Abstract
ODFM is a data management system that integrates comprehensive omics information for microorganisms associated with various fermented foods, additive ingredients, and seasonings (e.g. kimchi, Korean fermented vegetables, fermented seafood, solar salt, soybean paste, vinegar, beer, cheese, sake, and yogurt). The ODFM archives genome, metagenome, metataxonome, and (meta)transcriptome sequences of fermented food-associated bacteria, archaea, eukaryotic microorganisms, and viruses; 131 bacterial, 38 archaeal, and 28 eukaryotic genomes are now available to users. The ODFM provides both the Basic Local Alignment Search Tool search-based local alignment function as well as average nucleotide identity-based genetic relatedness measurement, enabling gene diversity and taxonomic analyses of an input query against the database. Genome sequences and annotation results of microorganisms are directly downloadable, and the microbial strains registered in the archive library will be available from our culture collection of fermented food-associated microorganisms. The ODFM is a comprehensive database that covers the genomes of an entire microbiome within a specific food ecosystem, providing basic information to evaluate microbial isolates as candidate fermentation starters for fermented food production.
Subject terms: Bacterial genomics, Archaeal genomics
Introduction
Advances in next-generation sequencing technology have led to the rapid expansion of microbial genome sequence data. Easy access, as well as convenient analytical tools, have enabled the exploration of microbial communities in various environmental samples. However, efficient resource usage is becoming increasingly difficult because of the rapid accumulation of sequencing data. Environmental microbiomes in fermented foods, the mammalian gut, and soils comprise not only bacteria, but also archaea, eukaryotic microorganisms, and viruses1–3. These microbial entities are all essential in determining the microbial signature and thus, the inherent characteristics of a given ecosystem. In this context, a comprehensive database covering all genomes of a microbiome within a specific ecosystem would aid in improving our understanding of the complex interactions among the microbial populations.
Fermented foods are an integral part of the global human diet. Microbial entities in fermented foods include bacteria, archaea, yeasts, and viruses. Microbial activities, as well as the type of raw materials, ultimately determine the nutritional and organoleptic properties, quality, and safety of the fermentation product4,5. Given that consumers and manufacturers alike are interested in tasty, high-quality foods6 as well as the reliability of geographic origins (i.e. no false indication of the origin of the product)1, providing standardised microbial profiles and/or genome information for key microorganisms during the fermentation process is important for ensuring the high quality of fermentation products.
Kimchi is a traditional Korean food prepared by fermentation of vegetables, such as kimchi cabbage, along with various added ingredients and seasonings. The global annual consumption of kimchi is 1,500,000 tons1. Like other fermented foods, kimchi shows the presence of a distinct microbial community4,5. Taxonomic studies using culture-dependent and -independent (e.g. bacterial 16 S rRNA gene sequencing) approaches have revealed that lactic acid bacteria (LAB), including Leuconostoc, Lactobacillus, and Weissella, are mainly responsible for kimchi fermentation7–9.
We have developed the Omics Database of Fermentative Microbes (ODFM), a data management system that integrates comprehensive omics information for fermentative microorganisms at the World Institute of Kimchi funded by the Korean government. The ODFM offers not only curated omics sequences of fermented food-associated bacteria, archaea, eukaryotic microorganisms, and viruses, but also several analytical tools that enable gene diversity and taxonomic analyses of an input query on the database at the whole genome level. Our knowledgebase is valuable to researchers who are interested in the functions and spatiotemporal dynamics of microbiomes in fermented foods. In particular, it provides basic information to evaluate microbial strains isolated from fermented foods as candidate starters in terms of food safety and sanitation.
Results
System design and data registration
The ODFM is a web-based application developed in compliance with the HyperText Markup Language (HTML) 5 web standards and, thus, is supported by most web browsers. The program was designed based on the Representational State Transfer (REST) service architecture to support use on various devices, including desktop computers and mobile devices. To support stable web service in a cloud-based service environment, the ODFM is hosted on four servers (web, web application, database, and storage servers). Key specifications for each server are summarised in Table 1.
Table 1.
Features of the ODFM.
| Category | Features |
|---|---|
| System environment | |
| Operating system | Centos (v6.5) |
| Java runtime environment | Java Development Kit (v1.8) |
| Web application | Apache (v2.2.15) and Tomcat server (v7.0) |
| Database operation | MySQL (v5.7) |
| Database content | |
| Genome | |
| Bacteria | 62 complete and 69 draft genomes covering 96 (sub)species |
| Archaea | 7 complete and 31 draft genomes covering 36 species |
| Unicellular eukaryotes | 14 complete and 14 draft genomes covering 9 species |
| Metagenome | 10 total and 60 viral metagenomes (kimchi) |
| Metataxonome | 113 bacterial metataxonomes (kimchi, fermented seafood, and soybean paste) |
| (Meta)transcriptome | 5 metatranscriptome (kimchi) and 4 archaeal transcriptome |
| Metabolome | 7 metabolomes (kimchi and fermented seafood) |
| Functionality | |
| Browsing | JBrowse and GView Java package |
| Tools | Sequence similarity search (BLAST) and genomic relatedness analysis (ANI) |
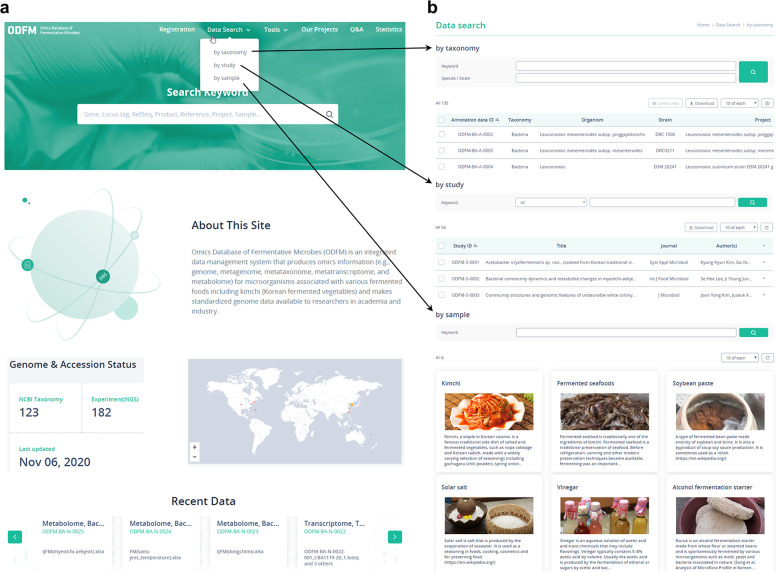
The software architecture of the ODFM consists of client, server, and database modules. The client module uses Google’s AngularJS (version 1.7) as a front-end framework to support cross browsing. The server module operates on JAVA (https://www.oracle.com/technetwork/java/index.html)-implemented Spring framework (https://spring.io) and is additionally equipped with the open-source programs Python (https://www.python.org/) and FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/). The database module uses MySQL (https://www.mysql.com) to manage the database, while the ODFM user interface architecture consists of Registration, Data Search, Tools, Our Projects, Statistics, and Q&A (Fig. 1a).
Fig. 1.
Search functions in the ODFM knowledgebase. (a) Front page of the ODFM knowledgebase. (b) Basic search functions of the ODFM. By selecting one of three search functions on the data search tab, users can search omics data categorised by taxonomy, study, and sample.
To improve data search and the accuracy of search results, submitted genome data and metadata are stored on the storage server and the database server, respectively. The system administrator processes the verification, approval, and release of the registered data. In the data verification step, validation of the file format and conversion of the registered data files are processed in batch using a back-end module. Once the processing is completed, the registered data are presented to the user in the web browser. The ODFM provides an interface to report data, including sequence files, annotated files, and results of sequence quality control according to the data file types. In addition, JBrowse and GView are integrated into the system to provide microbial genome analysis services via the genome browser. A diagram describing the data registration is provided in Supplementary Fig. S1.
Database content
The ODFM database currently contains 131 complete/draft bacterial genome sequences covering 38 genera with 96 (sub)species, of which 24 (sub)species belong to the genus Lactobacillus10, 12 to the genus Leuconostoc11–16, seven to the genus Acetobacter17, six to the genus Staphylococcus18, five to the genus Enterococcus, four (sub)species to each of the genera Pediococcus and Weissella19,20, three to the genus Lactococcus, two (sub)species to each of the genera Brachybacterium, Clostridium, Corynebacterium, and Pseudomonas, and one species to each of the genera Alishewanella21, Bacillus22,23, Brevibacterium, Dietzia, Escherichia, Glutamicibacter, Hafnia, Halomonas, Lentibacillus, Listeria, Megasphaera, Microbacterium, Morganella, Mycetocola, Oceanobacillus, Paracoccus, Pectinatus, Pistricoccus, Propionibacterium, Salimicrobium24, Streptococcus, Tetragenococcus, and Vibrio. As for archaea, 38 complete/draft genome sequences of extremely halophilic archaea (19 genera with 36 species) are available, including seven species belonging to the genus Haloarcula25, four belonging to the genus Halorubrum26, three belonging to each of the genera Haloferax and Natronomonas, two belonging to each of the genera Halapricum27, Halobacterium28, Halolamina29 and Haloplanus, and one species belonging to each of the genera Haladaptatus30, Halalkalicoccus, Halarchaeum, Halobellus31, Halococcus, Halogeometricum, Halopenitus, Halorhabdus, Halostella, Haloterrigena32, and Natrinema33. As for eukaryotic microorganisms, 28 genome sequences are currently available, including five undesirable white colony-forming yeasts of the species Candida, Hanseniaspora, Kazachstania, Pichia, and Yarrowia. These spoilage yeasts can grow on the surface of the kimchi and affect its odour, appearance, and texture34. Genome sequences of Brettanomyces-, Penicillium-, and Saccharomyces-belonging species isolated from beer, cheese, and sake, respectively, are available. The database also contains 70 metagenomes, 113 bacterial metataxonomes, nine (meta)transcriptomes, and seven metabolomes for various fermented foods (Table 1). The viral metagenomic sequences have been deposited in the European Bioinformatics Institute (EMBL-EBI) database35 and are available under accession number PRJEB23957. Details on ODFM database contents are provided in Supplementary Table S11,7,9–14,16–34,36–108.
Functional omics archive for fermented food-associated microorganisms
The primary purpose of the ODFM is to provide integrative functional omics information on fermented food-associated microorganisms. The ODFM and online resource provide omics information for microbial isolates from food materials (e.g. kimchi, fermented seafood, solar salt, soybean paste, vinegar, beer, cheese, sake, and yogurt). Recent microbial community analyses based on metataxonomics have revealed that hundreds of bacterial operational taxonomic units/amplicon sequence variants can be detected in fermented foods, and that the number of species varies according to the fermentation process1,7. To cover the entire microbial populations involved in food fermentation, since 2018, we have been constantly isolating and sequencing fermented food-associated microorganisms, and updating the database with new data to expand the ODFM archive.
Search function
For easy access of omics information, several search tools with simple (i.e. exact-match keyword) and lexical (i.e. partial-match keyword) search options are available on the front page (Fig. 1a). These tools allow users to search for different combinations of search terms. Users can search microbial taxa at the species and strain levels. Once a taxon name is provided by users, the system returns categorised search results (Supplementary Fig. S2a). Results are presented in a tabular format, with each row depicting a microbial taxon that contains the query gene. In the annotation data detail page, a split function in each row shows detailed information on the submitters, isolation sources, sequencing, and annotation results (Supplementary Fig. S2b). The annotation results presented in columns link to both available datasets and additional functions, such as genome viewer. For easy integration of omics data, the ODFM provides three basic search functions. Users can search omics data by taxonomy (i.e. bacteria, archaea, eukaryotic microorganisms, and viruses), study (i.e. publicly available studies highlighting the fermentative microbes), and sample (e.g. kimchi, fermented seafood, solar salt, soybean paste, vinegar, beer, cheese, sake, and yogurt) (Fig. 1b).
Genome browsing function
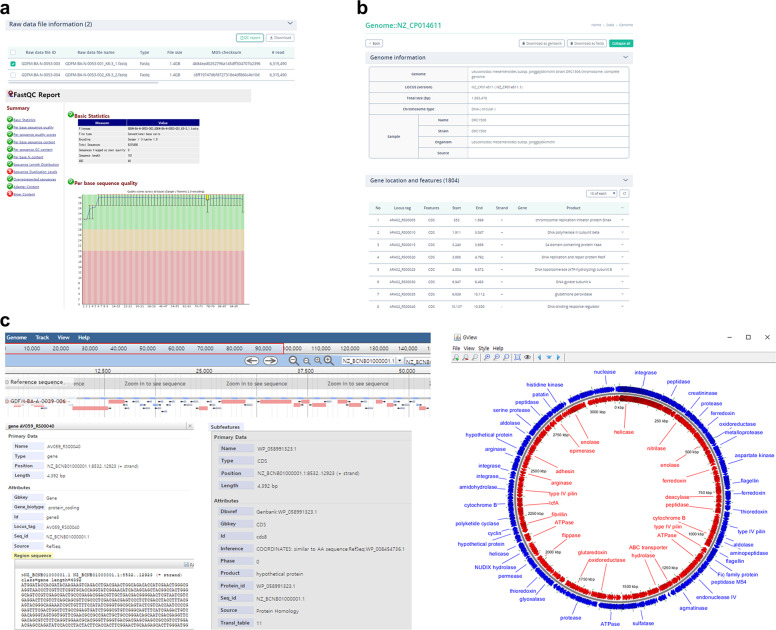
In the data search page, we provide several browsing functions for annotation data of the database resources. The raw data file information tab provides basic information on raw data (i.e. information regarding the experiment, library preparation, sequencing, and FASTQ file). The QC report tab utilises FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/) to facilitate simple quality control checks of raw sequence data (Fig. 2a). On the sequence information tab, the browser provides basic (i.e. FASTA) as well as detailed information about annotation data (Fig. 2b). The program uses the GenBank (gbk) file format and returns annotation results, such as location and product name of the gene/coding sequence (CDS). Furthermore, the ODFM provides linear and circular genome views representing graphical genome data based on GFF file information (Fig. 2c). The linear view utilises JBrowse109, enabling users to browse local annotation results, while the circular view provides microbial genome visualisation in a circular context with an interactive pan and zoom interface using the GView JAVA package110.
Fig. 2.
Genome browsing function in the ODFM. (a) Screen image of raw data details comprising raw data information and QC information tabs. The raw data file information tab provides basic information on raw data. The QC report tab utilises FastQC to facilitate simple quality control checks of raw sequence data. (b) Details on annotation results, including location and product name of the gene/CDS in table format by using the GenBank (gbk) file format. (c) The ODFM converts the GFF file to graphical genome data with linear (left) and circular genome views (right).
Archive expansion
We are currently seeking ways to expand the ODFM archive. Though the system currently does not allow data registration by other researchers, we are willing to accommodate deposition of fermented food-associated omics data generated by others upon request. In addition to the genome resources, the ODFM currently covers various outputs of genome annotation results generated from other databases, such as Kyoto Encyclopaedia of Genes and Genomes (KEGG)111 and Clusters of Orthologous Groups of proteins (COGs)112. In our projects tab on the front page, a list of fermented food-associated microbial studies is provided (Supplementary Fig. S3).
Comparative genomics
The microbial resources included in the ODFM are all candidate culture starters for fermented foods. Given that metabolic capabilities (e.g. lactate, lactose, and citrate metabolism) and resistance to bacteriophages, but not antibiotic resistance and virulence potential, are desirable functions for a candidate starter113,114, preliminary screening by means of comparative genomics analysis between query and subject genome resources can be a practical way to select strains for the production of fermented foods. Accordingly, several open-source analytical tools for comparative genomics analysis are integrated in the ODFM.
Sequence similarity search against the ODFM
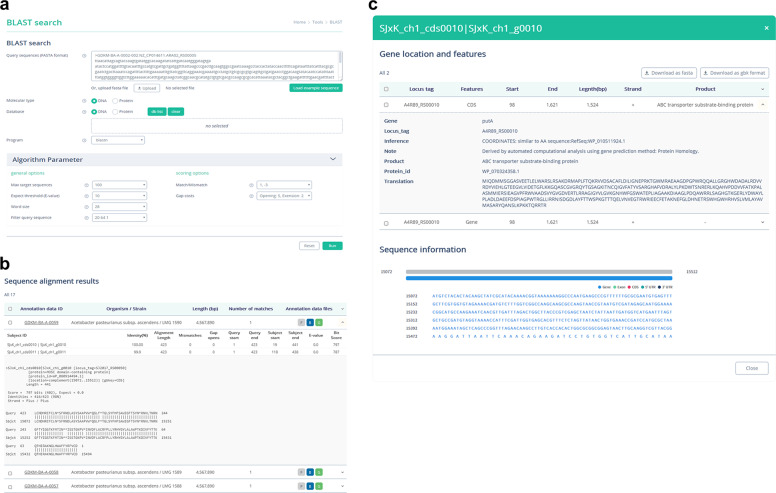
A sequence similarity search against public nucleotide databases is generally the first step in the identification of microbial isolates. The ODFM provides the Basic Local Alignment Search Tool (BLAST)115 search-based local alignment function. Once nucleotide/amino acid sequences are provided by users, the system aligns the query sequences with the local DNA/protein database (Fig. 3a). The system returns alignment results with statistical indicators, including bit score and E-value (Fig. 3b), and provides sequence/CDS information for the annotated data in a downloadable text format (Fig. 3c). Given that the ODFM comprises primarily fermentation-associated microbial genomes, this function is particularly helpful for an initial similarity search of strong candidate fermentative starter strains.
Fig. 3.
BLAST search function in the ODFM (a) Screen image of the BLAST search tab. Users can provide nucleotide/amino acid sequences, and select a BLAST program (blastn, blastp, blastx, tblastnl, or tblastx), expected threshold, and filter query sequence (true or false). (b,c) The system returns alignment results in order of match (b), and provides sequence/CDS information for the annotated data as a downloadable text format (c).
Genetic relatedness analysis
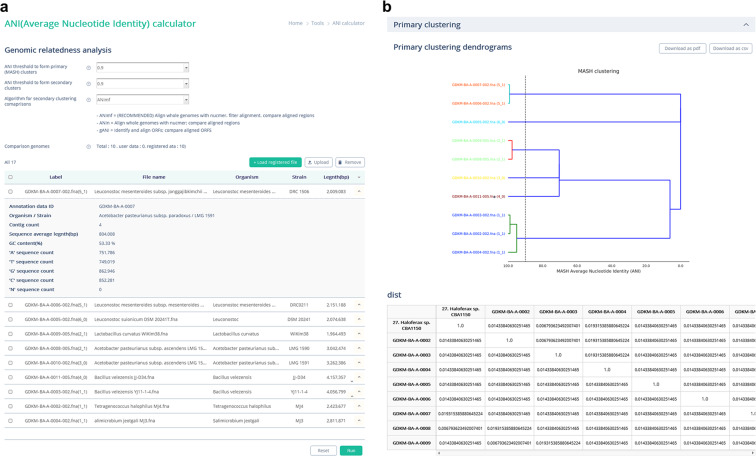
For average nucleotide identity (ANI)-based genome clustering and genetic relatedness measurement, the dRep tool116 was integrated into the ODFM. The ANI value is calculated from two genome sequences. Users can create comparative datasets by selecting FASTA files from the registered data in the ODFM or by uploading their own sequences (Fig. 4a). The query genome sequence (complete or draft) is cut into 1,020 bp-long sequences117, and each fragment is annotated against the whole sequence of the subject genome. Using the MinHash distance to estimate similarity between genomes118, the dRep tool calculates nucleotide identity between each of the query fragments and the subject genome and returns the ANI value, allowing for simple and standardised procedures for genome-related analysis of microbial isolates with closely related strains. The results are provided as a downloadable table and image (Fig. 4b).
Fig. 4.
ANI calculator function in the ODFM. (a) Screen image of the ANI calculator tab. Users can form comparative datasets by selecting FASTA files from the registered data in the ODFM or by uploading their own sequences. (b) The calculation results are returned as a downloadable table and image.
Discussion
We developed the ODFM, a web-based knowledgebase featuring archival and analytical functions for genome data for bacteria, archaea, eukaryotic microorganisms, and viruses associated with fermented foods. The ODFM is freely available on the website https://odfm.wikim.re.kr. This easily accessible online-browsable resource facilitates rapid and functional explorations of genomes of fermentation-associated microorganisms. Genome sequence and annotation results for reference microorganisms, as well as analytical results, are directly downloadable. All microbial strains registered in the ODFM will be made available. Our team operates a culture collection of fermented food-associated microorganisms, the Microorganism and Gene Bank (https://mgb.wikim.re.kr), at the World Institute of Kimchi.
By using the omics resources combined with the search tools, users are able to evaluate microbial strains isolated from fermented foods as candidate starters, and/or select microbial strain(s) among the deposited resources for use as starters. However, the process of fermentation is difficult to control because the fermentation phenotypes of different isolates are influenced by environmental conditions (e.g., temperature, humidity, and type of ingredients) and interactions with other, pre-colonised microbial communities. Previous studies have reported findings that allow linking of certain fermentative microbes with expected key features/metabolites in kimchi fermentation. Leuconostoc and Lactobacillus species are the major mannitol- and gamma-aminobutyric acid-producing LAB, respectively119. Lactococcus and some Lactobacillus species are homo-fermentative LAB responsible for the production of lactate from pyruvate by lactate dehydrogenase120. Leuconostoc mesenteroides, Lactobacillus sakei, and Weissella koreensis convert pyruvate to diacetyl/acetoin by using acetolactate synthase, acetolactate decarboxylase, and diacetyl reductase and thus contribute to the flavour of kimchi15,20,121.
We expect the ODFM to provide a framework for the analysis of genome characteristics of microorganisms isolated from various fermented foods. To increase the usage of the data and information contained in the ODFM knowledgebase, we will continuously improve the features and performance of each function. By adding categories of fermented foods based on global consumer preferences and encompassing microbial resources, our long-term goal with the ODFM is to facilitate the genomic characterisation of food microorganisms and their application as fermentation starters, as well as further functional probiotics and biological agents.
Supplementary information
Acknowledgements
This research was supported by a grant from the World Institute of Kimchi (KE2101-2) funded by the Ministry of Science and ICT, Korea.
Author contributions
S.W.R. and S.H.L. designed the study. T.W.W., S.W.A., S.Y., J.Y.K., Y.B.K., Y.K., J.-M.H., H.J., Y.-E.C., S.H.L., and S.W.R. conducted the system design and data processing. T.W.W., S.W.A., S.H.L., and S.W.R. wrote the manuscript.
Data availability
The ODFM is licensed under a Creative Commons Attribution 4.0 International License. The genome sequences in the ODFM are freely available on the website https://odfm.wikim.re.kr. All genome sequences have been deposited in NCBI GenBank and are available under the accession numbers listed in Supplementary Table S1. The viral metagenomic sequences have been deposited in the European Bioinformatics Institute (EMBL-EBI) database35 and are available under accession number PRJEB23957. We plan to deposit additional genome sequences for fermentative microbes to a member of the INSDC (http://www.insdc.org/) to promote sharing activities in the genomics community.
Code availability
The code used to build the systemic architecture of the GDKM is available on GitHub: https://github.com/yang4851/gdkm.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tae Woong Whon, Seung Woo Ahn.
Contributor Information
Se Hee Lee, Email: leesehee@wikim.re.kr.
Seong Woon Roh, Email: swroh@wikim.re.kr.
Supplementary information
The online version contains supplementary material available at 10.1038/s41597-021-00895-x.
References
- 1.Jung MJ, et al. Viral community predicts the geographical origin of fermented vegetable foods more precisely than bacterial community. Food Microbiol. 2018;76:319–327. doi: 10.1016/j.fm.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 2.Kim MS, Bae JW. Lysogeny is prevalent and widely distributed in the murine gut microbiota. Isme J. 2018;12:1127–1141. doi: 10.1038/s41396-018-0061-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bar-On YM, Phillips R, Milo R. The biomass distribution on Earth. Proc Natl Acad Sci USA. 2018;115:6506–6511. doi: 10.1073/pnas.1711842115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tamang JP, Watanabe K, Holzapfel WH. Review: Diversity of Microorganisms in Global Fermented Foods and Beverages. Front Microbiol. 2016;7:377. doi: 10.3389/fmicb.2016.00377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patra JK, Das G, Paramithiotis S, Shin HS. Kimchi and Other Widely Consumed Traditional Fermented Foods of Korea: A Review. Front Microbiol. 2016;7:1493. doi: 10.3389/fmicb.2016.01493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tuomola E, Crittenden R, Playne M, Isolauri E, Salminen S. Quality assurance criteria for probiotic bacteria. Am J Clin Nutr. 2001;73:393S–398S. doi: 10.1093/ajcn/73.2.393s. [DOI] [PubMed] [Google Scholar]
- 7.Jung JY, et al. Metagenomic analysis of kimchi, a traditional Korean fermented food. Appl Environ Microbiol. 2011;77:2264–2274. doi: 10.1128/AEM.02157-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Park EJ, et al. Bacterial community analysis during fermentation of ten representative kinds of kimchi with barcoded pyrosequencing. Food Microbiol. 2012;30:197–204. doi: 10.1016/j.fm.2011.10.011. [DOI] [PubMed] [Google Scholar]
- 9.Lee SH, Jung JY, Jeon CO. Bacterial community dynamics and metabolite changes in myeolchi-aekjeot, a Korean traditional fermented fish sauce, during fermentation. Int J Food Microbiol. 2015;203:15–22. doi: 10.1016/j.ijfoodmicro.2015.02.031. [DOI] [PubMed] [Google Scholar]
- 10.Lee, S. H., Jung, M. Y., Song, J. H., Lee, M. & Chang, J. Y. Complete Genome Sequence of Lactobacillus curvatus Strain WiKim38 Isolated from Kimchi. Genome Announc5, 10.1128/genomeA.00273-17 (2017). [DOI] [PMC free article] [PubMed]
- 11.Jung JY, Lee SH, Jeon CO. Complete genome sequence of Leuconostoc carnosum strain JB16, isolated from kimchi. J Bacteriol. 2012;194:6672–6673. doi: 10.1128/JB.01805-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jung JY, Lee SH, Jeon CO. Complete genome sequence of Leuconostoc gelidum strain JB7, isolated from kimchi. J Bacteriol. 2012;194:6665. doi: 10.1128/JB.01806-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee SH, Jung JY, Lee SH, Jeon CO. Complete genome sequence of Leuconostoc kimchii strain C2, isolated from Kimchi. J Bacteriol. 2011;193:5548. doi: 10.1128/JB.05707-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JS, et al. Genome sequence analysis of potential probiotic strain Leuconostoc lactis EFEL005 isolated from kimchi. J Microbiol. 2015;53:337–342. doi: 10.1007/s12275-015-5090-8. [DOI] [PubMed] [Google Scholar]
- 15.Chun, B. H., Kim, K. H., Jeon, H. H., Lee, S. H. & Jeon, C. O. Pan-genomic and transcriptomic analyses of Leuconostoc mesenteroides provide insights into its genomic and metabolic features and roles in kimchi fermentation. Sci Rep7, 10.1038/s41598-017-12016-z (2017). [DOI] [PMC free article] [PubMed]
- 16.Chun BH, Lee SH, Jeon HH, Kim DW, Jeon CO. Complete genome sequence of Leuconostoc suionicum DSM 20241T provides insights into its functional and metabolic features. Stand Genomic Sci. 2017;12:38. doi: 10.1186/s40793-017-0256-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jia B, et al. Complete Genome Sequences of Two Acetic Acid-Producing Acetobacter pasteurianus Strains (Subsp. ascendens LMG 1590T and Subsp. paradoxus LMG 1591T) Front Bioeng Biotechnol. 2017;5:33. doi: 10.3389/fbioe.2017.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sung JS, Chun J, Choi S, Park W. Genome sequence of the halotolerant Staphylococcus sp. strain OJ82, isolated from Korean traditional salt-fermented seafood. J Bacteriol. 2012;194:6353–6354. doi: 10.1128/JB.01653-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SH, Jung JY, Lee SH, Jeon CO. Complete genome sequence of Weissella koreensis KACC 15510, isolated from kimchi. J Bacteriol. 2011;193:5534. doi: 10.1128/JB.05704-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jeong SE, et al. Genomic and metatranscriptomic analyses of Weissella koreensis reveal its metabolic and fermentative features during kimchi fermentation. Food Microbiol. 2018;76:1–10. doi: 10.1016/j.fm.2018.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Jung J, Chun J, Park W. Genome sequence of extracellular-protease-producing Alishewanella jeotgali isolated from traditional Korean fermented seafood. J Bacteriol. 2012;194:2097. doi: 10.1128/JB.00153-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jung WY, Jung JY, Lee HJ, Jeon CO. Functional Characterization of Bacterial Communities Responsible for Fermentation of Doenjang: A Traditional Korean Fermented Soybean Paste. Front Microbiol. 2016;7:827. doi: 10.3389/fmicb.2016.00827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lee, H. J., Chun, B. H., Jeon, H. H., Kim, Y. B. & Lee, S. H. Complete Genome Sequence of Bacillus velezensis YJ11-1-4, a Strain with Broad-Spectrum Antimicrobial Activity, Isolated from Traditional Korean Fermented Soybean Paste. Genome Announc5, 10.1128/genomeA.01352-17 (2017). [DOI] [PMC free article] [PubMed]
- 24.Lee SH, Jung JY, Jeon CO. Draft genome sequence of Salimicrobium sp. strain MJ3, isolated from Myulchi-Jeot, Korean fermented seafood. J Bacteriol. 2012;194:6695. doi: 10.1128/JB.01808-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yun JH, et al. Complete genome sequence of Haloarcula sp. CBA1115 isolated from non-purified solar salts. Mar Genomics. 2015;23:19–21. doi: 10.1016/j.margen.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Lee HW, et al. Draft genome sequence of Halorubrum halophilum B8T, an extremely halophilic archaeon isolated from salt-fermented seafood. Mar. Genomics. 2014;18 Pt B:117–118. doi: 10.1016/j.margen.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 27.Song HS, et al. Draft genome sequence of Halapricum salinum CBA1105T, an extremely halophilic archaeon isolated from solar salt. Mar Genomics. 2014;18 Pt B:133–134. doi: 10.1016/j.margen.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Lim SK, et al. Genomic Analysis of the Extremely Halophilic Archaeon Halobacterium noricense CBA1132 Isolated from Solar Salt That Is an Essential Material for Fermented Foods. J Microbiol Biotechnol. 2016;26:1375–1382. doi: 10.4014/jmb.1603.03010. [DOI] [PubMed] [Google Scholar]
- 29.Lee MH, et al. Draft genome sequence of Halolamina rubra CSA1107T, an agarolytic haloarchaeon isolated from solar salt. Mar. Genomics. 2014;18:127–128. doi: 10.1016/j.margen.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Lee HW, et al. Draft genome sequence of the extremely halophilic archaeon Haladaptatus cibarius type strain D43T isolated from fermented seafood. Stand Genomic Sci. 2015;10:53. doi: 10.1186/s40793-015-0051-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee MH, et al. Draft genome sequence of the agarolytic haloarchaeon Halobellus rufus type strain CBA1103. FEMS Microbiol Lett. 2015;362:1–3. doi: 10.1093/femsle/fnu005. [DOI] [PubMed] [Google Scholar]
- 32.Cha IT, et al. Genome sequence of the haloarchaeon Haloterrigena jeotgali type strain A29T isolated from salt-fermented food. Stand Genomic Sci. 2015;10:49. doi: 10.1186/s40793-015-0047-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim YB, et al. Novel haloarchaeon Natrinema thermophila having the highest growth temperature among haloarchaea with a large genome size. Sci Rep. 2018;8:7777. doi: 10.1038/s41598-018-25887-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim JY, et al. Community structures and genomic features of undesirable white colony-forming yeasts on fermented vegetables. J Microbiol. 2019;57:30–37. doi: 10.1007/s12275-019-8487-y. [DOI] [PubMed] [Google Scholar]
- 35.2020. European Nucleotide Archive. PRJEB23957
- 36.Jung MY, Lee SH, Lee M, Song JH, Chang JY. Lactobacillus allii sp. nov. isolated from scallion kimchi. Int J Syst Evol Microbiol. 2017;67:4936–4942. doi: 10.1099/ijsem.0.002327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim, H. I. et al. Draft Genome Sequence of Lactobacillus sakei Strain wikim 22, Isolated from Kimchi in Chungcheong Province, South Korea. Genome Announc2, 10.1128/genomeA.01296-14 (2014). [DOI] [PMC free article] [PubMed]
- 38.Jung MY, Lee C, Seo MJ, Roh SW, Lee SH. Characterization of a potential probiotic bacterium Lactococcus raffinolactis WiKim0068 isolated from fermented vegetable using genomic and in vitro analyses. BMC Microbiol. 2020;20:136. doi: 10.1186/s12866-020-01820-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chun BH, Kim KH, Jeon HH, Lee SH, Jeon CO. Pan-genomic and transcriptomic analyses of Leuconostoc mesenteroides provide insights into its genomic and metabolic features and roles in kimchi fermentation. Sci Rep. 2017;7:11504. doi: 10.1038/s41598-017-12016-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Whon TW, et al. Oceanobacillus kimchii sp. nov. isolated from a traditional Korean fermented food. J Microbiol. 2010;48:862–866. doi: 10.1007/s12275-010-0214-7. [DOI] [PubMed] [Google Scholar]
- 41.Shin NR, et al. Genome sequence of Corynebacterium nuruki S6-4T, isolated from alcohol fermentation starter. J Bacteriol. 2011;193:4257. doi: 10.1128/JB.05354-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim KH, et al. Acetobacter oryzifermentans sp. nov., isolated from Korean traditional vinegar and reclassification of the type strains of Acetobacter pasteurianus subsp. ascendens (Henneberg 1898) and Acetobacter pasteurianus subsp. paradoxus (Frateur 1950) as Acetobacter ascendens sp. nov., comb. nov. Syst Appl Microbiol. 2018;41:324–332. doi: 10.1016/j.syapm.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 43.Park SK, Roh SW, Whon TW, Bae JW. Genome sequence of Brachybacterium squillarum M-6-3T, isolated from salt-fermented seafood. J Bacteriol. 2011;193:6416–6417. doi: 10.1128/JB.06183-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim J, Roh SW, Bae JW. Draft genome sequence of Dietzia alimentaria 72T, belonging to the family Dietziaceae, isolated from a traditional Korean food. J Bacteriol. 2011;193:6791. doi: 10.1128/JB.06229-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jung MJ, Roh SW, Kim MS, Whon TW, Bae JW. Genome sequence of Lentibacillus jeotgali GrbiT, isolated from traditional Korean salt-fermented seafood. J Bacteriol. 2011;193:6414–6415. doi: 10.1128/JB.06139-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim J, et al. Paracoccus jeotgali sp. nov., isolated from Korean salted and fermented shrimp. J Microbiol. 2019;57:444–449. doi: 10.1007/s12275-019-8704-8. [DOI] [PubMed] [Google Scholar]
- 47.Kim KH, Lee SH, Chun BH, Jeong SE, Jeon CO. Tetragenococcus halophilus MJ4 as a starter culture for repressing biogenic amine (cadaverine) formation during saeu-jeot (salted shrimp) fermentation. Food Microbiol. 2019;82:465–473. doi: 10.1016/j.fm.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 48.Cleenwerck I, Vandemeulebroecke K, Janssens D, Swings J. Re-examination of the genus Acetobacter, with descriptions of Acetobacter cerevisiae sp. nov. and Acetobacter malorum sp. nov. Int J Syst Evol Microbiol. 2002;52:1551–1558. doi: 10.1099/00207713-52-5-1551. [DOI] [PubMed] [Google Scholar]
- 49.Lisdiyanti P, et al. Systematic study of the genus Acetobacter with descriptions of Acetobacter indonesiensis sp. nov., Acetobacter tropicalis sp. nov., Acetobacter orleanensis (Henneberg 1906) comb. nov., Acetobacter lovaniensis (Frateur 1950) comb. nov., and Acetobacter estunensis (Carr 1958) comb. nov. J Gen Appl Microbiol. 2000;46:147–165. doi: 10.2323/jgam.46.147. [DOI] [PubMed] [Google Scholar]
- 50.Huang CH, Chang MT, Huang L, Chua WS. Molecular discrimination and identification of Acetobacter genus based on the partial heat shock protein 60 gene (hsp60) sequences. J Sci Food Agric. 2014;94:213–218. doi: 10.1002/jsfa.6231. [DOI] [PubMed] [Google Scholar]
- 51.Tohno M, et al. Description of Lactobacillus iwatensis sp. nov., isolated from orchardgrass (Dactylis glomerata L.) silage, and Lactobacillus backii sp. nov. Int J Syst Evol Microbiol. 2013;63:3854–3860. doi: 10.1099/ijs.0.051920-0. [DOI] [PubMed] [Google Scholar]
- 52.Fraunhofer ME, et al. Characterization of beta-glucan formation by Lactobacillus brevis TMW 1.2112 isolated from slimy spoiled beer. Int J Biol Macromol. 2018;107:874–881. doi: 10.1016/j.ijbiomac.2017.09.063. [DOI] [PubMed] [Google Scholar]
- 53.Long GY, Gu CT. Lactobacillus jixianensis sp. nov., Lactobacillus baoqingensis sp. nov., Lactobacillus jiayinensis sp. nov., Lactobacillus zhaoyuanensis sp. nov., Lactobacillus lindianensis sp. nov., Lactobacillus huananensis sp. nov., Lactobacillus tangyuanensis sp. nov., Lactobacillus fuyuanensis sp. nov., Lactobacillus tongjiangensis sp. nov., Lactobacillus fujinensis sp. nov. and Lactobacillus mulengensis sp. nov., isolated from Chinese traditional pickle. Int J Syst Evol Microbiol. 2019;69:2340–2353. doi: 10.1099/ijsem.0.003474. [DOI] [PubMed] [Google Scholar]
- 54.Geissler AJ, Behr J, von Kamp K, Vogel RF. Metabolic strategies of beer spoilage lactic acid bacteria in beer. Int J Food Microbiol. 2016;216:60–68. doi: 10.1016/j.ijfoodmicro.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 55.Bergsveinson, J., Thomson, E., Jacoby, D., Coady, Y. & Ziola, B. Genome Sequence of Megasphaera cerevisiae NSB1, a Bacterium Isolated from a Canning Line and Able To Grow in Beer with High Alcohol Content. Genome Announc5, 10.1128/genomeA.01686-16 (2017). [DOI] [PMC free article] [PubMed]
- 56.Kramer T, et al. Comparative genetic and physiological characterisation of Pectinatus species reveals shared tolerance to beer-associated stressors but halotolerance specific to pickle-associated strains. Food Microbiol. 2020;90:103462. doi: 10.1016/j.fm.2020.103462. [DOI] [PubMed] [Google Scholar]
- 57.Kern CC, Usbeck JC, Vogel RF, Behr J. Optimization of Matrix-Assisted-Laser-Desorption-Ionization-Time-Of-Flight Mass Spectrometry for the identification of bacterial contaminants in beverages. J Microbiol Methods. 2013;93:185–191. doi: 10.1016/j.mimet.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 58.Kajala, I. et al. Lactobacillus backii and Pediococcus damnosus isolated from 170-year-old beer recovered from a shipwreck lack the metabolic activities required to grow in modern lager beer. FEMS Microbiol Ecol94, 10.1093/femsec/fix152 (2018). [DOI] [PubMed]
- 59.Storari, M., Wuthrich, D., Bruggmann, R., Berthoud, H. & Arias-Roth, E. Draft Genome Sequences of Clostridium tyrobutyricum Strains FAM22552 and FAM22553, Isolated from Swiss Semihard Red-Smear Cheese. Genome Announc3, 10.1128/genomeA.00078-15 (2015). [DOI] [PMC free article] [PubMed]
- 60.Collins MD, Falsen E, Akervall E, Sjoden B, Alvarez A. Corynebacterium kroppenstedtii sp. nov., a novel corynebacterium that does not contain mycolic acids. Int J Syst Bacteriol. 1998;48:1449–1454. doi: 10.1099/00207713-48-4-1449. [DOI] [PubMed] [Google Scholar]
- 61.Martino GP, Quintana IM, Espariz M, Blancato VS, Magni C. Aroma compounds generation in citrate metabolism of Enterococcus faecium: Genetic characterization of type I citrate gene cluster. Int J Food Microbiol. 2016;218:27–37. doi: 10.1016/j.ijfoodmicro.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/IAI.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bonham, K. S., Wolfe, B. E. & Dutton, R. J. Extensive horizontal gene transfer in cheese-associated bacteria. Elife6, 10.7554/eLife.22144 (2017). [DOI] [PMC free article] [PubMed]
- 64.Oguntoyinbo FA, et al. Halomonas nigrificans sp. nov., isolated from cheese. Int J Syst Evol Microbiol. 2018;68:371–376. doi: 10.1099/ijsem.0.002515. [DOI] [PubMed] [Google Scholar]
- 65.Zhuravleva DE, et al. Complete Genome Sequence of Lactobacillus hilgardii LMG 7934, Carrying the Gene Encoding for the Novel PII-Like Protein PotN. Curr Microbiol. 2020;77:3538–3545. doi: 10.1007/s00284-020-02161-6. [DOI] [PubMed] [Google Scholar]
- 66.Hynonen U, et al. Functional characterization of probiotic surface layer protein-carrying Lactobacillus amylovorus strains. BMC Microbiol. 2014;14:199. doi: 10.1186/1471-2180-14-199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pepper, S. J. & Britz, M. L. An Acid Up-Regulated Surface Protein of Lactobacillus paracasei Strain GCRL 46 is Phylogenetically Related to the Secreted Glucan- (GpbB) and Immunoglobulin-Binding (SibA) Protein of Pathogenic Streptococci. Int J Mol Sci20, 10.3390/ijms20071610 (2019). [DOI] [PMC free article] [PubMed]
- 68.Somerville V, et al. Long-read based de novo assembly of low-complexity metagenome samples results in finished genomes and reveals insights into strain diversity and an active phage system. BMC Microbiol. 2019;19:143. doi: 10.1186/s12866-019-1500-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ennahar S, et al. Production of pediocin AcH by Lactobacillus plantarum WHE 92 isolated from cheese. Appl Environ Microbiol. 1996;62:4381–4387. doi: 10.1128/AEM.62.12.4381-4387.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tschoeke, D. A. et al. Exploring the Genome of Cheese Starter Lactic Acid Bacterium Lactococcus lactis subsp. lactis CECT 4433. Genome Announc2, 10.1128/genomeA.01142-14 (2014). [DOI] [PMC free article] [PubMed]
- 71.Frantzen CA, et al. Genomic Characterization of Dairy Associated Leuconostoc Species and Diversity of Leuconostocs in Undefined Mixed Mesophilic Starter Cultures. Front Microbiol. 2017;8:132. doi: 10.3389/fmicb.2017.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Makela P, Schillinger U, Korkeala H, Holzapfel WH. Classification of ropy slime-producing lactic acid bacteria based on DNA-DNA homology, and identification of Lactobacillus sake and Leuconostoc amelibiosum as dominant spoilage organisms in meat products. Int J Food Microbiol. 1992;16:167–172. doi: 10.1016/0168-1605(92)90011-q. [DOI] [PubMed] [Google Scholar]
- 73.Guo X, et al. Detection and Genomic Characterization of a Morganella morganii Isolate From China That Produces NDM-5. Front Microbiol. 2019;10:1156. doi: 10.3389/fmicb.2019.01156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li J, et al. Mycetocola zhujimingii sp. nov., isolated from faeces of Tibetan antelopes (Pantholops hodgsonii) Int J Syst Evol Microbiol. 2019;69:1117–1122. doi: 10.1099/ijsem.0.003280. [DOI] [PubMed] [Google Scholar]
- 75.Quintieri L, et al. Biofilm and Pathogenesis-Related Proteins in the Foodborne P. fluorescens ITEM 17298 With Distinctive Phenotypes During Cold Storage. Front Microbiol. 2020;11:991. doi: 10.3389/fmicb.2020.00991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Quintieri, L., Caputo, L., De Angelis, M. & Fanelli, F. Genomic Analysis of Three Cheese-Borne Pseudomonas lactis with Biofilm and Spoilage-Associated Behavior. Microorganisms8, 10.3390/microorganisms8081208 (2020). [DOI] [PMC free article] [PubMed]
- 77.Bertuzzi, A. S. et al. Genome Sequence of Staphylococcus saprophyticus DPC5671, a Strain Isolated from Cheddar Cheese. Genome Announc5, 10.1128/genomeA.00193-17 (2017). [DOI] [PMC free article] [PubMed]
- 78.Wels, M. et al. Draft Genome Sequence of Streptococcus thermophilus C106, a Dairy Isolate from an Artisanal Cheese Produced in the Countryside of Ireland. Genome Announc3, 10.1128/genomeA.01377-15 (2015). [DOI] [PMC free article] [PubMed]
- 79.Toh H, et al. Complete genome sequence of Lactobacillus acetotolerans RIB 9124 (NBRC 13120) isolated from putrefied (hiochi) Japanese sake. J Biotechnol. 2015;214:214–215. doi: 10.1016/j.jbiotec.2015.09.006. [DOI] [PubMed] [Google Scholar]
- 80.Gong L, et al. A New Isolate of Pediococcus pentosaceus (SL001) With Antibacterial Activity Against Fish Pathogens and Potency in Facilitating the Immunity and Growth Performance of Grass Carps. Front Microbiol. 2019;10:1384. doi: 10.3389/fmicb.2019.01384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.He Q, et al. Comparative genomic analysis of Enterococcus faecalis: insights into their environmental adaptations. BMC Genomics. 2018;19:527. doi: 10.1186/s12864-018-4887-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Iartchouk, O., Kozyavkin, S., Karamychev, V. & Slesarev, A. Complete Genome Sequence of Lactobacillus acidophilus FSI4, Isolated from Yogurt. Genome Announc3, 10.1128/genomeA.00166-15 (2015). [DOI] [PMC free article] [PubMed]
- 83.Laino, J. E., Hebert, E. M., Savoy de Giori, G. & LeBlanc, J. G. Draft Genome Sequence of Lactobacillus delbrueckii subsp. bulgaricus CRL871, a Folate-Producing Strain Isolated from a Northwestern Argentinian Yogurt. Genome Announc3, 10.1128/genomeA.00693-15 (2015). [DOI] [PMC free article] [PubMed]
- 84.Bai Y, et al. Complete genome sequence of Streptococcus thermophilus MN-BM-A01, a strain with high exopolysaccharides production. J Biotechnol. 2016;224:45–46. doi: 10.1016/j.jbiotec.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 85.Roh SW, et al. Complete genome sequence of Halalkalicoccus jeotgali B3T, an extremely halophilic archaeon. J Bacteriol. 2010;192:4528–4529. doi: 10.1128/JB.00663-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yim KJ, et al. Draft genome sequence of the extremely halophilic archaeon Halococcus sediminicola CBA1101T isolated from a marine sediment sample. Mar Genomics. 2014;18:145–146. doi: 10.1016/j.margen.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Gallone B, et al. Domestication and Divergence of Saccharomyces cerevisiae Beer Yeasts. Cell. 2016;166:1397–1410 e1316. doi: 10.1016/j.cell.2016.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Salazar AN, et al. Chromosome level assembly and comparative genome analysis confirm lager-brewing yeasts originated from a single hybridization. BMC Genomics. 2019;20:916. doi: 10.1186/s12864-019-6263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nguyen HV, Gaillardin C. Evolutionary relationships between the former species Saccharomyces uvarum and the hybrids Saccharomyces bayanus and Saccharomyces pastorianus; reinstatement of Saccharomyces uvarum (Beijerinck) as a distinct species. FEMS Yeast Res. 2005;5:471–483. doi: 10.1016/j.femsyr.2004.12.004.. [DOI] [PubMed] [Google Scholar]
- 90.Rainieri S, Kodama Y, Nakao Y, Pulvirenti A, Giudici P. The inheritance of mtDNA in lager brewing strains. FEMS Yeast Res. 2008;8:586–596. doi: 10.1111/j.1567-1364.2008.00363.x. [DOI] [PubMed] [Google Scholar]
- 91.Hewitt SK, Donaldson IJ, Lovell SC, Delneri D. Sequencing and characterisation of rearrangements in three S. pastorianus strains reveals the presence of chimeric genes and gives evidence of breakpoint reuse. PLoS One. 2014;9:e92203. doi: 10.1371/journal.pone.0092203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Tafer, H., Sterflinger, K. & Lopandic, K. Draft Genome Sequence of the Interspecies Hybrid Saccharomyces pastorianus Strain HA2560, Isolated from a Municipal Wastewater Treatment Plant. Genome Announc6, 10.1128/genomeA.00341-18 (2018). [DOI] [PMC free article] [PubMed]
- 93.Gibson B, Liti G. Saccharomyces pastorianus: genomic insights inspiring innovation for industry. Yeast. 2015;32:17–27. doi: 10.1002/yea.3033. [DOI] [PubMed] [Google Scholar]
- 94.Bing J, Han PJ, Liu WQ, Wang QM, Bai FY. Evidence for a Far East Asian origin of lager beer yeast. Curr Biol. 2014;24:R380–381. doi: 10.1016/j.cub.2014.04.031. [DOI] [PubMed] [Google Scholar]
- 95.Jung JY, et al. Effects of Leuconostoc mesenteroides starter cultures on microbial communities and metabolites during kimchi fermentation. Int J Food Microbiol. 2012;153:378–387. doi: 10.1016/j.ijfoodmicro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 96.Jeong SH, Jung JY, Lee SH, Jin HM, Jeon CO. Microbial succession and metabolite changes during fermentation of dongchimi, traditional Korean watery kimchi. Int J Food Microbiol. 2013;164:46–53. doi: 10.1016/j.ijfoodmicro.2013.03.016. [DOI] [PubMed] [Google Scholar]
- 97.Jeong SH, et al. Effects of red pepper powder on microbial communities and metabolites during kimchi fermentation. Int J Food Microbiol. 2013;160:252–259. doi: 10.1016/j.ijfoodmicro.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 98.Jeong SH, Lee SH, Jung JY, Choi EJ, Jeon CO. Microbial succession and metabolite changes during long-term storage of Kimchi. J Food Sci. 2013;78:M763–769. doi: 10.1111/1750-3841.12095. [DOI] [PubMed] [Google Scholar]
- 99.Lee SH, Jung JY. & Jeon, C. O. Source Tracking and Succession of Kimchi Lactic Acid Bacteria during Fermentation. J Food Sci. 2015;80:M1871–1877. doi: 10.1111/1750-3841.12948. [DOI] [PubMed] [Google Scholar]
- 100.Jung MY, et al. Role of jeotgal, a Korean traditional fermented fish sauce, in microbial dynamics and metabolite profiles during kimchi fermentation. Food Chem. 2018;265:135–143. doi: 10.1016/j.foodchem.2018.05.093. [DOI] [PubMed] [Google Scholar]
- 101.Jung JY, et al. Metatranscriptomic analysis of lactic acid bacterial gene expression during kimchi fermentation. Int J Food Microbiol. 2013;163:171–179. doi: 10.1016/j.ijfoodmicro.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 102.Song HS, et al. Microbial niches in raw ingredients determine microbial community assembly during kimchi fermentation. Food Chem. 2020;318:126481. doi: 10.1016/j.foodchem.2020.126481. [DOI] [PubMed] [Google Scholar]
- 103.Jung JY, Lee SH, Jeon CO. Microbial community dynamics during fermentation of doenjang-meju, traditional Korean fermented soybean. Int J Food Microbiol. 2014;185:112–120. doi: 10.1016/j.ijfoodmicro.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 104.Chun BH, Kim KH, Jeong SE, Jeon CO. The effect of salt concentrations on the fermentation of doenjang, a traditional Korean fermented soybean paste. Food Microbiol. 2020;86:103329. doi: 10.1016/j.fm.2019.103329. [DOI] [PubMed] [Google Scholar]
- 105.Jung JY, Lee SH, Lee HJ, Jeon CO. Microbial succession and metabolite changes during fermentation of saeu-jeot: traditional Korean salted seafood. Food Microbiol. 2013;34:360–368. doi: 10.1016/j.fm.2013.01.009. [DOI] [PubMed] [Google Scholar]
- 106.Lee SH, Jung JY, Jeon CO. Effects of temperature on microbial succession and metabolite change during saeu-jeot fermentation. Food Microbiol. 2014;38:16–25. doi: 10.1016/j.fm.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 107.Lee SH, Jung JY, Jeon CO. Microbial successions and metabolite changes during fermentation of salted shrimp (saeu-jeot) with different salt concentrations. PLoS One. 2014;9:e90115. doi: 10.1371/journal.pone.0090115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jung JY, Lee HJ, Chun BH, Jeon CO. Effects of Temperature on Bacterial Communities and Metabolites during Fermentation of Myeolchi-Aekjeot, a Traditional Korean Fermented Anchovy Sauce. PLoS One. 2016;11:e0151351. doi: 10.1371/journal.pone.0151351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Buels R, et al. JBrowse: a dynamic web platform for genome visualization and analysis. Genome Biol. 2016;17:66. doi: 10.1186/s13059-016-0924-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Petkau A, Stuart-Edwards M, Stothard P, Van Domselaar G. Interactive microbial genome visualization with GView. Bioinformatics. 2010;26:3125–3126. doi: 10.1093/bioinformatics/btq588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kanehisa M, Sato Y, Kawashima M, Furumichi M, Tanabe M. KEGG as a reference resource for gene and protein annotation. Nucleic Acids Res. 2016;44:D457–D462. doi: 10.1093/nar/gkv1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tatusov RL, et al. The COG database: an updated version includes eukaryotes. BMC Bioinformatics. 2003;4:41. doi: 10.1186/1471-2105-4-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Kelleher P, Murphy J, Mahony J, van Sinderen D. Next-generation sequencing as an approach to dairy starter selection. Dairy Sci Technol. 2015;95:545–568. doi: 10.1007/s13594-015-0227-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Bennedsen M, Stuer-Lauridsen B, Danielsen M, Johansen E. Screening for antimicrobial resistance genes and virulence factors via genome sequencing. Appl Environ Microbiol. 2011;77:2785–2787. doi: 10.1128/AEM.02493-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Camacho C, et al. BLAST+: architecture and applications. BMC Bioinformatics. 2009;10:421. doi: 10.1186/1471-2105-10-421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Olm MR, Brown CT, Brooks B, Banfield JF. dRep: a tool for fast and accurate genomic comparisons that enables improved genome recovery from metagenomes through de-replication. Isme J. 2017;11:2864–2868. doi: 10.1038/ismej.2017.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Goris J, et al. DNA-DNA hybridization values and their relationship to whole-genome sequence similarities. Int J Syst Evol Microbiol. 2007;57:81–91. doi: 10.1099/ijs.0.64483-0. [DOI] [PubMed] [Google Scholar]
- 118.Ondov BD, et al. Mash: fast genome and metagenome distance estimation using MinHash. Genome Biol. 2016;17:132. doi: 10.1186/s13059-016-0997-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Jung JY, Lee SH, Jeon CO. Kimchi microflora: history, current status, and perspectives for industrial kimchi production. Appl Microbiol Biotechnol. 2014;98:2385–2393. doi: 10.1007/s00253-014-5513-1. [DOI] [PubMed] [Google Scholar]
- 120.Jia B, et al. Catalytic, Computational, and Evolutionary Analysis of the d-Lactate Dehydrogenases Responsible for d-Lactic Acid Production in Lactic Acid Bacteria. J Agric Food Chem. 2018;66:8371–8381. doi: 10.1021/acs.jafc.8b02454. [DOI] [PubMed] [Google Scholar]
- 121.Kim KH, et al. Genomic and metabolic features of Lactobacillus sakei as revealed by its pan-genome and the metatranscriptome of kimchi fermentation. Food Microbiol. 2020;86:103341. doi: 10.1016/j.fm.2019.103341. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- 2020. European Nucleotide Archive. PRJEB23957
Supplementary Materials
Data Availability Statement
The ODFM is licensed under a Creative Commons Attribution 4.0 International License. The genome sequences in the ODFM are freely available on the website https://odfm.wikim.re.kr. All genome sequences have been deposited in NCBI GenBank and are available under the accession numbers listed in Supplementary Table S1. The viral metagenomic sequences have been deposited in the European Bioinformatics Institute (EMBL-EBI) database35 and are available under accession number PRJEB23957. We plan to deposit additional genome sequences for fermentative microbes to a member of the INSDC (http://www.insdc.org/) to promote sharing activities in the genomics community.
The code used to build the systemic architecture of the GDKM is available on GitHub: https://github.com/yang4851/gdkm.