Background
We know that varices have the thinnest wall at the gastroesophageal (GE) junction and, with an increase in portal pressure beyond 12 mm Hg, they can rupture and cause a torrential bleed. To salvage this situation, we use injections of n-butyl-2-cyanoacrylate to achieve hemostasis.
Conventionally, cyanoacrylate injections are used as 1 mL aliquots with a 21-gauge needle, and the injection of glue is followed by an injection of distilled water to flush the needle. Therefore, 1 mL of distilled water is used routinely. In addition, glue is always mixed with lipiodol as a solvent in the ratio 1:1 or 1: 1.6.1 This is traditionally done for 2 reasons: first, to avoid glue solidifying in the needle itself; second, because lipiodol is radio-opaque, it can be traced under fluoroscopy to confirm the filling of the varix and to detect embolization. Lipiodol also allows us to salvage the injection needle so it can be reused in the same patient for a repeat injection. However, with availability of good, cheaper, disposable needles, we have stopped reusing the needle for subsequent glue injections.
In our practice, in very early years, we saw few cases of pulmonary embolism after glue and lipiodol injection. Since then, we have stopped mixing lipiodol with glue.2 No pulmonary embolism has been noted in our experience since then.
Our thought process has been a little different. In our recently published experience with 2299 cases, we hypothesized that because the flow of blood in portal hypertension is from below upward (ie, from the GE junction toward the esophagus), if we block the varix at the GE junction, we could probably achieve better obliteration of the varices and less recurrent bleeding. Therefore, we included all the gastroesophageal varices (GOV) 1, along with GOV 2 and isolated gastric varices (IGV) 1. However, we included only those GOV 1 varices that extended more than 2 cm beyond the GE junction or those that could be noted bulging in the gastric lumen on retroflexion of the endoscope. We had very good results with our protocol.
Second, n-butyl-2-cyanoacrylate glue polymerizes fast when it comes in contact with blood or body fluids. The speed of polymerization is around 5 to 10 seconds after contact with blood. It does not solidify in the needle if injected rapidly. Our experience is with an Indian brand called Endocryl (Samarth Life Sciences, Mumbai, India), and in our experience of 2299 cases we did not have needle blockade during the first injection. Large-volume glue that causes more embolization quoted in the medical literature is absolutely true when lipiodol is used. Our protocol is 0.5 mL per aliquot, without lipiodol, followed by rapid injection of distilled water in a little more quantity to allow the glue to polymerize a larger area in the varix. Without lipiodol, the glue does not tend to flow away. That is what we have shared in our article.
Indications
All GOV 1 varices extended more than 2 cm beyond the Z line into the gastric part or were in a hiatus hernia; if seen extending into the gastric cardia on retroflexion of the endoscope, they were deemed GOV 2 varices or IGV 1 varices. In our protocol, if the patient had both esophageal and gastric varices, then regardless of the source—whether the bleed was from esophageal or gastric varices—as a protocol, we injected the gastric varices first and then did esophageal variceal ligation for the esophageal varices.
Method
We used a regular gastroscope for all patients. A minimum of 10 ampoules of glue, 0.5 mL per ampoule, were kept ready with ten 23-gauge glue injection needles of 170 cm length. The gastric varices were identified looking at the GE junction and with the endoscope in retroflexion.
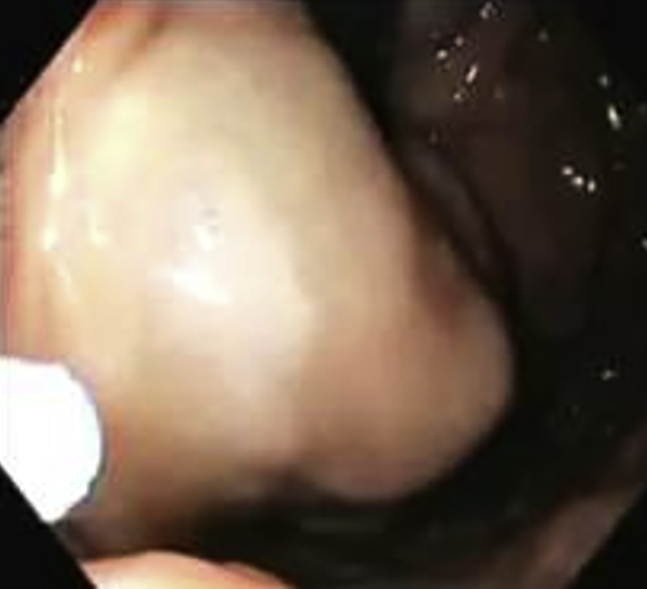
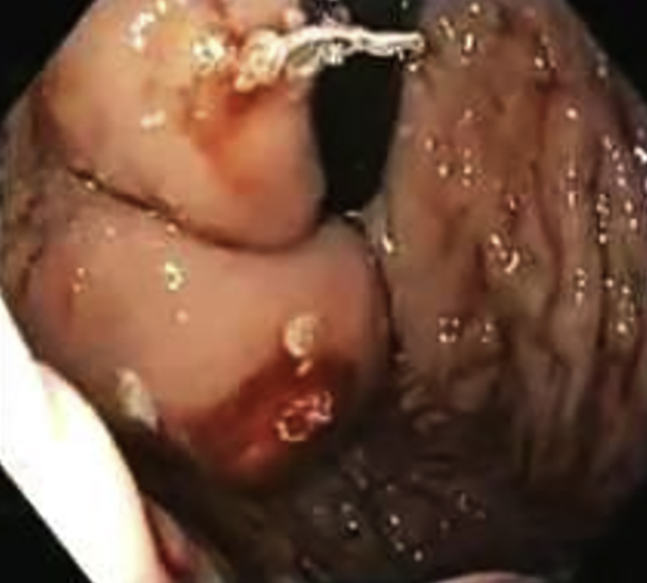
Our technique of injecting the gastric varices is as follows. The first injection is always at the farthest point of the gastric varix where it arises from the gastric wall (Figure 1, Figure 2). Next, we follow it toward the most bulging part. In our experience, when we inject the most bulging part of the varix first, we have severe back bleeding from the injection site. We committed this mistake very early in our practice. Learning from our mistakes, we started injecting at the farthest point and blocked the blood flow in the rest of the varix, subsequently progressing toward the most prominent bulging part. Using this technique, we experienced less back bleeding.
Figure 1.
Injection starting from farthest end.
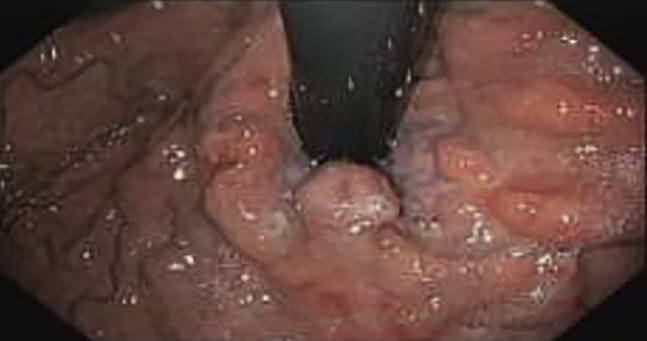
Figure 2.
Engorged and solid varix.
Another improvisation we made is to flush a maximum of 3 mL distilled water through the injection needle after injecting the glue. The amount of distilled water per varix was decided depending on the size of the varix. For larger varices, a maximum of 3 mL was used per injection; for smaller ones, 1.5 to 2 mL was used. The endpoint of any injection was to ensure that the varix bulges outward and becomes engorged and solid (Video 1, available online at www.giejournal.org).
The number of injections used per session was not restricted. We used as many injections as were needed to obliterate all visible gastric varices. We achieved successful hemostasis in 99.2% in the index procedure, and complete obliteration of varices without ulcerations was achieved in 95.3% at end of the third session (Fig. 3).
Figure 3.
Result: obliterated gastric varices with fibrosis.
There were no major contraindications for the procedure. Very sick patients, especially those with Child C status, encephalopathy, respiratory distress, and poor cardiac performance status with bleed, were prophylactically intubated to avert an on-table disaster.
We had a few adverse events in our series. The rate of aspiration due to massive bleed was 0.9%, bleeding from glue ulcers was 2.8%, mortality was 0.13%, and uncontrolled bleed was 0.8%, managed with transjugular intrahepatic portosystemic shunt and EUS-guided coil and glue. Most importantly, we did not have any pulmonary embolism in our cases. This was attributed to avoiding lipiodol, which causes embolization.3
Troubleshooting
We would like to highlight a few important tips to avoid mishaps during the procedure.
-
1.
Protection of the eyes from splashing of glue during injection is very important. The assistant should be thoroughly trained. Both the doctor and assistants should wear goggles, especially during the learning curve. If glue splashes into the eyes, immediate thorough lavage of the eyes with copious amount of water should be done, followed by applying methyl cellulose eye drops every 4 hours for a couple of days. Sometimes the glue will form a cast on the eye and will fall off with time, but ulcerations and damage to the sclera can happen. Therefore, caution is necessary.
-
2.
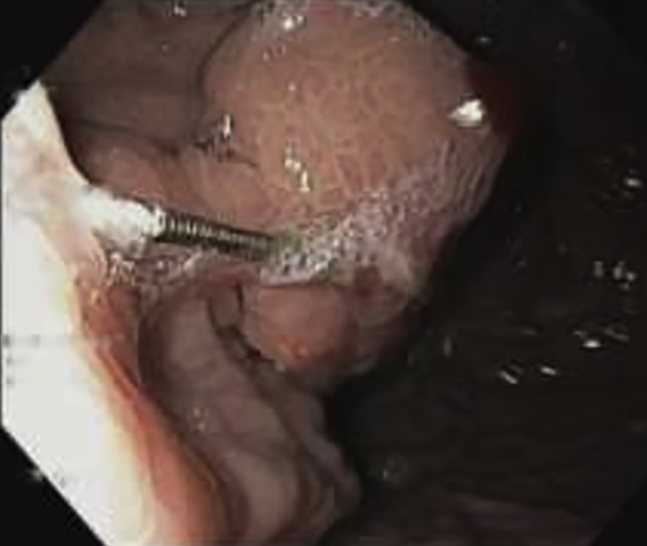
The needle may get stuck in the glue in the varix during injection. This happens because the glue solidifies very quickly, and we may be slow in pulling the needle back. This is best avoided with proper training. However, if the needle gets stuck, we must not attempt to remove the needle with any forceps unless a major part of the needle is protruding in the gastric lumen. The best method is to give a firm tug on the sheath to dislodge the sheath from the needle, leaving the needle in the glue in the varix. The needle will fall off in due time with glue cast. One small improvisation we have made is as follows: While injecting glue and distilled water, we gently jiggle the needle sheath with up and down movements. This has helped us prevent the needle from getting stuck in the varix (Fig. 4).
-
3.
If bleeding from the injection site occurs, we have to avoid the tendency to try and suction the blood. The best option is to stay away from the spurter, keep watching it, prepare more glue, and inject rapidly in the same varix or any feeding varix that is visible in the vicinity. The best option sometimes is to inject in the same point from which the bleeding is occurring (Figure 5, Figure 6).
Figure 4.
Needle impacted in varix after glue injection.
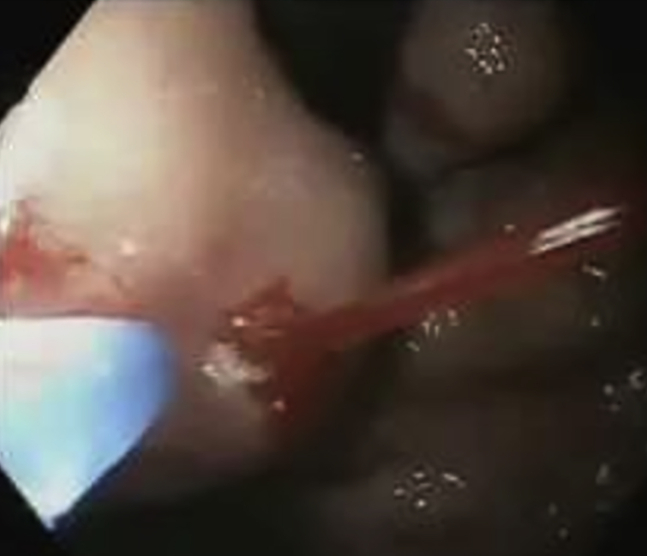
Figure 5.
Active spurt from gastroesophageal varices 2.
Figure 6.
Hemostasis secured after glue injection.
In summary, we can conclude from our experience that glue is an excellent modality for management of gastric varices4 (GOV 1, 2, and IGV). The amount of glue per session should not be a restricting factor; use as much glue as needed to ensure hemostasis and achieve complete obliteration of varices in the long run. Using a small glue bolus with more distilled water ensures complete filling of the varix and causes fewer ulcerations, less recurrent bleeding, and more fibrosis. We have fewer adverse events such as pulmonary aspiration and a very low incidence of death.
Acknowledgments
Supported by a Robert W. Summers grant from the American Society for Gastrointestinal Endoscopy.
Disclosure
All authors disclosed no financial relationships.
Footnotes
If you would like to chat with an author of this article, you may contact Dr Desai at drp_desai@hotmail.com.
Supplementary data
Procedure.
Video 2. Active spurt from varix.
References
- 1.Kumar A., Singh S., Madan K. Undiluted N-butyl cyanoacrylate is safe and effective for gastric variceal bleeding. Gastrointest Endosc. 2010;72:721–727. doi: 10.1016/j.gie.2010.06.015. [DOI] [PubMed] [Google Scholar]
- 2.Seewald S., Ang T.L., Imazu H. A standardized injection technique and regimen ensures success and safety of N-butyl-2-cyanoacrylate injection for the treatment of gastric fundal varices (with videos) Gastrointest Endosc. 2008;68:447–454. doi: 10.1016/j.gie.2008.02.050. [DOI] [PubMed] [Google Scholar]
- 3.Saraswat V.A., Verma A. Gluing gastric varices in 2012: lessons learnt over 25 years. J Clin Exp Hepatol. 2012;2:55–69. doi: 10.1016/S0973-6883(12)60088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cheng L.F., Wang Z.Q., Li C.Z. Low incidence of complications from endoscopic gastric variceal obturation with butyl cyanoacrylate. Clin Gastroenterol Hepatol. 2010;8:760–766. doi: 10.1016/j.cgh.2010.05.019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Procedure.