS-CoV-2 vaccines, developed by Pfizer and Moderna, are currently approved, with efficacy reaching 94–95% in clinical trials.1 , 2 Solid organ transplant (SOT) recipients were excluded from those trials, thus, the vaccine's immunogenicity in this population is unknown. Despite the lack of data, vaccination of liver transplant (LT) recipients has been recommended by professional societies.[3], [4], [5] We assessed immunogenicity and safety of 2 doses of the Pfizer-BioNTech BNT162b2 SARS-CoV-2 vaccine among LT recipients.
Patients and methods
Study design
From December 2020, prioritized vaccination of SOT recipients was launched in Israel with the Pfizer-BioNTech BNT162b2 SARS-CoV-2 vaccine, administered in 2 doses given 3 weeks apart. The trial design included a study group of LT recipients followed in our SOT outpatient clinic, and a control group of healthcare workers with no major comorbidities. All participants completed the full vaccination schedule. Exclusion criteria included age <18, inability to provide informed consent, and pregnancy. Participants signed a written informed consent. The Study was approved by the Tel-Aviv Sourasky Medical Center Institutional Review Board.
Patient information
Participants filled in a questionnaire on early 7 days post-vaccination side effects: local (pain, redness, swelling, and lymphadenopathy) and systemic (fever, chills, headache, fatigue, myalgia, arthralgia, nausea and vomiting, diarrhea) on a scale (0-4).
Clinical data was obtained from patients' medical records and routine blood tests up to 3 months prior to the date of the first vaccination. Estimated glomerular filtration rate (eGFR) was calculated with the MDRD formula and adjusted to body surface area (Mosteller calculation).6 Chronic kidney disease (CKD) categories were defined according to KDIGO recommendations.7
SARS-CoV-2 antibodies test
Blood samples were taken 10–20 days after the second vaccination. LIAISON SARS-CoV-2 S1/S2 IgG chemiluminescent assay against a recombinant Spike (S) protein (S1/S2) (DiaSorin S.p.A., Saluggia, Italy) was used according to the manufacture instructions. Results below 12.0 AU/ml were considered negative, 12.0-15.0 AU/ml borderline, and >15 AU/ml positive. A borderline result (1 participant) was considered negative. Detection of IgG antibodies directed against the SARS-CoV-2 nucleocapsid (N) protein was performed with an Architect i2000SR analyzer (Abbot Diagnostics, IL, USA) and Abbott chemistry kit according to the manufacture instructions. A cut-off of 1.4 index (S/C) was used.8
Statistical analysis
Data are displayed as mean (SD) for the continuous variables and as number of patients and percentage in each group for categorical variables. For categorical variables, the Chi-Square statistic was used to assess the statistical significance between groups. Continuous variables were first tested for normal distribution using the Kolmogorov-Smirnov test and Q-Q plots and then compared by t test if normally distributed or by Kruskal Wallis/Mann-Whitney test if abnormally distributed. We fitted binary logistic regression models for the risk of negative serology test including the significant variables found in univariate analysis. p <0.05 was considered statistically significant for all analyses. IBM SPSS Statistics for Windows, version 22 (IBM Corp., Armonk, N.Y., USA) was used for all statistical analyses.
Results
Participant characteristics
Eighty LT recipients and 25 controls were enrolled. The mean age of LT recipients was significantly older than controls (60.1 [ ± 12.8] vs. 52.7 [ ± 11.5] years, respectively, p = 0.013), and female sex was more prevalent in the controls (30% vs. 68%, p = 0.002). The mean time period between administration of the vaccinations and antibody testing after the second dose was similar in both groups 14.8 (±3.2) vs. 15.8 (±2.9) days (p = 0.196) (study and control groups, respectively).
The median time from LT to the first vaccination was 5 years (range 5 months to 37 years). Three patients were transplanted in the 6 months prior to the study and 9 patients in the past year. The most common indications were viral (39, 48.7%), followed by hepatocellular carcinoma (26, 32.5%) and non-alcoholic fatty liver disease (16, 20%). Other indications are listed in Table 1 .
Table 1.
Baseline characteristics for LT recipients and comparison of recipients with negative and positive SARS-CoV-2 S1/S2 IgG serology.
| Parameter | All LT recipients (n = 80) | Negative (n = 42) | Positive (n = 38) | p value |
|---|---|---|---|---|
| Age† (years) | 60.1 ± 12.8 | 63.22 ± 11.9 | 57.78 ± 11.9 | 0.04 |
| Sex, female (%)†† | 24 (30) | 14 (33.3) | 10 (26.3) | 0.63 |
| BMI† | 26.3 ± 4.6 | 25.83 ± 3.9 | 26.8 ± 5.6 | 0.33 |
| Months after first transplantation† | 76.6 ± 74.1 | 71.78 ± 71.2 | 89.2 ± 66.3 | 0.28 |
| Etiology of liver disease∗ | 0.18 | |||
| Viral | 39 (HCV 26, HBV 13) | 20 | 19 | |
| NASH/ALD | 16/3 | 13 | 6 | |
| AIH/PBC/PSC | 6/3/7 | 8 | 8 | |
| Other | 8∗∗ | 2 | 6 | |
| HCC, (%)†† | 26 (32.5) | 14 (33.3) | 12 (31.5) | 0.811 |
| HTN, (%)†† | 45 (56.2) | 25 (62.5) | 20 (51.2) | 0.36 |
| DM, (%)†† | 26 (32.5) | 15 (37.5) | 11 (28.2) | 0.47 |
| High dose steroids last 12 months | 16 (20) | 13 (30.9) | 3 (7.8) | 0.010 |
| Prednisone, (%)†† | 24 (30) | 16 (38) | 8 (21) | 0.086 |
| Daily dose (mg)††† | 5 (2.5-15) | |||
| CNIs, (%)†† | 75 (93.7) | 40 (95.2) | 35 (92.1) | 0.055 |
| Tacrolimus/cyclosporine | 65/10 | |||
| Drug level (ng/ml)††† | 5.9 (2.2-12.8)/72.5 (33-180) | |||
| Everolimus, (%)†† | 18 (22.5) | 9 (21.4) | 11 (28.9) | 0.612 |
| Drug level (ng/ml)††† | 3.45 (1.6-8) | |||
| Azathioprine (%) | 4 (5) | 1 (2.3) | 3 (7.8) | 0.358 |
| MMF, (%)†† | 40 (50) | 25 (59.5) | 15 (39.4) | 0.069 |
| Daily dose (mg)††† | 720 (250-2,000) | |||
| Hb† (g/dl) | 13.4 ± 1.6 | 13.11 ± 1.7 | 14.13 ± 1.5 | 0.04 |
| WBC† (103/μl) | 6.23 ± 2.1 | 5.83 ± 2.2 | 6.74 ± 1.6 | 0.054 |
| PMC† (103/μl) | 3.79 ± 1.5 | 3.63 ± 1.6 | 4.02 ± 1.4 | 0.307 |
| Lymphocyte† (103/μl) | 1.66 ± 0.8 | 1.59 ± 0.8 | 1.75 ± 0.7 | 0.427 |
| Platelet† (103/μl) | 156.6 ± 60.8 | 151.9 ± 60.2 | 168.3 ± 70.3 | 0.282 |
| eGFR† (ml/min) | 64.6 (±26.1) | 56.32 ± 22.4 | 75.17 ± 11.6 | 0.001 |
| Serum albumin† (g/L) | 40.8 ± 10.2 | 39.9 ± 11.0 | 41.8 ± 8.0 | 0.433 |
Date presented as: †Mean ± SD, ††number (percentage), †††Median (range). For all categorical variables, the Chi-Square statistic was used. Continuous variables were compared by using a t test if normally distributed or by Kruskal Wallis/Mann-Whitney test if non-normally distributed. p <0.05 was considered statistically significant for all analyses. ∗Two patients had multiple etiologies (HBV+HCV and HCV + congenital hepatic fibrosis). ∗∗ Other etiologies: Acute liver failure- 2, cryptogenic cirrhosis - 3, Wilson's disease- 1, secondary sclerosing cholangitis- 1 and congenital hepatic fibrosis - 1.
AIH, autoimmune hepatitis; ALD, alcohol-related liver disease; CNIs, calcineurin inhibitors; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate; HCC, hepatocellular carcinoma; HTN, hypertension; MMF, mycophenolate mofetil; NASH, non-alcoholic steatohepatitis; PBC, primary biliary cholangitis; PMC, polymorphonuclear cell; PSC, primary sclerosing cholangitis, WBC, white blood cell.
Calcineurin inhibitors (CNIs) were used as the backbone of the immunosuppressive regimen in 94% of the patients. Everolimus was used in 18 patients (22.5%), mostly in combination with CNIs (16 patients). Forty patients (50%) received mycophenolate mofetil (MMF). Median CNIs and everolimus serum levels and median daily MMF dose and range are presented in Table 1. The majority of patients (50, 62.5%) were treated with a combination of 2 immunosuppressive medications, 17 patients (21.2%) received triple therapy. Two participants were off immunosuppressive treatment. Acute rejection occurred in the year prior to enrollment in 9 patients, 3 required treatment with high dose methyl-prednisolone and 6 were managed with oral prednisone (high dose, 40 mg).
Comorbidities were common among LT recipients, with 63% diagnosed with at least 1 co-morbidity, including hypertension (56%), hyperlipidemia (37%) and diabetes (32.5%). The majority of patients had preserved renal function and mean eGFR was 64.8 ml/min (±24.7). Nonetheless, CKD stage 3, 4 and 5 were diagnosed in 42.5% (34) of patients. All LT recipients had stable graft function prior to vaccination.
SARS-CoV-2 vaccination immunogenicity
All the patients and controls had a negative SARS-CoV-2 N-protein IgG serology test. Protective levels of SARS-CoV-2 S1/S2 IgG antibodies were detected in all of the controls. Only 38/80 LT recipients (47.5%) had positive serology (p <0.001). Furthermore, in LT recipients with a positive serological response, the mean SARS-CoV-2 S1/S2 IgG titer was significantly lower compared to the control group (95.41 [ ± 92.4] vs. 200.5 [ ± 65.1] AU/ml, p <0.001).
Risk factors for negative serology among LT recipients
Comparison of the clinical and laboratory data for the LT recipients with positive and negative SARS-CoV-2 S1/S2 IgG serology is presented in Table 1. Participants with negative serology were significantly older (mean age 63 years (±11.9) compared with 57 years (±11.9) in those with positive serology, p = 0.04), and had significantly lower eGFR (mean 56.32 ± 22.4 ml/min vs. 75.17 ± 11.6 ml/min in the positive serology group, p = 0.001). They also had a higher prevalence of treatment with high dose steroids in the last 12 months and a trend toward more frequent treatment with MMF and CNIs. Comparison of tacrolimus level or MMF dose did not yield statistically significant differences in immunogenicity. Time from transplant was not associated with reduced serological response (9 patients <1 year from LT, 3 patients <6 months from LT). No significant difference in gender, body mass index, etiology of liver disease or comorbidities was noted and we found no correlation between symptoms following the vaccinations and immunogenicity.
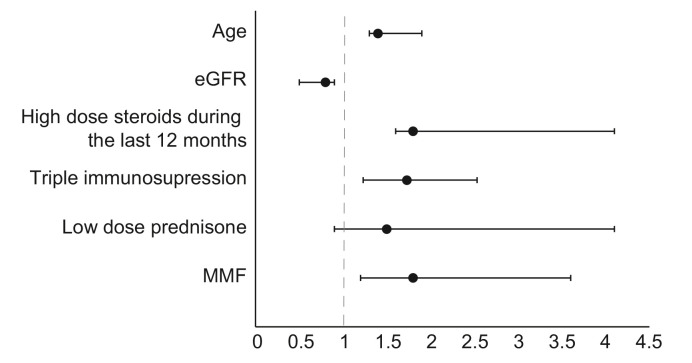
In a multivariate analysis, factors related significantly to negative serologic response were older age, use of high dose prednisone in the past 12 months, MMF and triple therapy immunosuppression. Use of low dose steroids showed a trend towards reduced immunogenicity but was not statistically significant. Higher eGFR was inversely related to a negative response (Fig. 1 ).
Fig. 1.
Multivariate analysis of the risk for negative serology in the liver transplant recipients’ group.
Note: dash line represents OR =1. Age (OR 1.3; 95% CI 1.17–1.95; p = 0.021), lower eGFR (OR 0.8; 95% CI 0.47–0.95; p = 0.034), high dose prednisone in the past 12 months (OR 1.8; 95% CI 1.58–4.61; p = 0.041), triple therapy immunosuppression (OR 1.73; 95% CI 1.21–2.52; p = 0.019), low dose steroids (OR 1.5, 95% CI 0.91–4.1, p = 0.089), MMF (OR 1.8; 95% CI 1.15–3.47; p = 0.037). The analysis was done using binary logistic regression model. p <0.05 was considered statistically significant for all analyses. eGFR, estimated glomerular filtration rate; MMF, mycophenolate mofetil; OR, odds ratio.
SARS-CoV-2 vaccination side effects
The vaccine was well tolerated and no major adverse events occurred in any participant. Information regarding side effects was provided by 71 LT recipients and 21 controls. Injection site reactions occurred at a similar frequency in both groups following the first and second dose (43/71, 60.5% vs. 15/21, 71%, p = 0.68; 38/71, 53.5% vs. 15/21, 71%, p = 0.14 respectively). The only reported local symptom was mild-to-moderate pain at the injection site.
The frequency of systemic events was similar in both groups after the first vaccination (14/71, 19.7% vs. 6/21, 28% for LT recipients and controls respectively, p = 0.74). Following the second vaccination systemic symptoms occurred significantly less among LT recipients compared to controls (18/71, 25% vs. 18/21, 85.7% respectively, p <0.001). The most common systemic side effect was fatigue, followed by headache and myalgia. During the follow-up period (7–10 weeks after the second vaccination), the LT recipients experienced no events of suspected or confirmed graft rejection, compromised graft function, neurological events or severe allergic reaction.
Discussion
We show significantly inferior serological response to the Pfizer-BioNTech BNT162b2 SARS-CoV-2 vaccine among LT recipients, with only 47.5% developing a positive neutralizing antibody titer. Among those who developed antibodies, the anti-Spike IgG titer was significantly lower in comparison to controls. Our results are in line with previous reports showing inferior vaccination response in SOT recipients in general and following a single SARS-CoV-2 vaccine dose.9 , 10
Treatment with MMF or high dose steroids in the year prior to vaccination had a significant effect on immunogenicity as previously shown in vaccination studies in SOT recipients.10 , 11 Other factors related to an inferior serological response were older age and reduced eGFR. This is also in line with other recent preliminary reports regarding the impact of older age on COVID-19 vaccine response.10 , 12 There were no major adverse events or graft rejections.
Our study included only LT recipients, and thus does not necessarily reflect immunogenicity among other SOT recipients (i.e. lung, kidney) that are known to have a lower post-vaccination response.9 , 11
Study limitations include a small sample size and short follow-up period. We did not test for the T-cell mediated response. Further studies are warranted to determine how lower immunogenicity and lower antibody titers might affect COVID-19 morbidity and mortality. Our findings raise concerns regarding the vulnerability of vaccinated LT recipients to COVID-19 infection, and define a recipient group at highest risk of immunization failure (older age, intense immunosuppression and kidney failure). Currently, effective measures to improve immunogenicity to the COVID-19 vaccine in this population remain unknown and are urgently needed. For the time being, our results support current recommendations that emphasize the importance of vaccinating patients pre-transplant. Finally, the reduced immunity must be considered when counseling patients with regards to practicing personal protective measures.
Abbreviations
CNIs, calcineurin inhibitors; COVID-19, coronavirus disease 2019; eGFR, estimated glomerular filtration rate; LT, liver transplant; MMF, mycophenolate mofetil; SOT, solid organ transplant.
Financial support
The authors received no financial support to produce this manuscript.
Authors’ contributions
Liane Rabinowich-concept and design, patient requiting and blood tests, data collection, writing of article. Ayelet Grupper-data collection, patient recruiting and blood tests, statistical analysis. Roni Baruch-data collection. Merav Ben-Yehoyada-data collection and laboratory assays. Tami Halperin-laboratory assays. Dan Turner-laboratory assays. Eugene Katchman-laboratory assays. Sharon Levi-data collection. Inbal Houri-data collection. Nir Lubezky-paper review. Oren Shibolet-patient recruiting, data collection, critical review of the data, writing of article. Helena Katchman-concept and design, patient recruiting and blood tests, data collection, writing of article.
Data availability statement
Due to the sensitive nature of the medical information, patients were assured that raw data would remain confidential and would not be shared.
Conflict of interest
The authors declare no conflicts of interest that pertain to this work.
Please refer to the accompanying ICMJE disclosure forms for further details.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jhep.2021.04.020.
Supplementary data
The following are the supplementary data to this article:
References
- 1.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. C4591001 clinical trial group. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020 Dec 31;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., et al. COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021 Feb 4;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cornberg M., Buti M., Eberhardt C.S., Grossi P.A., Shouval D. EASL position paper on the use of COVID-19 vaccines in patients with chronic liver diseases, hepatobiliary cancer and liver transplant recipients. J Hepatol. 2021 Apr;74(4):944–951. doi: 10.1016/j.jhep.2021.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.American Society of Transplantation: COVID-19: VACCINE FAQ SHEET. https://www.myast.org/covid-19-vaccine-faq-sheet. Last entered 24/03/2021.
- 5.Fix O.K., Blumberg E.A., Chang K.M., Chu J., Chung R.T., Goacher E.K., et al. AASLD COVID-19 Vaccine Working Group AASLD expert panel consensus statement: vaccines to prevent COVID-19 infection in patients with liver disease. Hepatology. 2021 Feb 12 doi: 10.1002/hep.31751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Levey A.S., Bosch J.P., Lewis J.B., Greene T., Rogers N., Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999 Mar 16;130(6):461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 7.KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. https://kdigo.org/guidelines/ckd-evaluation-and-management/Last entered 24/03/2021. [DOI] [PubMed]
- 8.Perkmann T., Perkmann-Nagele N., Breyer M.K., Breyer-Kohansal R., Burghuber O.C., Hartl S., et al. Side-by-Side comparison of three fully automated SARS-CoV-2 antibody assays with a focus on specificity. Clin Chem. 2020 Nov 1;66(11):1405–1413. doi: 10.1093/clinchem/hvaa198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckerle I., Rosenberger K.D., Zwahlen M., Junghanss T. Serologic vaccination response after solid organ transplantation: a systematic review. PloS One. 2013;8(2) doi: 10.1371/journal.pone.0056974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boyarsky B.J., Werbel W.A., Avery R.K., Tobian A.A.R., Massie A.B., Segev D.L., et al. Immunogenicity of a single dose of SARS-CoV-2 messenger RNA vaccine in solid organ transplant recipients. JAMA. 2021 Mar 15 doi: 10.1001/jama.2021.4385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baluch A., Humar A., Eurich D., Egli A., Liacini A., Hoschler K., et al. Randomized controlled trial of high-dose intradermal versus standard-dose intramuscular influenza vaccine in organ transplant recipients. Am J Transpl. 2013 Apr;13(4):1026–1033. doi: 10.1111/ajt.12149. [DOI] [PubMed] [Google Scholar]
- 12.Müller L., Andrée M., Moskorz W., Drexler I., Walotka L., Grothmann R., et al. Age-dependent immune response to the Biontech/Pfizer BNT162b2 COVID-19 vaccination. medRxiv preprint. March 5, 2021 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Due to the sensitive nature of the medical information, patients were assured that raw data would remain confidential and would not be shared.