Abstract
Alcohol use disorder (AUD) and anxiety disorders are frequently comorbid and share mechanisms that could be therapeutic targets. To facilitate mechanistic studies, we adapted an inhibitory avoidance-based “2-hit” rat model of posttraumatic stress disorder (PTSD) and identified predictors and biomarkers of comorbid alcohol (ethanol)/PTSD-like symptoms in these animals. Stressed Wistar rats received a single footshock on two occasions. The first footshock occurred when rats crossed into the dark chamber of a shuttle box. Forty-eight hours later, rats received the second footshock in a familiar (FAM) or novel context (NOV). Rats then received 4 weeks of two-bottle choice (2BC) ethanol access. During subsequent abstinence, PTSD-like behavior responses, GABAergic synaptic transmission in the central amygdala (CeA), and circulating cytokine levels were measured. FAM and NOV stress more effectively increased 2BC drinking in males and females, respectively. Stressed male rats, especially drinking-Vulnerable individuals (≥0.8g/kg average 2-hr ethanol intake with >50% ethanol preference), showed higher fear overgeneralization in novel contexts, increased GABAergic transmission in the CeA, and a profile of increased G-CSF, GM-CSF, IL-13, IL-6, IL-17a, leptin and IL-4 that discriminated between stress context (NOV>FAM>Control). However, drinking-resilient males showed the highest G-CSF, IL-13, and leptin levels. Stressed females showed increased acoustic startle and decreased sleep maintenance, indicative of hyperarousal, with increased CeA GABAergic transmission in NOV females. This paradigm promotes key features of PTSD, including hyperarousal, fear generalization, avoidance, and sleep disturbance, with comorbid ethanol intake, in a sex-specific fashion that approximates clinical comorbidities better than existing models, and identifies increased CeA GABAergic signaling and a distinct pro-hematopoietic, proinflammatory, and pro-atopic cytokine profile that may aid in treatment.
Keywords: PTSD, AUD, stress, sex differences, GABA, central amygdala, cytokine
Introduction
Alcohol use disorder (AUD) is a stress surfeit disorder; amplified stress signaling during alcohol (ethanol) use or withdrawal promotes greater drinking and relapse, respectively1. AUD is comorbid with stress- and anxiety-related disorders2, 3, including posttraumatic stress disorder (PTSD) and this comorbidity can differ between the sexes4, 5. Individuals with stress/anxiety disorders have increased alcohol withdrawal symptoms and relapse risk6 and use alcohol to cope7. Common precipitants, such as psychogenic stress8, 9, and molecular mechanisms, such as extended amygdala GABAergic neurotransmission10, 11 and proinflammatory cytokines12–21, may be implicated in both syndromes22 but remain understudied.
To identify novel molecular mechanisms, more robust animal models of AUD-stress/anxiety comorbidities are needed. Many existing models involve Pavlovian fear conditioning protocols, in which animals form associations between footshocks and otherwise neutral stimuli7, 23. In reality, stress/anxiety disorders also involve fear overgeneralization, non-associative hyperarousal and fear24, and operant components25. Whereas Pavlovian conditioning putatively drives the “re-experiencing” symptoms of PTSD, operant conditioning drives avoidance26. Models that incorporate non-Pavlovian attributes may hold advantages over pure classical conditioning models23. Inhibitory avoidance (IA), footshock-based procedures have been used to generate PTSD-like behaviors in rodents27, 28 and involve both operant and Pavlovian learning under conflict29. When a rodent crosses from an illuminated chamber to a dark one29 they escape from aversive light but then receive an aversive footshock in the darkened chamber. Thus, the IA response evokes negative reinforcement and punishment, respectively, a conflicted outcome that may differ from classical conditioning.
Here, we characterize comorbid AUD- and PTSD-like symptoms that result from variants of a “2-hit” IA procedure that generates lasting hyperarousal, fear generalization, and extinction-resistant avoidance behavior28. In the original “2-hit” procedure, a rat receives a single shock on two separate occasions with the first shock in an IA context (i.e., shuttle box) and the second in a different, novel context28. The literature is inconsistent whether ethanol intake is increased more by an anticipated vs. unanticipated stressor7. Therefore, we compared effects of receiving the second footshock in the original IA chamber, reflecting stress re-exposure in a familiar (FAM) context, versus in an unfamiliar apparatus, or stress re-exposure in a novel (NOV) context. We then sought to apply this novel translationally-relevant behavioral model to identify whether sex differences in physiology and biomarkers of PTSD/AUD comorbidity exist. We evaluated impacts of the 2-hit paradigms on: 1) voluntary ethanol intake via chronic intermittent 2-bottle choice (2BC) access; 2) translationally-relevant PTSD-related behavioral responses; 3) inhibitory GABAergic transmission in the central nucleus of the amygdala (CeA), due to its role in anxiety and since its elevation is considered a hallmark of alcohol dependence across species10, 11; and 4) circulating cytokine profiles, due to their hypothesized biomarker and mechanistic roles in AUD and PTSD12–18, 20. We hypothesized that model conditions that effectively promote PTSD/AUD phenotypes would lead to elevated CeA GABA signaling10, 11 and peripheral inflammation during abstinence from ethanol12–21 and that re-experiencing stress in a novel environment would lead to greater effects28. Finally, given sex-specific responses to stress and prevalence of stress disorders, we predicted nuanced sex differences, whereby females would show greater vulnerability to develop hyperarousal symptoms and PTSD/AUD comorbidity5, 30, 31.
Materials and Methods
More detailed methods are in Supplemental.
Animals
Male and female Wistar rats (n=96, Charles River Laboratories) weighed 425±3.7 g and 247±2.1 g, respectively, when experiments began. Rats (12:12 L/D cycle, food and water ad libitum) were pair-housed, separated by a perforated clear plexiglass divider to permit individual 2-bottle choice (2BC) drinking while reducing isolation stress32. The procedures followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition) and were approved by The Scripps Research Institute Institutional Animal Care and Use Committee.
Footshock Paradigm
Rats were randomly assigned to treatment groups by cage, within ethanol condition. The first shock was given after the rat crossed from the illuminated to dark chamber of an IA shuttle box (see Fig.1a&b for timeline). The second footshock occurred 48hr later, whereby half of the rats received the second footstock in the same familiar apparatus as the first (FAM). The other half of the rats received the second footshock in a novel, environmentally-distinct, single-chambered apparatus (NOV) (Fig.1b). Each 3-mA footshock was delivered over 2 sec. Controls (CTL) were handled by experimenters and naive to stress.
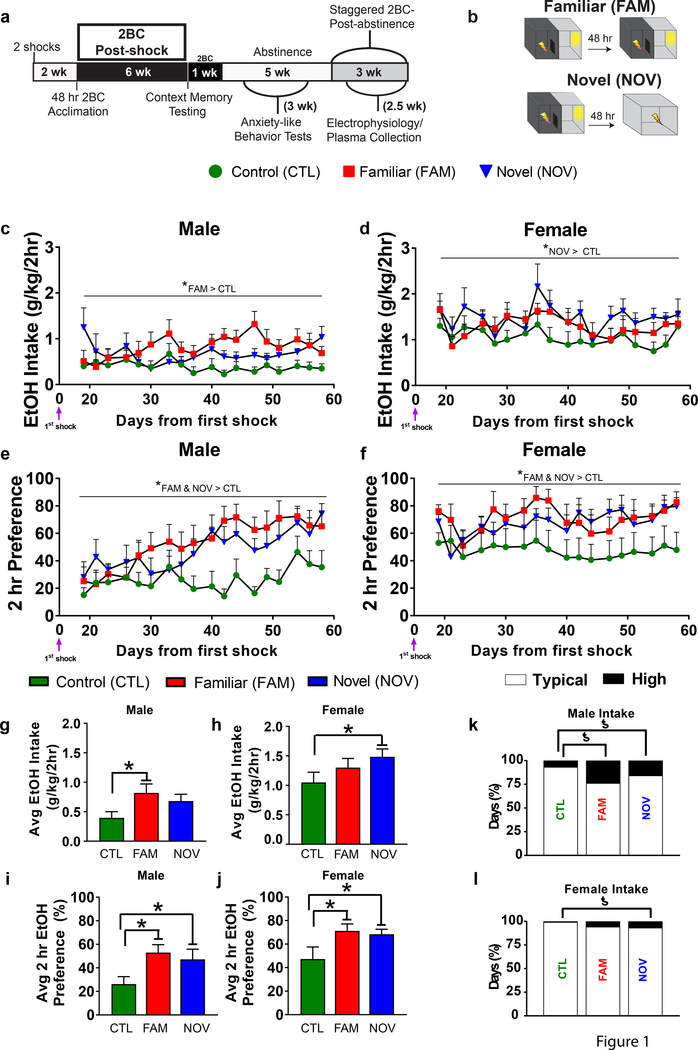
Fig. 1. The “2-hit” stress paradigm increased ethanol intake in rats with intermittent, limited ethanol access.
(a) Experimental timeline with post-shock limited access (2-hr) 2BC sessions. (b) Rats were naive to stress (CTL) or administered “2-hit” stress with the second shock in a familiar (FAM) or novel (NOV) context. (c,d) Two-way ANOVA with repeated measures (Session) indicated a main effect of Stress on post-shock ethanol intake (F2,42=4.119, P=0.023). Within-sex planned comparisons revealed that the increase was significant in FAM males (P=0.039) and NOV females (P=0.034), but not NOV males (P=0.159) or FAM females (P=0.363). Females drank more ethanol (g/kg) than males (F1,42=31.717, P<0.001, main effect of Sex). Intraclass correlations demonstrated strong, stable individual differences in post-shock ethanol intake (ICC(2,41)=0.92 for males, 0.9 for females). (e,f) Ethanol preference was increased in stressed males (e) and females (f) during the post-shock phase, regardless of the stress context (Stress main effect: F2,41=6.894, P=0.003). Within-sex planned comparisons confirmed that all stress-treated males and females showed significantly elevated ethanol preference (all P’s<0.05). Females had greater ethanol preferences than males (Sex main effect: F1,42=11.383, P=0.002, without Stress*Sex interaction: F1,42=0.026, P=0.974). Intraclass correlations revealed strong, stable individual differences in post-shock ethanol preference (ICC(2,41)=0.95 for males, 0.94 for females). (g,h) Bar graphs summarizing mean male (g) and female (h) post-shock intakes from the line graphs in previous panels. To investigate whether 2-hit stress increased ethanol intake without regard to second stressor context, we also combined FAM and NOV groups (yellow bars) and found a significant increase in males (P=0.044) and a trend for an increase in females (P=0.051). (i,j) Increased ethanol preference also was seen for males (P=0.009) and females (P=0.014) in the combined Stress group. (k,i) Contingency graphs. The percentage of high drinking (>1.96 standard deviations above the respective CTL mean) vs. typical intake days (144 observations/group). (k) FAM and NOV males as well as (l) NOV females had increased rates of high drinking days. n=8 rats/group. *P<0.05, within-sex planned comparisons; ƾP<0.05, two-sided Fisher’s exact test; @P=0.051.
2BC Testing
Beginning 2 weeks after the first footshock, half of the rats received 48-hr acclimation to ethanol (20% v/v) followed by chronic, intermittent (MWF: Mondays, Wednesdays, Fridays), limited 2BC access (2-hr) to ethanol at scotophase onset (Fig.1a). These parameters were chosen because limited access sessions can promote greater binge-like drinking than continuous access33 while ethanol exposure prior to footshock may impair 2BC escalation23. The second cohort received dual water bottle access, in lieu of 2BC, to determine which post-stress behavioral phenotypes required concurrent ethanol access.
Abstinence Behavior Testing
After 18 2BC sessions, rats received 7 days of abstinence before behavioral testing began. In counter-balanced order, rats completed elevated plus-maze, novelty-induced hypophagia, and bottle-brush tests during the next 1.5 weeks of abstinence, followed by sleep-phase analysis, social investigation, acoustic startle, and finally fear overgeneralization testing across the subsequent 16 days of abstinence (see Fig.1a for timeline). All non-automated procedures and data collection were carried out by treatment-blind experimenters, and the order of animals was randomized for each behavioral test.
Elevated Plus-Maze (EPM)
Time and entries in the open arm, closed arm and center of a 5-min EPM test were recorded34.
Novelty-Induced Hypophagia
Latency to eat and intake of sucrose-rich pellets35 in an unfamiliar double-size cage were measured. Rats received home cage pre-acclimation to the food 24-hr earlier.
Bottle-Brush Irritability
An experimenter rotated a bottle-brush (10 trials, 5 phases, 3 sec each) before the rat in an unfamiliar test cage. Two raters scored aggressive- and defensive-like behaviors36.
Comprehensive Lab Animal Monitoring System (CLAMS)
Since sleep disturbances are a hallmark of PTSD37, we assessed diurnal sleep maintenance in a Comprehensive Lab Animal Monitoring System (CLAMS; Columbus Instruments, Columbus, OH, USA). Such noninvasive, activity-based measurements correlate well with EEG-defined sleep38. OXYmax-CLAMS units39, 40 were used to infer sleep parameters of habituated rats from photocell-defined motor activity across (11-hr) their inactive phase (lights on).
Acoustic Startle
Exaggerated acoustic startle responses are present in human PTSD patients and indicate hyperarousal24. During a 30-min session (75 pseudorandomized trials), an SR-LAB Startle Response system41 measured startle responses to acoustic stimuli (80- to 120-dB) and no-stimulus control trials.
Social Investigation
We used a social investigation paradigm42 to measure stress-induced avoidance. Time near a dish containing clean bedding was measured for 3 min; then, time near a new dish containing soiled bedding from same-sex, unfamiliar conspecifics was measured over the next 3 min42.
Context Memory
Latency to cross (maximum 360 sec) in the original IA shuttle box was measured 61 days post-shock.
Fear Overgeneralization
Latency to cross (maximum 10 min) to the dark compartment of a novel shuttle box (different dimensions, material and color from the IA apparatus) was measured 96 days post-shock (Fig.2b).
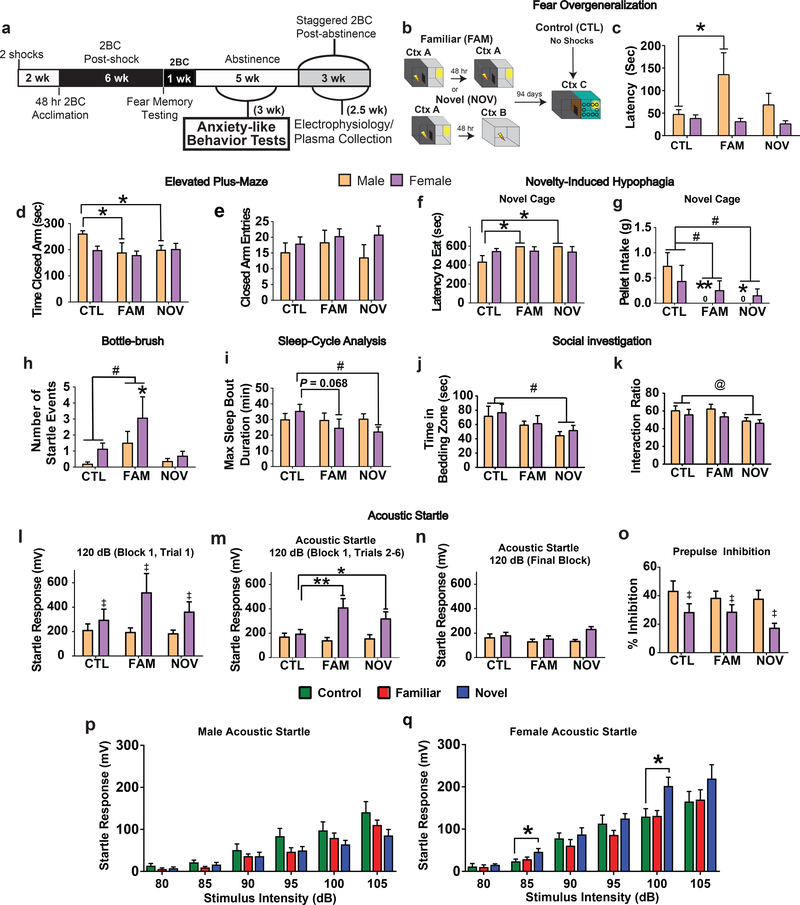
Fig. 2. Effects of prior “2-hit” stress with familiar (FAM) versus novel (NOV) context on anxiety-related behaviors during abstinence.
Rats were tested across 3 weeks in the following order beginning after at least 1 week of abstinence: elevated plus-maze, novelty-induced hypophagia, bottle-brush irritability, and then in counter-balanced order, by CLAMS and social investigation, and then acoustic startle and finally fear overgeneralization. (a) Timeline illustrating behavioral tests relative to two-bottle choice limited access. (b) Diagram of fear overgeneralization testing. Stress rats received a second footshock in a familiar context (Ctx A) or novel (Ctx B) box. Ninety-four days later, latency to cross to the dark compartment of a distinct, novel box (Ctx C) was measured. (c) FAM males showed longer latencies (P=0.009, planned comparisons). (d) Both FAM males (P=0.019, within-sex planned comparisons) and NOV males (P=0.049, within-sex planned comparison) spent significantly less times in the closed arms of the elevated plus-maze. There was no interaction with Sex (F1,42=1.376, P<0.264) and a trend for a Sex main effect (F1,41=2.455, P=0.098). (e) We did not observe any significant effects of Stress or Sex on the number of entries to the closed arms (all P’s>0.05). (f) NOV and FAM males did not consume any pellets in novelty-induced hypophagia testing, producing maximum and significantly greater intake latencies (P’s≤0.008, planned comparisons). (g) A main effect of Stress (F2,41=4.309, P=0.02), reflected reduced food intake in NOV and FAM rats (P’s≤0.037, Dunnett’s t-test) during novelty-induced hypophagia testing. Planned comparisons suggest males (P’s≤0.011) rather than females (P’s≥0.3) drive the effect. (h). In bottle-brush testing, there was a significant main effect of stress (F2,42=4.324, P=0.02) wherein FAM rats showed increased startle compared with controls (P=0.034, Dunnett’s t-test). Within-sex planned comparisons showed that the effect was driven by females (P=0.045) vs. males (P=0.168). (i) Stress reduced the longest bout of sleep in females (NOV, P=0.028; FAM, P=0.068; planned comparisons) during the first 11-hr of the light phase of a Comprehensive Lab Animal Monitoring System testing. (j) For social investigation/avoidance, NOV rats spent less time in the zone containing soiled bedding from unfamiliar same-sex conspecifics (P=0.019, Dunnett’s t-test; significant main effect of Stress, F2,41=3.695, P=0.033). (k) There was also a main effect of Stress (F2,41=3.329, P=0.046) on percentage of time in the bedding zone (interaction bedding zone time/(interaction zone time+acclimation zone time)*100), and NOV rats favored the unsoiled bedding (P=0.05, Dunnett’s t-test). *P<0.05, **P=0.01 within-sex planned comparisons, #P<0.05, Dunnett’s t-test, @P=0.05, Dunnett’s t-test, ‡P<0.05, main effect of Sex. n=8 rats/group, except NOV males where n=7 as one NOV male was removed before behavior studies due to illness. All data shown as mean±SEM. (l-q) Acoustic startle response was also assessed. (l) The very first trial of acoustic startle testing was considered separately, as is commonly done, and did not yield significant Stress (F2,41=0.867, P=0.428) or interaction effects (F1,41=1.046, P=0.361) in two-way ANOVA. Females showed an overall greater response than males (F1,41=7.914, P=0.007, main effect of Sex). (m) During the first block of 120-dB stimuli, NOV females (P=0.045, within-sex planned comparisons) and FAM females (P=0.001, within-sex planned comparisons), but not males of either stress group (P’s>0.6) exhibited significantly increased startle responses (Stress*Sex interaction: F2,41=4.126, P=0.023, two-way ANOVA). Females still had higher responses (Sex: F1,41=18.457, P<0.001), with no main effect of Stress (F2,41=2.345, P=0.109). (n) Groups did not differ in their responses to the final block of 120-dB stimuli (all P’s>0.05, two-way ANOVA with within-sex planned comparisons). (o) There were no Stress or interaction effects on prepulse inhibition (all P’s>0.05, two-way ANOVA with within-sex planned comparisons), but females showed a lower prepulsed response (Sex: F1,41=11.992, P=0.001). (p) Whereas NOV and FAM males also showed normal responses to less intense acoustic stimuli (all within-sex planned comparisons P’s>0.05), (q) within-sex planned comparisons showed that NOV females also exhibited exaggerated startle to 85-dB (P=0.005) and 100-dB stimuli (P=0.003), yielding Stress*Sex interactions (85-dB: F2,41=3.326, P=0.046; 100-dB: F2,41=5.740, P=0.006). *P<0.05, **P=0.001, within-sex planned comparison, #P<0.05, Dunnett’s t-test, @P<0.07, Dunnett’s t-test, ‡P<0.05, main effect of Sex. n=8 rats /group, except NOV males where n=7. All data are shown as mean±SEM.
Vulnerability
Only a subset of people with PTSD show comorbid AUD43; thus, we established criteria for identifying drinking-Vulnerable vs. Resilient subjects. Vulnerable rats were designated as those exceeding previous NIAAA criteria for moderate ethanol intake (≥0.8 g/kg/2-hr averaged over 4-wk)44, 45 in conjunction with an ethanol-preferring phenotype (>50% preference ratios). This intake level also is relevant to current NIAAA criteria of 4 or 5 drinks in a 2-hr window for a woman or man respectively, assuming each drink contains ~14 g ethanol46 and an average human weight of 70 kg47. We used weeks 3 thru 6 of the postshock drinking phase for vulnerability analysis.
Amygdala slice preparation and electrophysiological recordings
Preparation of acute brain slices and electrophysiological recordings were performed as previously described48 from a subset of 24-hr abstinent rats from each group that were under deep isoflurane anesthesia. Elevated CeA GABA transmission represents a hallmark of alcohol dependence across species10, 11, and acute ethanol application increase CeA GABA signaling48. Thus, we recorded pharmacologically-isolated GABAA receptor mediated miniature inhibitory postsynaptic currents (mIPSCs) from 51 neurons in the medial subdivision of the CeA using whole-cell voltage clamp mode. Data were analyzed using Mini Analysis (Synaptosoft Inc., Fort Lee, NJ) with 3-min bins of gap-free recording48 and only currents >5 pA accepted for analysis.
Cytokine and stress hormone assays
At the time of euthanasia, 24-hr following the final post-abstinence 2BC test, heparin-treated nocturnal trunk blood was collected from isoflurane-anesthetized rats and centrifuged. Plasma was stored (−80oC) until being assayed in duplicate using 27-plex cytokine (RECYMAG65K27PMX) and 2-plex corticosterone/adrenocorticotropic hormone (ACTH) (RSHMAG-69K) Luminex magnetic bead panels. The comprehensive cytokine panel includes candidates from prior human or animal model PTSD- and AUD-related studies12–18, 21.
Estrous Cycle
Estrous stage was determined by vaginal lavage at euthanasia and not earlier to limit stressful or reproductive effects of vaginocervical stimulation49.
Statistical Analyses
Behavior, electrophysiology, and 2BC were analyzed using 2-way ANOVA with between-subjects repeated measures followed by within-sex planned comparisons (simple effects of Sex*Stress interactions)50, 51 comparing NOV and FAM rats to their unstressed control. Significant omnibus tests were followed with post-hoc Dunnett’s t-test relative to controls. Outliers were determined using Grubbs’ test. Since many patients with PTSD engage in sporadic bouts of heavy drinking rather than increased daily drinking52, we quantified heavy drinking days as those 1.96 SD greater than the control mean. IA latencies were assessed using 3-way ANOVA with repeated measures examining change in latency to cross to the dark compartment during the first shock, second shock, and context memory testing. “Stress” and “Sex” are capitalized throughout when referring to main effects. Linear discriminant function analyses were performed on standardized (% control), log-transformed scores (to normalize and homogenize variance) within each sex to identify cytokine profiles that significantly discriminated subjects per Stress history or drinking-Vulnerability/Resilience. Cytokines that loaded significantly on discriminating functions per Wilks’ lambdas within the LDA were further interpreted using intercorrelations (Pearson r) and pairwise difference analysis (LSD within univariate ANOVAs). Interferon gamma (interferon-γ) and human growth-regulated oncogene/keratinocyte chemoattractant (GRO/KC) were undetectable and excluded from analyses. All tests were two-sided. P≤0.05 was considered statistically significant. All data are presented as mean ± standard error of the mean (SEM). Minimum sample size was determined using a power analysis, and n is reported in the figure legends. See Supplemental for details.
Results
“2-hit” stress increases voluntary ethanol intake
A Stress main effect indicated increased ethanol intake (Fig.1). The efficacy of NOV vs. FAM stress was sex-dependent; within-sex planned comparisons showed that FAM stress increased intake by males (Fig.1c&g), whereas NOV stress did so in females (Fig.1d&h). However, if stress conditions were combined, increased intake remained significant in males and approached significance in females, reflecting the lack of a Stress*Sex interaction. Each stressor also strengthened ethanol preference in males (Fig.1e&i) and females (Fig.1f&j). Stress did not increase average total fluid or water intake, so stress-mediated increases in ethanol intake were specific to ethanol (Fig.S1a–d).
We also examined the frequency of extreme intake days (>1.96 SD vs. control mean). NOV and FAM males had greater rates of high intake days (>1.21 g/kg/2-hr) than CTL males (Fig.1k). NOV females also had more frequent high intake days (>2.57 g/kg/2-hr, Fig.1l).
The IA-based “2-hit” stress model generated anxiety-like phenotypes
Elevated Plus-Maze (EPM)
FAM and NOV males spent significantly less time in the closed arms than controls (Fig.2d), with trends for more open arm time (Fig.S2d) and entries (Fig.S2e). Conventionally, this would be interpreted as less unconditioned avoidance of the anxiogenic-like open arms. We hypothesized instead that males might be generalizing their conditioned fear of the IA box’s dark compartment to the dark, closed arms of the EPM53–55. Accordingly, FAM males took significantly longer than controls to enter the dark compartment of a novel shuttle box (Fig.2c). Thus, FAM males may overgeneralize fear to different, but reminiscent, contexts.
Novelty-induced hypophagia
NOV and FAM males had increased latencies to eat (Fig.2f), and Stressed subjects from both sexes (main effect) ate less than controls in the novel environment. No such Stress effects were seen during home cage acclimation to the food.
Bottle-brush test
FAM males and females showed more startle responses to the brush (Fig.2h, Supplemental Table 1) and did not differ on other measures (Supplemental Table 1).
CLAMS sleep analysis
NOV stress significantly reduced the duration of the longest sleep bout in females, with a similar trend in FAM females (Fig.2i). Stress did not alter average bout duration (Fig.S2g), number of bouts (Fig.S2h), or total sleep time (Fig.S2i).
Social investigation
NOV stress significantly reduced time in the conspecific-soiled bedding zone (Fig.2j) with a similar trend on the interaction ratio (Fig.2k). Stress history did not affect time near clean bedding, a non-social, control measure (Fig.S2j).
Acoustic startle
Consistent with the bottle brush startle phenotype, FAM and NOV stress females had increased acoustic startle responses during the first block of 120-dB stimuli (Fig.2m), but not the first trial (Fig.2l). NOV (Fig.2q), but not FAM (Fig.2q), females also exhibited elevated startle responses at lower intensity stimuli, including 85- and 100-dB. Stress did not affect acoustic startle of males (Fig.2l–n,p) or pre-pulse inhibition (Fig.2o).
Ethanol history
As we were studying PTSD/AUD comorbidity, all above groups were studied during abstinence from 2BC ethanol access. To determine the requirement for 2BC access in PTSD-like phenotypes, a cohort of “2-hit” stress rats that instead received 2BC access to water were studied in the same tests (Fig.S3). No-ethanol stress groups again showed significantly decreased plus-maze closed arm time (Fig.S3a; FAM and NOV) and reduced maximum sleep bout duration (Fig.S3f; FAM) with trends for novelty-induced hypophagia (Fig.S3d, NOV female) and increased fear overgeneralization in the novel context (Fig.S3o; FAM males). Unlike 2BC groups, decreased social interaction and increased startle to bottle brush or isolated acoustic stimuli were not seen in stressed, no-ethanol animals. However, no-ethanol NOV females uniquely showed increased total defensive behaviors in the bottle brush test, decreased total sleep time and impaired startle prepulse inhibition (Fig.S3l). Thus, 2BC ethanol access influenced some, but not all, anxiety-like phenotypes, exacerbating some, while reducing others.
Vulnerable males show comorbid alcohol use- and anxiety-disorder like comorbidity
Drinking-Vulnerability was more prevalent in stressed rats (21/32; 66%) than CTLs (4/16; 25%; P=0.0135, Fisher’s exact test). Of stressed males, 4/8 FAM and 3/7 NOV were Vulnerable vs. 0/8 CTLs. All but 2 stressed females (2/16) met drinking-Vulnerable criteria versus half of CTL females (4/8). Because of the greater baseline drinking (and vulnerability) in control females, further vulnerability subgroup analyses were male-focused.
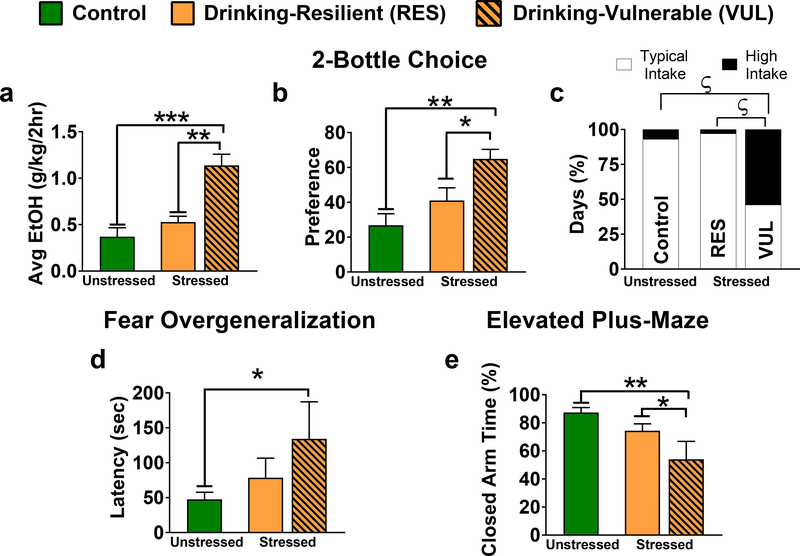
Vulnerable males exhibited higher ethanol intake (Fig.3a) and preference (Fig.3b) than both Resilient and unstressed CTL males during the post-shock phase. Vulnerable males also showed more extreme high intake days (>1.14 g/kg/2-hr) than CTL and Resilient males, which did not differ (Fig.3c). Only Vulnerable males significantly overgeneralized fear compared with CTL males as indicated by increased latency in a novel shuttle box (Fig.3d) and decreased closed arm time in the EPM (Fig.3e). Vulnerable males showed higher average drinking during post-context memory testing (Fig.S4e&f). Vulnerable males did not differ from Control or Resilient counterparts on any other behavioral parameters (Supplemental Table 2).
Fig. 3. Drinking-Vulnerable males exhibit increased two-bottle choice (2BC) drinking with heightened fear overgeneralization.
Drinking-Vulnerable rats were defined as those with average ethanol intakes equal to or exceeding 0.8g/kg/2-hr over 4 wk44, 45 and ethanol preferences above 50%. For analyses, 7 Stressed males were Vulnerable, 8–9 were Resilient (1 Resilient rat was removed before anxiety-related behavior testing due to illness) and 8 were controls. Only 2 females met criteria for vulnerability, so we focused within-sex planned companions to contrast Vulnerable males with control (CTL) and Resilient males. (a) Vulnerable males had higher ethanol intakes and preferences than Resilient males (P=0.002) and CTL males (P<0.001). (b) Vulnerable males also had a significantly higher ethanol preference than Resilient (P=0.021) and CTL counterparts (P=0.001). (c) Graphical depictions of contingency tables for percentage of drinking days that were high intake days (>1.96 standard deviations from the mean of the CTL group for the respective sex) and typical intake days per treatment. Due to differences in sample size among groups, the total number of observation days differ (CTL, total days=96; Resilient, total days=108; Vulnerable, total days=84) thus results are standardized as percent to allow comparison. Vulnerable males had a significantly greater rate of high intake days than Resilient (P<0.001, 2-sided Fisher’s exact test) and CTL counterparts (P<0.001, 2-sided Fisher’s exact test), whereas CTL and Resilient males did not differ. (d) Males were tested in a highly modified, and novel inhibitory avoidance box to asses fear overgeneralization (i.e. potentially inappropriate generalization of fear to a novel context). Vulnerable males generalized fear to the novel context as demonstrated by a significant increase in latency to cross to the dark compartment (P=0.014). (e) Just as Vulnerable males overgeneralized in the novel box by avoiding the dark compartment, they also spent a significantly lower percentage of time during elevated plus-maze testing in the dark, closed arms than control (P=0.001) or Resilient (P=0.043) counterparts. *P<0.05, **P<0.01, ***P<0.001; planned comparisons; ƾP<0.001, Fisher’s exact test. All data are shown as mean±SEM.
Stress increased CeA GABA activity
To assess whether our 2-hit model increased GABAA-receptor mediated synaptic transmission in the CeA, we recorded action-potential independent mIPSCs from 51 CeA neurons from a random subset of rats from each group. FAM and NOV males showed significantly increased mIPSC amplitudes compared with CTL (Fig.4d), and mIPSC frequency (but not amplitude) was significantly elevated in FAM females (Fig.4h), Stress did not alter mIPSC kinetics (Fig.4e,f,j,k). In neuronal recordings (n=122) from animals that received Stress but had no history of ethanol, FAM males had significantly elevated mIPSC frequency and faster kinetics (rise time) compared to CTL males, while CeA GABA transmission did not differ between the other groups (Supplemental Table 3).
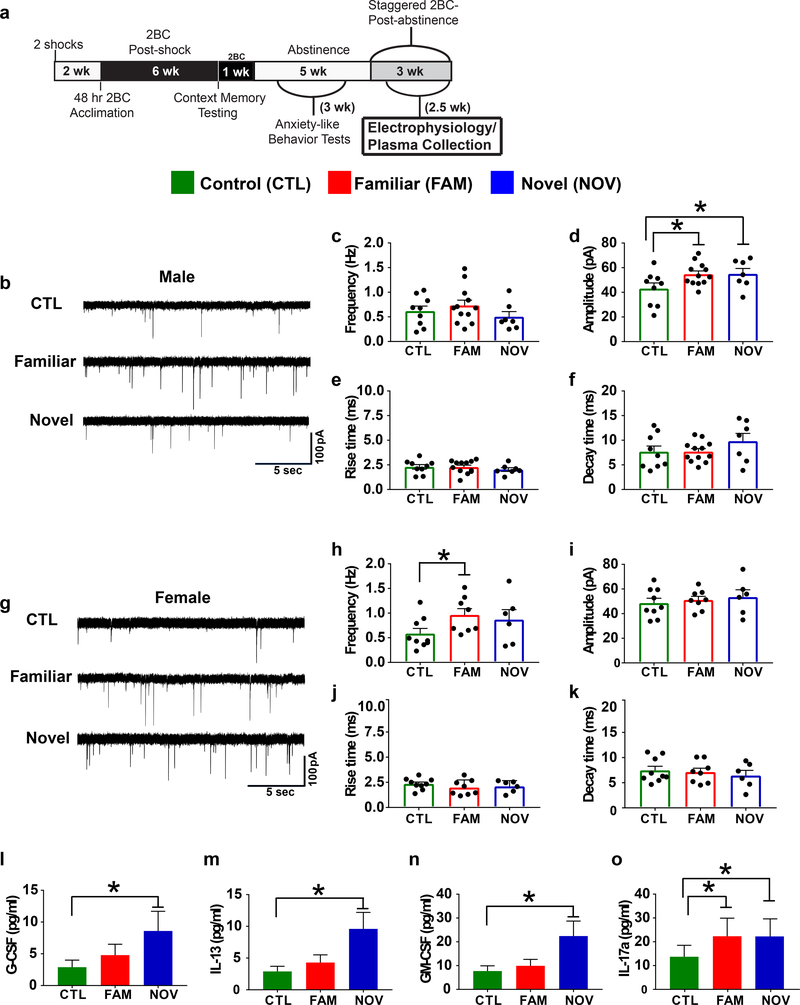
Fig. 4. Stress increased central amygdala (CeA) inhibitory GABAergic transmission and elevated peripheral cytokines.
(a) Timeline showing when physiological endpoints were measured (electrophysiology and plasma collection for cytokine analysis). (b-k) We examined CeA miniature inhibitory postsynaptic currents (mIPSCs). Representative mISPCs for males (b) and females (g) performed 24-hr into abstinence, 104–123 days post-first stressor (n=3–4 rats/group, # cells/group=6–12 (see scatter plots). (d) Within-sex planned comparisons demonstrated that among males, FAM (P=0.025) and NOV (P=0.044) stress significantly increased mISPC amplitude. (h) Conversely, FAM females exhibited increased mIPSC frequency (P=0.034). Frequency was not affected in males (c) and amplitude was not affected in females (i), andthere were no differences in rise (e,j) or decay (f,k) times in either sex (all P’s>0.05, planned comparisons). (i-o) Graphs showing backlog transformed cytokine and chemokine concentrations with Luminex plate (n=2) as a covariate (n=7–8 rats/group; 1 FAM and 1 CTL male removed as outliers, n=7 NOV males). Least significant difference analyses indicated that NOV males showed significantly elevated plasma concentrations of G-CSF, IL-13 and GM-CSF compared with controls (P’s≤0.04), while plasma IL-17a levels were elevated in both NOV (P=0.034) and FAM (P=0.041) males. *P<0.05, within-sex planned comparisons. All data are shown as mean±SEM.
Cytokine profile of past stress
Linear discriminant analysis (LDA) revealed sex differences in stress-induced cytokine profiles. In males, a function significantly loaded on by higher G-CSF, IL-13, GM-CSF, IL-6, IL-17A, leptin and IL-4 levels (0.45<rs<0.62, Ps<0.05; Table 1) discriminated Stress history groups, with NOV>FAM>CTL (z-scores of 7.1+0.4 vs. 3.2+0.7 vs. 0.0+0.4, respectively; F2,18=48.16, p<0.001; 24% of variance; all pairwise Ps<0.001). These 7 cytokines also intercorrelated (M+SEM r=0.61+0.08). Follow-up univariate analyses confirmed (Fig.4) higher G-CSF (214+37 vs. 100+16%), GM-CSF (153+21 vs. 100+8%), IL-13 (202+32 vs. 100+8%), and IL-17A (176+42 vs. 100+12%) in NOV males vs. controls (Ps<0.05), with similar trends for IL-6 (111+7 vs. 100+0%, P=0.06), leptin (106.5+3.6 vs. 100+1.7%, P=0.078), and IL-4 (176+42 vs. 100+12, P=0.079). FAM males also had elevated IL-17A (123+8 vs. 100+5%, P<0.05) and a trend for higher IL-13 (139+31 vs. 100+8%, P=0.06). Other cytokine markers were non-significantly intermediate. These cytokines were not elevated in Stressed females, yielding Stress*Sex interactions for GM-CSF, IL-13, IL-4 and IL-17A (e.g., for NOV stress, F1,25=4.16, 6.75, 3.50 and 3.20, respectively, 0.01<P<0.08). LDA did not identify a cytokine profile that discriminated females by Stress history (raw data in Supplemental Table 4).
Table 1.
Cytokine stress and resilience profiles from linear discriminant analysis (LDA)
| “Stress” LDA Profile | “Resilience” LDA Profile | ||||
|---|---|---|---|---|---|
|
| |||||
| Cytokine | r-value | P-value | Cytokine | r-value | P-value |
|
| |||||
| G-CSF | 0.617 | 0.003 | G-CSF | 0.647 | 0.002 |
| IL-13 | 0.586 | 0.005 | Leptin | 0.473 | 0.03 |
| GM-CSF | 0.561 | 0.008 | IL-13 | 0.467 | 0.033 |
| IL-6 | 0.470 | 0.032 | TNFα | 0.413 | |
| IL-17A | 0.468 | 0.032 | RANTES | 0.382 | |
| Leptin | 0.467 | 0.033 | IL-17A | 0.350 | |
| IL-4 | 0.451 | 0.04 | IL-6 | 0.348 | |
| TNFα | 0.406 | Eotaxin | 0.343 | ||
| IL-5 | 0.394 | GM-CSF | 0.337 | ||
| IP-10 | 0.373 | IP-10 | 0.310 | ||
| MIP2 | 0.330 | MIP-2 | 0.309 | ||
| Eotaxin | 0.305 | EGF | 0.306 | ||
| EGF | 0.291 | IL-4 | 0.219 | ||
| IL-1β | 0.273 | IL-12p70 | 0.219 | ||
| MCP1 | 0.198 | IL-1α | 0.211 | ||
| IL-10 | 0.186 | LIX | 0.197 | ||
| IL-1α | 0.166 | MCP-1 | 0.185 | ||
| IL-12p70 | 0.138 | Fractalkine | 0.156 | ||
| RANTES | 0.111 | IL-5 | 0.120 | ||
| LIX | 0.110 | VEGF | 0.116 | ||
| VEGF | 0.051 | IL-1β | 0.033 | ||
| Fractalkine | 0.050 | IL-10 | −0.007 | ||
| MIP-1α | 0.009 | IL-18 | −0.007 | ||
| IL-18 | 0.031 | MIP-1α | −0.011 | ||
| IL-2 | 0.079 | IL-2 | −0.149 | ||
Note: Values show the strength of correlation (Pearson’s r) of each cytokine to separate LDA functions that discriminated male rats according to either their Stress history (left) or drinking-Resilience (right). Significant correlations (“loadings”) are indicated with their corresponding P-values. Note that significance vs. non-significance shown for a given cytokine (vs. P<0.05) was the same whether calculated within the LDA from each individual cytokine’s Wilks’ lambda or externally as the correlation of the cytokine to the discriminant function score. Stress LDA scores differed significantly across stress groups with NOV>FAM>CTL. Drinking-Resilience associated with significantly higher Resilience LDA scores. See text and Fig.4 for univariate group differences for the significantly predictive cytokines (shown in bold). n=21 male rats.
Cytokine profile of drinking resilience
LDA revealed a unique cytokine profile for drinking-Resilient males, significantly loaded on by higher GM-CSF, leptin, and IL-13 levels (0.46<rs<0.66, Ps<0.05; Table 1), with Resilient>>Vulnerable>CTL (z-scores of 4.1+0.4 vs. 1.7+0.4 vs. 0.0+0.4, respectively; F2,18=27.90, p<0.001; all pairwise Ps<0.009). Follow-up univariate analyses confirmed that drinking-Resilient subjects had higher G-CSF (P<0.04; 10+3.5 vs. 2.9+1.0 pg/ml), IL-13 (p<0.005; 10.0+2.6 vs. 2.9+0.8 pg/ml), and leptin (P=0.05; 73.1+9.3 vs. 58.2+7.6 ng/ml), levels than controls, whereas drinking-Vulnerable subjects did not (4.1+1.4 pg/ml, 4.1+1.1 pg/ml, and 57.4+7.3 ng/ml, respectively). This yielded significantly higher IL-13 levels between drinking-Resilient vs. Vulnerable males (P<0.03). However, cytokine levels did not individually correlate with the amount of ethanol consumed for male animals.
Body weight and HPA-stress hormones
There were no effects of stress on weight gain, body weight (Fig.S5e&f), or point-sampled ACTH or corticosterone levels at sacrifice (Fig.S5a–c). Plasma corticosterone levels correlated with ethanol intake during the last 2BC session, 2 days before euthanasia (Fig.S5d; Spearman rs=0.423; Pearson r=0.494, Ps≤0.003).
At the time of euthanasia, most females were in proestrus or estrus (21/24). Comparisons between proestrus vs. estrus were limited by sample size, as there were only 3 CTL, 2 NOV and 1 FAM females in proestrus, but descriptively large differences were not seen for terminal endpoints.
Discussion
We adapted a novel rat model of lasting comorbid AUD/PTSD symptoms from a “2-hit” IA-based traumatic memory consolidation model28. Stressed rats developed increased ethanol intake, preference and heavy drinking days with PTSD-like symptoms, increased CeA GABAergic synaptic transmission and novel proinflammatory cytokine profiles during abstinence. Our translationally-relevant findings also demonstrate the importance of stressor context, sex, and individual factors, in vulnerability to specific comorbid AUD/PTSD symptomatology.
In females, NOV stress more robustly escalated ethanol intake severity and promoted behavioral responses translationally-relevant to PTSD. NOV females had briefer longest sleep bouts and increased startle responses, paralleling common clinical PTSD symptoms that may share common mechanisms24, 37. In contrast, in males, FAM stress more robustly promoted ethanol intake and preference with PTSD-like fear overgeneralization24, interpreted from increased avoidance of dark compartments in an unfamiliar shuttle box and EPM. These sex differences resonate with previous findings that females show different responses and strategies in fear learning, EPM, and other common anxiety-related paradigms31. Perhaps stressed males in this protocol more often overgeneralized than females due to the long (>6 wk) post-shock interval56, multiple IA trials56, lack of olfactory cues57, or ethanol history58. Male rats also overgeneralize more than females in contexts more dissimilar to the original stress context59. Finsterwald et al. (2015) showed a second traumatic shock was necessary for male rats to develop PTSD-like avoidance responses in the IA model we adapted. To our knowledge, we are the first to try this approach in females, and a different protocol may result in more overgeneralization in females. To further study the distinct outcomes of elevated voluntary drinking and fear overgeneralization in males vs. elevated drinking and hyperarousal (sleep disturbances with increased startle) in females, we examined these sex-specific behavioral phenotypes in FAM, NOV, and CTL rats that never had access to ethanol. Stress alone elicited signs of fear overgeneralization and impaired sleep maintenance, but concurrent post-stress ethanol access influenced the expression of other behaviors, exacerbating social avoidance and modifying startle and defensive behavioral responding.
Cognitive and physical avoidance are key symptoms60 that predict clinical PTSD and “coping” use of alcohol60. Accordingly, drinking-Vulnerable male rats most strongly showed avoidance of trauma-reminiscent dark places. Their avoidance of unfamiliar, but trauma-reminiscent dark places resembles a prior finding that situational reminders induced avoidance of the dark side of novel shuttle boxes and EPM-closed arms in IA-trained male rats53. EPM closed arms thus may be a “low similarity” reminder of IA training, (conditioned) avoidance of which indicates a greater degree of fear overgeneralization than does dark compartment avoidance in a novel shuttle box53. NOV males appeared to show generalization in the “low similarity” environment, but not the subsequent higher similarity one, whereas FAM males generalized in both. This finding may mean that fear generalization was less extinction-resistant in NOV males than in FAM males, especially in the drinking-Resilient subgroup. In this view, extinction during EPM testing may have reduced later fear overgeneralization in the novel inhibitory avoidance context in NOV males. Because the overgeneralization effect was maintained in Vulnerable males from both groups, extinction-resistance may be a useful marker of persistent overgeneralization28, 61 and vulnerability to drink. Vulnerable males did not separate from the other groups on other behaviors suggesting their Vulnerable phenotype associates differentially with fear overgeneralization-like responses. We cannot rule out that acoustic startle testing may have differentially affected behavior of groups in subsequent fear overgeneralization testing, but we note that males did not show behavioral differences in acute response to acoustic startle.
Additional avoidance behavior also resulted from “2-hit” stress. Hyponeophagia increased after “2-hit” stress, suggesting an anxiogenic-like62, avoidant state63. Stressed rats also avoided odors of same sex-conspecifics. Similarly, female rats previously displayed reduced social interaction after exposure to footshock and “re-experiencing” context memory testing64. Disrupted social function is a significant clinical feature of PTSD60, and social phobias are commonly comorbid with alcohol use disorder43.
Only some people with PTSD show AUD comorbidity43. Therefore, we distinguished AUD-Vulnerable from AUD-Resilient subgroups using NIAAA criteria for intake exceeding moderate levels44, 45 and ethanol preference. About half of stressed males (47%) developed elevated ethanol drinking, especially with comorbid fear overgeneralization. Even higher vulnerability rates in stressed females, on the background of greater basal drinking in control females, confirm the “2-hit” model’s efficacy. The model also elevated rates of extreme high drinking days, translationally relevant because individuals with PTSD have elevated rates of heavy drinking days52, rather than necessarily daily drinking. Future work will examine interactions between our PTSD/AUD comorbidity model and ethanol dependence models.
The previously reported Pavlovian stress-enhanced fear learning (SEFL) model also elevates ethanol intake23. SEFL generates non-associative fear learning with freezing as its primary fear output. In contrast, our “2-hit” IA model engages different behaviors, learning mechanisms, and underlying neural circuitry29. The IA model involves a complex avoidance-avoidance conflict29 wherein the animal instrumentally controls the outcome. This contingency may enhance later ethanol action tendency or otherwise prepare the animal to learn associated outcomes of its actions. This conceptualization resonates with our finding that drinking-Vulnerability corresponded with over-avoidance. Other notable features of the present model include the long-term (>6 week) nature of outcomes; the lack of pre-stress and presence of post-stress intermittent, limited ethanol access; and the use of multi-modal contextual cues to assess overgeneralization.
Exposure to “2-hit” stress without history of ethanol increased CeA inhibitory GABAergic transmission only in FAM males. Notably, Stress and ethanol increased CeA GABAergic transmission; FAM males showed increased GABAA receptor function, while FAM females showed increased GABA release. Although voluntary intake without dependence is insufficient to alter GABAergic transmission in the CeA65, 66, the heightened ethanol intake of stressed subjects may contribute to the altered CeA GABA signaling observed here. We propose that the Stress and ethanol history synergistically led to increased CeA GABA synaptic transmission, which is anxiogenic10 and seen in other conditions with heightened anxiety and ethanol intake, such as alcohol dependence18, 67.
Stressed male subjects exhibited a distinct cytokine profile of increased circulating levels of specific pro-hematopoietic (G-CSF, GM-CSF), pro-inflammatory (IL-6, leptin), and pro-atopic, Th2/Th17-like cytokines (IL-13, IL-17A, IL-4 levels). Some prior reports have observed peripheral elevations of these cytokines in isolation in patients with PTSD68, 69 or other severe anxiety conditions70, 71. Elevated levels of G-CSF and GM-CSF could signal alterations in hematopoiesis, potentially favoring myelopoiesis versus lymphopoiesis, a phenomenon that has been described with chronic social stress in mice72. IL-6 levels have also been reported to be positively correlated with early-life adversity and anxiety73. Higher IL-6 levels could be linked to increased myelopoiesis, given that it is a major cytokine of granulocytes. Plasma levels of pro-atopic cytokines IL-4 and IL-13 may be associated with stress vulnerability74. Similarly, IL-17 levels also correspond with stress. Our findings show that these cytokines potentially serve as a strongly intercorrelated, lasting biomarker “cluster” caused by traumatic stress. The cytokine profile was unique to males and more pronounced in subjects with a history of 2-hit stress in the novel (putatively, unexpected) vs. familiar (putatively, expected) context. Most of these cytokines were not measured in a recent study that did not find a neuroimmune profile in comorbid PTSD-AUD75. Interestingly, several of these cytokines increase during ethanol withdrawal76–78 and central G-CSF reportedly increases motivation for drug reinforcers79. A subset of these cytokines (G-CSF, leptin, IL-13) were most elevated in drinking-Resilient subjects, which also indicates that their elevations were not artifacts secondary to heightened ethanol intake. This subset potentially might have anti-ethanol intake actions or reflect a lack of ethanol suppression of cytokine levels. Consistent with either interpretation, reduced G-CSF80, 81 levels are seen in actively-drinking alcohol-dependent individuals. The sexual dimorphisms in cytokine predictiveness or behavioral outcomes could be explained by many factors, including estrous cycle variability; lack of females in diestrus or metestrus; sexual dimorphism in potentially relevant third variables (e.g., greater baseline ethanol drinking and vulnerability by females, body weight, body composition, exploratory activity), and/or sexually dimorphic regulation of the implicated cytokines82–85.
In summary, IA-based “2-hit” stress coupled with intermittent, limited ethanol access modeled comorbid AUD and PTSD in a sex-dependent, context-sensitive manner. Importantly, these behavioral and physiological phenotypes are distinct from those generated by either model alone, a key finding missing from many other models of comorbidity. This synergism increases the translational utility of this model, as the key behavioral phenotypes of elevated ethanol drinking with sex-specific stress and anxiety behaviors map well onto clinical PTSD experiences. The data from this initial approach will allow targeted refinement of this model to more closely match the PTSD/AUD comorbidity seen in patients. Physiological findings, including sexually-distinct mechanisms of increased CeA GABA synaptic transmission and cytokine profiles of stress history, provide future opportunities to identify sex-specific biomarkers/mechanisms of vulnerability vs. resistance to comorbid drinking, and will help to define these previously neglected characteristics for females. Finally, translational use of this paradigm to identify the molecular and neurocircuitry bases of AUD-PTSD comorbidity and test targeted interventions is needed since no FDA-approved treatments for PTSD/AUD comorbidity exist86.
Supplementary Material
Acknowledgments
We thank Drs. Amanda J. Roberts for paradigm design advice and Sarah A. Laredo for help with figure art. This is manuscript number 29810 from The Scripps Research Institute.
Funding: Support for this study was provided by The National Institute on Alcohol Abuse and Alcoholism grants AA027700, AA013498, P60 AA006420, AA017447, AA021491, AA015566, K99 AA026638, and T32 AA007456, The National Institute on Drug Abuse grant R21 DA046865, as well as the Pearson Center for Alcoholism and Addiction Research.
Footnotes
Conflict of Interest: The authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Koob GF. Addiction is a Reward Deficit and Stress Surfeit Disorder. Front Psychiatry 2013; 4: 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kushner MG, Abrams K, Borchardt C. The relationship between anxiety disorders and alcohol use disorders: a review of major perspectives and findings. Clin Psychol Rev 2000; 20(2): 149–171. [DOI] [PubMed] [Google Scholar]
- 3.Zimmermann P, Wittchen HU, Hofler M, Pfister H, Kessler RC, Lieb R. Primary anxiety disorders and the development of subsequent alcohol use disorders: a 4-year community study of adolescents and young adults. Psychol Med 2003; 33(7): 1211–1222. [DOI] [PubMed] [Google Scholar]
- 4.Debell F, Fear NT, Head M, Batt-Rawden S, Greenberg N, Wessely S et al. A systematic review of the comorbidity between PTSD and alcohol misuse. Soc Psychiatry Psychiatr Epidemiol 2014; 49(9): 1401–1425. [DOI] [PubMed] [Google Scholar]
- 5.Gilpin NW, Weiner JL. Neurobiology of comorbid post-traumatic stress disorder and alcohol-use disorder. Genes Brain Behav 2017; 16(1): 15–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schellekens AF, de Jong CA, Buitelaar JK, Verkes RJ. Co-morbid anxiety disorders predict early relapse after inpatient alcohol treatment. Eur Psychiatry 2015; 30(1): 128–136. [DOI] [PubMed] [Google Scholar]
- 7.Becker HC, Lopez MF, Doremus-Fitzwater TL. Effects of stress on alcohol drinking: a review of animal studies. Psychopharmacology (Berl) 2011; 218(1): 131–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady KT, Sonne SC. The role of stress in alcohol use, alcoholism treatment, and relapse. Alcohol Res Health 1999; 23(4): 263–271. [PMC free article] [PubMed] [Google Scholar]
- 9.Pynoos RS, Steinberg AM, Piacentini JC. A developmental psychopathology model of childhood traumatic stress and intersection with anxiety disorders. Biol Psychiatry 1999; 46(11): 1542–1554. [DOI] [PubMed] [Google Scholar]
- 10.Gilpin NW, Herman MA, Roberto M. The Central Amygdala as an Integrative Hub for Anxiety and Alcohol Use Disorders. Biol Psychiat 2015; 77(10): 859–869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jimenez VA, Herman MA, Cuzon Carlson VC, Walter NA, Grant KA, Roberto M. Synaptic adaptations in the central amygdala and hypothalamic paraventricular nucleus associated with protracted ethanol abstinence in male rhesus monkeys. Neuropsychopharmacology 2019; 44(5): 982–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoge EA, Brandstetter K, Moshier S, Pollack MH, Wong KK, Simon NM. Broad spectrum of cytokine abnormalities in panic disorder and posttraumatic stress disorder. Depress Anxiety 2009; 26(5): 447–455. [DOI] [PubMed] [Google Scholar]
- 13.von Kanel R, Hepp U, Kraemer B, Traber R, Keel M, Mica L et al. Evidence for low-grade systemic proinflammatory activity in patients with posttraumatic stress disorder. J Psychiatr Res 2007; 41(9): 744–752. [DOI] [PubMed] [Google Scholar]
- 14.Passos IC, Vasconcelos-Moreno MP, Costa LG, Kunz M, Brietzke E, Quevedo J et al. Inflammatory markers in post-traumatic stress disorder: a systematic review, meta-analysis, and meta-regression. Lancet Psychiatry 2015; 2(11): 1002–1012. [DOI] [PubMed] [Google Scholar]
- 15.Wang W, Wang L, Xu H, Cao C, Liu P, Luo S et al. Characteristics of pro- and anti-inflammatory cytokines alteration in PTSD patients exposed to a deadly earthquake. J Affect Disord 2019; 248: 52–58. [DOI] [PubMed] [Google Scholar]
- 16.Hori H, Kim Y. Inflammation and post-traumatic stress disorder. Psychiatry Clin Neurosci 2019; 73(4): 143–153. [DOI] [PubMed] [Google Scholar]
- 17.Gonzalez-Reimers E, Santolaria-Fernandez F, Martin-Gonzalez MC, Fernandez-Rodriguez CM, Quintero-Platt G. Alcoholism: a systemic proinflammatory condition. World J Gastroenterol 2014; 20(40): 14660–14671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Crews FT, Lawrimore CJ, Walter TJ, Coleman LG, Jr, The role of neuroimmune signaling in alcoholism. Neuropharmacology 2017; 122: 56–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patel RR, Khom S, Steinman MQ, Varodayan FP, Kiosses WB, Hedges DM et al. IL-1β expression is increased and regulates GABA transmission following chronic ethanol in mouse central amygdala. Brain, behavior, and immunity 2019; 75: 208–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Warden AS, Wolfe SA, Khom S, Varodayan FP, Patel RR, Steinman MQ et al. Microglia control escalation of drinking in alcohol dependent mice: Genomic and synaptic drivers. Biol Psychiat 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cui C, Shurtleff D, Harris RA. Neuroimmune mechanisms of alcohol and drug addiction. Int Rev Neurobiol 2014; 118: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pickens RW, Svikis DS, McGue M, LaBuda MC. Common genetic mechanisms in alcohol, drug, and mental disorder comorbidity. Drug Alcohol Depend 1995; 39(2): 129–138. [DOI] [PubMed] [Google Scholar]
- 23.Meyer EM, Long V, Fanselow MS, Spigelman I. Stress increases voluntary alcohol intake, but does not alter established drinking habits in a rat model of posttraumatic stress disorder. Alcohol Clin Exp Res 2013; 37(4): 566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Golub Y, Mauch CP, Dahlhoff M, Wotjak CT. Consequences of extinction training on associative and non-associative fear in a mouse model of Posttraumatic Stress Disorder (PTSD). Behav Brain Res 2009; 205(2): 544–549. [DOI] [PubMed] [Google Scholar]
- 25.Bouton ME, Mineka S, Barlow DH. A modern learning theory perspective on the etiology of panic disorder. Psychol Rev 2001; 108(1): 4–32. [DOI] [PubMed] [Google Scholar]
- 26.Sauerhofer E, Pamplona FA, Bedenk B, Moll GH, Dawirs RR, von Horsten S et al. Generalization of contextual fear depends on associative rather than non-associative memory components. Behav Brain Res 2012; 233(2): 483–493. [DOI] [PubMed] [Google Scholar]
- 27.Corral-Frias NS, Lahood RP, Edelman-Vogelsang KE, French ED, Fellous JM. Involvement of the ventral tegmental area in a rodent model of post-traumatic stress disorder. Neuropsychopharmacology 2013; 38(2): 350–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Finsterwald C, Steinmetz AB, Travaglia A, Alberini CM. From Memory Impairment to Posttraumatic Stress Disorder-Like Phenotypes: The Critical Role of an Unpredictable Second Traumatic Experience. J Neurosci 2015; 35(48): 15903–15915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tinsley MR, Quinn JJ, Fanselow MS. The role of muscarinic and nicotinic cholinergic neurotransmission in aversive conditioning: comparing pavlovian fear conditioning and inhibitory avoidance. Learn Mem 2004; 11(1): 35–42. [DOI] [PubMed] [Google Scholar]
- 30.Wellman CL, Bangasser DA, Bollinger JL, Coutellier L, Logrip ML, Moench KM et al. Sex Differences in Risk and Resilience: Stress Effects on the Neural Substrates of Emotion and Motivation. J Neurosci 2018; 38(44): 9423–9432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shansky RM. Sex differences in PTSD resilience and susceptibility: Challenges for animal models of fear learning. Neurobiol Stress 2015; 1: 60–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boggiano MM, Cavigelli SA, Dorsey JR, Kelley CE, Ragan CM, Chandler-Laney PC. Effect of a cage divider permitting social stimuli on stress and food intake in rats. Physiol Behav 2008; 95(1–2): 222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Murphy JM, Gatto GJ, Waller MB, McBride WJ, Lumeng L, Li TK . Effects of scheduled access on ethanol intake by the alcohol-preferring (P) line of rats. Alcohol 1986; 3(5): 331–336. [DOI] [PubMed] [Google Scholar]
- 34.Lin D, Parsons LH. Anxiogenic-like effect of serotonin(1B) receptor stimulation in the rat elevated plus-maze. Pharmacol Biochem Behav 2002; 71(4): 581–587. [DOI] [PubMed] [Google Scholar]
- 35.Kreisler AD, Mattock M, Zorrilla EP. The duration of intermittent access to preferred sucrose-rich food affects binge-like intake, fat accumulation, and fasting glucose in male rats. Appetite 2018; 130: 59–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kimbrough A, de Guglielmo G, Kononoff J, Kallupi M, Zorrilla EP, George O. CRF1 Receptor-Dependent Increases in Irritability-Like Behavior During Abstinence from Chronic Intermittent Ethanol Vapor Exposure. Alcohol Clin Exp Res 2017; 41(11): 1886–1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ross RJ, Ball WA, Sullivan KA, Caroff SN. Sleep Disturbance as the Hallmark of Posttraumatic Stress Disorder. Am J Psychiat 1989; 146(6): 697–707. [DOI] [PubMed] [Google Scholar]
- 38.Zeng T, Mott C, Mollicone D, Sanford LD. Automated determination of wakefulness and sleep in rats based on non-invasively acquired measures of movement and respiratory activity. J Neurosci Methods 2012; 204(2): 276–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spierling SR, Kreisler AD, Williams CA, Fang SY, Pucci SN, Kines KT et al. Intermittent, extended access to preferred food leads to escalated food reinforcement and cyclic whole-body metabolism in rats: Sex differences and individual vulnerability. Physiol Behav 2018; 192: 3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rupprecht LE, Kreisler AD, Spierling SR, de Guglielmo G, Kallupi M, George O et al. Self-administered nicotine increases fat metabolism and suppresses weight gain in male rats. Psychopharmacology (Berl) 2018; 235(4): 1131–1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Markou A, Matthews K, Overstreet DH, Koob GF, Geyer MA. Flinders resistant hypocholinergic rats exhibit startle sensitization and reduced startle thresholds. Biol Psychiatry 1994; 36(10): 680–688. [DOI] [PubMed] [Google Scholar]
- 42.Duque-Wilckens N, Steinman MQ, Busnelli M, Chini B, Yokoyama S, Pham M et al. Oxytocin Receptors in the Anteromedial Bed Nucleus of the Stria Terminalis Promote Stress-Induced Social Avoidance in Female California Mice. Biol Psychiatry 2018; 83(3): 203–213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.McFarlane AC. Epidemiological evidence about the relationship between PTSD and alcohol abuse: the nature of the association. Addict Behav 1998; 23(6): 813–825. [DOI] [PubMed] [Google Scholar]
- 44.Turner TB, Bennett VL, Hernandez H. The beneficial side of moderate alcohol use. Johns Hopkins Med J 1981; 148(2): 53–63. [PubMed] [Google Scholar]
- 45.NIAAA (National Institute on Alcohol Abuse and Alcoholism). National Institutes of Health PHS, U.S. Department of Health and Human Services. The Sixth Special Report to the U.S. Congress on Alcohol and Health from the Secretary of Health and Human Services., vol. DHHS Publ. No. (ADM) 87–1519. U.S. Government Printing Office: Washington, D.C., 1987. [Google Scholar]
- 46.Alcohol Research: Current Reviews Editorial S. Drinking Patterns and Their Definitions. Alcohol Res 2018; 39(1): 17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Barker D, Gillum KT, Niemuth NA, Kodihalli S. Therapeutic efficacy of equine botulism heptavalent antitoxin against all seven botulinum neurotoxins in symptomatic guinea pigs. PLoS One 2019; 14(9): e0222670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Roberto M, Cruz MT, Gilpin NW, Sabino V, Schweitzer P, Bajo M et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol Psychiatry 2010; 67(9): 831–839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silva AL, Fry WH, Sweeney C, Trainor BC. Effects of photoperiod and experience on aggressive behavior in female California mice. Behav Brain Res 2010; 208(2): 528–534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosnow RL, Rosenthal R. Statistical procedures and the justification of knowledge in psychological science. American Psychologist 1989; 44(10): 1276–1284. [Google Scholar]
- 51.Steinman MQ, Duque-Wilckens N, Greenberg GD, Hao R, Campi KL, Laredo SA et al. Sex-Specific Effects of Stress on Oxytocin Neurons Correspond With Responses to Intranasal Oxytocin. Biol Psychiatry 2016; 80(5): 406–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kachadourian LK, Pilver CE, Potenza MN. Trauma, PTSD, and binge and hazardous drinking among women and men: findings from a national study. J Psychiatr Res 2014; 55: 35–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Viviani D, Haegler P, Jenck F, Steiner MA. Orexin neuropeptides contribute to the development and persistence of generalized avoidance behavior in the rat. Psychopharmacology (Berl) 2015; 232(8): 1383–1393. [DOI] [PubMed] [Google Scholar]
- 54.El-Kordi A, Kastner A, Grube S, Klugmann M, Begemann M, Sperling S et al. A single gene defect causing claustrophobia. Transl Psychiatry 2013; 3: e254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.File SE. The interplay of learning and anxiety in the elevated plus-maze. Behav Brain Res 1993; 58(1–2): 199–202. [DOI] [PubMed] [Google Scholar]
- 56.Lynch J, 3rd, Cullen PK, Jasnow AM, Riccio DC. Sex differences in the generalization of fear as a function of retention intervals. Learn Mem 2013; 20(11): 628–632. [DOI] [PubMed] [Google Scholar]
- 57.Keiser AA, Turnbull LM, Darian MA, Feldman DE, Song I, Tronson NC. Sex Differences in Context Fear Generalization and Recruitment of Hippocampus and Amygdala during Retrieval. Neuropsychopharmacology 2017; 42(2): 397–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Suh J, Ressler KJ. Common Biological Mechanisms of Alcohol Use Disorder and Post-Traumatic Stress Disorder. Alcohol Res 2018; 39(2): 131–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Daviu N, Andero R, Armario A, Nadal R. Sex differences in the behavioural and hypothalamic-pituitary-adrenal response to contextual fear conditioning in rats. Horm Behav 2014; 66(5): 713–723. [DOI] [PubMed] [Google Scholar]
- 60.North CS, Nixon SJ, Shariat S, Mallonee S, McMillen JC, Spitznagel EL et al. Psychiatric disorders among survivors of the Oklahoma City bombing. JAMA 1999; 282(8): 755–762. [DOI] [PubMed] [Google Scholar]
- 61.Schreiber AL, Lu YL, Baynes BB, Richardson HN, Gilpin NW. Corticotropin-releasing factor in ventromedial prefrontal cortex mediates avoidance of a traumatic stress-paired context. Neuropharmacology 2017; 113(Pt A): 323–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Dulawa SC, Hen R. Recent advances in animal models of chronic antidepressant effects: the novelty-induced hypophagia test. Neurosci Biobehav Rev 2005; 29(4–5): 771–783. [DOI] [PubMed] [Google Scholar]
- 63.Laricchiuta D, Petrosini L. Individual differences in response to positive and negative stimuli: endocannabinoid-based insight on approach and avoidance behaviors. Front Syst Neurosci 2014; 8: 238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Louvart H, Maccari S, Ducrocq F, Thomas P, Darnaudery M. Long-term behavioural alterations in female rats after a single intense footshock followed by situational reminders. Psychoneuroendocrinology 2005; 30(4): 316–324. [DOI] [PubMed] [Google Scholar]
- 65.Sparrow AM, Lowery-Gionta EG, Pleil KE, Li C, Sprow GM, Cox BR et al. Central neuropeptide Y modulates binge-like ethanol drinking in C57BL/6J mice via Y1 and Y2 receptors. Neuropsychopharmacology 2012; 37(6): 1409–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Varodayan FP, Soni N, Bajo M, Luu G, Madamba SG, Schweitzer P et al. Chronic ethanol exposure decreases CB1 receptor function at GABAergic synapses in the rat central amygdala. Addict Biol 2016; 21(4): 788–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Natividad LA, Buczynski MW, Herman MA, Kirson D, Oleata CS, Irimia C et al. Constitutive increases in amygdalar corticotropin-releasing factor and fatty acid amide hydrolase drive an anxious phenotype. Biol Psychiat 2017; 82(7): 500–510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Maloley PM, England BR, Sayles H, Thiele GM, Michaud K, Sokolove J et al. Post-traumatic stress disorder and serum cytokine and chemokine concentrations in patients with rheumatoid arthritis(). Semin Arthritis Rheum 2019; 49(2): 229–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spencer-Segal JL, Hyzy RC, Iwashyna TJ, Standiford TJ. Psychiatric Symptoms in Survivors of Acute Respiratory Distress Syndrome. Effects of Age, Sex, and Immune Modulation. Ann Am Thorac Soc 2017; 14(6): 960–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leff Gelman P, Mancilla-Herrera I, Flores-Ramos M, Saravia Takashima MF, Cruz Coronel FM, Cruz Fuentes C et al. The cytokine profile of women with severe anxiety and depression during pregnancy. BMC Psychiatry 2019; 19(1): 104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tang Z, Ye G, Chen X, Pan M, Fu J, Fu T et al. Peripheral proinflammatory cytokines in Chinese patients with generalised anxiety disorder. J Affect Disord 2018; 225: 593–598. [DOI] [PubMed] [Google Scholar]
- 72.Jin J, Wang X, Wang Q, Guo X, Cao J, Zhang X et al. Chronic psychological stress induces the accumulation of myeloid-derived suppressor cells in mice. PLoS One 2013; 8(9): e74497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Carpenter LL, Gawuga CE, Tyrka AR, Lee JK, Anderson GM, Price LH. Association between plasma IL-6 response to acute stress and early-life adversity in healthy adults. Neuropsychopharmacology 2010; 35(13): 2617–2623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Han A, Yeo H, Park MJ, Kim SH, Choi HJ, Hong CW et al. IL-4/10 prevents stress vulnerability following imipramine discontinuation. J Neuroinflammation 2015; 12: 197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Neupane SP, Bramness JG, Lien L. Comorbid post-traumatic stress disorder in alcohol use disorder: relationships to demography, drinking and neuroimmune profile. BMC Psychiatry 2017; 17(1): 312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Song C, Lin A, De Jong R, Vandoolaeghe E, Kenis G, Bosmans E et al. Cytokines in detoxified patients with chronic alcoholism without liver disease: increased monocytic cytokine production. Biol Psychiatry 1999; 45(9): 1212–1216. [DOI] [PubMed] [Google Scholar]
- 77.Nikou T, Ioannidis A, Zoga M, Tzavellas E, Paparrigopoulos T, Magana M et al. Alteration in the concentrations of Interleukin-7 (IL-7), Interleukin-10 (IL-10) and Granulocyte Colony Stimulating Factor (G-CSF) in alcohol-dependent individuals without liver disease, during detoxification therapy. Drug Alcohol Depend 2016; 163: 77–83. [DOI] [PubMed] [Google Scholar]
- 78.Aguiar-Nemer AS, Toffolo MC, da Silva CJ, Laranjeira R, Silva-Fonseca VA. Leptin influence in craving and relapse of alcoholics and smokers. J Clin Med Res 2013; 5(3): 164–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Calipari ES, Godino A, Peck EG, Salery M, Mervosh NL, Landry JA et al. Granulocyte-colony stimulating factor controls neural and behavioral plasticity in response to cocaine. Nat Commun 2018; 9(1): 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manzardo AM, Poje AB, Penick EC, Butler MG. Multiplex Immunoassay of Plasma Cytokine Levels in Men with Alcoholism and the Relationship to Psychiatric Assessments. Int J Mol Sci 2016; 17(4): 472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Santolaria F, Rodriguez-Lopez C, Martin-Hernandez B, Aleman-Valls MR, Gonzalez-Reimers E, Alonso-Socas MD et al. Similar inflammatory response in alcoholic and non-alcoholic sepsis patients. Eur Cytokine Netw 2011; 22(1): 1–4. [DOI] [PubMed] [Google Scholar]
- 82.Beatty WW, Fessler RG. Ontogeny of sex differences in open-field behavior and sensitivity to electric shock in the rat. Physiol Behav 1976; 16(4): 413–417. [DOI] [PubMed] [Google Scholar]
- 83.Drago F, Bohus B, Scapagnini U, de Wied D. Sexual dimorphism in passive avoidance behavior of rats: relation to body weight, age, shock intensity and retention interval. Physiol Behav 1980; 24(6): 1161–1164. [DOI] [PubMed] [Google Scholar]
- 84.Pare WP. Age, sex, and strain differences in the aversive threshold to grid shock in the rat. J Comp Physiol Psychol 1969; 69(2): 214–218. [PubMed] [Google Scholar]
- 85.Smith JT, Waddell BJ. Developmental changes in plasma leptin and hypothalamic leptin receptor expression in the rat: peripubertal changes and the emergence of sex differences. J Endocrinol 2003; 176(3): 313–319. [DOI] [PubMed] [Google Scholar]
- 86.Ralevski E, Olivera-Figueroa LA, Petrakis I. PTSD and comorbid AUD: a review of pharmacological and alternative treatment options. Subst Abuse Rehabil 2014; 5: 25–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.