Abstract
Aim/background
The most recent pandemic caused by the new coronavirus disease (COVID-19) urged dramatic changes in people's lives. Potentially, the COVID-19 pandemic affects physical and mental health as well as behavioral and social aspects. However, the direct impacts of the COVID-19 pandemic on health-related parameters are not yet known. The present study aimed to evaluate the effect of 16 weeks during the COVID-19 pandemic on health-related parameters of physically inactive women aged 50 to 70 years.
Methods
Thirty-four physically inactive women participated in the study. We performed tests to evaluate aerobic capacity and muscle strength, anthropometric measurements, blood pressure (BP), blood parameters, diet, and physical activity levels. All evaluations were carried out before and 16 weeks after the initial phase of the COVID-19 pandemic in Brazil (i.e., from March to July 2020).
Results
Systolic BP (p < .0001; effect size (ES) = 0.62), diastolic BP (p < .0001; ES = 0.71), grip strength of the right (p < .05; ES = 0.43) and left hand (p < .05; ES = 0.49), performance in six-minute walk test (p < .05; ES = 0.46), free time physical activity levels (p < .05; ES = 0.40), domestic physical activity levels (p < .05; ES = 0.39), platelet count (p < .0001; ES = 0.48), and mean corpuscular hemoglobin concentration (p < .0001; ES = 1.14) reduced in comparison to the period before the pandemic. In contrast, glycated hemoglobin levels (p < .0001; ES = 0.77), triglycerides (p < .05; ES = 0.40), and insulin levels (p < .05; ES = 0.60) increased in comparison to the period before the pandemic.
Conclusion
The COVID-19 pandemic negatively impacted the general health status of physically inactive women aged 50 to 70, potentially increasing their susceptibility to comorbidities, such as type 2 diabetes and hypertriglyceridemia.
Keywords: Fitness, Blood parameters, Physical inactivity, SARS-CoV-2, Aging
1. Introduction
The new coronavirus disease (COVID-19) is a pandemic disease caused by the severe acute respiratory syndrome virus (SARS-CoV-2), that infects the airways and shows a high level of spread and contagion, leading to aggravations in older adults and people with associated comorbidities, such as obesity and/or chronic non-communicable diseases (NCDs), such as type 2 diabetes mellitus, hypertension, and coronary heart disease, thus increasing the risk of mortality (Palmer et al., 2020; Zheng et al., 2020). Given this situation, social isolation is a crucial tool in this process to control the pandemic (Wilder-Smith and Freedman, 2020). However, social isolation presents itself as a trigger for decreasing leisure and physical activities, impairing physical capacity, and reducing social interaction (Jiang et al., 2019).
Changes in routine and decreased in-person contact are some of the social effects of the new coronavirus. As physical and social environment and mental health are associated, the persistence of the pandemic and the emergency can lead to increased loneliness, stress, anxiety, and depression. This set of factors can affect other aspects of the general health, such as physical activity, diet, smoking, and alcohol consumption, which are risk factors for several cardiovascular and metabolic diseases, such as hypertension, type 2 diabetes mellitus, dyslipidemia, and obesity (Shankar et al., 2011; Fritschi et al., 2016; Rodrigues, 2018; Schrempft et al., 2019; Domènech-Abella et al., 2020; Smith and Lim, 2020).
In this scenario, practice of physical activity emerges as an important and significant factor for decreasing glycated hemoglobin (HbA1c) levels, systolic blood pressure (SBP), diastolic blood pressure (DBP), body mass index (BMI), low-density lipoprotein (LDL), and total cholesterol (TC) (Parra-Sáncheza et al., 2015; Ferrer et al., 2018).
It is known that social isolation may be related to greater impairments in parameters related to the global health of older adults (Shankar et al., 2011; Xia and Li, 2018). However, we cannot conclude that social isolation, in the current pandemic scenario due to the new coronavirus, and its effects on health parameters in older people is similar to the social isolation reported in the literature, characterized by restrictions imposed on living in society against the will of individuals (Shankar et al., 2011; Xia and Li, 2018).
Based on what was elucidated above and because we are not aware of the existence of experimental studies in the literature on the effects of a period of 16 weeks during the COVID-19 pandemic on health-related parameters, the present study aims to analyze the effects of this pandemic period on blood pressure, anthropometric measurements, functional capacity, blood parameters, levels of physical activity, and quality of diet in women aged 50 to 70 years.
2. Methods
2.1. Experimental design and participants
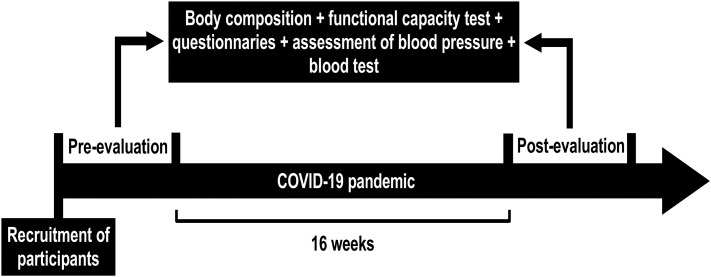
In total, 34 women participated in the study aged between 50 and 70 years. The inclusion criteria were: being between 50 and 70 years old, female, and being physically inactive, that is, not having performed physical exercises in the previous six months. Participants who smoked, with self-reported alcohol dependence, with chronic kidney disease, infectious diseases, and/or coronary heart disease were excluded from the study. Before the beginning of the research protocol, all participants were informed about the experimental protocol (objectives, methods, benefits, and risks) and voluntarily signed the informed consent form previously approved by the Ethics Committee of the School of Physical Education and Sport of Ribeirao Preto (EEFERP, USP), Ribeirao Preto, Brazil (Protocol No. 29187719.4.0000.5659). All subjects participated in evaluations before (pre) and after (post) the period of 16 weeks of the COVID-19 pandemic related to levels of physical activity, quality of food, body composition, blood collection, blood pressure measurement, and tests to evaluate aerobic capacity and muscle strength (Fig. 1 ). Pre-evaluations were carried out between January and February 2020, and post-evaluations were carried out between June and July 2020. As the protocol included physical fitness tests, all participants were required to present a medical report indicating fitness for physical activity.
Fig. 1.
Experimental design.
2.2. Sociodemographic data and clinical characteristics, levels of physical activity, and food intake
For the collection of sociodemographic data and clinical characteristics, a questionnaire prepared by the researchers themselves was used. To collect data related to the levels of physical activity, the Modified Baecke Questionnaire for Older Adults (MBQO) was used, through an interview and taking the previous 12 months as a reference. The questionnaire is divided into three sections: the first section presents questions related to domestic physical activities and the second and third sections analyze sports physical activities and free time physical activities, respectively. The scores equivalent to domestic physical activities, sports physical activities, and free time physical activities are established through the sum of the specific scores attributed to the questions grouped in each of the sections of the questionnaire (Ueno, 2013; Silva et al., 2020). Together with the MBQO to evaluate the participants' diet, the Brazilian Ministry of Health's Food Consumption Markers Form (FCMF) was applied, which consists of ten foods, food groups, or preparations that depict the frequency of consumption in seven days of the previous week (Brasil, 2008).
2.3. Blood collection
Blood samples were collected after 12-h of fasting in EDTA tubes, and plasma was separated by centrifugation and stored at −80 °C until analysis. Blood analyses of lipid and glycemic profiles and complete blood count were performed (Tjønna et al., 2008). At both times, blood samples were taken early in the morning.
2.4. Anthropometric measurements and blood pressure
All participants were in a food/liquid fasting state for evaluation. Body weight was measured using an electronic platform Fiziola TM scale with a precision of 0.1 kg and maximum capacity of 300 kg. A vertical shaft with a 0.5 cm graduation was used to measure body height. Waist circumference was measured with an inextensible tape at the largest circumference between the last rib and the iliac crest (Tjønna et al., 2008). Fat mass was measured using electrical bioimpedance, with participants in the supine position (Maltron BF-906) (Bera, 2014). Blood pressure was measured using a calibrated automatic arm device, which performs measurements using the oscillometric method (OMRON, model HEM-7113). The measurement was performed after the participant rested for 5 min in a sitting position, following the 7th Brazilian Guideline on Hypertension (Malachias et al., 2016).
2.5. Strength measurement and aerobic capacity
In the present study, the six-minute walk test (6MWT) adapted by Rikli and Jones (2001) was used to evaluate aerobic capacity. In this test, the participants cover a rectangular course (18.28/4.57 m) for 6 min. Participants were instructed to walk as quickly as possible, except in cases of dizziness, pain of any kind, nausea, and/or excessive fatigue (Rikli and Jones, 2001).
To measure strength, the participants performed a handgrip strength test (HGS) in both hands - handgrip strength of the left hand (HGSL) and handgrip strength of the right hand (HGSR) - using a hydraulic hand dynamometer (Baseline®). The test was performed with the participants in an orthostatic position, with the elbow flexed at 90° and close to the body. Before making valid attempts, the participants performed one repetition in each hand to familiarize themselves with the device and the test. After familiarization with the device and test, three attempts were made with a duration of 3 s of grip and 10 s of interval. Attempts were made consecutively with each hand (Sousa-Santos and Amaral, 2017). The highest peak strength (kgf) recorded between the three attempts of each hand was considered for analysis.
2.6. Statistical analyses
The Shapiro Wilk test was used to analyze normality of all variables. Variables with normal distribution were analyzed by the Student's paired t-test and those that did not present normal distribution were analyzed by the Wilcoxon test for non-parametric data. The data are presented as mean ± standard deviation of the mean (SD). The significance level adopted was p < .05. The data were analyzed using IBM SPSS Statistics for Windows software (Version 22.0, Armonk, NY: IBM Corp). The Cohen test (d) was used as a measure of effect size (ES) for dependent data. The effect was considered null for the range of 0.0–0.1, small for 0.2–0.4, medium for 0.5–0.7, and large for 0.8-≥1.0 (Cohen, 1992).
3. Results
The participants (n = 34) were 58.5 ± 6.0 years of age, height 1.59 ± 0.06 m, body weight 81.2 ± 16.6 kg, and with 11.6 ± 3 years of study.
3.1. Anthropometric measurements and blood pressure
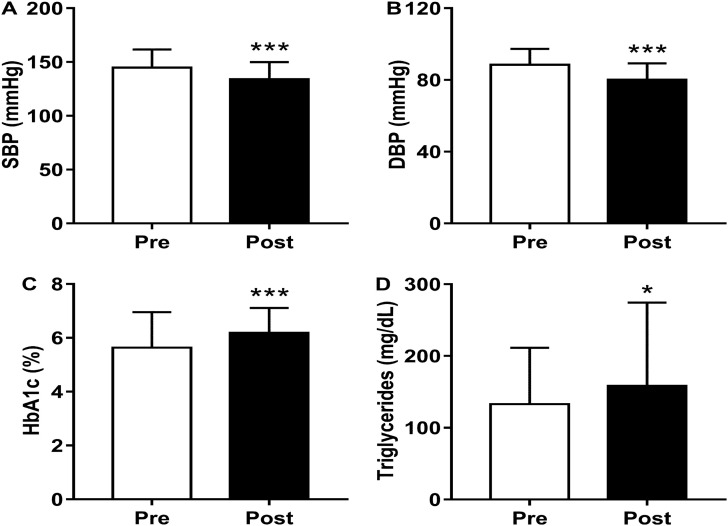
There were no significant alterations between the measurement time points, before and after isolation, in fat mass (−1.1%, p = .776), BMI (0%, p = .923), and WC (+1.8%, p = .121) (Table 1 ). There was a reduction in SBP (Δ = −7.4%, p < .0001; ES = 0.62) and DBP (−9.4%, p < .0001; ES = 0.71) (Fig. 2 ).
Table 1.
Anthropometry, physical activity levels, and functional capacity of women aged 50 to 70 years before and after 16 weeks of social isolation.
| Pre | Post | Δ% | p | Effect size | |
|---|---|---|---|---|---|
| Fat mass (%) | 43.7 ± 9.7 | 43.2 ± 8.8 | −1.1 | 0.776 | 0.05 |
| BMI (kg/m2) | 31.9 ± 5.6 | 31.9 ± 5.7 | 0 | 0.923 | 0.02 |
| WC (cm) | 100.1 ± 9.4 | 101.9 ± 12.2 | 1.8 | 0.121 | 0.17 |
| Domestic physical activities - MBQO (score) | 2.01 ± 0.28 | 1.90 ± 0.29 | −5.8 | 0.046 | 0.39 |
| Free time physical activities - MBQO (score) | 0.61 ± 1.16 | 0.10 ± 0.31 | −83.2 | 0.026 | 0.40 |
| Sports physical activities - MBQO (score) | 0.815 ± 1.893 | 0.758 ± 2.061 | −7.1 | 0.972 | 0.01 |
| Total MBQO (score) | 3.44 ± 2.32 | 2.76 ± 2.28 | −19.9 | 0.189 | 0.24 |
| FCMF (score) | 18.7 ± 8.5 | 19.2 ± 8.0 | 2.6 | 0.689 | 0.06 |
| 6MWT (m) | 527 ± 46 | 504 ± 54 | −4.4 | 0.017 | 0.46 |
| HGSL (kgf) | 29.9 ± 9.1 | 26.5 ± 4.9 | −5.6 | 0.037 | 0.49 |
| HGSR (kgf) | 30.3 ± 5.3 | 28.2 ± 4.4 | −6.9 | 0.003 | 0.43 |
BMI: body mass index; WC: waist circumference; MBQO: modified Baecke questionnaire for Older Adults; FCMF: Food Consumption Markers Form; 6MWT: six-minute walk test; HGSR: palmar grip strength of the right hand; HGSL: palmar grip strength of the left hand; kgf: kilogram strength; Δ%: percentage variation between the means of the post in relation to the pre measurement time point. Data presented as mean ± SD. Student's t-test (Wilcoxon test was applied for non-parametric data. p < .05). Cohen's d effect size for dependent data.
Fig. 2.
Effects of 16-weeks of social isolation on (A) systolic blood pressure (SBP, mmHg), (B) diastolic blood pressure (DBP, mmHg), (C) glycosylated hemoglobin (HbA1c, %), and (D) triglycerides (mg/dL). Data presented as mean ± SD. *p < .05; ***p < .0001. Student's t-test (Wilcoxon test was applied for non-parametric data). Cohen's d effect size for dependent data.
3.2. Blood analyses
There was an increase in HbA1c levels (+9.7%, p < .0001; ES = 0.77), TG (+19.2%, p = .025; ES = 0.40), and insulin (+39.8%, p = .001; ES = 0.60), but there were no alterations in glucose levels (+1.3%, p = .229; ES = 0.21). There were no significant alterations in TC levels (+8.03%, p = .985), HDL (+0.4%, p = .742), and LDL (−2.6%, p = .165) (Table 2 and Fig. 2).
Table 2.
Lipid and glycemic profiles, and complete blood count of women aged 50 to 70 years before and after 16 weeks of social isolation.
| Pre | Post | Δ% | p | Effect size | |
|---|---|---|---|---|---|
| Total cholesterol (mg/dL) | 222 ± 38 | 223 ± 41 | 8.3 | 0.985 | 0.03 |
| HDL-cholesterol (mg/dL) | 52.4 ± 11.3 | 52.3 ± 11.6 | 0.4 | 0.742 | 0.06 |
| LDL-cholesterol (mg/dL) | 143.7 ± 36 | 140.2 ± 40 | −2.6 | 0.165 | 0.09 |
| Glucose (mg/dL) | 106.2 ± 29 | 107.5 ± 43 | 1.3 | 0.229 | 0.21 |
| Insulin (μUI/mL) | 10.6 ± 8.0 | 14.8 ± 9.3 | 39.8 | 0.001 | 0.60 |
| Platelets (/mm3) | 238 ± 53 | 214 ± 46 | −10.1 | <0.0001 | 0.48 |
| Hemoglobin (g/dL) | 13.9 ± 1.1 | 14.2 ± 1.1 | 2.1 | <0.01 | 0.27 |
| Hematocrit (%) | 40.2 ± 3.1 | 42.7 ± 3.2 | 6.0 | <0.0001 | 0.79 |
| MCV (fL) | 87.8 ± 4.9 | 92.3 ± 4.5 | 5.1 | <0.0001 | 0.87 |
| MCH (pg) | 30.3 ± 2.0 | 30.7 ± 1.8 | 1.3 | 0.050 | 0.35 |
| MCHC (g/dL) | 34.5 ± 0.9 | 33.3 ± 1.2 | −3.5 | <0.0001 | 1.14 |
| RDW (%) | 12.3 ± 1.0 | 12.4 ± 0.8 | 0.3 | 0.864 | 0.03 |
| Leucocytes (x103μL) | 6.06 ± 1.35 | 6.45 ± 1.52 | 6.5 | 0.041 | 0.37 |
| Segmented neutrophils (x103μL) | 3.10 ± 0.96 | 3.42 ± 1.08 | 10.3 | 0.027 | 0.31 |
| Lymphocytes (x103μL) | 2.22 ± 0.53 | 2.35 ± 0.62 | 5.8 | 0.050 | 0.35 |
| Monocytes (x103μL) | 0.468 ± 0.148 | 0.442 ± 0.131 | −5.7 | 0.174 | 0.24 |
| Eosinophils (x103μL) | 0.192 ± 0.131 | 0.187 ± 0.146 | −2.7 | 0.781 | 0.05 |
| Basophils (x103μL) | 0.082 ± 0.049 | 0.089 ± 0.098 | 8.1 | 0.788 | 0.10 |
HDL: high-density lipoprotein; LDL: low-density lipoprotein; MCV: mean corpuscular volume; RDW: red blood cell width; MCH: mean corpuscular hemoglobin; MCHC: mean corpuscular hemoglobin concentration. Δ%: percentage variation between the means of the post in relation to the pre measurement time point. Data presented as mean ± SD. Student's t-test (Wilcoxon test was applied for non-parametric data. p < .05). Cohen's d effect size for dependent data.
There were significant reductions in the amounts of platelets (−10.1%, p < .0001; ES = 0.48) and in the MCHC (−3.5%, p < .0001; ES = 1.14), but not monocytes (−5.7, p = .174; ES = 0.24) and eosinophils (2.7%, p = .781). In addition, there were significant increases in hemoglobin levels (+2.1%, p < .01; ES = 0.27), hematocrit (+6.0%, p < .0001; ES = 0.79), MCV (+5.1%, p < .0001; ES = 0.87), leucocytes (+6.5%, p = .041; ES = 0.37), and segmented neutrophils (+10.3%, p = .027; ES = 0.31). There were no alterations in MCH levels (+1.3%, p = .050; ES = 0.35), RDW (+0.3%, p = .864), lymphocytes (+5.8%, p = .050), and basophils (+8.1%, p = .788) (Table 2).
3.3. Strength and aerobic capacity
There was a significant reduction in the aerobic capacity evaluated by the distance covered in the 6MWT (−4.4%, p = .017; ES = 0.46) and in the muscular strength evaluated by the HGSL (−5.6%, p = .037; ES = 0.49) and HGSR (−6.9%, p = .003; ES = 0.43) (Table 1).
3.4. Physical activity levels and food intake
The participants presented a significant reduction in the levels of domestic physical activities (−5.8%, p = .046; ES = 0.39) and free time physical activities (−83.2%, p = .026; ES = 0.40). There was no significant alteration in sports physical activities score (−7.1%; p = .189), the total QBMI score (−19.9%; p = .972; ES = 0.24), and food, evaluated by the FCMF (+2.6%, p = .689) (Table 1).
4. Discussion
The present study aimed to analyze the effects of a period of 16 weeks during the COVID-19 pandemic on blood pressure, anthropometric measurements, functional capacity, blood parameters, levels of physical activity, and quality of diet in women aged 50 to 70 years. Sixteen weeks of the COVID-19 pandemic resulted in important alterations in parameters related to the health of women aged 50 to 70 and physically inactive.
The subjects presented, even before the pandemic, low levels of physical activity, as identified by the total MBQO score (Ueno, 2013; Silva et al., 2020). Therefore, as expected, there was no alteration in the total MBQO or in the levels of sports physical activities. There were percentage reductions in the levels of free time and domestic physical activities, 83.2 and 5.8%, respectively, which were not determinants for a significant decrease in the total MBQO score because while the score of domestic physical activity contributes to 68.8% of the total MBQO score, the score for free time physical activity contributes only 3.6%. The reduction, mainly in the practice of free time physical activity, may have probably occurred due to social isolation and other strategies adopted to contain the spread of the COVID-19 (Woods et al., 2020).
Interestingly, there was a reduction in SBP and DBP, which was not expected, considering that reduced levels of physical activity and sedentary behavior increase the risk for the development of several metabolic and cardiovascular disorders, such as hypertension, for example (Lacombe et al., 2019). Blood pressure (BP) is the product of cardiac output (CO) and peripheral vascular resistance (RVP) (BP = CO x RVP), and variation in any of those parameters affects BP. However, in the present study, such parameters were not evaluated. Psychosocial stress is also a risk factor for hypertension (Liu et al., 2017; Johnson, 2019). As previously observed, hypertensive patients present higher psychosocial stress when compared to normotensive people (Liu et al., 2017). In this context, we hypothesize that there was a reduction in psychosocial stress during 16-week period of the COVID-19 pandemic, potentially due to the reduction in the volume and quantity of activities performed by the subjects. However, the determining factors that caused the reduction in BP values after this period of the pandemic remain unknown and specific studies on the subject should be carried out.
In the glycemic profile, there was no significant increase in glucose levels. However, glycated hemoglobin increased significantly by 9.7% and insulin levels increased significantly by 39.8%. This could be explained by the reductions in physical activity and not by the diet's qualitative aspects since the FCMF showed a 2.6% increase in healthy food consumption without significant changes. In this context, studies have demonstrated that physical inactivity is one of the factors that can cause an increase in insulin levels (Barnard et al., 1998; Davies et al., 2018). We highlight that these alterations in the glycemic profile can result in the development and/or worsening of the clinical condition of type 2 diabetes mellitus.
An interesting finding in the blood parameters (Fig. 2) was that the TG concentration showed a significant increase, from ~132 mg/dL at the measurement time point before isolation to ~158 mg/dL after 16 weeks, which is above acceptable values for healthy subjects. Elevated levels of TG are known to increase the predisposition to cardiovascular diseases (Toth, 2016). Besides, the pandemic scenario of COVID-19 can intensify the effects of several factors, such as inappropriate eating habits, physical inactivity, stress, chronic diseases, genetic predispositions, and age (Navar, 2019).
There were increases in the levels of hemoglobin, hematocrit, MCV, and reductions in MCHC and the number of platelets in the present study regarding red blood cells. Some of these alterations could be explained by hypovolemia, as occurs during dehydration/hypohydration, with an increase in hemoglobin and erythrocytes' proportion to blood volume (Walker et al., 1990). However, the determining factors that caused all these changes after this period of the pandemic remain unknown and specific studies should be carried out.
The leukogram is divided into granulocytes [segmented neutrophils, eosinophils, and basophils] and agranulocytes [lymphocytes and monocytes]. In the present study, we showed that for these hematological markers there was a significant increase of 6.5% for leukocytes and 10.3% for segmented neutrophils. Shimamiya et al. (2004), in a study on confinement effects for 10 days not associated with the COVID-19, showed that blood parameters, including leukocyte subpopulations, demonstrated proliferative reactions, in addition to changes in leukocyte distribution, which can be explained by the reaction of possible stress in the immune system caused by confinement (Shimamiya et al., 2004).
Regarding functional capacity, the participants showed a significant reduction in muscle strength, assessed by the handgrip strength of both hands, and in aerobic capacity, which was assessed by the distance covered in the six-minute walk test. The handgrip strength test is a test in which the strength values obtained are associated with several parameters of health and quality of life in older adults, with low levels of strength production increasing the risk for cardiometabolic diseases, functional disability, fracture, morbidity, and mortality from all causes (McGrath et al., 2018).
A study in Brazilian subjects proposed normative values for different age groups. In women aged 50 to 59 years, the mean HGS values (50th percentile) were 23.0 kgf for the right hand, 21.1 kgf for the same hand in women aged 60 to 69 years, 21.6 kgf for the left hand in women aged 50 to 59 years, and 20.4 kgf in women aged 60 to 69 years (Amaral et al., 2019). The values in our study would be classified above the median in relation to the aforementioned study. In that same study, from the age of 30 to 39, reductions in grip strength occurred every decade up to 80 years or over (Costa et al., 2016). The reduction in handgrip strength in the participants of the present study was ~5.6% in the left hand and ~6.9% in the right. Thus, we suggest that the scenario of the COVID-19 pandemic contributed to accelerate the decline in strength of the participants, something natural and due to the aging process, which under normal conditions occurs gradually over years and not months.
The 6MWT, which is easy to apply and low cost, can predict VO2peak and assess aerobic capacity in healthy middle-aged and older people (American Thoracic Society, 2002). In the present study, the mean distance walked by the participants was ~527 m before the COVID-19 pandemic and ~504 m after 16 weeks from the beginning of the COVID-19 pandemic - with a significant reduction of 4.4%. Mazo et al. (2015) proposed percentile cut-off values for the distance covered in the 6MWT for Brazilian women aged 60 to 69 years. According to these cut-off values, the participants in the current study would be classified as showing regular performance before the pandemic, between the 25th and 75th percentiles, while after 16 weeks from the beginning of the COVID-19 pandemic there was a reduction in the distance covered, the classification of the participants was downgraded, becoming weak, between the 10th and 25th percentiles (Mazo et al., 2015). Trapé et al. (2017) evaluated the effects of 12 weeks of intervention with multicomponent training on health parameters of physically inactive Brazilian women aged 50 to 80 years. The distance reached in 6MWT by the participants of their study before the intervention was 512 m - it was 527 m in the present study. The intervention significantly increased the distance walked by the participants, from 512 to 559 m.
Rikli and Jones (2001) proposed that distance values below 597 m for American women aged 60 to 64 would be considered weak. Therefore, according to this classification, the participants in the present study performed poorly (Rikli and Jones, 2001). Thus, with the results regarding the parameters related to functional capacity, the present study demonstrates that the COVID-19 pandemic is capable of providing a favorable scenario for morbidities and a worse health prognosis.
For LDL-cholesterol and TC levels, although there was no statistical difference between before and after measurement time points, the mean values of both parameters extrapolate the levels considered acceptable before and after the period of 16 weeks, as shown in Table 2. Studies indicate an inverse relationship between reduced levels of LDL-cholesterol and total cholesterol with cardiovascular risk and mortality from all causes (Liang et al., 2017). Other studies also suggest that high levels of LDL-cholesterol may result in increased risks of atherosclerotic cardiovascular diseases (Abdullah et al., 2018; Gidding and Allen, 2019).
Regarding HDL-cholesterol levels (mg/dL), in the current study it was observed that there was no statistical difference, with values of 52 mg/dL. It is known that ideal categorical levels are above >60 mg/dL, and that a healthy and balanced diet, together with the regular practice of physical activities, especially aerobic exercise, contribute to ideal levels of HDL-c. Nicholls and Nelson (2019) support the evidence that HDL-c has beneficial and protective properties against cardiovascular risk. In a survey of middle-aged adults, Singh-Manoux et al. (2008) demonstrated that low HDL-c levels are related to cardiovascular diseases, stroke, and neurodegenerative processes (Singh-Manoux et al., 2008; Nicholls and Nelson, 2019).
In the anthropometric measurements, we found no differences for the values of BMI, percentage of fat mass, and WC. However, at both measurement time points these variables showed values above those recommended, indicating an increased prevalence of obesity (Ross et al., 2020). Recent studies have shown that obesity can worsen infection and the prognosis of patients infected with the COVID-19 (Tartof et al., 2020).
We emphasize that among the main comorbidities associated with the COVID-19, in addition to obesity, there are other NCDs, such as diabetes, cardiovascular diseases, dyslipidemia, and chronic obstructive pulmonary diseases, which can be prevented, controlled, and even treated by regular physical exercise (Lacombe et al., 2019).
The strength of this study is the evaluation of numerous health variables before and after a period of 16 weeks during the COVID-19 pandemic. As a limitation, we can point out that we did not use a gold standard method to evaluate the participants' diet.
In summary, we suggest that the 16 weeks during the COVID-19 pandemic negatively affected the global health of physically inactive women aged 50 to 70, even though there was a reduction in blood pressure. These results have fundamental implications due to the tremendous negative potential in public health that the current situation can bring about if strategies are not adopted to guarantee people's health in pandemic times. Some of these strategies are to encourage physical activity and social interaction. For these, virtual interactions may represent a critical tool for when in-person contact is reduced to essential.
Funding
This work was supported by the grant #2017/21361-2 (São Paulo Research Foundation, FAPESP) and by the Coordination of Superior Level Staff Improvement - Brazil (CAPES) - Academic Excellence Program (PROEX)- Finance Code #001, Graduate Support Program (PROAP) - Finance Code #001.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
The authors thank all the volunteers for their participation, and the entire team involved in data collection.
Section editor: Cheryl Conover
References
- Abdullah S.M., Defina L.F., Leonard D., et al. Long-term association of low-density lipoprotein cholesterol with cardiovascular mortality in individuals at low 10-year risk of atherosclerotic cardiovascular disease. Circulation. 2018;138:2315–2325. doi: 10.1161/CIRCULATIONAHA.118.034273. [DOI] [PubMed] [Google Scholar]
- ATS committee on proficiency standards for clinical pulmonary function laboratories. Am. J. Respir. Crit. Care Med. 2002;166(1):111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- Amaral C.A., Amaral T.L.M., Monteiro G.T.R., Vasconcellos M.T.L., Portela M.C. Hand grip strength: reference values for adults and elderly people of Rio Branco, Acre, Brazil. PLoS One. 2019;14(1) doi: 10.1371/journal.pone.0211452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard R.J., Roberts C.K., Varon S.M., Berger J.J. Diet-induced insulin resistance precedes other aspects of the metabolic syndrome. J. Appl. Physiol. 1998;84(4):1311–1315. doi: 10.1152/jappl.1998.84.4.1311. [DOI] [PubMed] [Google Scholar]
- Bera T.K. Bioelectrical impedance methods for noninvasive health monitoring: a review. J. Med. Eng. Technol. 2014;2014(2):1–28. doi: 10.1155/2014/381251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brasil. Ministério da Saúde, Secretaria de Atenção à Saúde, Departamento de Atenção Básica . Secretaria de Atenção à Saúde, Departamento de Atenção Básica - Ministério da Saúde; Brasília: 2008. Protocolos do Sistema de Vigilância Alimentar e Nutricional - SISVAN/Ministério da Saúde. [Google Scholar]
- Cohen J. A power primer. Psychol. Bull. 1992;112(1):155–159. doi: 10.1037/0033-2909.112.1.155. [DOI] [PubMed] [Google Scholar]
- Costa J.P., Vitorino R., Silva G.M., Vogel C., Duarte A.C., Rocha-Santos T. A synopsis on aging theories, mechanisms and future prospects. Ageing Res. Rev. 2016;29:90–112. doi: 10.1016/j.arr.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies K.A.B., Sprung V.S., Norman J.A., et al. Short-term decreased physical activity with increased sedentary behaviour causes metabolic derangements and altered body composition: effects in individuals with and without a first-degree relative with type 2 diabetes. Diabetologia. 2018;61:1282–1294. doi: 10.1007/s00125-018-4603-5. [DOI] [PubMed] [Google Scholar]
- Domènech-Abella J., Switsers L., Mundó J., Dierckx E., Sarah Dury S., Donder L. The association between perceived social and physical environment and mental health among older adults: mediating effects of loneliness. Aging Ment. Health. 2020:1–7. doi: 10.1080/13607863.2020.1727853. [DOI] [PubMed] [Google Scholar]
- Ferrer M.D., Capó X., Martorell M., et al. Regular practice of moderate physical activity by older adults ameliorates their anti-inflammatory status. Nutrients. 2018;10(11):1780. doi: 10.3390/nu10111780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritschi C., Park H., Richardson A., Park C., Collins E.G., Mermelstein R., Riesche L., Quinn L. Association between daily time spent in sedentary behavior and duration of hyperglycemia in type 2 diabetes. Biol. Res. Nurs. 2016;18(2):160–166. doi: 10.1177/1099800415600065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidding S.S., Allen N.B. Cholesterol and atherosclerotic cardiovascular disease: a lifelong problem. J. Am. Heart Assoc. 2019;e012924:8. doi: 10.1161/JAHA.119.012924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang F., Deng L., Zhang L., Cai Y., Cheung C.W., Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J. Gen. Intern. Med. 2019;35(5):1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson H.M. Anxiety and hypertension: is there a link? A literature review of the comorbidity relationship between anxiety and hypertension. Curr. Hypertens. Rep. 2019;21(9):66. doi: 10.1007/s11906-019-0972-5. [DOI] [PubMed] [Google Scholar]
- Lacombe J., Armstrong M.E.G., Wright F.L., Charlie Foster G. The impact of physical activity and an additional behavioural risk factor on cardiovascular disease, cancer and all-cause mortality: a systematic review. BMC Public Health. 2019;19(1):900. doi: 10.1186/s12889-019-7030-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y., Vetrano D.L., Qiu C. Serum total cholesterol and risk of cardiovascular and non-cardiovascular mortality in old age: a population-based study. BMC Geriatr. 2017;17(294):1–7. doi: 10.1186/s12877-017-0685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M.Y., Li N., Li W.A., Khan H. Association between psychosocial stress and hypertension: a systematic review and meta-analysis. Neurol. Res. 2017;39(6):573–580. doi: 10.1080/01616412.2017.1317904. [DOI] [PubMed] [Google Scholar]
- Malachias M., Plavnik F.L., Machado C.A., Malta D., Scala L.C.N., Fuchs S. 7th Brazilian guideline of arterial hypertension: chapter 1 - concept, epidemiology and primary prevention. Arq. Bras. Cardiol. 2016;107(3):1–6. doi: 10.5935/abc.20160151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazo G.Z., Petreca D.R., Sandreschi P.F., Benedetti T.R.B. Valores normativos da aptidão física para idosas brasileiras de 60 a 69 anos de idade. Rev. Bras. Med. Esporte. 2015;21(4):318–322. doi: 10.1590/1517-869220152104134470. [DOI] [Google Scholar]
- McGrath R.P., Kraemer W.J., Snih S.A., Peterson M.D. Handgrip strength and health in aging adults. Sports Med. 2018;48(9):1993–2000. doi: 10.1007/s40279-018-0952-y. [DOI] [PubMed] [Google Scholar]
- Navar A.M. The evolving story of triglycerides and coronary heart disease risk. JAMA. 2019;321(4):347–349. doi: 10.1001/jama.2018.20044. [DOI] [PubMed] [Google Scholar]
- Nicholls S., Nelson A.J. HDL and cardiovascular disease. Pathology. 2019;51(2):142–147. doi: 10.1016/j.pathol.2018.10.017. [DOI] [PubMed] [Google Scholar]
- Palmer K., Monaco A., Kivipelto M., et al. The potential long-term impact of the COVID-19 outbreak on patients with non-communicable diseases in Europe: consequences for healthy ageing. Aging Clin. Exp. Res. 2020;32(7):1189–1194. doi: 10.1007/s40520-020-01601-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parra-Sáncheza J., Moreno-Jiménez M., Nicola C.M., et al. Evaluación de un programa de ejercicio físico supervisado en pacientes sedentarios mayores de 65 años con diabetes mellitus tipo 2. Aten. Primaria. 2015;47(9):555–562. doi: 10.1016/j.aprim.2015.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rikli R., Jones C.J. 1st edition. Human Kinetics; Champaign, IL: 2001. Senior Fitness Test Manual. Human Kinetics. [Google Scholar]
- Rodrigues R.M. Solidão, um fator de risco. Rev. Port. Med. Geral. Fam. 2018;34:334–338. doi: 10.32385/rpmgf.v34i5.12073. [DOI] [Google Scholar]
- Ross R., Neeland I.J., Yamashita S., et al. Waist circumference as a vital sign in clinical practice: a consensus statement from the IAS and ICCR working group on visceral obesity. Nat. Rev. Endocrinol. 2020;16(3):177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrempft S., Jackowska M., Hamer M., Steptoe A. Associations between social isolation, loneliness, and objective physical activity in older men and women. BMC Public Health. 2019;19(1):74. doi: 10.1186/s12889-019-6424-y5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankar A., McMunn A., Banks J., Steptoe A. Loneliness, social isolation, and behavioral and biological health indicators in older adults. Health Psychol. 2011;30(4):377–385. doi: 10.1037/a0022826. [DOI] [PubMed] [Google Scholar]
- Shimamiya T., Nobuyuki T., Yoshimitsu H., Sonoe W., Hirotake K., Motohiko M. Effects of 10-day confinement on the immune system and psychological aspects in humans. J. Appl. Physiol. 2004;97:920–924. doi: 10.1152/japplphysiol.00043.2004. [DOI] [PubMed] [Google Scholar]
- Silva F.G., Oliveira C.B., Hisamatsu T.M., et al. Critical evaluation of physical activity questionnaires translated to Brazilian-Portuguese: a systematic review on cross-cultural adaptation and measurements properties. Braz. J. Phys. Ther. 2020;24(3):187–218. doi: 10.1016/j.bjpt.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh-Manoux A., Gimeno D., Kivimaki M., Brunner E., Marmot M.G. Low HDL cholesterol is a risk factor for deficit and decline in memory in midlife the whitehall II study. Arterioscler. Thromb. Vasc. Biol. 2008;28:1556–1562. doi: 10.1161/ATVBAHA.108.163998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith B.J., Lim M.H. How the COVID-19 pandemic is focusing attention on loneliness and social isolation. Public Health Res. Pract. 2020;30(2) doi: 10.17061/phrp3022008. [DOI] [PubMed] [Google Scholar]
- Sousa-Santos A.R., Amaral T.F. Differences in handgrip strength protocols to identify sarcopenia and frailty - a systematic review. BMC Geriatr. 2017;17(238):1–21. doi: 10.1186/s12877-017-0625-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tartof S.Y., Qian L., Hong V., et al. Obesity and mortality among patients diagnosed with COVID-19: results from an integrated health care organization. Ann. Intern. Med. 2020:M20–3742. doi: 10.7326/M20-3742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjønna A.E., Lee S.J., Rognmo O., et al. Aerobic interval training SV. continuous moderate exercise as a treatment for the metabolic syndrome - “a pilot study”. Circulation. 2008;118(4):346–354. doi: 10.1161/CIRCULATIONAHA.108.772822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth P.P. Triglyceride-rich lipoproteins as a causal factor for cardiovascular disease. Vasc. Health Risk Manag. 2016;12:171–183. doi: 10.2147/VHRM.S104369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trapé A.A., Lizzi E.A.S., Gonçalves T.C.P., et al. Effect of multicomponent training on blood pressure, nitric oxide, redox status, and physical fitness in older adult women: influence of endothelial nitric oxide synthase (NOS3) haplotypes. Oxidative Med. Cell. Longev. 2017;2017:1–12. doi: 10.1155/2017/2578950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UENO, D . Universidade Estadual Paulista; 2013. Validação do Questionário Baecke Modificado para Idosos e Proposta de Valores Normativos. Dissertação. [Google Scholar]
- Walker H.K., Hall W.D., Willis J., Hurst J.W. 3rd edition. Butterworths; Boston: 1990. Clinical Methods: The History, Physical, and Laboratory Examinations. [PubMed] [Google Scholar]
- Wilder-Smith A., Freedman D.O. Isolation, quarantine, social distancing and community containment: pivotal role for old-style public health measures in the novel coronavirus (2019-nCoV) outbreak. J. Travel. Med. 2020;27(2):1–4. doi: 10.1093/jtm/taaa020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woods J.A., Hutchinson N.T., Powers S.K., et al. The COVID-19 pandemic and physical activity. J. Sport Health Sci. 2020;2(2):55–64. doi: 10.1016/j.smhs.2020.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia N., Li H. Loneliness, social isolation, and cardiovascular health. Antioxid. Redox Signal. 2018;28(9):837–851. doi: 10.1089/ars.2017.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng Z., Peng F., Xu B., Zhao J., Liu H., et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. Infect. 2020;81(2):e16–e25. doi: 10.1016/j.jinf.2020.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]