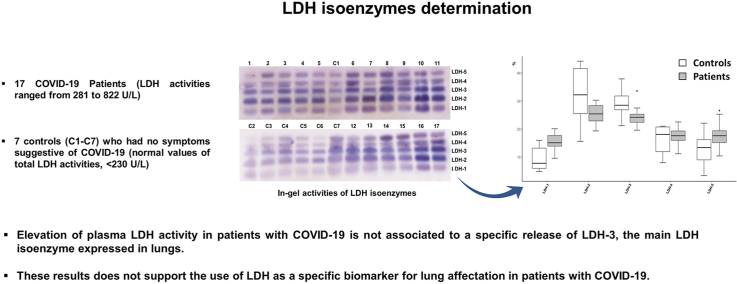
Abstract
Objectives
We aimed to determine whether the plasma profile of lactate dehydrogenase (LDH) isoenzymes is altered in patients with COVID-19, and whether this is attributable to a specific release of LDH-3, the main LDH isoenzyme expressed in lungs.
Design
We collected fresh plasma aliquots from 17 patients (LDH range, 281–822 U/L) and seven controls (LDH < 230 U/L). In-gel relative activity of the different LDH isoenzymes was determined by electrophoresis and densitometric analysis.
Results
Despite the expected higher total LDH activity levels in patients (p < 0.001), the in-gel relative activities of LDH isoenzymes did not differ between patients and controls (all p > 0.05). We found no correlation between total plasma LDH activity and the in-gel relative activities of the different LDH isoenzymes, including LDH-3. Likewise, there was no correlation between LDH-3 and various routine haematological and serum parameters that have been previously reported to be altered in COVID-19 (such as lymphocyte count, albumin, alanine and aspartate aminotransferase, creatinine, C-reactive protein, or ferritin).
Conclusions
Our findings suggest that elevation of plasma LDH activity in patients with COVID-19 is not associated to a specific release of LDH-3 into the bloodstream, and do not support the use of LDH as a specific biomarker for lung affectation in patients with COVID-19.
Keywords: COVID-19, SARS-CoV-2, Lactate dehydrogenase, LDH isoenzymes
Graphical abstract
Highlights
-
•
Plasma lactate dehydrogenase (LDH) isoenzyme profile is not altered in COVID-19.
-
•
The main lung LDH isoenzyme, LDH3, is not increased in COVID-19.
-
•
There is no association between plasma LDH-3 and COVID-19 biomarkers.
1. Introduction
Identification of laboratory predictors of progression towards severity and fatality is needed for an efficient management of patients with coronavirus disease 2019 (COVID-19) [1,2]. In this effect, several biochemical analytes that show abnormal values in severely affected patients have been proposed as disease biomarkers, including among others serum [[3], [4], [5], [6], [7], [8], [9]] or nasopharyngeal lactate dehydrogenase (LDH) activity [10].
LDH (EC 1.1.1.27) is a hydrogen transfer cytoplasmatic enzyme that catalyses the oxidation of l-lactate to pyruvate with nicotinamide-adenine dinucleotide (NAD)+ as hydrogen acceptor, the final reaction in the anaerobic glycolysis pathway [11]. This enzyme is composed of four subunits by the combination of two proteins, M and H, which are expressed mainly in skeletal and heart muscle. Five different isoenzymes, LDH-1 (H4), LDH-2 (H3M), LDH-3 (H2M2), LDH-4 (HM3) and LDH-5 (M4), are detected in plasma by electrophoresis, and since they are – at least partly – tissue-specific, analysis of the isoenzyme profile could help to identify the main source of LDH release and thus to get insight into the biological significance of this biomarker. Due to specific isoenzyme differences in efficiency for conversion of pyruvate to lactate [12], the major type of LDH expressed in a particular tissue depends on its metabolic demands – LDH-1 and LDH-2 are predominantly expressed in the heart, kidneys, and erythrocytes, LDH-4 and LDH-5 in the liver and skeletal muscle, and LDH isoenzymes of intermediate mobility in spleen, lymph nodes, leukocytes, and platelets [11]. In turn, LDH-3 is prevalent in lung tissue and total serum LDH has been proposed as a tumour biomarker for lung cancer [12].
Severe infections may cause tissue damage induced by cytokine production with subsequent release of LDH into the bloodstream [13]. In this context, it has been proposed [6] that the main LDH isoform in lung tissue, LDH-3, could be released in greater amounts in the more severely affected patients with COVID-19, since a severe form of interstitial pneumonia (often evolving into acute respiratory distress syndrome) is the hallmark of this disease [14]. However, to our best knowledge, the contribution of the different LDH isoenzymes to the total LDH elevation that is usually observed in COVID-19 has not been determined.
It was the purpose of this study to evaluate whether the plasma profile of the different LDH isoenzymes is altered in patients with COVID-19 compared with controls, and whether this is associated with a specific elevation of LDH-3.
2. Methods
This study was approved by the Institutional Review Board of “12 de Octubre” University Hospital of Madrid (Spain) (No. #20/222). We obtained fresh plasma aliquots from supernatants of peripheral blood samples collected in lithium heparinized tubes that were previously centrifuged for 10 min at 1500×g in our Emergency/Core Laboratory – of note, there is not an optimal storage condition for all LDH isoenzymes [15] and preserving frozen samples can destroy the activity of some LDH isoenzymes activity [11].
We selected patients diagnosed with COVID-19 using real-time reverse transcription polymerase chain reaction within four days after hospital admission. Eligibility was based on their total plasma LDH activity with the aim of covering a wide range of elevated levels in LDH activities, and on their clinical and laboratory data in order to obtain a mixed study sample regarding the COVID-19 severity [3,16] (Table 1). Patient’s plasma LDH activities ranged from 281 to 822 U/L. Seven control fresh plasma aliquots were obtained from blood samples belonging to adult (>50-year-old) outpatients attending our center, who had no symptoms suggestive of COVID19 and showed normal values of total LDH activities (<230 U/L, range 112–194 U/L).
Table 1.
Demographic, clinical and laboratory data of 17 COVID-19 patients.
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (years) | 47 | 71 | 50 | 48 | 70 | 83 | 65 | 61 | 70 | 73 | 68 | 87 | 62 | 66 | 94 | 63 | 57 |
| Sex | F | M | M | M | M | M | F | M | M | M | M | F | F | M | M | M | M |
| Overweight/obesity1 | ++ | + | ++ | + | NR | + | – | ++ | – | – | ++ | NR | – | NR | + | ++ | NR |
| Hypertension | N | Y | Y | N | Y | Y | Y | N | Y | Y | Y | Y | N | N | Y | Y | N |
| Onset to sample (days)2 | 11 | 24 | 16 | 14 | 23 | 30 | 12 | 31 | 15 | 37 | 38 | 4 | 5 | 38 | 57 | 52 | 51 |
| Dyspnea3 | ++ | ++ | ++ | + | ++ | – | ++ | ++ | – | + | ++ | + | – | ++ | – | ++ | ++ |
| Fever peak (°C) | NR | 38.2 | 38.8 | 41.0 | NR | 38.4 | 37.8 | 39.0 | 38.0 | NR | 38.9 | 38.5 | 37.3 | 37.5 | 37.7 | 37.8 | 37.5 |
| Pneumonia | Y | N | N | Y | Y | Y | Y | N | Y | Y | Y | Y | N | Y | Y | Y | Y |
| Outcome4 | DIS | ICU | DIS | ICU | DEC | HOS | HOS | HOS | HOS | DIS | ICU | HOS | DIS | DIS | HOS | ICU | ICU |
| Blood cells counts (·103/μL) | |||||||||||||||||
| Leukocytes | 3.9 | 24.0 | 7.0 | 10.7 | 10.8 | 10.4 | 7.1 | 9.4 | 8.5 | 5.9 | 11.0 | 3.3 | 2.6 | 8.6 | 8.3 | 14.3 | 17.1 |
| Neutrophils | 1.7 | 16.6 | 4.1 | 8.9 | 8.2 | 8.2 | 5.8 | 6.3 | 6.9 | 4.3 | 8.5 | 2.4 | 1.4 | 4.4 | 7.5 | 9.0 | 13.8 |
| Lymphocytes | 1.8 | 4.4 | 2.0 | 1.0 | 1.1 | 0.9 | 0.7 | 1.0 | 0.6 | 0.9 | 1.2 | 0.4 | 1.0 | 2.0 | 0.4 | 2.1 | 1.5 |
| Eosinophils | 0.2 | 0.2 | 0.1 | 0.1 | 0.8 | 0.4 | 0.1 | 1.3 | 0.1 | 0.1 | 0.7 | 0.1 | 0.1 | 1.2 | 0.3 | 2.2 | 0.3 |
| Monocytes | 0.3 | 2.8 | 0.7 | 0.7 | 0.5 | 0.9 | 0.3 | 0.6 | 0.7 | 0.5 | 0.6 | 0.4 | 0.2 | 0.9 | 0.3 | 0.8 | 1.3 |
| Platelets | 461 | 153 | 361 | 411 | 135 | 383 | 200 | 158 | 593 | 354 | 314 | 162 | 35 | 406 | 19 0 | 405 | 521 |
| Hemoglobin (g/dL) | 13.6 | 12.3 | 15.8 | 9.9 | 10.3 | 13.8 | 12.7 | 11.8 | 10.5 | 13.2 | 10.3 | 11.6 | 11.0 | 11.3 | 12.5 | 9.4 | 8.7 |
| Albumin (g/dL) | 3.4 | 2.6 | 3.4 | 2.5 | 1.7 | 2.8 | 3.1 | 3.0 | 2.7 | 3.1 | 2.7 | 3.2 | 3.3 | 3.6 | 2.9 | 2.9 | 2.6 |
| ALT (U/L) | 118 | 51 | 102 | 68 | 18 | 31 | 154 | 34 | 55 | 86 | 44 | 9 | 267 | 381 | 11 | 38 | 47 |
| AST (U/L) | 32 | 34 | 52 | 65 | 37 | 29 | 152 | 19 | 39 | 64 | 34 | 18 | 127 | 158 | 20 | 40 | 41 |
| Creatinine (mg/dL) | 0.43 | 0.95 | 0.88 | 0.56 | 0.47 | 0.65 | 0.68 | 0.53 | 1.25 | 0.75 | 0.62 | 1.67 | 1.83 | 0.55 | 1.21 | 1.33 | 0.64 |
| CRP (mg/dL) | 0.30 | 0.30 | 0.34 | 11.2 | 19.3 | 5.19 | 0.49 | 13.4 | 3.45 | 0.19 | 6.11 | 4.20 | 4.56 | 1.47 | 8.41 | 0.83 | 9.15 |
| Ferritin (ng/ml) | 1016 | 1102 | 3031 | 957 | 1037 | 928 | 1627 | 1019 | 968 | 1454 | 1416 | 875 | 604 | 2197 | 527 | 563 | 519 |
| LDH (U/L) | 281 | 301 | 371 | 400 | 417 | 438 | 524 | 535 | 335 | 603 | 822 | 367 | 371 | 393 | 395 | 490 | 534 |
1 + overweight (i.e., body mass index 25–29.9 kg/m2); ++ obesity (i.e., body mass index ≥ 30 kg/m2); - normal weigh (i.e., body mass index 18.5–24.9 kg/m2). 2 Period of time (in days) from the onset of symptoms to blood/plasma testing. 3 ++ severe; + mild; - no dyspnea. 4 Outcome assessment at one week after sampling. DIS, discharged; ICU, hospitalized in intensive care unit; HOS, hospitalized no ICU; DEC, deceased. M, male; F, female; Y, yes; N, no; ALT, alanine-aminotransferase; AST, aspartate-aminotransferase; CRP, C-reactive protein; LDH, lactate dehydrogenase. NR, not recorded.
Total plasma LDH was measured by an optimized standardized method of the Clinical Chemistry German Society [17] in a Cobas 8000 Modular Autoanalyzer (Roche diagnostics, Basel, Switzerland) which measures the initial oxidation rate of NADH in the reaction between pyruvate and NADH to form l-lactate and NAD+ catalysed by LDH. Samples with ‘haemolysis index’ > 5 (as calculated by the autoanalyzer) were discarded to prevent false elevations in plasma LDH activity due in vitro haemolysis.
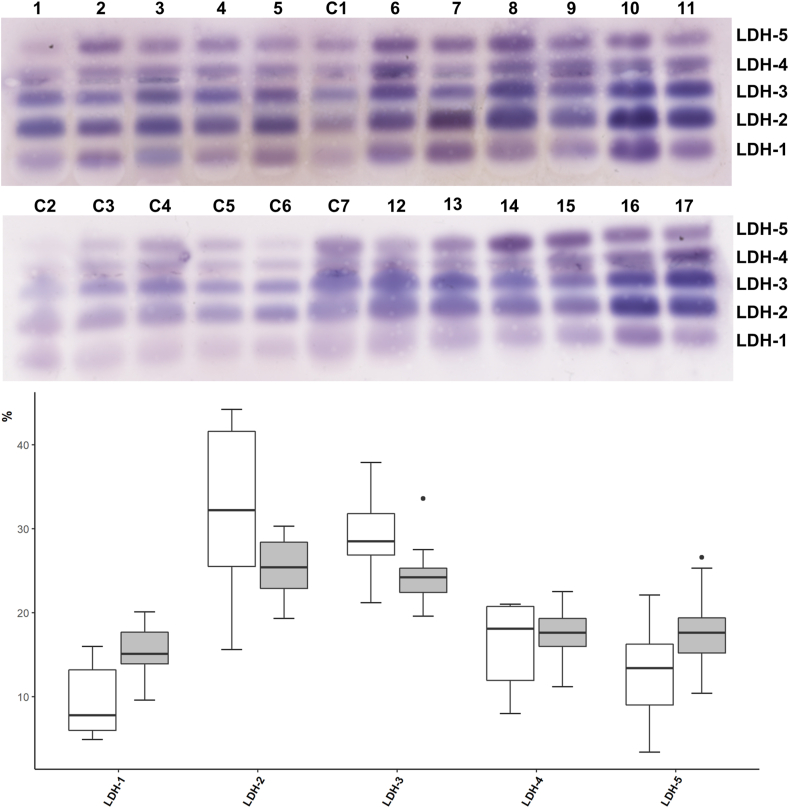
The five plasma LDH isoenzymes were separated by electrophoresis in agarose gel using the SAS-1 LD Vis Kit in the SAS-1 Plus instrument (Helena Biosciences Europe, UK) that uses a modification of the method described by Preston et al. [18] to detect the LDH activity (i.e., in-gel oxidation of Lactate to Pyruvate by LDH is detected with a colorimetric reagent containing lactate as a substrate, NAD + as an acceptor of electrons, and staining by a tetrazolium salt). LDH-1 isoenzyme runs anodic and muscle LDH-5 runs cathodic, with intermediate positions for the remainder of heterotetrameric isoenzymes (LDH2-4). Electrophoresis was performed at 80 V and 15 °C for 20 min following incubation at 45 °C for 25 min with the reagents required for the identification of LDH isoenzymes. Electrophoretic bands were relatively quantified by densitometry using Image J freeware program (Fig. 1), and the in-gel relative activity of each LDH isoenzyme was expressed as a percentage of the total in-gel LDH activity.
Fig. 1.
Electrophoretic separation and in-gel activity of lactate dehydrogenase (LDH) isoenzymes in patients with COVID-19.Upper panel: Electrophoretic separation of LDH isoenzymes plasma from 17 patients with COVID-19 (lanes 1–17) and 7 controls (lanes C1–C7). Lower panel: Box-plots of in-gel activity of the five LDH isoenzymes in patients (grey colour) and controls (white).
Plasma albumin was measured by an immunoturbidimetric method. Plasma alanine (ALT) and aspartate transaminase (AST) were measured following the IFCC recommendations [19], creatinine was measured with a colorimetric test based on the Jaffé method [20], and C-reactive protein (CRP) and ferritin were measured with an immunoturbidimetric test using latex coated particles with monoclonal anti-CRP antibodies and anti-ferritin antibodies, respectively. All these aforementioned measurements were done in a Cobas 8000 Modular Autoanalyzer (Roche Diagnotics). The haematological parameters were assayed in a DxH 900 hematology analyser (Beckman Coulter; Brea, CA).
We used the Mann-Whitney U test to compare between patients and controls total LDH activity, as well as the relative activity levels of the different LDH isoenzymes. We used the Spearman correlation to determine the association within the patients’ group between the relative activity of the different LDH isoenzymes and total plasma LDH activity, and also between LDH-3 isoenzyme relative activity and some blood/plasma analytes that have been shown to be altered in patients with COVID-19 (i.e., albumin [7,8], AST [7,8], ALT [7,8], creatinine [3,7,8], CRP [4,[7], [8], [9]], ferritin [7,8,14], haemoglobin concentration [7], leukocyte count and cell subpopulations [4,[7], [8], [9]], and platelet count [7] Data analysis and graphical output were carried out with R (4.0.1) statistical software and the level of statistical significance was set at 0.05.
3. Results
Total LDH activity was significantly higher in patients than in controls (p < 0.001). Yet, we found no significant differences between patients and controls for the in-gel relative activity of LDH isoenzymes (all p > 0.05, Fig. 1).
In the group of patients no significant correlation were found between plasma total LDH activity (U/L) and the percentage of in-gel activity of the different LDH isoenzymes – including the predominant form in lungs, LDH3 – (See Supplemental Data Fig. S1). Likewise, no significant correlations were found between LDH-3 relative activity and the various haematological and serum biochemical parameters we studied (See Supplemental Data Fig. S2).
4. Discussion
Our results suggest that the plasma LDH elevation that is typically found in patients with COVID19 is not due to a specific increase of the predominant LDH isoenzyme in lungs, LDH-3, in detriment of any other LDH isoenzyme. Indeed, we found no significant differences in LDH-3 levels between patients and controls. Likewise, none of the other LDH isoenzymes analysed seems to be a major contributor to the increase of total plasma LDH levels observed in COVID-19 patients, as we failed to find any correlation between the activity level of the different LDH isoenzymes and total plasma LDH activity. Furthermore, we intended to explore whether LDH-3 relative activity was associated with various blood and plasma parameters that had been previously reported to be altered in patients with COVID-19 [4,[7], [8], [9]], but failed to find any significant correlation.
The small cohort of patients is a limitation of the study, but the required use of fresh plasma samples to avoid the loss of LDH isoenzyme activity poses a major problem to schedule the electrophoretic assay [11,15]. However, we intended to get a representative sample of COVID-19 status, by collecting patients’ samples encompassing a range of total LDH activities according to previously reported values in patients with COVID-19 [6], as well as by selecting patients with a wide spectrum of clinical and laboratory parameters associated with COVID-19 severity [3,7] (Table 1).
4.1. Conclusion
The elevation of plasma LDH activity, a routine laboratory analyte with a promising value for prognostic and stratifying strategies in COVID-19, does not appear to be due to the specific release into bloodstream of the prevalent LDH-3 isoenzyme in lungs as previously suggested [6].
Author contributions
Conceptualization, P.S-L., J.A. and MA.M.; methodology and statistics, P.S-L, ON.C., A.D. and COVID-19 ’12 Octubre’ Hospital Clinical Biochemistry Study Group; formal analysis, P.S-L., ON.C. A.L. and MA.M.; writing—original draft preparation, P.S-L and MA.M.; writing—review and editing, all listed authors; supervision, A.L, J.A. and MA.M; project administration, A.L. and MA.M.
Declaration of competing interest
None.
Acknowledgments
The authors would like to thank Paloma Puerta and Rocío Garrido-Moraga for their technical assistance.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.plabm.2021.e00226.
Contributor Information
Miguel A. Martín, Email: mamcasanueva.imas12@h12o.es.
COVID-19 ’12 Octubre’ Hospital Clinical Biochemistry Study Group:
Alejandro Santos-Lozano, Cecilia Cueto-Felgueroso, Alba Fernández-del Pozo, and Montserrat de Miguel-Reyes
Funding
This work was supported by research grants of Spanish Instituto de Salud Carlos III (ISCIII), COV20-00181 and PI17-02052 and and the European Regional Development Fund (ERDF/FEDER a way to achieve Europe)
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Lippi G., Plebani M. The critical role of laboratory medicine during coronavirus disease 2019 (COVID-19) and other viral outbreaks. Clin. Chem. Lab. Med. 2020;58:1063–1069. doi: 10.1515/cclm-2020-0240. [DOI] [PubMed] [Google Scholar]
- 2.Bohn M.K., Lippi G., Horvath A., Sethi S., Koch D., Ferrari M., Wang C.-B., Mancini N., Steele S., Adeli K. Molecular, serological, and biochemical diagnosis and monitoring of COVID-19: IFCC taskforce evaluation of the latest evidence. Clin. Chem. Lab. Med. 2020;58:1037–1052. doi: 10.1515/cclm-2020-0722. [DOI] [PubMed] [Google Scholar]
- 3.Cecconi M., Piovani D., Brunetta E., Aghemo A., Greco M., Ciccarelli M., Angelini C., Voza A., Omodei P., Vespa E., Pugliese N., Parigi T.L., Folci M., Danese S., Bonovas S. Early predictors of clinical deterioration in a cohort of 239 patients hospitalized for covid-19 infection in lombardy, Italy. J. Clin. Med. 2020;9 doi: 10.3390/jcm9051548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ferrari D., Motta A., Strollo M., Banfi G., Locatelli M. Routine blood tests as a potential diagnostic tool for COVID-19. Clin. Chem. Lab. Med. 2020;58:1095–1099. doi: 10.1515/cclm-2020-0398. [DOI] [PubMed] [Google Scholar]
- 5.Han Y., Zhang H., Mu S., Wei W., Jin C., Xue Y., Tong C., Zha Y., Song Z., Gu G. Lactate dehydrogenase, a risk factor of severe COVID-19 patients. MedRxiv. 2020 doi: 10.1101/2020.03.24.20040162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henry B.M., Aggarwal G., Wong J., Benoit S., Vikse J., Plebani M., Lippi G. Lactate dehydrogenase levels predict coronavirus disease 2019 (COVID-19) severity and mortality: a pooled analysis. Am. J. Emerg. Med. 2020 doi: 10.1016/j.ajem.2020.05.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henry B.M., de Oliveira M.H.S., Benoit S., Plebani M., Lippi G. Hematologic, biochemical and immune biomarker abnormalities associated with severe illness and mortality in coronavirus disease 2019 (COVID-19): a meta-analysis. Clin. Chem. Lab. Med. 2020;58:1021–1028. doi: 10.1515/cclm-2020-0369. [DOI] [PubMed] [Google Scholar]
- 8.Lippi G., Plebani M. Laboratory abnormalities in patients with COVID-2019 infection. Clin. Chem. Lab. Med. 2020;58:1131–1134. doi: 10.1515/cclm-2020-0198. [DOI] [PubMed] [Google Scholar]
- 9.Zhang Z.-L., Hou Y.-L., Li D.-T., Li F.-Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand. J. Clin. Lab. Invest. 2020:1–7. doi: 10.1080/00365513.2020.1768587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharote M.A. Role of Nasopharyngeal lactate dehydrogenase as a possible economical mass screening test for the detection and segregation of SARS-CoV-2 (COVID-19) cases in India. Indian J. Med. Sci. 2020;72:21–24. doi: 10.25259/IJMS_25_2020. [DOI] [Google Scholar]
- 11.Rifai N. sixth ed. Elsevier; St. Louis, Missouri: 2018. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics. [Google Scholar]
- 12.Jurisic V., Radenkovic S., Konjevic G. The actual role of LDH as tumor marker, biochemical and clinical aspects. Adv. Exp. Med. Biol. 2015;867:115–124. doi: 10.1007/978-94-017-7215-0_8. [DOI] [PubMed] [Google Scholar]
- 13.Erez A., Shental O., Tchebiner J.Z., Laufer-Perl M., Wasserman A., Sella T., Guzner-Gur H. Diagnostic and prognostic value of very high serum lactate dehydrogenase in admitted medical patients. Isr. Med. Assoc. J. 2014;16:439–443. [PubMed] [Google Scholar]
- 14.Guan W., Ni Z., Hu Y., Liang W., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., Du B., Li L., Zeng G., Yuen K.-Y., Chen R., Tang C., Wang T., Chen P., Xiang J., Li S., Wang J., Liang Z., Peng Y., Wei L., Liu Y., Hu Y., Peng P., Wang J., Liu J., Chen Z., Li G., Zheng Z., Qiu S., Luo J., Ye C., Zhu S., Zhong N. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kreutzer H.H., Fennis W.H. Lactic dehydrogenase isoenzymes in blood serum after storage at different temperatures. Clin. Chim. Acta. 1964;9:64–68. doi: 10.1016/0009-8981(64)90045-2. [DOI] [PubMed] [Google Scholar]
- 16.Li L.-Q., Huang T., Wang Y.-Q., Wang Z.-P., Liang Y., Huang T.-B., Zhang H.-Y., Sun W., Wang Y. COVID-19 patients’ clinical characteristics, discharge rate, and fatality rate of meta-analysis. J. Med. Virol. 2020;92:577–583. doi: 10.1002/jmv.25757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rick W., Schmidt E. Recommendations of the German society for clinical Chemistry. Z. Klin. Chem. Klin. Biochem. 1974;12:7–8. [Google Scholar]
- 18.Preston J.A., Briere R.O., Batsakis J.G. Rapid electrophoretic separation of lactate dehydrogenase isoenzymes on cellulose acetate. Am. J. Clin. Pathol. 1965;43:256–260. doi: 10.1093/ajcp/43.3.256. [DOI] [PubMed] [Google Scholar]
- 19.Bowers G.N., Bergmeyer H.U., Hørder M., Moss D.W. Approved recommendation (1978) on IFCC methods for the measurement of catalytic concentration of enzymes. Clin. Chim. Acta. 1979;98:163F–174F. doi: 10.1016/0009-8981(79)90176-1. [DOI] [PubMed] [Google Scholar]
- 20.Jaffe M. About the precipitate which picric acid produces in normal urine and about a new reaction of creatinine. Journal of Physiological Chemistry. 1886;10:391–400. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.