Abstract
The cerebral cortex is a central structure in the mammalian brain that enables higher cognitive functions and intellectual skills. It is the hallmark of the mammalian nervous system with enormous complexity, consisting of a large number of neurons and glia that are diverse in morphology, molecular expression, biophysical properties, circuit connectivity and physiological function. Cortical neurons and glia are generated by neural progenitor cells during development. Ensuring the correct cell cycle kinetics, fate behavior and lineage progression of neural progenitor cells is essential to determine the number and types of neurons and glia in the cerebral cortex, which together constitute neural circuits for brain function. In this review, we discuss recent findings on mammalian cortical progenitor cell types and their lineage behaviors in generating neurons and glia, cortical evolution and expansion, and advances in brain organoid technology that allow the modeling of human cortical development under normal and disease conditions.
As part of the central nervous system, the cerebral cortex originates from the neural tube in the neuroectoderm. During early embryonic development, neuroepithelial cells proliferate to give rise to radial glial cells, the founder neural progenitor cells responsible for generating the vast majority of neurons and glia in the cerebral cortex. Radial glial progenitors (RGPs) in the dorsal telencephalon produce glutamatergic excitatory neurons that migrate radially to constitute the future cortex, as well as glia, including astrocytes and oligodendrocytes. In the ventral telencephalon, RGPs generate g-aminobutyric acid (GABA)-ergic inhibitory interneurons and glia that migrate tangentially to the future cerebral cortex [1]. The cerebral cortex also contains other cell types with different origins. In particular, microglia are the resident immune cells and derive from the mesoderm, colonizing the developing cortex during early embryonic development [2]. Similarly, the endothelial cells that contribute to blood vessel development also arise from the mesoderm [3].
Cortical neural progenitor cell types
RGPs are the primary neural progenitor cells that exist transiently in the developing cortex. They possess a characteristic bipolar morphology with the cell body located in the ventricular zone (VZ). They grow a long basal radial glial fiber reaching the pia and a short apical endfoot anchored at VZ surface. RGPs are organized as a peudostratified cell layer and actively divide with the cell cycle synchronized with interkinetic nuclear migration. In this process, the division of RGPs occurs exclusively at the VZ surface, where the centrosomes reside. Initially, RGPs undergo a series of symmetrical divisions to amplify themselves before entering a neurogenic and gliogenic phase, where they divide asymmetrically to generate neurons and glia in a progressive manner. In the mouse, the amplification phase takes place around embryonic day (E) 9–11, while the second phase extends from around E12 into early the post-natal stages of development. As a result, RGPs exhibit three distinct and sequential phases of development: amplification, neurogenesis and gliogenesis.
During cortical neurogenesis and gliogenesis, RGPs divide to produce, respectively, neurons and glia either directly or indirectly via transit amplifying progenitors (TAPs) that divide predominantly outside of the VZ (e.g., the subventricular zone, SVZ). Various types of TAPs have been identified, including intermediate progenitors (IPs; also called basal progenitors), outer subventricular zone RGPs (oRGs; also called basal RGPs, intermediate RGPs or transit RGPs), and short neural precursors (SNPs) [4]. IPs typically present a multipolar morphology and reside in the SVZ, where they divide [5–7]. oRGs show a unipolar morphology with a long basal radial glial fiber, but no apical endfoot, and reside predominantly in the outer SVZ (oSVZ), where they divide [8–10]. Finally, SNPs possess the apical endfoot and a short basal radial glial fiber, and may represent the emerging IPs in the VZ [11,12]. The number and types of TAPs vary greatly across species and have been linked to cortical expansion and evolution. In particular, IPs constitute the predominant type of TAPs in the developing mouse cortex, while oRGs have been shown to be abundant in the developing cortex of higher mammals, such as primates and ferrets [9,13–16]. Notably, while RGPs and IPs have been well-characterized based on transcription factor signatures, lineage progression, and neural outputs, largely due to genetic labeling and tracing studies in mice [17,18], the precise features of oRGs and SNPs remain to be defined.
The emergence of TAPs not only increases the capacities of neurogenesis by RGPs, but by providing another level of spatial and temporal control, they also facilitate the generation of neural diversity in the developing cortex. Compared with RGPs, TAPs are more restricted in their proliferative potential and output potency. The lineage progression from RGPs to TAPs to neurons are features common to the production of both glutamatergic excitatory and GABAergic inhibitory interneurons in the cortex, even though the underlying transcription factor regulatory programs are distinct. Notably, a similar lineage strategy is employed in gliogenesis. Upon the completion of neurogenesis, a fraction of RGPs proceed to generate glia either directly or indirectly via intermediate glial precursor cells which, in common with TAPs, show limited proliferative potential and output potency [19–21]. Therefore, despite the spatial and temporal, as well as transcriptional, differences in cortical excitatory and inhibitory neurogenesis, and gliogenesis, the basic hierarchical lineage organization with RGPs generating TAPs or intermediate precursors through asymmetric division, which in turn give rise to differentiated neuronal or glial progenies, is conserved between cortical regions and across species.
Cortical neural progenitor behavior and lineage output
Cortical progenitors exhibit distinct division patterns in a spatially and temporally specific manner. RGPs undergo a series of symmetric proliferative divisions before transferring into a phase of asymmetric neurogenic and then gliogenic divisions, which take place at the VZ surface exclusively. IPs typically undergo a limited series of renewing divisions in the SVZ, giving rise to neuronal, but not glial, progenies. By contrast, oRGs can divide symmetrically or asymmetrically to self-renew while producing neurons or IPs in the oSVZ. Notably, progenitor behavior is not synchronized across, or even within, cortical domains, with transitions between phases taking place across developmental times. Therefore, to assess progenitor behavior and output, it is necessary to carry out analysis at single cell resolution in a temporal specific manner. Indeed, clonal analysis has been crucial for understanding cortical progenitor behavior and lineage potential. In vertebrates, this is best performed in the developing mouse cortex due to the availability of genetic tools that allow for specific and reliable labeling of defined progenitors. Given that different types of progenitors coexist, it is essential to know the identity of progenitors subject to analysis, and the timing of labeling.
Several approaches have been developed and applied to the clonal analysis of cortical progenitors. Among these methods, mosaic analysis with double markers (MADM) has proved to be a particularly powerful. By marking sister lineages with fluorescent reporters of different color, the MADM system provides access in vivo to both the long-term lineage output and the individual fate behavior of marked progenitors in a spatially and temporally specific manner [22]. While it is labor intensive, systematic and quantitative clonal analyses using MADM have provided unprecedented insights into the behavior and lineage potential of neural progenitors in the developing mouse cortex [23]. These findings suggest that, while RGPs are not synchronous in the timing of the transitions between the three phases of development, they exhibit highly organized and predictable behavior during lineage progression (Figure 1). Specifically, during their proliferative amplification phase (~E10–11), the size correlation of sister MADM clones show that RGPs follow a seemingly rigid pattern of lineage progression in which early progenitors undergo a defined number of symmetric cell divisions before entry into their neurogenic phase. Following entry into neurogenesis, individual RGPs then undergo a sequence of asymmetric divisions, leading to the output of a relatively defined number (~8–9) of neurons, occupying both the deep and superficial layers of the cortex with an evolving fate bias correlated with the timing of their specification.
Figure 1. Highly organized behavior and lineage program of RGPs in the developing mouse cortex.
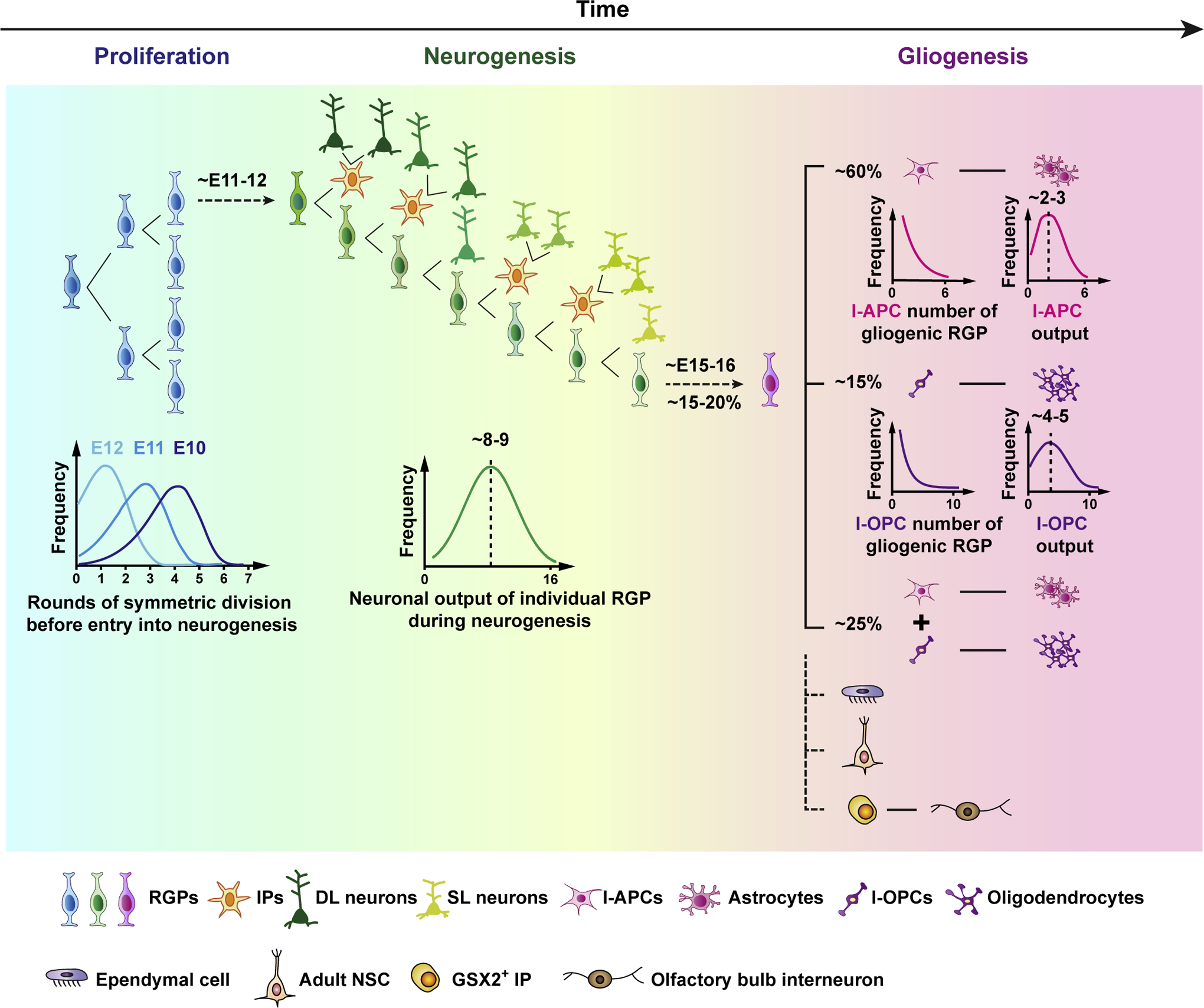
A schematic representation of mouse cortical RGP behavior and lineage progression. RGPs first amplify through a defined sequence of symmetric divisions before switching abruptly into a neurogenic phase (left). While the timing of the transition varies across the ensemble of RGPs, it is synchronized by division number within an individual RGP lineage in the amplification phase (left, lower). In the neurogenic phase, deep layer (DL) and then superficial layer (SL) excitatory neurons are generated through asymmetric RGP cell divisions, either directly or indirectly through IPs with more restricted division and neuronal output potentials (middle). Overall, individual RGPs give rise to a “unitary” number of neuronal output with a Gaussian-like distribution peaked at around 8–9 neurons (middle, lower). At the end of neurogenesis, a fraction (~20%) of RGPs switch abruptly into a gliogenic phase, giving rise to astrocytes and/or oligodendrocytes either directly or indirectly via fate-restricted intermediate astrocyte precursor cells (I-APCs) or intermediate oligodendrocyte precursor cells (I-OPCs) (right). While the total number of I-APCs and I-OPCs generated by individual gliogenic RGPs appears to be governed by defined probabilistic rules, the glial outputs of individual I-APCs and I-OPCs are relatively well-defined. A small number of RGPs also give rise to ependymal cells, adult ventricular-subventricular zone neural stem cells (i.e., type B1 cells), and GSX2+ IPs that give rise to GABAergic interneurons in the olfactory bulb.
Neuronal generation by RGPs occurs progressively in an “inside-out” manner with deep layer neurons produced first and superficial layer neurons later. Notably, this behavior is evident at both the population and clonal level, suggesting that temporal fate restriction, lineage progression and the histogenesis of neuronal outputs occur within the framework of a largely equipotent RGP population. Consistent with these findings, when cultured, isolated RGPs produce both deep and superficial layer neurons and in an orderly manner, indicating that temporal progression of neuronal fate is largely an intrinsic feature of RGPs [24,25]. Such temporal restriction of neurogenic fate potential is also consistent with early studies suggesting that late stage progenitors (likely RGPs and IPs) cannot generate early born, deep layer, neurons, even upon transplantation into the early stage cortex [26,27]. However, these findings contrast with a more recent study showing that, when transplanted into early stage cortex, late stage RGPs alone are capable of producing early-born, deep layer neurons, suggesting that the output potential of RGPs may be modulated by local environmental cues [28]. Together, these reports emphasize that the intrinsic versus environmental regulation of RGPs and their lineage potential remains an important area for future research.
Upon the completion of neurogenesis, a fraction (~15–20%) of RGPs in the mouse cortex proceeds to gliogenesis, while the others exit cell cycle and differentiate. Notably, although the switch between neurogenesis and gliogenesis for individual RGPs is abrupt, the timing of this transition varies across the population of RGPs, starting at ~E14 and completing by ~E17 with a peak ~E15–16. Although variations in the timing of the transition into and out of the neurogenic phase may influence the precise number of neuronal progeny, the neuronal output of individual RGPs is well-approximated by a Gaussian-like distribution with a peak at ~8–9 neurons (i.e., the coherent program model) [23] (Figure 2). This suggests that the neurogenic potential of individual RGPs is not random, but has a relatively defined “unitary” output (at least with regard to the number of neuronal progeny, i.e., clonal size). A strong indication of the defined unitary output is the inverse correlation between deep layer (DL) and superficial layer (SL) neurons generated by individual RGPs.
Figure 2. Lineage variations of RGPs during neurogenesis and possible underlying mechanisms in the developing mouse cortex.

(a) Statistical variations in the neuronal output of individual RGPs labeled at ~E12.5 based on the Emx1-CreER;RCL-Gfp (left) and Emx1-CreER;MADM (right) reporter with respect to the number and laminar position reported by Llorca et al. and Gao et al. Laminar-restricted: DL, deep layer (5–6) neurons only; SL, superficial layer (2–4) neurons only; Translaminar: DL and SL. Note that, at this induction time, some RGPs will have yet to enter into neurogenesis, while others may have already completed a significant fraction of their neurogenic program (see Fig. 1). (b) Two possible sources of RGP neurogenesis lineage variations have been proposed: the heterogeneous neurogenesis configuration model (left; Llorca et al.), and the coherent neurogenesis program model (right; Gao et al.). In the former, based particularly on the existence of laminar-restricted clones, RGPs are proposed to be pre-specified into distinct subtypes, each generating a diverse but defined neuronal output. For example, RGPD generates DL neurons only, RGPS generates SL neurons only, and RGPSD generates both DL and SL neurons. During neurogenesis, different subtypes of RGPs with distinct division and neurogenesis behaviors are programmed to generate neurons independently across the RGP population, implying a model of high complexity and specificity. In particular, as detailed by Llorca et al., a model of some 27 parameters is required to capacity the repertoire of observed sublineage sizes and compositions pointing to a complex underlying program of transcriptional control. By contrast, in the latter model, RGPs are considered to be largely equipotent, unfolding the same neurogenic program, and bearing the same proliferative and neurogenic potential. Small variations in the total neuronal output from the ~8–9 neuron average reflect heterogeneity in the proliferative capacity of daughter IPs, while variations in the neuronal composition of individual RGP clones reflect the timing of entry into and exit neurogenesis, with DL neurons enriched in RGP lineages that enter into neurogenesis early, and SL neurons enriched in those that enter late. The coherent neurogenesis program model, which predicts well the progressive output of DL and SL neurons at both the clonal and population level with regard to the number and laminar position across the entire neurogenic phase, also has the benefit of parsimony, depending on few parameters linked to the measured distribution of entry times into, and the length of, the neurogenic phase.
Although early tracing studies, based on the results of MADM tracing, has placed emphasis on equipotency of RGPs, both in proliferative and sublineage potential, a recent study has suggested that RGPs may be heterogeneous, with progenitors becoming restricted either to laminar or translaminar neuronal outputs – the “heterogenous configuration model” [29] (Figure 2). Here, three different clonal labeling methods, including MADM, retroviral labeling and a Emx1-CreER;RCL-Gfp genetic reporter, were used to probe the output of individual RGPs. Analysis of the Emx1-CreER;RCL-Gfp dataset induced at E12.5 showed that translaminar clones (~80% of the total clones analyzed) display a Gaussian-like distribution with a clear peak at ~8 neurons, similar to the results from MADM tracing reported previously [23] and in this study. By contrast, laminar-restricted clones (~20% of the total clones analyzed; this ratio is significantly smaller in the MADM dataset) exhibited a much smaller size of ~3–4 neurons, implicating a discrete RGP type with different proliferative and neurogenic potential. However, given the relatively high sensitivity of Emx1-CreER;RCL-Gfp labeling, it remains possible that the few laminar-restricted clones may not arise from bone-fide RGPs, but emerge from sublineage-restricted IPs with EMX1 expression. Notably, it has been shown that a small fraction of IPs can undergo more than one round of division [6,30].
Indeed, the capacity of RGPs to generate laminar-restricted neurons has been actively debated in the field. In particular, it has been suggested that CUX2+ RGPs are fated to produce superficial layer neurons only, whereas CUX2- RGPs produce deep layer neurons only [31,32]. This idea has been challenged by a series of clonal lineage tracing studies showing that individual RGPs labeled at the start of neurogenesis (~E12) generate both deep and superficial layer neurons [23,33–36]. However, since tracing studies do not sample the lineage potential of every RGP in the developing cortex, the existence of a small subset of RGPs with the capacity to generate only laminar-restricted neuronal output cannot be rigorously ruled out. If such fate-restricted RGPs do exist, they must be very scarce. Consistent with this conclusion, evidence for fate-restricted RGPs has not been found in single-cell transcriptomic studies [37,38]. Combining a high-temporal resolution labeling method and single-cell RNA sequencing analysis, the transcriptional trajectories of successive RGPs and their daughter neurons have been mapped out. These analyses indicate that a core set of temporally patterned genes drive RGPs from intrinsic genetic programs to states that are more susceptible to environment-derived signals [38].
Besides the size of neuronal output, the precise subtypes of neurons generated by individual RGPs remain to be fully characterized. A combination of information, including the morphology, biophysical properties, gene expression, synaptic connectivity, and circuit function, needs to be gathered to decipher neuronal subtypes, in conjunction with the area and laminar localization in the cortex [39].
Following entry into the gliogenic phase, RGPs divide to generate astrocytes and oligodendrocytes. A systematic and quantitative clonal analysis using MADM tracing showed that, in mouse, ~60% of gliogenic RGPs give rise to astrocytes alone, ~15% oligodendrocytes alone, and ~25% both astrocytes and oligodendrocytes [21]. Notably, these ratios are found consistently, regardless of the exact time when RGPs transition from neurogenesis to gliogenesis, indicating that they are a defined feature of gliogenic RGP lineage progression. The existence of the three types of gliogenic clones raises the question of whether gliogenic RGPs are heterogeneous with different lineage potential (i.e., unipotent and bipotent), or whether RGPs are equipotent with fixed probabilities of producing astrocytes or oligodendrocytes. In other words, unipotent gliogenic clones represent the output of “biopotent” RGPs that, by chance, fail to generate one of the glial cell types. Interestingly, in-depth analysis of the size distribution and the relative frequency of the three different types of gliogenic clones suggest that the latter is the case [21].
Furthermore, in common with fate behavior during the neurogenic phase, astrogenesis and oligogenesis by RGPs occur predominantly through the generation of intermediate fate-restricted astrocyte or oligodendrocyte precursor cells (i.e., gliogenic TAPs; intermediate astrocyte precursor cells, I-APCs or intermediate oligodendrocyte precursor cells, I-OPCs) that largely produce the same subtype of astrocytes or oligodendrocytes, forming local clusters of glial cells in the cortex [21]. Notably, the production of I-APCs and I-OPCs by RGPs appears to be stochastic, with RGPs choosing between intermediate precursor cell output and cell cycle exit with fixed probability. On the other hand, the output of individual I-APCs or I-OPCs is relatively well defined. In particular, a single I-APC generates ~2–3 astrocytes and a single I-OPC generates ~4–5 oligodendrocytes on average. These findings suggest that, while the glial output of individual RGPs is highly variable, the output of the ensemble of RGPs during the gliogenic phase is defined. Interestingly, despite the evidence for equipotency in the proliferative potential of RGPs, single-cell analysis shows evidence of glial cell heterogeneity in the cortex [40–42].
A significant proportion of cortical oligodendrocytes arise from RGPs in the ventral telencephalon [43], which migrate tangentially to arrive at the cortex. Glia originating from the dorsal and ventral telencephalon may exhibit distinct properties; yet, the overall cellular processes underlying the gliogenesis in the dorsal and ventral telencephalon is likely similar.
RGPs also give rise to ependymal cells that form the lining of the lateral ventricle [44]. While the vast majority of RGPs exit the cell cycle and become depleted as they go through neurogenesis and gliogenesis, a small fraction of RGPs transform into the adult neural stem cells (i.e., type B1 cells) in the ventricular-subventricular zone within the lateral ventricle that function to refresh neurons in the olfactory bulb. Notably, adult neural stem cells appear to be more abundant in the ventrolateral wall of the lateral ventricle, indicating potential regional differences of RGPs [45]. Moreover, a recent study reported the emergence of GSX2+ IPs in the dorsal telencephalon at the late developmental stage, which give rise to GABAergic interneurons in the olfactory bulb [46]. Together, these findings suggest that, alongside the conserved dynamics of the majority RGPs that generate cortical neurons and glia, there is a fine regulation of minor RGP lineages in the developing cortex that will require further targeted clonal analyses to dissect.
Cortical progenitors in expansion and gyrification
Our current understanding of mammalian cortical development is largely built on extensive research on the rodent cortex. However, the murine and human cortices exhibit striking differences. In particular, the human cortex is greatly expanded with a much higher density of neurons and complex folding (i.e., gyrification or gyrencephalic), whereas the murine cortex is relatively small with no folding (i.e., smooth or lissencephalic). These differences call for research on the mechanisms underlying cortical expansion and gyrification [47].
Given that RGPs are the primary neural progenitors in the developing cortex, an increase in RGP number would result in an expansion of the cortex. This could be achieved by enhanced rates of RGP proliferation and/or prolonged RGP lifespan. The emergence of TAPs, such as IPs and oRGs, also plays a crucial role in cortical expansion and evolution. The mammalian cerebral cortex is thought to have evolved from a common ancestor of mammals and reptiles. Despite sharing functional and cytoarchitectonic characteristics with the mammalian cortex, the adult turtle cortex consists of only three basic layers, of which only the middle layer contains densely packed excitatory neurons, and the outer and inner layers are neuropilar and mainly synaptic, with some inhibitory interneurons and glial cells [48,49] (Figure 3). Even though thalamic inputs to cortical neurons and cortical outputs to the striatum and brain stem are segregated in the turtle cortex, as in mammals, the reptilian cortex is considered to lack an equivalent of the superficial layers (2–4) of the mammalian cortex. Notably, while TBR2+ IPs are present in the developing turtle cortex, they do not form a prominent SVZ, suggesting that the SVZ may be important for cortical expansion and six-layer histogenesis [50]. On the other hand, the existence of TBR2+ IPs in the developing turtle cortex indicates that indirect neurogenesis through IPs (or TAPs) is evolved prior to its presence in mammals. Moreover, TBR2+ IPs contribute to cortical neuron generation even in the simplified turtle cortex with a single layer of excitatory neurons. This is consistent with the findings that TBR2+ IPs give rise to both deep and superficial layer neurons significantly and similarly in the six-layer mouse cortex [13,15,51].
Figure 3. Comparison of the architectonic structures of the embryonic and mature cortices in three representative species.

Schematic illustrations of the embryonic and adult cortical organizations in reptiles, rodents and primates. (a) In reptiles, neurogenesis occurs primarily by RGPs in the ventricular zone (VZ). Some TBR2+ IPs also exist in the VZ, but there is no obvious subventricular zone (SVZ). The mature cortex is a tri-laminar structure with one prominent excitatory neuron layer in the middle. (b) In rodents, in addition to the VZ consisting of RGPs, the SVZ is prominent with ample IPs. The extensive indirect neurogenesis through IPs contributes to the production of both DL and SL neurons, and the formation of a six-layer cortex. (c) In primates, in addition to the increase in RGPs in the VZ, the SVZ is further expanded, including IPs and oRGs that predominantly constitutes the inner (iSVZ) and outer subventricular zone (oSVZ), respectively. oRGs also provide additional scaffolds supporting and guiding neuronal migration. Variations in neural progenitor cell types and consequently neurogenesis, as well as cell migration, may lead to a distinct functional spatial organization of the cortex in different species. For example, the neural map of orientation selectivity in the visual cortex shows a ‘salt-and-pepper’ organization in the mouse, whereas it is ‘clustered’ in primates and other higher mammals. IZ, intermediate zone; CP, cortical plate.
While the developing mouse cortex possesses a prominent SVZ with ample TBR2+ IPs, it contains rare oRGs [52]. In line with this, very little expression of the oRG markers such as HOPX can be detected in the embryonic mouse cortex. On the other hand, in the developing ferret and primate cortices, oRGs are abundant and occupy predominantly the oSVZ. Similar to RGPs in the VZ, oRGs divide asymmetrically to self-renew and generate TBR2+ IPs or neurons. oRGs may also divide symmetrically to proliferate. In addition, human oRGs provide an extra source of pre-oligodendrocyte precursor cells (Pre-OPC), and consequently expand the white matter and myelination in the human brain [53]. In this regard, human oRGs are similar to gliogenic RGPs in the late embryonic stage mouse cortex in morphology and lineage output. In comparison, TBR2+ IPs do not appear to contribute to gliogenesis, at least in the mouse cortex [15,51]. Notably, oRGs undergo mitotic somal translocation, where the soma rapidly ascends along its own radial fiber toward the pia before cytokinesis, leading to the outward expansion of the oSVZ boundary. Distinct from TBR2+ IPs, oRGs also provide additional radial glial fiber scaffolds that support and thereby affect the route of neuronal migration. The expansion of oRGs in the oSVZ has been thought to be critical for cortical folding, as indicated by the correlation between the relative abundance of oRGs in the oSVZ and the degree of folding [54,55]. Consistent with this, genetic manipulations leading to an increase in oRG abundance promote cortical folds in mice and ferrets [10,56–59]. On the other hand, cortical folds do not occur in the absence of a clear expansion of oRGs [60,61].
Cortical gyrification is remarkably consistent across individuals and even across some species, and often coupled to cytoarchitectonic boundaries, indicating that it is a tightly regulated biological process. A number of mechanisms have been proposed to be related to cortical gyrification, including the axonal tension theory, the surface expansion theory, the differential tangential expansion theory, and the relative radial versus tangential expansion theory [14,62–66]. Two key aspects of gyrification are the nature and pattern of the forces that drive the folding, and the biological origins of the forces. The axonal tension theory postulates that axons connecting different parts of the cortex pull to form the gyri and consequently the sulci. Recently studies have challenged the active role of the axonal connection and tension in cortical gyrification. The expansion theories impinge on the drastic increase in the neurogenesis capacity that is driven by the number and types of neural progenitors in the developing cortex. In principle, there are two major sources of neurogenesis: RGP division at the VZ surface and TAP division in the SVZ, and oRG division in the oSVZ and IP division in the inner SVZ (iSVZ). An increase in the number of pseudostratified RGPs would lead to a lateral expansion of founder neurogenic units (i.e., radial units). On the other hand, an increase in the number or proliferative potential of TAPs (oRGs and IPs) would lead to a radial increase in neuronal output. Interestingly, a recent study suggests that the relative increase in the surface area versus the thickness of the cortex is a critical determinant of cortical gyrafication [64]. This idea is consistent with the notion that cortical gyrafication is unlikely due to a single evolutionary adaptation, like the cortical volume increase or the abundance of oRGs, but a function of multiple developmental processes, including neuronal migration and outgrowth. Manipulation of inter-neuronal adhesion via FLRT affects neuronal migration and causes cortical folding [60].
The stereotyped gyrification of the cortex suggests that the patterns of folding may be precisely regulated during development and across evolution. Local variations in the rate of neurogenesis, radial glial fiber divergence and abundance of oRGs have been suggested to drive the defined folding patterns. This can be achieved by patterned gene expressions in the prospective locations of gyri and sulci [67]. Systematic dissection of gene expression and function during cortical development and across species will help to establish the link between gene expression and cortical gyrification.
While RGPs, oRGs, and IPs exist commonly in the developing cortices, they exhibit distinct behaviors and properties in different species. RGPs undergo ~11 rounds of cell divisions in mice [68], at least ~28 cell divisions in primates, and potentially far more in human [69]. IPs largely undergo one round of division in mice, with multiple rounds in primates and human. These differences in the properties and behaviors across species may be reflected in significant changes in gene expression during evolution. Rapid advances in sequencing are providing a wealth of information into species-specific gene expression patterns in cortical development [58,70–77]. Future efforts on the functional interrogation of genetic and epigenetic variations will be essential to achieve a deep understanding of the mechanisms of cortical expansion and evolution.
The expansion and evolution of the cortex not only entail an increase in neuronal number and cortical gyrification, but also affect the functional organization of the cortex. Neurons in the cortex are organized into functional neural maps. This is best exemplified in the primary sensory cortex such as the primary visual cortex. While the neural map in higher mammals such as carnivores and primates is clustered, it displays a striking ‘salt-and-pepper’ pattern in rodents [78]. These differences in functional neural map organization may stem from the variations in neural progenitor behavior and neuronal migration during early cortical development.
Cerebral organoids to model human cortical development and disease
The human cortex represents one of the greatest achievements during evolution. Understanding human cortical development under normal and disease conditions is a major goal in neurobiology. Human genetics and high-throughput transcriptome-wide profiling studies have yielded a plethora of gene expression information on human cortical development. The underlying mechanisms, however, remain poorly understood, in part due to the inaccessibility of developing human brain tissue, especially to experimental manipulation. To understand the molecular and cellular bases of human cortical development, there is an urgent need to develop viable experimental models that can recapitulate the growth characteristics and anatomical organization of the tissue [79,80]. In this context, organoid technology provides an exciting opportunity to investigate the mechanisms of human cortical development and model diseases.
Organoids are self-assembled three-dimensional suspension cultures, derived from human pluripotent stem cells (hPSCs), including human embryonic stem cells (hESCs) and human induced pluripotent stem cells (hiPSCs), and resemble various features of human development and organization. Brain organoid technologies derive from earlier work on cultures of embryoid bodies, multicellular aggregates of PSCs similar to that of the pregastrulation embryo and capable of undergoing developmental specification. Pioneering studies by the Sasai group paved the way to this expanding field of research. Building on initial work on using hESCs to generate self-organizing optic cup-like structures displaying features of retinal architecture, Sasai and colleagues differentiated hESCs as self-organizing three-dimensional serum-free floating cultures of embryoid body-like aggregates, with quick reaggregation, that contained spatially organized cortical structures, including VZ-like, SVZ-like, and cortical plate (CP)-like regions [81]. In 2013, brain organoids containing a developing cortex as well as other brain regions were generated by Lancaster, Knoblich, and colleagues using hiPSCs derived from a patient with microcephaly [82]. Around the same time, inductive signaling molecules to mimic endogenous patterning (i.e., dorsal and ventral forebrain differentiation) were used to generate cortical region-specific organoids [83].
Since then, the protocols for the effective generation of whole brain organoids or brain region-specific organoids were progressively refined and established. Some protocols take advantage of the remarkable ability of PSCs to spontaneously acquire neural identity in the absence of serum, growth factors, or other inductive signals (i.e., undirected protocols). This promotes the generation of diverse brain regions and cell populations. However, while the diversity is attractive, it can lead to a relatively variable outcome and the generation of non-ectodermal cell types. To overcome this, other protocols direct neural induction towards the desired brain region by mimicking endogenous patterns through the addition of exogenous cues (i.e., directed protocols), such as SMAD signaling inhibition to prevent mesoderm and endoderm formation, WNT signaling inhibition to avoid posteriorization, and sonic hedgehog signaling inhibition to promote dorsal telencephalon identities. The directed protocols allow for a high degree of consistency and predictability within and across experiments. Along these lines, additional growth factors or signaling molecules including inhibitors or activators can be included in the culture medium to affect stem/progenitor cell behavior and cortical tissue generation. Organoid complexity, consistency, and efficacy can also be enhanced by applying extracellular matrix and implementing various bioengineering techniques, such as constant agitation using shakers or spinning bioreactors [84], and growing organoids on microfilament scaffolds [85]. Organoid technology is evolving rapidly, with emphasis now placed on, for example, the development of organoid fusion paradigms to investigate the interaction of different brain regions [86], or the integration of non-neural cells such as endothelial cells and microglia [87]. One can anticipate significant advances in the near future, leading to the generation of more consistent, mature, and architectonically complex cerebral organoids.
While two-dimensional cultures of embryoid bodies generate self-organized neural rosettes with features of the embryonic neuroepithelium and substantial neurogenesis [88], a major advantage of organoids is the presence of in vivo-like cell-cell interaction and organization, which are essential to the development of the complex cortex. Indeed, cerebral organoids recapitulate a number of key features of early human cortical development at molecular, cellular, structural and functional levels [82,83,89–92]. In this context, cerebral organoids provide a unique platform to carry out gene function studies, either by genetically eliminating genes or introducing mutations using versatile gene editing tools. Such studies should, in principle, allow the molecular and cellular processes underlying human cortical development to be dissected.
Organoids also hold promise for modeling brain diseases in vitro in a three-dimensional multicellular environment, as they are amenable to studies that require live tissues. Moreover, in conjunction with patient-derived iPSC technology, they provide a unique opportunity to model complex polygenic disorders, including those with unidentified risk loci [93]. Cerebral organoids have been actively used to model malformations of human cortical development such as microcephaly and lissencephaly [82,94,95]. The recent Zika virus outbreak has highlighted the potential uses of organoids to model the impact of infectious diseases on human cortical development [84,96]. Organoids have also been used to model neuropsychiatric diseases such as autism spectrum disorder, schizophrenia, bipolar disorder, and Rett syndrome, as well as neurodegenerative diseases such as Alzheimer’s disease and brain tumorigenesis [97–103]. Neuropsychiatric diseases often manifest defects postnatally, with alterations in dendrite and axon outgrowth, circuitry assembly, or brain network function. To what degree brain organoids can be used to model these diseases, or neurodegenerative diseases, critically depend on future developments to generate organoids resembling more mature stages of the human brain, which remains an overriding challenge in brain organoid technology.
In addition, while brain organoids represent promising tools to study previously inaccessible aspects of human brain development and disease, their limitations cannot be neglected. Besides their relative immaturity (i.e., corresponding to the period from early to mid-gestational stages of human brain development), a recent study suggested that cells in organoids exhibit enhanced expression of genes associated with cellular stress pathways, electron transport pathways, and endoplasmic reticulum dysfunction [104]. Therefore, one should be cautious to interpret disease phenotypes in organoids that might be influenced by metabolic stress pathways.
Conclusions and outlook
Extensive progress has been made on the development of the cerebral cortex, especially on the early processes of neural progenitor cell specification and histogenesis. A systematic and quantitative understanding of progenitor cell types, their proliferative behaviors and lineage outputs is fundamental to understanding cortical development. RGPs represent the founder neural progenitor cells in the developing cortex and execute a highly organized developmental program both in terms of lineage progression and neuronal and glial output. During development, RGPs progress sequentially from amplification to neurogenesis and then gliogenesis. Within individual phases, the fate behavior of individual RGPs is organized and predictable with regard to proliferative potential and statistical neuronal and glial output. These observations suggest that the behavior of RGPs is highly regulated. A crucial step moving forward is to delineate the molecular control of the organized RGP behavior and lineage progression. This endeavor will benefit from the rapid advances in high-throughput in-depth single-cell OMICS and gene editing approaches.
RGPs divide to give rise to neurons or glia, as well as TAPs that further divide to amplify the neuronal and glial output. The abundance and diversity of TAPs play a critical role in cortical expansion and evolution. It is, therefore, necessary to fully understand the behaviors and lineages of the various TAP cell types. Moreover, while RGPs and TAPs exist commonly across species, they exhibit species-specific features. A compounding issue is that some TAPs are defined morphologically, especially in non-genetically tractable species, which leads to difficulties in identifying and tracing them reliably for in-depth analyses. Given the rapid growth of transcriptome information across species, and the advancement of gene editing technology, it will be important to establish new genetically tractable systems to examine the behaviors and lineages of RGPs and TAPs systematically and quantitatively to achieve a mechanistic understanding of cortical expansion and evolution.
One of the ultimate goals of neurobiology is to understand human cortical development. Cerebral organoids are exciting and promising tools to study inaccessible aspect of human cortical development and disease. However, one must acknowledge the strengths and limitations of organoid modeling systems. The absences of cell types and structures critical for normal brain development and function, the relative immaturity in neural differentiation and circuit formation, the variations in cellular and tissue architecture, and the need for more comprehensive characterization to match fetal tissue will need to be overcome to realize their full potential to model human brain development and disease.
Acknowledgements
We thank members of the Shi lab for insightful discussions and inputs. Research in the Shi lab was supported by Beijing Outstanding Young Scientist Program (BJJWZYJH01201910003012), Simons Foundation (SFARI #567854), TRI-SCI, National Institute of Health, and Howard Hughes Medical Institute. B.D.S acknowledges funding through a Royal Society E.P. Abraham Research Professorship (RP\R1\180165) and the Wellcome Trust (098357/Z/12/Z). We apologize to all our colleagues whose work we did not cite due to limited space but have been invaluable to our understanding of cortical progenitors and development.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
Nothing declared.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
* Of special interest
** Of outstanding interest
- 1.Wonders CP, Anderson SA: The origin and specification of cortical interneurons. Nat Rev Neurosci 2006, 7:687–696. [DOI] [PubMed] [Google Scholar]
- 2.Swinnen N, Smolders S, Avila A, Notelaers K, Paesen R, Ameloot M, Brone B, Legendre P, Rigo JM: Complex invasion pattern of the cerebral cortex bymicroglial cells during development of the mouse embryo. Glia 2013, 61:150–163. [DOI] [PubMed] [Google Scholar]
- 3.Dzierzak E, Bigas A: Blood Development: Hematopoietic Stem Cell Dependence and Independence. Cell Stem Cell 2018, 22:639–651. [DOI] [PubMed] [Google Scholar]
- 4.Florio M, Huttner WB: Neural progenitors, neurogenesis and the evolution of the neocortex. Development 2014, 141:2182–2194. [DOI] [PubMed] [Google Scholar]
- 5.Haubensak W, Attardo A, Denk W, Huttner WB: Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci U S A 2004, 101:3196–3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Noctor SC, Martinez-Cerdeno V, Ivic L, Kriegstein AR: Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci 2004, 7:136–144. [DOI] [PubMed] [Google Scholar]
- 7.Miyata T, Kawaguchi A, Saito K, Kawano M, Muto T, Ogawa M: Asymmetric production of surface-dividing and non-surface-dividing cortical progenitor cells. Development 2004, 131:3133–3145. [DOI] [PubMed] [Google Scholar]
- 8.Hansen DV, Lui JH, Parker PR, Kriegstein AR: Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464:554–561. [DOI] [PubMed] [Google Scholar]
- 9.Fietz SA, Kelava I, Vogt J, Wilsch-Brauninger M, Stenzel D, Fish JL, Corbeil D, Riehn A, Distler W, Nitsch R, et al. : OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat Neurosci 2010, 13:690–699. [DOI] [PubMed] [Google Scholar]
- 10.Reillo I, de Juan Romero C, Garcia-Cabezas MA, Borrell V: A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex 2011, 21:1674–1694. [DOI] [PubMed] [Google Scholar]
- 11.Stancik EK, Navarro-Quiroga I, Sellke R, Haydar TF: Heterogeneity in ventricular zone neural precursors contributes to neuronal fate diversity in the postnatal neocortex. J Neurosci 2010, 30:7028–7036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hevner RF: Intermediate progenitors and Tbr2 in cortical development. J Anat 2019, 235:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kowalczyk T, Pontious A, Englund C, Daza RA, Bedogni F, Hodge R, Attardo A, Bell C, Huttner WB, Hevner RF: Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb Cortex 2009, 19:2439–2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Reillo I, de Juan Romero C, García-Cabezas M, Borrell V: A role for intermediate radial glia in the tangential expansion of the mammalian cerebral cortex. Cereb Cortex 2011, 21:1674–1694. [DOI] [PubMed] [Google Scholar]
- 15.Lv X, Ren SQ, Zhang XJ, Shen Z, Ghosh T, Xianyu A, Gao P, Li Z, Lin S, Yu Y, et al. : TBR2 coordinates neurogenesis expansion and precise microcircuit organization via Protocadherin 19 in the mammalian cortex. Nat Commun 2019, 10:3946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Betizeau M, Cortay V, Patti D, Pfister S, Gautier E, Bellemin-Menard A, Afanassieff M, Huissoud C, Douglas RJ, Kennedy H, et al. : Precursor diversity and complexity of lineage relationships in the outer subventricular zone of the primate. Neuron 2013, 80:442–457. [DOI] [PubMed] [Google Scholar]
- 17.Englund C, Fink A, Lau C, Pham D, Daza RA, Bulfone A, Kowalczyk T, Hevner RF: Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J Neurosci 2005, 25:247–251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Götz M, Stoykova A, Gruss P: Pax6 controls radial glia differentiation in the cerebral cortex. Neuron 1998, 21:1031–1044. [DOI] [PubMed] [Google Scholar]
- 19.Clavreul S, Abdeladim L, Hernandez-Garzon E, Niculescu D, Durand J, Ieng SH, Barry R, Bonvento G, Beaurepaire E, Livet J, et al. : Cortical astrocytes develop in a plastic manner at both clonal and cellular levels. Nat Commun 2019, 10:4884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge WP, Miyawaki A, Gage FH, Jan YN, Jan LY: Local generation of glia is a major astrocyte source in postnatal cortex. Nature 2012, 484:376–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shen Z, Lin Y, Yang J, Jörg DJ, Peng Y, Zhang X, Xu Y, Hernandez L, Ma J, Simons BD, et al. : Distinct progenitor behavior underlying neocortical gliogenesis related to tumorigenesis. bioRxiv:2020.2010.2013.338459. [DOI] [PubMed]
- 22.Zong H, Espinosa JS, Su HH, Muzumdar MD, Luo L: Mosaic analysis with double markers in mice. Cell 2005, 121:479–492. [DOI] [PubMed] [Google Scholar]
- **23.Gao P, Postiglione MP, Krieger TG, Hernandez L, Wang C, Han Z, Streicher C, Papusheva E, Insolera R, Chugh K, et al. : Deterministic progenitor behavior and unitary production of neurons in the neocortex. Cell 2014, 159:775–788. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study took advantage of MADM and performed a systematic and quantitative analysis of individual RGP division behavior and lineage progression in the developing mouse cortex, and demonstrated that individual RGPs conforms to a highly coherent and defined developmental program of proliferation, neurogenesis and gliogenesis.
- 24.Gaspard N, Bouschet T, Hourez R, Dimidschstein J, Naeije G, van den Ameele J, Espuny-Camacho I, Herpoel A, Passante L, Schiffmann SN, et al. : An intrinsic mechanism of corticogenesis from embryonic stem cells. Nature 2008, 455:351–357. [DOI] [PubMed] [Google Scholar]
- 25.Shen Q, Wang Y, Dimos JT, Fasano CA, Phoenix TN, Lemischka IR, Ivanova NB, Stifani S, Morrisey EE, Temple S: The timing of cortical neurogenesis is encoded within lineages of individual progenitor cells. Nat Neurosci 2006, 9:743–751. [DOI] [PubMed] [Google Scholar]
- 26.Frantz GD, McConnell SK: Restriction of late cerebral cortical progenitors to an upper-layer fate. Neuron 1996, 17:55–61. [DOI] [PubMed] [Google Scholar]
- 27.Desai AR, McConnell SK: Progressive restriction in fate potential by neural progenitors during cerebral cortical development. Development 2000, 127:2863–2872. [DOI] [PubMed] [Google Scholar]
- **28.Oberst P, Fievre S, Baumann N, Concetti C, Bartolini G, Jabaudon D: Temporal plasticity of apical progenitors in the developing mouse neocortex. Nature 2019, 573:370–374. [DOI] [PubMed] [Google Scholar]; This study isolated specific populations of RGPs and IPs in the developing mouse cortex, and transplanted them into younger embryos. They found that later stage RGPs are temporally plastic and maintain the capacity to reacquire an earlier developmental program, whereas IPs are committed to a fixed neurogenic competence.
- **29.Llorca A, Ciceri G, Beattie R, Wong FK, Diana G, Serafeimidou-Pouliou E, Fernandez-Otero M, Streicher C, Arnold SJ, Meyer M, et al. : A stochastic framework of neurogenesis underlies the assembly of neocortical cytoarchitecture. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study performed clonal analyses of RGPs in the developing mouse cortex, focusing on the variations of individual clones in laminar distribution and thus proposing a model of heterogeneous RGPs with stochastic lineage configurations.
- 30.Mihalas AB, Hevner RF: Clonal analysis reveals laminar fate multipotency and daughter cell apoptosis of mouse cortical intermediate progenitors. Development 2018, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *31.Franco SJ, Gil-Sanz C, Martinez-Garay I, Espinosa A, Harkins-Perry SR, Ramos C, Muller U: Fate-restricted neural progenitors in the mammalian cerebral cortex. Science 2012, 337:746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study reported the identification of CUX2+ RGPs in the developing mouse cortex selectively produce superficial layer neurons and proposed that distinct subsets of RGPs exist to produce deep versus superficial layer neurons (i.e., laminar fate-restricted RGPs).
- 32.Marin O: Brain development: The neuron family tree remodelled. Nature 2012, 490:185–186. [DOI] [PubMed] [Google Scholar]
- *33.Eckler MJ, Nguyen TD, McKenna WL, Fastow BL, Guo C, Rubenstein JLR, Chen B: Cux2-positive radial glial cells generate diverse subtypes of neocortical projection neurons and macroglia. Neuron 2015, 86:1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study showed that CUX2+ RGPs are not fate-restricted to produce superficial corticocortical projection neurons, but generate both superficial and deep layer neurons.
- 34.Guo C, Eckler MJ, McKenna WL, McKinsey GL, Rubenstein JL, Chen B: Fezf2 expression identifies a multipotent progenitor for neocortical projection neurons, astrocytes, and oligodendrocytes. Neuron 2013, 80:1167–1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cadwell CR, Scala F, Fahey PG, Kobak D, Mulherkar S, Sinz FH, Papadopoulos S, Tan ZH, Johnsson P, Hartmanis L, et al. : Cell type composition and circuit organization of clonally related excitatory neurons in the juvenile mouse neocortex. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *36.Kaplan ES, Ramos-Laguna KA, Mihalas AB, Daza RAM, Hevner RF: Neocortical Sox9+ radial glia generate glutamatergic neurons for all layers, but lack discernible evidence of early laminar fate restriction. Neural Dev 2017, 12:14. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study used Sox9-CreER mouse line and performed MADM analysis of cortical RGPs and observed no evidence on fate-restriced RGPs to produce only deep or superficial layer neurons.
- 37.Oberst P, Agirman G, Jabaudon D: Principles of progenitor temporal patterning in the developing invertebrate and vertebrate nervous system. Current Opinion in Neurobiology 2019, 56:185–193. [DOI] [PubMed] [Google Scholar]
- *38.Telley L, Agirman G, Prados J, Amberg N, Fievre S, Oberst P, Bartolini G, Vitali I, Cadilhac C, Hippenmeyer S, et al. : Temporal patterning of apical progenitors and their daughter neurons in the developing neocortex. Science 2019, 364. [DOI] [PubMed] [Google Scholar]; This study performed single-cell RNA sequencing to trace the transcriptional trajectories of the progressive generations of RGPs and daughter neurons in the developing mouse cortex and showed that early temporal molecular birthmarks, together with later environmental-dependent signals, contribute to the neuronal diversity in the adult cortex.
- 39.Tasic B, Yao Z, Graybuck LT, Smith KA, Nguyen TN, Bertagnolli D, Goldy J, Garren E, Economo MN, Viswanathan S, et al. : Shared and distinct transcriptomic cell types across neocortical areas. Nature 2018, 563:72–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lanjakornsiripan D, Pior BJ, Kawaguchi D, Furutachi S, Tahara T, Katsuyama Y, Suzuki Y, Fukazawa Y, Gotoh Y: Layer-specific morphological and molecular differences in neocortical astrocytes and their dependence on neuronal layers. Nat Commun 2018, 9:1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Marques S, Zeisel A, Codeluppi S, van Bruggen D, Mendanha Falcao A, Xiao L, Li H, Haring M, Hochgerner H, Romanov RA, et al. : Oligodendrocyte heterogeneity in the mouse juvenile and adult central nervous system. Science 2016, 352:1326–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study performed three-dimensional morphological reconstruction and molecular cluster analyses, and showed that astrocytes in the mouse cortex exhibit layer-specific morphological and molecular differences, depending on neuronal layer establishment.
- *42.Bayraktar OA, Bartels T, Holmqvist S, Kleshchevnikov V, Martirosyan A, Polioudakis D, Ben Haim L, Young AMH, Batiuk MY, Prakash K, et al. : Astrocyte layers in the mammalian cerebral cortex revealed by a single-cell in situ transcriptomic map. Nat Neurosci 2020, 23:500–509. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study performed single cell RNA sequeince of the oligodendrocyte lineages in different regions of the mouse central nervous system and revealed the heterogenity of oligodendroctye lineages and transcriptional profiles.
- 43.Kessaris N, Fogarty M, Iannarelli P, Grist M, Wegner M, Richardson WD: Competing waves of oligodendrocytes in the forebrain and postnatal elimination of an embryonic lineage. Nat Neurosci 2006, 9:173–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ortiz-Alvarez G, Daclin M, Shihavuddin A, Lansade P, Fortoul A, Faucourt M, Clavreul S, Lalioti ME, Taraviras S, Hippenmeyer S, et al. : Adult Neural Stem Cells and Multiciliated Ependymal Cells Share a Common Lineage Regulated by the Geminin Family Members. Neuron 2019, 102:159–172 e157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mizrak D, Levitin HM, Delgado AC, Crotet V, Yuan J, Chaker Z, Silva-Vargas V, Sims PA, Doetsch F: Single-Cell Analysis of Regional Differences in Adult V-SVZ Neural Stem Cell Lineages. Cell Rep 2019, 26:394–406.e395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Y, Liu G, Guo T, Liang XG, Du H, Yang L, Bhaduri A, Li X, Xu Z, Zhang Z, et al. : Cortical Neural Stem Cell Lineage Progression Is Regulated by Extrinsic Signaling Molecule Sonic Hedgehog. Cell Rep 2020, 30:4490–4504 e4494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Miller DJ, Bhaduri A, Sestan N, Kriegstein A: Shared and derived features of cellular diversity in the human cerebral cortex. Curr Opin Neurobiol 2019, 56:117–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *48.Tosches MA, Yamawaki TM, Naumann RK, Jacobi AA, Tushev G, Laurent G: Evolution of pallium, hippocampus, and cortical cell types revealed by single-cell transcriptomics in reptiles. Science 2018, 360:881–888. [DOI] [PubMed] [Google Scholar]; This study used single-cell messenger RNA sequencing and identified molecular similarities in neuronal cell types between the reptilian and mammalian pallial region.
- 49.Briscoe SD, Ragsdale CW: Homology, neocortex, and the evolution of developmental mechanisms. Science 2018, 362:190–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cardenas A, Villalba A, de Juan Romero C, Pico E, Kyrousi C, Tzika AC, Tessier-Lavigne M, Ma L, Drukker M, Cappello S, et al. : Evolution of Cortical Neurogenesis in Amniotes Controlled by Robo Signaling Levels. Cell 2018, 174:590–606 e521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vasistha NA, Garcia-Moreno F, Arora S, Cheung AF, Arnold SJ, Robertson EJ, Molnar Z: Cortical and Clonal Contribution of Tbr2 Expressing Progenitors in the Developing Mouse Brain. Cereb Cortex 2015, 25:3290–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalebic N, Gilardi C, Stepien B, Wilsch-Brauninger M, Long KR, Namba T, Florio M, Langen B, Lombardot B, Shevchenko A, et al. : Neocortical Expansion Due to Increased Proliferation of Basal Progenitors Is Linked to Changes in Their Morphology. Cell Stem Cell 2019, 24:535–550 e539. [DOI] [PubMed] [Google Scholar]
- 53.Huang W, Bhaduri A, Velmeshev D, Wang S, Wang L, Rottkamp CA, Alvarez-Buylla A, Rowitch DH, Kriegstein AR: Origins and Proliferative States of Human Oligodendrocyte Precursor Cells. Cell 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dehay C, Kennedy H, Kosik KS: The outer subventricular zone and primate-specific cortical complexification. Neuron 2015, 85:683–694. [DOI] [PubMed] [Google Scholar]
- 55.Llinares-Benadero C, Borrell V: Deconstructing cortical folding: genetic, cellular and mechanical determinants. Nat Rev Neurosci 2019, 20:161–176. [DOI] [PubMed] [Google Scholar]
- 56.Wang L, Hou S, Han YG: Hedgehog signaling promotes basal progenitor expansion and the growth and folding of the neocortex. Nat Neurosci 2016, 19:888–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stahl R, Walcher T, De Juan Romero C, Pilz GA, Cappello S, Irmler M, Sanz-Aquela JM, Beckers J, Blum R, Borrell V, et al. : Trnp1 regulates expansion and folding of the mammalian cerebral cortex by control of radial glial fate. Cell 2013, 153:535–549. [DOI] [PubMed] [Google Scholar]
- 58.Florio M, Albert M, Taverna E, Namba T, Brandl H, Lewitus E, Haffner C, Sykes A, Wong FK, Peters J, et al. : Human-specific gene ARHGAP11B promotes basal progenitor amplification and neocortex expansion. Science 2015, 347:1465–1470. [DOI] [PubMed] [Google Scholar]
- 59.Nonaka-Kinoshita M, Reillo I, Artegiani B, Martinez-Martinez MA, Nelson M, Borrell V, Calegari F: Regulation of cerebral cortex size and folding by expansion of basal progenitors. EMBO J 2013, 32:1817–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Del Toro D, Ruff T, Cederfjall E, Villalba A, Seyit-Bremer G, Borrell V, Klein R: Regulation of Cerebral Cortex Folding by Controlling Neuronal Migration via FLRT Adhesion Molecules. Cell 2017, 169:621–635 e616. [DOI] [PubMed] [Google Scholar]
- 61.Shao W, Yang J, He M, Yu XY, Lee CH, Yang Z, Joyner AL, Anderson KV, Zhang J, Tsou MB, et al. : Centrosome anchoring regulates progenitor properties and cortical formation. Nature 2020, 580:106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Striedter GF, Srinivasan S, Monuki ES: Cortical folding: when, where, how, and why? Annu Rev Neurosci 2015, 38:291–307. [DOI] [PubMed] [Google Scholar]
- 63.Van Essen DC: A tension-based theory of morphogenesis and compact wiring in the central nervous system. Nature 1997, 385:313–318. [DOI] [PubMed] [Google Scholar]
- 64.Mota B, Herculano-Houzel S: BRAIN STRUCTURE. Cortical folding scales universally with surface area and thickness, not number of neurons. Science 2015, 349:74–77. [DOI] [PubMed] [Google Scholar]
- 65.Garcia KE, Kroenke CD, Bayly PV: Mechanics of cortical folding: stress, growth and stability. Philos Trans R Soc Lond B Biol Sci 2018, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Smart IH, McSherry GM: Gyrus formation in the cerebral cortex of the ferret. II. Description of the internal histological changes. J Anat 1986, 147:27–43. [PMC free article] [PubMed] [Google Scholar]
- 67.de Juan Romero C, Borrell V: Genetic maps and patterns of cerebral cortex folding. Current Opinion in Cell Biology 2017, 49:31–37. [DOI] [PubMed] [Google Scholar]
- 68.Takahashi T, Nowakowski RS, Caviness VS Jr.,: The cell cycle of the pseudostratified ventricular epithelium of the embryonic murine cerebral wall. J Neurosci 1995, 15:6046–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kornack DR, Rakic P: Changes in cell-cycle kinetics during the development and evolution of primate neocortex. Proc Natl Acad Sci U S A 1998, 95:1242–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Loo L, Simon JM, Xing L, McCoy ES, Niehaus JK, Guo J, Anton ES, Zylka MJ: Single-cell transcriptomic analysis of mouse neocortical development. Nat Commun 2019, 10:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan X, Dong J, Zhong S, Wei Y, Wu Q, Yan L, Yong J, Sun L, Wang X, Zhao Y, et al. : Spatial transcriptomic survey of human embryonic cerebral cortex by single-cell RNA-seq analysis. Cell Res 2018, 28:730–745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bakken TE, Miller JA, Ding SL, Sunkin SM, Smith KA, Ng L, Szafer A, Dalley RA, Royall JJ, Lemon T, et al. : A comprehensive transcriptional map of primate brain development. Nature 2016, 535:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johnson MB, Sun X, Kodani A, Borges-Monroy R, Girskis KM, Ryu SC, Wang PP, Patel K, Gonzalez DM, Woo YM, et al. : Aspm knockout ferret reveals an evolutionary mechanism governing cerebral cortical size. Nature 2018, 556:370–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nowakowski TJ, Bhaduri A, Pollen AA, Alvarado B, Mostajo-Radji MA, Di Lullo E, Haeussler M, Sandoval-Espinosa C, Liu SJ, Velmeshev D, et al. : Spatiotemporal gene expression trajectories reveal developmental hierarchies of the human cortex. Science 2017, 358:1318–1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Suzuki IK, Gacquer D, Van Heurck R, Kumar D, Wojno M, Bilheu A, Herpoel A, Lambert N, Cheron J, Polleux F, et al. : Human-Specific NOTCH2NL Genes Expand Cortical Neurogenesis through Delta/Notch Regulation. Cell 2018, 173:1370–1384 e1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fiddes IT, Lodewijk GA, Mooring M, Bosworth CM, Ewing AD, Mantalas GL, Novak AM, van den Bout A, Bishara A, Rosenkrantz JL, et al. : Human-Specific NOTCH2NL Genes Affect Notch Signaling and Cortical Neurogenesis. Cell 2018, 173:1356–1369 e1322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhu Y, Sousa AMM, Gao T, Skarica M, Li M, Santpere G, Esteller-Cucala P, Juan D, Ferrandez-Peral L, Gulden FO, et al. : Spatiotemporal transcriptomic divergence across human and macaque brain development. Science 2018, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ohki K, Chung S, Ch’ng YH, Kara P, Reid RC: Functional imaging with cellular resolution reveals precise micro-architecture in visual cortex. Nature 2005, 433:597–603. [DOI] [PubMed] [Google Scholar]
- 79.Muchnik SK, Lorente-Galdos B, Santpere G, Sestan N: Modeling the Evolution of Human Brain Development Using Organoids. Cell 2019, 179:1250–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Di Lullo E, Kriegstein AR: The use of brain organoids to investigate neural development and disease. Nat Rev Neurosci 2017, 18:573–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Eiraku M, Watanabe K, Matsuo-Takasaki M, Kawada M, Yonemura S, Matsumura M, Wataya T, Nishiyama A, Muguruma K, Sasai Y: Self-organized formation of polarized cortical tissues from ESCs and its active manipulation by extrinsic signals. Cell Stem Cell 2008, 3:519–532. [DOI] [PubMed] [Google Scholar]
- **82.Lancaster MA, Renner M, Martin CA, Wenzel D, Bicknell LS, Hurles ME, Homfray T, Penninger JM, Jackson AP, Knoblich JA: Cerebral organoids model human brain development and microcephaly. Nature 2013, 501:373–379. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study introduced a powerful method for generating cerebral organoids and used it to model the pathogenesis of microcephaly.
- 83.Kadoshima T, Sakaguchi H, Nakano T, Soen M, Ando S, Eiraku M, Sasai Y: Self-organization of axial polarity, inside-out layer pattern, and species-specific progenitor dynamics in human ES cell-derived neocortex. Proc Natl Acad Sci U S A 2013, 110:20284–20289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qian X, Jacob F, Song MM, Nguyen HN, Song H, Ming GL: Generation of human brain region-specific organoids using a miniaturized spinning bioreactor. Nat Protoc 2018, 13:565–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lancaster MA, Corsini NS, Wolfinger S, Gustafson EH, Phillips AW, Burkard TR, Otani T, Livesey FJ, Knoblich JA: Guided self-organization and cortical plate formation in human brain organoids. Nat Biotechnol 2017, 35:659–666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Birey F, Andersen J, Makinson CD, Islam S, Wei W, Huber N, Fan HC, Metzler KRC, Panagiotakos G, Thom N, et al. : Assembly of functionally integrated human forebrain spheroids. Nature 2017, 545:54–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hutter-Schmid B, Kniewallner KM, Humpel C: Organotypic brain slice cultures as a model to study angiogenesis of brain vessels. Front Cell Dev Biol 2015, 3:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shi Y, Kirwan P, Smith J, Robinson HP, Livesey FJ: Human cerebral cortex development from pluripotent stem cells to functional excitatory synapses. Nat Neurosci 2012, 15:477–486, s471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Qian X, Nguyen HN, Song MM, Hadiono C, Ogden SC, Hammack C, Yao B, Hamersky GR, Jacob F, Zhong C, et al. : Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165:1238–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Otani T, Marchetto MC, Gage FH, Simons BD, Livesey FJ: 2D and 3D Stem Cell Models of Primate Cortical Development Identify Species-Specific Differences in Progenitor Behavior Contributing to Brain Size. Cell Stem Cell 2016, 18:467–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Amiri A, Coppola G, Scuderi S, Wu F, Roychowdhury T, Liu F, Pochareddy S, Shin Y, Safi A, Song L, et al. : Transcriptome and epigenome landscape of human cortical development modeled in organoids. Science 2018, 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *92.Velasco S, Kedaigle AJ, Simmons SK, Nash A, Rocha M, Quadrato G, Paulsen B, Nguyen L, Adiconis X, Regev A, et al. : Individual brain organoids reproducibly form cell diversity of the human cerebral cortex. Nature 2019, 570:523–527. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study compared a large scRNA-seq dataset derived from human brain organoids and human cerebral cortex, and showed that a high degree of reproducibility of cell type compositions and developmental trajectories of the human cerebral cortex could be modeled using human brain organoids.
- 93.Amin ND, Pasca SP: Building Models of Brain Disorders with Three-Dimensional Organoids. Neuron 2018, 100:389–405. [DOI] [PubMed] [Google Scholar]
- 94.Bershteyn M, Nowakowski TJ, Pollen AA, Di Lullo E, Nene A, Wynshaw-Boris A, Kriegstein AR: Human iPSC-Derived Cerebral Organoids Model Cellular Features of Lissencephaly and Reveal Prolonged Mitosis of Outer Radial Glia. Cell Stem Cell 2017, 20:435–449 e434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Iefremova V, Manikakis G, Krefft O, Jabali A, Weynans K, Wilkens R, Marsoner F, Brandl B, Muller FJ, Koch P, et al. : An Organoid-Based Model of Cortical Development Identifies Non-Cell-Autonomous Defects in Wnt Signaling Contributing to Miller-Dieker Syndrome. Cell Rep 2017, 19:50–59. [DOI] [PubMed] [Google Scholar]
- 96.Garcez PP, Loiola EC, Madeiro da Costa R, Higa LM, Trindade P, Delvecchio R, Nascimento JM, Brindeiro R, Tanuri A, Rehen SK: Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352:816–818. [DOI] [PubMed] [Google Scholar]
- 97.Ogawa J, Pao GM, Shokhirev MN, Verma IM: Glioblastoma Model Using Human Cerebral Organoids. Cell Rep 2018, 23:1220–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Bian S, Repic M, Guo Z, Kavirayani A, Burkard T, Bagley JA, Krauditsch C, Knoblich JA: Genetically engineered cerebral organoids model brain tumor formation. Nat Methods 2018, 15:631–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Choi H, Song J, Park G, Kim J: Modeling of Autism Using Organoid Technology. Mol Neurobiol 2017, 54:7789–7795. [DOI] [PubMed] [Google Scholar]
- 100.Brennand KJ, Simone A, Jou J, Gelboin-Burkhart C, Tran N, Sangar S, Li Y, Mu Y, Chen G, Yu D, et al. : Modelling schizophrenia using human induced pluripotent stem cells. Nature 2011, 473:221–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Papaspyropoulos A, Tsolaki M, Foroglou N, Pantazaki AA: Modeling and Targeting Alzheimer’s Disease With Organoids. Front Pharmacol 2020, 11:396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Mellios N, Feldman DA, Sheridan SD, Ip JPK, Kwok S, Amoah SK, Rosen B, Rodriguez BA, Crawford B, Swaminathan R, et al. : MeCP2-regulated miRNAs control early human neurogenesis through differential effects on ERK and AKT signaling. Mol Psychiatry 2018, 23:1051–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Kathuria A, Lopez-Lengowski K, Vater M, McPhie D, Cohen BM, Karmacharya R: Transcriptome analysis and functional characterization of cerebral organoids in bipolar disorder. [DOI] [PMC free article] [PubMed]
- 104.Bhaduri A, Andrews MG, Mancia Leon W, Jung D, Shin D, Allen D, Jung D, Schmunk G, Haeussler M, Salma J, et al. : Cell stress in cortical organoids impairs molecular subtype specification. Nature 2020, 578:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]


