Abstract
Background
Little is known about the psychological outcomes of germline multigene panel testing, particularly among diverse patients and those with moderate‐risk pathogenic variants (PVs).
Methods
Study participants (N = 1264) were counseled and tested with a 25‐ or 28‐gene panel and completed a 3‐month postresult survey including the Multidimensional Impact of Cancer Risk Assessment (MICRA).
Results
The mean age was 52 years, 80% were female, and 70% had cancer; 45% were non‐Hispanic White, 37% were Hispanic, 10% were Asian, 3% were Black, and 5% had another race/ethnicity. Approximately 28% had a high school education or less, and 23% were non–English‐speaking. The genetic test results were as follows: 7% had a high‐risk PV, 6% had a moderate‐risk PV, 35% had a variant of uncertain significance (VUS), and 52% were negative. Most participants (92%) had a total MICRA score ≤ 38, which corresponded to a mean response of “never,” “rarely,” or only “sometimes” reacting negatively to results. A multivariate analysis found that mean total MICRA scores were significantly higher (more uncertainty/distress) among high‐ and moderate‐risk PV carriers (29.7 and 24.8, respectively) than those with a VUS or negative results (17.4 and 16.1, respectively). Having cancer or less education was associated with a significantly higher total MICRA score; race/ethnicity was not associated with the total MICRA score. High‐ and moderate‐risk PV carriers did not differ significantly from one another in the total MICRA score, uncertainty, distress, or positive experiences.
Conclusions
In a diverse population undergoing genetic counseling and multigene panel testing for hereditary cancer risk, the psychological response corresponded to test results and showed low distress and uncertainty. Further studies are needed to assess patient understanding and subsequent cancer screening among patients from diverse backgrounds.
Lay Summary
Multigene panel tests for hereditary cancer have become widespread despite concerns about adverse psychological reactions among carriers of moderate‐risk pathogenic variants (mutations) and among carriers of variants of uncertain significance.
This large study of an ethnically and economically diverse cohort of patients undergoing panel testing found that 92% “never,” “rarely,” or only “sometimes” reacted negatively to results.
Somewhat higher uncertainty and distress were identified among carriers of high‐ and moderate‐risk pathogenic variants, and lower levels were identified among those with a variant of uncertain significance or a negative result.
Although the psychological response corresponded to risk, reactions to testing were favorable, regardless of results.
Keywords: genetic counseling, genetic techniques, genetic testing, hereditary neoplastic syndromes, psycho‐oncology, psychosocial factors
Short abstract
Multigene panel tests for hereditary cancer have become widespread despite concerns that carriers of moderate‐risk pathogenic variants or uncertain variants could have adverse psychological reactions. This large cohort study of patients undergoing panel testing has found that the psychological response is favorable, regardless of genetic test results.
Introduction
With advances in next‐generation sequencing, hereditary cancer multigene panel testing is becoming widespread, particularly at cancer genetic counseling clinics, where panels have mostly replaced single‐gene or single‐syndrome genetic testing. 1 , 2 Panels offer convenience 3 and potential cost‐effectiveness 4 by allowing for the simultaneous assessment of multiple cancer predisposition genes and syndromes. Using panel tests may reduce the need for future testing as family histories evolve and additional cancer associations with known susceptibility genes are identified over time. Consensus statements and guidelines from professional societies support the use of panels for patients meeting standard genetic testing criteria. 5 , 6 , 7 , 8 However, panel testing poses challenges as well, including the identification of variants of uncertain significance (VUSs) as well as pathogenic variants (PVs) in off‐target genes or in moderate‐risk genes for which the clinical impact is less fully defined.
Moderate‐risk genes are defined as those that confer a 2‐ to 5‐fold relative risk of cancer when altered by a PV and include variants within genes such as ATM and CHEK2. 2 Identification of PVs in moderate‐risk genes can be clinically significant, with implications for patient screening or preventive measures beyond that which would have been indicated by a family history alone. 9 , 10 Associated cancer risks and age‐specific penetrance are less well understood for moderate‐risk genes than high‐risk genes, and screening guidelines for moderate‐risk variants rely heavily on expert opinion. 2 , 11 Some experts have expressed concern that testing moderate‐risk genes may lead to patient confusion, uncertainty, and psychological distress. 12 , 13 , 14
Because of these concerns, further studies of the psychosocial and behavioral impact of panel testing and the identification of moderate‐risk PVs are needed to inform optimal consent models and counseling approaches. 14 One small study from Spain comparing carriers of high‐ and moderate‐risk PVs found higher distress and uncertainty in moderate‐risk carriers than high‐risk carriers. 15 Although other studies have measured psychological responses among patients undergoing cancer panel testing, they were not designed to measure outcomes specifically in moderate‐risk PV carriers. 16 , 17 , 18 , 19 , 20 Additionally, individuals of diverse ethnic or socioeconomic backgrounds have not been well represented in previous studies. 16 , 17 , 19
We conducted a multicenter, prospective study of a hereditary cancer panel in a diverse cohort of 1264 participants responding to a survey 3 months after disclosure of the results. 21 We hypothesized that panel testing would be received favorably overall but that high‐risk PV carriers would have higher levels of distress than other participants, whereas moderate‐risk PV carriers would have higher levels of uncertainty than other participants. The results of our study will inform cancer genetic counseling and testing strategies.
Materials and Methods
Participants
We enrolled 2000 patients undergoing genetic counseling and multigene panel testing between July 2014 and November 2016. Participants were recruited at 3 cancer genetics clinics: the USC Norris Comprehensive Cancer Center and Hospital (USC Norris), the Los Angeles County + USC Medical Center (LAC+USC), and the Stanford University Cancer Institute (Stanford; Fig. 1). Patients were eligible if they had a ≥2.5% probability of a PV based on a probability model such as BRCAPRO, PENN II, or PREMM 1,2,6 (Prediction Model for MLH1,MSH2, or MSH6) or met National Comprehensive Cancer Network genetic testing guidelines. 2 Patients were excluded if they had previous testing for any genes on the panel or if there was a known PV in the family and no other indication for genetic testing. The study was approved by the institutional review boards of the University of Southern California and Stanford. The methods of this study have been previously reported in detail. 21
Figure 1.
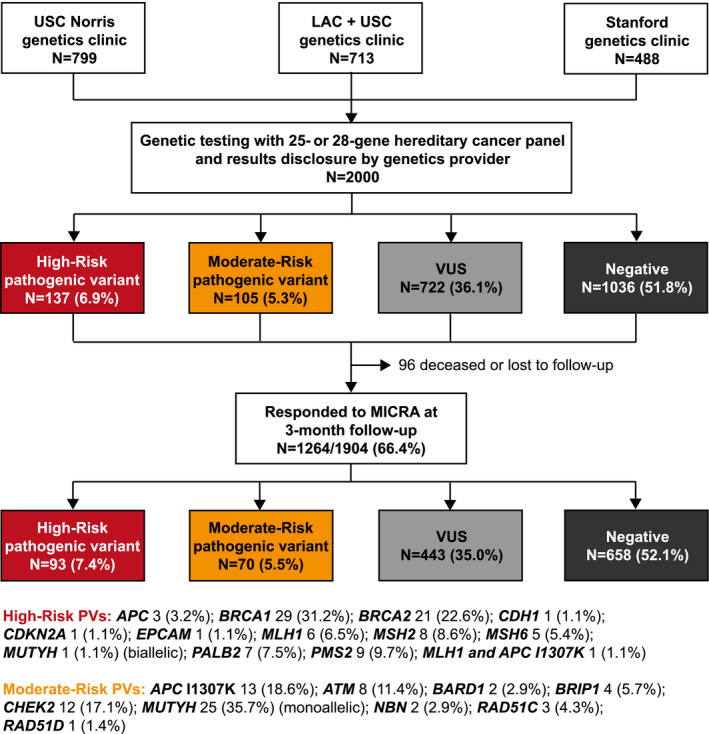
Flow diagram depicting the recruitment of participants and genetic test results among the 1264 participants completing 3 months of follow‐up. LAC+USC indicates Los Angeles County + USC Medical Center; MICRA, Multidimensional Impact of Cancer Risk Assessment; PV, pathogenic variant; Stanford, Stanford University Cancer Institute; USC Norris, USC Norris Comprehensive Cancer Center and Hospital; VUS, variant of unknown significance.
Study Procedures
All participants underwent pre‐test counseling with a board‐certified genetic counselor (CGC) or an advanced practice genetics nurse practitioner (APNG), and 688 patients (34%) also met with a physician specializing in cancer genetics. Participants completed a baseline questionnaire at the time of enrollment. Testing was performed with a 25‐ or 28‐gene panel (Myriad Genetic Laboratories, Inc) that included the following: APC, ATM, BARD1, BMPR1A, BRCA1, BRCA2, BRIP1, CDH1, CDK4, CDKN2A (p14ARF and p16INK4a), CHEK2, EPCAM, MLH1, MSH2, MSH6, MUTYH, NBN, PALB2, PMS2, PTEN, RAD51C, RAD51D, SMAD4, STK11, and TP53. In July 2016, GREM1, POLD1, and POLE were added.
Participants at LAC+USC received results during an in‐person session with a CGC. Participants at USC Norris and Stanford were notified by phone, and those with a positive result were invited for an in‐person appointment with a CGC or APNG and a physician. A follow‐up questionnaire was administered 3 months after disclosure by mail, online with Web Progeny (version 10), or in person.
Measures
The Multidimensional Impact of Cancer Risk Assessment (MICRA) was administered to measure distress, uncertainty, and positive experiences after the receipt of genetic test results. 22 This tool has been used extensively in cancer genetics research studies 18 , 23 , 24 , 25 and addresses concerns specific to the testing experience that may not be captured by general distress measures. 22 , 25 MICRA is a 25‐item assessment regarding feelings that the individual may have experienced in the past week on a Likert scale (never [0], rarely [1], sometimes [3], or often [5]). The distress subscale (6 items; range, 0‐30) evaluates sadness, feeling upset, guilt, anxiety, problems with enjoying life, and loss of control. The uncertainty subscale (9 items; range, 0‐45) evaluates uncertainty about cancer risk, difficulty in making decisions, frustration, cancer worry, effects on work and family, concern about insurance, family communication, and family conflict. The positive experience subscale (4 items; range, 0‐20) evaluates relief, happiness, and family communication and is scored in reverse so that that the most positive score is 0 and the least positive score is 20. A total MICRA score is calculated as the sum of the distress, uncertainty, and positive subscale scores (range, 0‐95). The remaining 6 MICRA questions are not scored and include “understanding clearly my choices for cancer prevention or early detection” and “feeling regret about getting my test results.”
Because there are no recognized thresholds for a “high” MICRA level, 25 we developed a green‐amber‐red scale corresponding to the average response to a question to enable a qualitative evaluation of the MICRA score. The cool dark green corresponds to an average reply of “never” (0) to “rarely” (1) experiencing a negative emotion, light green and amber correspond to “rarely” (1) to “sometimes” (3), and hot red corresponds to “sometimes” (3) to “often” (5). Each participant's MICRA score and subscore are plotted on a graph, which shows a higher density of participants with a more darkly shaded mark.
Determination of Test Result Group
Participants were grouped according to genetic test results. PV and likely pathogenic carriers were placed in the high‐ and moderate‐risk groups according to the level of the associated cancer risk by gene as shown in Figure 1. 26 One participant had both an MLH1 PV and an APC I1307K variant and was placed in the high‐risk group. The VUS group consisted of participants with 1 or more VUSs and no PVs or likely PVs. The negative group included participants with no reported variants (only benign or likely benign findings).
Statistical Analysis
Differences in MICRA scores among result categories were tested through multivariate negative binomial regression using a natural‐log link function. This method was chosen to account for highly skewed distributions with overrepresentation of 0 score responses. The models were adjusted for potential confounders, including sex, race/ethnicity, age in years, cancer affected status, clinic site, education level, and language ability. Unscored MICRA questions were analyzed by pairwise trend tests and adjusted for multiple comparisons by a false discovery rate correction. P values less than .05 were considered significant for all statistical tests. All tests were conducted with SAS software (version 9.4) and R software (version 3.6.1 or later).
A post hoc power analysis of mean total MICRA differences was conducted by simulation. With variance estimates and sample sizes from the study, a sample for 2 genetic test result groups was generated, and significance was determined. This procedure was replicated 10,000 times, and the proportion of times that significance was concluded was recorded. We recorded power above 99.9% to detect a difference of 4 between the negative and VUS risk groups and more than 80% power to detect a difference of 2.1. We also recorded power of approximately 72% to detect a difference of 5.9, as was seen in the data, between high‐ and moderate‐risk PV groups. A similar process was conducted to determine power to detect differences between race/ethnic groups. We recorded a high power to detect differences between non‐Hispanic Whites and Hispanics and between non‐Hispanic Whites and Asians (99.9% and 92%, respectively); the power was between 29% and 75% for all other comparisons.
Results
Study Population and Genetic Test Results
The primary study included 2000 cancer genetic counseling participants undergoing hereditary cancer panel testing at USC Norris, LAC+USC, and Stanford. The overall response rate to the 3‐month follow‐up survey was 70.2% (1336 of 1904 possible respondents); this included 66.4% (1264 of 1904) who answered at least 1 MICRA question. One or more MICRA subscores could be generated for 99.0% (1251 of 1264), and a total MICRA score was calculated for 95.3% of the respondents (1205 of 1264).
Responders and nonresponders did not differ significantly by genetic test results. However, the response rate was significantly higher among females; older individuals; and those who were more educated, were English‐speaking, were non‐Hispanic White, and had no personal history of cancer. The response rate was highest among Stanford participants (71% vs 67% at USC Norris and 63% at LAC+USC; Supporting Table 1).
Among the 1264 respondents in this analysis, 93 (7.4%) had a high‐risk PV, 70 (5.5%) had a moderate‐risk PV, 443 (35.0%) had a VUS, and 658 (52.1%) had a negative result (Fig. 1).
The majority of the responders (79.8%) were female, and the mean age was 52.2 ± 13.6 years (range, 17‐92 years). The responders represented a diverse population; 37.3% were Hispanic, 27.7% had a high school education or less, 22.7% were non–English‐speaking, and an additional 28.2% spoke English and another language fluently. Most of the study population (69.9%) had a cancer history, and some had more than 1 cancer diagnosis; 35.4% of the sample had a history of breast cancer, and 13.8% had a history of colorectal cancer. The demographic characteristics of the responders are shown in Table 1, and responders are compared with nonresponders in Supporting Table 1.
TABLE 1.
Demographics and Characteristics for Patients Who Completed at Least 1 MICRA Survey Question (N = 1264)
| Characteristic | Value | |
|---|---|---|
| Age at testing, mean (SD), y | 52.2 (13.6) | |
| Sex, No. (%) | Female | 1009 (79.8) |
| Male | 255 (20.2) | |
| Race/ethnicity, No. (%) | Non‐Hispanic White | 567 (44.9) |
| Hispanic | 472 (37.3) | |
| Asian | 123 (9.7) | |
| Black or African American | 40 (3.2) | |
| Multiple/other a | 62 (4.9) | |
| Clinic site, No. (%) | USC Norris | 506 (40.0) |
| LAC+USC | 415 (32.8) | |
| Stanford | 343 (27.1) | |
| Education, No. (%) | High school or less | 350 (27.7) |
| Trade/vocational school | 44 (3.5) | |
| Some college | 231 (18.3) | |
| College degree or more | 573 (45.3) | |
| Missing | 66 (5.2) | |
| Language ability, No. (%) | English only | 621 (49.1) |
| English and another language | 356 (28.2) | |
| Another language only | 287 (22.7) | |
| Cancer history, No. (%) | Affected | 884 (69.9) |
| Unaffected | 380 (30.1) | |
| Cancer site, No. (%) b | Breast or DCIS | 448 (35.4) |
| Colon/rectum | 175 (13.8) | |
| Ovary | 71 (5.6) | |
| Uterus | 44 (3.5) | |
| Gastric | 27 (2.1) | |
| Pancreas | 23 (1.8) | |
| Prostate | 23 (1.8) | |
| Other | 168 (13.3) | |
| Genetic test result, No. (%) | High‐risk pathogenic variant | 93 (7.4) |
| Moderate‐risk pathogenic variant | 70 (5.5) | |
| VUS | 443 (35.0) | |
| Negative | 658 (52.1) | |
Abbreviations: DCIS, ductal carcinoma in situ; LAC+USC, Los Angeles County + USC Medical Center; MICRA, Multidimensional Impact of Cancer Risk Assessment; Stanford, Stanford University Cancer Institute; USC Norris, USC Norris Comprehensive Cancer Center and Hospital; VUS, variant of uncertain significance.
Multiple/other includes 49 patients with more than 1 race, 2 Alaskan Natives, 4 Native Hawaiians or Pacific Islanders, 2 Whites with an unspecified Hispanic status, and 5 patients of other race/ethnicity.
The groups are not exclusive; patients may have had more than 1 cancer.
Psychosocial Impact
We calculated each participant's total MICRA score, which is the sum of all negative feelings (uncertainty, distress, and reverse‐scored positive experiences; Fig. 2). The high‐risk PV group had the highest total MICRA scores (median, 27), and it was followed by the moderate‐risk PV group (median, 23), the VUS group (median, 16), and the negative group (median, 15; Fig. 2 and Table 2). As shown in Figure 2, participants with a negative or VUS result had a median MICRA score in the cool green range, which corresponded to “never” or “rarely” experiencing negative feelings. The moderate‐ and high‐risk PV groups had a median MICRA score in the light green range, which corresponded to “rarely” to “sometimes” experiencing negative feelings. The proportion of all participants with a total MICRA score in the green range (≤38) was 92.4%, and they included 74.4% of the participants with a high‐risk PV, 82.4% of those with a moderate‐risk PV, 94.1% of the VUS group, and 94.9% of the negative group. The MICRA uncertainty, distress, and positive experience subscores are also summarized in Figure 3 and Table 2, and they all followed the same trend as the total MICRA score with decreasing scores in the same order by result category.
Figure 2.
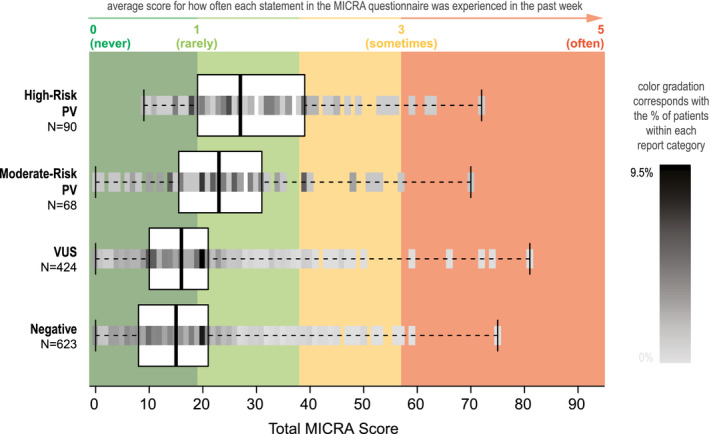
Distribution of total MICRA scores according to genetic test results. The median total MICRA score (thick vertical line) and the interquartile range (box) are shown for each genetic test result category, with a mark of varying shading gradation representing the percentage of patients with each MICRA score across the continuum of possible scores. Red, amber, and green coloring corresponds to a participant's average response to MICRA questions on a scale of 1 to 5. For example, because there are 19 questions on the MICRA scale, a total MICRA score of 19 corresponds to an average response of 1 on the MICRA (or “rarely” experiencing a negative emotion). MICRA indicates Multidimensional Impact of Cancer Risk Assessment; PV, pathogenic variant; VUS, variant of unknown significance.
TABLE 2.
Total and Subscale MICRA Scores According to Genetic Test Results: Multivariate Analysis
| Total MICRA Scores (0‐95) | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Result Category | No. | Mean (SD) | Median | Range | Estimate (Exponentiated) | Wald 95% CI (Exponentiated) | P |
| Negative | 623 | 16.1 (10.97) | 15 | 0‐75 | Reference | — | — |
| VUS | 424 | 17.4 (11.61) | 16 | 0‐81 | 0.075 (1.078) | –0.015 to 0.165 (0.985 to 1.179) | .103 |
| Moderate‐risk PV | 68 | 24.8 (14.03) | 23 | 0‐70 | 0.445 (1.560) | 0.270 to 0.620 (1.310 to 1.859) | <.001 |
| High‐risk PV | 90 | 29.7 (13.5) | 27 | 9‐72 | 0.617 (1.853) | 0.464 to 0.769 (1.590 to 2.158) | <.001 |
| High vs moderate PV | — | — | .125 | ||||
| MICRA Uncertainty Scores (0‐45) | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Result Category | No. | Mean (SD) | Median | Range | Estimate (Exponentiated) | Wald 95% CI (Exponentiated) | P |
| Negative | 637 | 6.4 (7.18) | 4 | 0‐35 | Reference | — | — |
| VUS | 429 | 7.4 (7.89) | 5 | 0‐43 | 0.155 (1.168) | 0.004 to 0.307 (1.004 to 1.359) | .045 |
| Moderate‐risk PV | 69 | 10.3 (8.6) | 10 | 0‐34 | 0.480 (1.616) | 0.184 to 0.775 (1.202 to 2.171) | .002 |
| High‐risk PV | 92 | 12.2 (8.82) | 10.5 | 0‐41 | 0.656 (1.927) | 0.399 to 0.912 (1.490 to 2.489) | <.001 |
| High vs moderate PV | — | — | .351 | ||||
| MICRA Distress Scores (0‐30) | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Result Category | No. | Mean (SD) | Median | Range | Estimate (Exponentiated) | Wald 95% CI (Exponentiated) | P |
| Negative | 648 | 1.7 (3.58) | 0 | 0‐28 | Reference | — | — |
| VUS | 437 | 2.1 (4.14) | 0 | 0‐30 | 0.144 (1.155) | –0.095 to 0.382 (0.909 to 1.465) | .238 |
| Moderate‐risk PV | 70 | 5 (5.87) | 4 | 0‐26 | 1.327 (3.770) | 0.882 to 1.773 (2.416 to 5.888) | <.001 |
| High‐risk PV | 93 | 6.8 (6.09) | 5 | 0‐26 | 1.486 (4.419) | 1.097 to 1.876 (2.995 to 6.527) | <.001 |
| High vs moderate PV | — | — | .574 | ||||
| MICRA Positive Experience Scores (0‐20) | Multivariate Analysis | ||||||
|---|---|---|---|---|---|---|---|
| Result Category | No. | Mean (SD) | Median | Range | Estimate (Exponentiated) | Wald 95% CI (Exponentiated) | P |
| Negative | 629 | 7.9 (6.45) | 8 | 0‐20 | Reference | — | — |
| VUS | 426 | 8.1 (6.36) | 8 | 0‐20 | 0.038 (1.039) | –0.093 to 0.170 (0.911 to 1.185) | .57 |
| Moderate‐risk PV | 68 | 9.5 (5.35) | 10 | 0‐20 | 0.220 (1.246) | –0.039 to 0.479 (0.962 to 1.614) | .096 |
| High‐risk PV | 90 | 10.8 (4.77) | 11 | 0‐20 | 0.394 (1.483) | 0.166 to 0.621 (1.181 to 1.861) | <.001 |
| High vs moderate PV | — | — | .300 | ||||
Abbreviations: MICRA, Multidimensional Impact of Cancer Risk Assessment; PV, pathogenic variant; VUS, variant of unknown significance.
Figure 3.
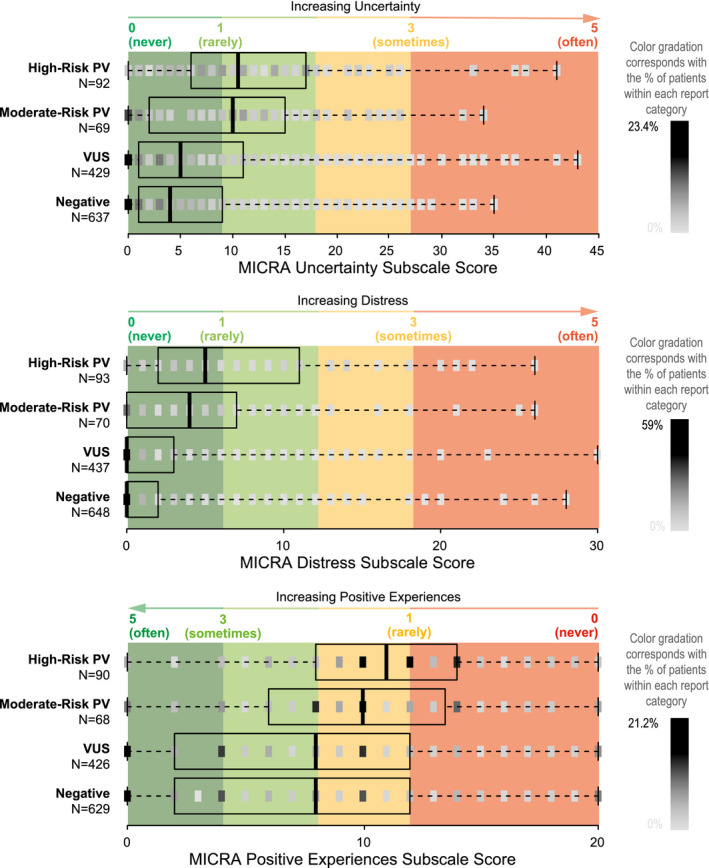
Distribution of scores for the uncertainty, distress, and positive experience subscales according to the genetic test result category. The median (thick vertical line) and the interquartile range (box) are shown, with a mark of varying shading gradation representing the percentage of patients with each MICRA score across the continuum of possible scores. Red, amber, and green coloring corresponds to a participant's average response to MICRA questions on a scale of 1 to 5. For example, because there are 9 questions on the uncertainty scale, a total uncertainty score of 9 corresponds to an average response of 1 (or “rarely” experiencing uncertainty). MICRA indicates Multidimensional Impact of Cancer Risk Assessment; PV, pathogenic variant; VUS, variant of unknown significance.
Multivariate modeling was performed to address the effects of possible confounders of MICRA scores, including age, sex, race/ethnicity, clinic site, education level, language ability, cancer affected status, and genetic test results. When we controlled for these factors, total MICRA scores for high‐ and moderate‐risk PV carriers were significantly higher than scores for those with negative results (the reference group). The model parameter estimates exponentiated to an expected ratio of 1.85 (95% CI, 1.590‐2.158; P < .001) between high‐risk PV carriers and negative participants and to an expected ratio of 1.56 (95% CI, 1.310‐1.859; P < .001) between moderate‐risk PV carriers and negative participants (Table 2).
After multivariate adjustments, we found that those affected with cancer had a higher total MICRA score than those without cancer (expected ratio, 1.269; 95% CI, 1.155‐1.392; P < .001). Those with at least a college education had lower MICRA scores than those with a high school education or less (expected ratio, 0.852; 95% CI, 0.748‐0.969; P = .015; Table 3). Other factors such as sex, language spoken, clinic site, and race/ethnicity did not significantly influence the total MICRA score.
TABLE 3.
Negative Binomial Multivariate Analysis of Total MICRA Scores
| Variable | Category | Estimate (Exponentiated) | Wald 95% CI (Exponentiated CI) | P |
|---|---|---|---|---|
| Age | –0.005 (0.995) | –0.008 to –0.002 (0.992 to 0.998) | .004 | |
| Sex | Male | Reference | — | — |
| Female | –0.098 (0.907) | –0.198 to 0.003 (0.820 to 1.003) | .058 | |
| Race/ethnicity | Non‐Hispanic White | Reference | — | — |
| Asian | 0.110 (1.116) | –0.045 to 0.264 (0.956 to 1.302) | .164 | |
| Black or African American | 0.145 (1.156) | –0.093 to 0.383 (0.911 to 1.467) | .233 | |
| Hispanic | –0.005 (0.995) | –0.148 to 0.138 (0.862 to 1.148) | .943 | |
| Study site | USC Norris | Reference | — | — |
| LAC+USC | –0.066 (0.936) | –0.199 to 0.068 (0.820 to 1.070) | .334 | |
| Stanford | 0.056 (1.058) | –0.047 to 0.159 (0.954 to 1.172) | .286 | |
| Education | High school or less | Reference | — | — |
| Trade or vocational school | –0.126 (0.882) | –0.358 to 0.106 (0.699 to 1.112) | .288 | |
| Some college | –0.159 (0.853) | –0.294 to –0.025 (0.745 to 0.975) | .020 | |
| College degree or more | –0.160 (0.852) | –0.290 to –0.031 (0.748 to 0.969) | .015 | |
| Language | English Only | Reference | — | — |
| And English | 0.033 (1.034) | –0.086 to 0.151 (0.918 to 1.163) | .589 | |
| No English | 0.096 (1.101) | –0.074 to 0.265 (0.929 to 1.303) | .268 | |
| Cancer history | Not affected | Reference | — | — |
| Affected | 0.238 (1.269) | 0.144 to 0.331 (1.155 to 1.392) | <.001 | |
| Test result | Negative | Reference | — | — |
| VUS | 0.075 (1.078) | –0.015 to 0.165 (0.985 to 1.179) | .103 | |
| Moderate‐risk pathogenic variant | 0.445 (1.560) | 0.270 to 0.620 (1.310 to 1.859) | <.001 a | |
| High‐risk pathogenic variant | 0.617 (1.853) | 0.464 to 0.769 (1.590 to 2.158) | <.001 a |
Abbreviations: LAC+USC, Los Angeles County + USC Medical Center; MICRA, Multidimensional Impact of Cancer Risk Assessment; Stanford, Stanford University Cancer Institute; USC Norris, USC Norris Comprehensive Cancer Center and Hospital; VUS, variant of uncertain significance..
Estimates are on a log scale, and results greater than 0 represent an increase with respect to the reference. Larger estimates represent a larger increase in MICRA with respect to the reference but are not on a linear scale.
VUS versus moderate risk, P < .001; VUS versus high risk, P < .001; and high risk versus moderate risk, P = .125.
MICRA subscale scores were compared in the same multivariate model. The high‐risk PV and moderate‐risk PV groups had higher scores than the negative group for uncertainty (P < .001 and P = .002) and distress (P < .001 and P < .001), and the high‐risk group had higher positive experience scores than the negative group; this meant that they had less of a positive experience (P < .001; Table 2).
Moderate‐risk PV and high‐risk PV groups were not significantly different from each other in their total MICRA scores (P = .125). High‐ and moderate‐risk groups also did not differ significantly from each other in uncertainty (P = .351), distress (P = .574), or positive experiences (P = .300; Table 2).
The VUS group showed no significant differences from the negative group in total MICRA scores in the multivariate model with an expected ratio of 1.078 (95% CI, 0.985‐1.179; P = .103; Table 2). The median uncertainty score was 5 in the VUS group and 4 in the negative group on a 45‐point scale; this was a modest but statistically significant difference with an expected ratio of 1.168 (95% CI, 1.004‐1.359; P = .045). The VUS and negative groups did not differ significantly in their levels of distress (P = .238) and positive experiences (P = .570).
Patient Perceptions
Participant responses to unscored MICRA questions are shown in Figure 4. Most participants in all 4 groups never regretted getting their genetic test results (Fig. 4A). There were no significant differences for this question by result category.
Figure 4.
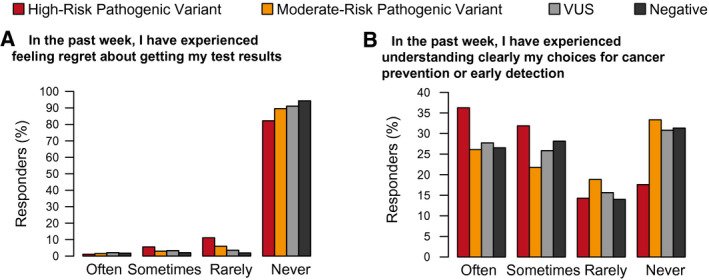
Responses to 2 questions that are not included in the Multidimensional Impact of Cancer Risk Assessment subscales. VUS indicates variant of unknown significance.
Reported levels of understanding of cancer screening and prevention choices differed by group, with the high‐risk PV group having the highest level of understanding in comparison with the moderate‐risk PV, VUS, and negative groups (P = .025, P = .024, and P = .024, respectively; data not shown). Notably, 51.5% of the individuals in the moderate‐risk PV group indicated that they rarely or never understood their screening/prevention choices, whereas 31.2% in the high‐risk PV group did (Fig. 4B). There was no significant difference in understanding between the VUS and negative groups (P = .941).
Discussion
This study reports psychosocial outcomes among ethnically and socioeconomically diverse individuals undergoing genetic counseling and receiving genetic panel test results. The majority of the participants had low levels of uncertainty and distress. Because there are no clinically recognized cutoffs associated with the MICRA measure, 24 , 25 we display our results with a red‐amber‐green color scale. We demonstrate that a substantial majority of the individuals were in the green zone, where they “rarely” or “never” experienced negative emotions associated with genetic testing.
Participants carrying a high‐ or moderate‐risk PV had significantly higher uncertainty, distress, and total MICRA scores and significantly fewer positive experiences than participants with a VUS or negative result, and this supports the hypothesis of higher distress and uncertainty among PV carriers. Previous studies have reported similar findings, with high‐risk PV carriers having higher total MICRA scores 18 , 22 , 24 , 25 as well as higher MICRA subscale scores of distress, 18 , 22 , 24 , 25 positive experiences, 22 , 25 and uncertainty 22 , 24 , 25 than those with negative test results. Although scores varied somewhat in previous study populations, the overall trends remained the same between single‐gene and panel testing, and this suggests that panel testing does not elicit a different response. This is consistent with our prior work showing no increase in cancer‐related worry associated with testing more genes. 27
Our study is one of the first to demonstrate that moderate‐risk PV carriers experience results similarly to those with a high‐risk PV without higher levels of uncertainty. The high‐ and moderate‐risk PV groups did not differ significantly in total or subset MICRA scores; this is contrary to our stated hypothesis that moderate‐risk carriers would have higher uncertainty than high‐risk carriers. One notable difference was that the high‐risk group had endorsed greater understanding of options for cancer screening and prevention than the moderate‐risk group, and this perhaps reflects that guidelines for lower penetrant genes were less established. Knowledge and familiarity with moderate‐penetrance genes grew during and after the study period, and this led to the incorporation of screening recommendations into the National Comprehensive Cancer Network guidelines. 28 , 29 These recommendations guide clinicians in more straightforward counseling, which may improve care for moderate‐risk PV carriers.
One concern raised about multigene panels is the high VUS rate and the possibility of associated psychological distress. Lumish et al 18 found that participants with a VUS had somewhat higher MICRA distress scores. Bradbury et al 16 found that patients with a VUS had a decrease in uncertainty and a slight increase in distress from baseline pretest levels to the 6‐month follow‐up. Reassuringly, we found that total MICRA scores did not differ between participants with a VUS and those with negative results. We did identify a slight increase in the uncertainty score among participants in the VUS group versus the negative‐result group, which could have been driven by the inherent uncertainty of a VUS.
The considerable strengths of this study include a large, racially and socioeconomically diverse cohort. Language and race/ethnicity were not significantly associated with MICRA scores; however, a lower education level was associated with higher MICRA scores, and this suggests that the educational level should be considered in formulating a counseling strategy. Lumish et al 18 found no differences in MICRA scores among Hispanics or Asians, but Blacks did have higher distress levels. Our study did not find a significant difference in total MICRA scores between Asian, Hispanic, and non‐Hispanic White participants, but Black participants were less well represented; this limited our ability to assess their experience. As such, this topic certainly deserves further exploration.
Additionally, our findings showed that patients affected by cancer had higher total MICRA scores than unaffected patients; this differed from findings by Lumish et al, 18 who found higher distress among unaffected patients undergoing panel testing. Future studies are warranted to understand how panel testing may affect individuals without a cancer diagnosis as well as patients with cancer at various points in their diagnosis, treatment, and survivorship course.
All participants had genetic counseling and testing at cancer genetics clinics at public and private academic hospitals with cancer genetics providers. The results do not reflect testing in community settings, testing without pretest and posttest counseling, or direct‐to‐consumer testing. 7 , 30 The perspectives of nonresponders to the 3‐month follow‐up could not be included in this analysis, and those individuals were more likely to be non‐White and have a lower level of education. As mentioned previously, Black participants were fewer in number; this reflected regional demographics but limited our ability to report on the specifics of these participants' experience with panel testing. Also, a post hoc power analysis revealed high power to detect differences in MICRA scores between negative and VUS groups but less power to detect differences between high‐ and moderate‐PV groups. Further studies of larger cohorts of PV carriers will be important to extend this study's findings.
This study offers novel, significant insights into psychological outcomes after multigene panel testing and helps to identify concerns of patients undergoing panel testing. The overall reaction to panel testing corresponded to test results and did not show concerning levels of uncertainty or distress. Although PV carriers did experience more psychological distress than those with VUS or negative results, the absolute level of distress and uncertainty was low in all respondents. Moderate‐risk PV carriers had reactions similar to those of high‐risk PV carriers, and the uncertainty surrounding moderate‐risk PVs was not significantly higher. Patients' educational level should be considered when one is approaching panel testing and strategies for effective counseling used to meet the psychological needs that may arise. Results from this analysis will help to identify concerns of patients undergoing panel testing and support their needs.
Funding Support
We acknowledge the following sources of research support: Myriad Genetics, the National Institutes of Health (grants KL2 TR000131, NCI R35 CA197461, R01 CA197350, and P30 CA14089), the Anton B. Burg Foundation, the Jane and Kris Popovich Chair in Cancer Research, and a gift from Daniel and Maryann Fong.
Conflict of Interest Disclosures
John Kidd and Krystal Brown are employed by Myriad Genetics. Uri Ladabaum is an advisor to Universal Dx and Lean Medical and is a consultant to Medtronic, Guardant, Check‐Cap, Leerink, Freenome, Motus, and Clinical Genomics. James M. Ford has research funding from Genentech, AstraZeneca, and Puma Biotechnology. Stephen B. Gruber has an ownership interest in Brogent International LLC and has received research funding from Myriad Genetics. Allison W. Kurian reports relationships with Ambry Genetics, Myriad Genetics, Color Genomics, GeneDx, BioReference, Invitae, and Genentech outside the submitted work. Gregory E. Idos has received research funding from Myriad Genetics. The other authors made no disclosures.
Author Contributions
Julie O. Culver: Conceptualization, data curation, formal analysis, investigation, methodology, project administration, supervision, validation, visualization, writing–original draft, and writing–review and editing. Charité N. Ricker: Conceptualization, methodology, data curation, writing–original draft, and writing–review and editing. Joseph Bonner: Conceptualization, methodology, formal analysis, investigation, visualization, writing–original draft, and writing–review and editing. John Kidd: Conceptualization, methodology, data curation, formal analysis, validation, writing–original draft, and writing–review and editing. Duveen Sturgeon: Data curation and writing–review and editing. Rachel Hodan: Data curation and writing–review and editing. Kerry Kingham: Data curation and writing–review and editing. Katrina Lowstuter: Data curation and writing–review and editing. Nicolette M. Chun: Data curation and writing–review and editing. Alexandra Lebensohn: Data curation and writing–review and editing. Courtney Rowe‐Teeter: Data curation and writing–review and editing. Peter Levonian: Data curation and writing–review and editing. Katlyn Partynski: Data curation and writing–review and editing. Karlena Lara‐Otero: Data curation and writing–review and editing. Christine Hong: Methodology, project administration, data curation, and writing–review and editing. Jennifer Morales Pichardo: Project administration, data curation, and writing–review and editing. Meredith A. Mills: Project administration, data curation, and writing–review and editing. Krystal Brown: Conceptualization, methodology, data curation, visualization, and writing–review and editing. Caryn Lerman: Conceptualization, methodology, data interpretation, writing–original draft, writing–review and editing, and funding acquisition. Uri Ladabaum: Data curation and writing–review and editing. Kevin J. McDonnell: Data curation and writing–review and editing. James M. Ford: Data curation, writing–review and editing, and funding acquisition. Stephen B. Gruber: Conceptualization, methodology, data curation, data interpretation, writing–original draft, writing–review and editing, and funding acquisition. Allison W. Kurian: Conceptualization, methodology, data curation, data interpretation, writing–original draft, writing–review and editing, and funding acquisition. Gregory E. Idos: Conceptualization, methodology, data curation, data interpretation, writing–original draft, writing–review and editing, and funding acquisition.
Supporting information
Table S1
Culver JO, Ricker CN, Bonner J, Kidd J, Sturgeon D, Hodan R, Kingham K, Lowstuter K, Chun NM, Lebensohn A, Rowe‐Teeter C, Levonian P, Partynski K, Lara‐Otero K, Hong C, Morales Pichardo J, Mills MA, Brown K, Lerman C, Ladabaum U, McDonnell KJ, Ford JM, Gruber SB, Kurian AW, Idos GE. Psychosocial outcomes following germline multigene panel testing in an ethnically and economically diverse cohort of patients. Cancer.2021. 10.1002/cncr.33357
We thank Blanca Ovalle, Serina Ovalle, and Angelica Mora for their tremendous help with the recruitment and observation of study participants at the Los Angeles County + USC Medical Center. We also thank Cindy Shu Yi Ma for her assistance with data verification at the Stanford University Cancer Institute.
Contributor Information
Julie O. Culver, Email: jculver@med.usc.edu.
Gregory E. Idos, Email: gidos@coh.org.
References
- 1. Kurian AW, Ward KC, Hamilton AS, et al. Uptake, results, and outcomes of germline multiple‐gene sequencing after diagnosis of breast cancer. JAMA Oncol. 2018;4:1066‐1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Daly MB, Pilarski R, Yurgelun MB, et al. NCCN Guidelines Insights: Genetic/Familial High‐Risk Assessment: Breast, Ovarian, and Pancreatic, Version 1.2020. J Natl Compr Canc Netw. 2020;18:380‐391. [DOI] [PubMed] [Google Scholar]
- 3. Ricker C, Culver JO, Lowstuter K, et al. Increased yield of actionable mutations using multi‐gene panels to assess hereditary cancer susceptibility in an ethnically diverse clinical cohort. Cancer Genet. 2016;209:130‐137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Sun L, Brentnall A, Patel S, et al. A cost‐effectiveness analysis of multigene testing for all patients with breast cancer. JAMA Oncol. 2019;5:1718‐1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Heald B, Hampel H, Church J, et al. Collaborative Group of the Americas on Inherited Gastrointestinal Cancer position statement on multigene panel testing for patients with colorectal cancer and/or polyposis. Fam Cancer. 2020;19:223‐239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Konstantinopoulos PA, Norquist B, Lacchetti C, et al. Germline and somatic tumor testing in epithelial ovarian cancer: ASCO guideline. J Clin Oncol. 2020;38:1222‐1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Manahan ER, Kuerer HM, Sebastian M, et al. Consensus guidelines on genetic testing for hereditary breast cancer from the American Society of Breast Surgeons. Ann Surg Oncol. 2019;26:3025‐3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. American College of Obstetricians and Gynecologists . Hereditary cancer syndromes and risk assessment, committee opinion, number 793. Accessed August 29, 2020. https://www.acog.org/clinical/clinical‐guidance/committee‐opinion/articles/2019/12/hereditary‐cancer‐syndromes‐and‐risk‐assessment
- 9. Desmond A, Kurian AW, Gabree M, et al. Clinical actionability of multigene panel testing for hereditary breast and ovarian cancer risk assessment. JAMA Oncol. 2015;1:943‐951. [DOI] [PubMed] [Google Scholar]
- 10. Vysotskaia V, Kaseniit KE, Bucheit L, Ready K, Price K, Johansen Taber K. Clinical utility of hereditary cancer panel testing: impact of PALB2, ATM, CHEK2, NBN, BRIP1, RAD51C, and RAD51D results on patient management and adherence to provider recommendations. Cancer. 2020;126:549‐558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tung N, Domchek SM, Stadler Z, et al. Counselling framework for moderate‐penetrance cancer‐susceptibility mutations. Nat Rev Clin Oncol. 2016;13:581‐588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cragun D, Radford C, Dolinsky JS, Caldwell M, Chao E, Pal T. Panel‐based testing for inherited colorectal cancer: a descriptive study of clinical testing performed by a US laboratory. Clin Genet. 2014;86:510‐520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Domchek SM, Bradbury A, Garber JE, Offit K, Robson ME. Multiplex genetic testing for cancer susceptibility: out on the high wire without a net? J Clin Oncol. 2013;31:1267‐1270. [DOI] [PubMed] [Google Scholar]
- 14. Hamilton JG, Robson ME. Psychosocial effects of multigene panel testing in the context of cancer genomics. Hastings Cent Rep. 2019;49(suppl 1):S44‐S52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esteban I, Vilaro M, Adrover E, et al. Psychological impact of multigene cancer panel testing in patients with a clinical suspicion of hereditary cancer across Spain. Psychooncology. 2018;27:1530‐1537. [DOI] [PubMed] [Google Scholar]
- 16. Bradbury AR, Egleston BL, Patrick‐Miller LJ, et al. Longitudinal outcomes with cancer multigene panel testing in previously tested BRCA1/2 negative patients. Clin Genet. 2020;97:601‐609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bradbury AR, Patrick‐Miller LJ, Egleston BL, et al. Patient feedback and early outcome data with a novel tiered‐binned model for multiplex breast cancer susceptibility testing. Genet Med. 2016;18:25‐33. [DOI] [PubMed] [Google Scholar]
- 18. Lumish HS, Steinfeld H, Koval C, et al. Impact of panel gene testing for hereditary breast and ovarian cancer on patients. J Genet Couns. 2017;26:1116‐1129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sie AS, Prins JB, van Zelst‐Stams WA, Veltman JA, Feenstra I, Hoogerbrugge N. Patient experiences with gene panels based on exome sequencing in clinical diagnostics: high acceptance and low distress. Clin Genet. 2015;87:319‐326. [DOI] [PubMed] [Google Scholar]
- 20. Bredart A, Kop JL, Dick J, et al. Psychosocial problems in women attending French, German and Spanish genetics clinics before and after targeted or multigene testing results: an observational prospective study. BMJ Open. 2019;9:e029926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Idos GE, Kurian AW, Ricker C, et al. Multicenter prospective cohort study of the diagnostic yield and patient experience of multiplex gene panel testing for hereditary cancer risk. JCO Precis Oncol. 2019;2019:PO.18.00217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cella D, Hughes C, Peterman A, et al. A brief assessment of concerns associated with genetic testing for cancer: the Multidimensional Impact of Cancer Risk Assessment (MICRA) questionnaire. Health Psychol. 2002;21:564‐572. [PubMed] [Google Scholar]
- 23. Esteban I, Lopez‐Fernandez A, Balmana J. A narrative overview of the patients' outcomes after multigene cancer panel testing, and a thorough evaluation of its implications for genetic counselling. Eur J Med Genet. 2019;62:342‐349. [DOI] [PubMed] [Google Scholar]
- 24. Smit AK, Newson AJ, Best M, et al. Distress, uncertainty, and positive experiences associated with receiving information on personal genomic risk of melanoma. Eur J Hum Genet. 2018;26:1094‐1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bjornslett M, Dahl AA, Sorebo O, Dorum A. Psychological distress related to BRCA testing in ovarian cancer patients. Fam Cancer. 2015;14:495‐504. [DOI] [PubMed] [Google Scholar]
- 26. Robson M, Domchek S. Broad application of multigene panel testing for breast cancer susceptibility—Pandora's box is opening wider. JAMA Oncol. Published online October 3, 2019. doi: 10.1001/jamaoncol.2019.4004 [DOI] [PubMed] [Google Scholar]
- 27. Katz SJ, Ward KC, Hamilton AS, Abrahamse P, Hawley ST, Kurian AW. Association of germline genetic test type and results with patient cancer worry after diagnosis of breast cancer. JCO Precis Oncol. 2018;2018:PO.18.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Daly MB, Pilarski R, Axilbund JE, et al. Genetic/Familial High‐Risk Assessment: Breast and Ovarian, Version 2.2015. J Natl Compr Canc Netw. 2016;14:153‐162. [DOI] [PubMed] [Google Scholar]
- 29. Gupta S, Provenzale D, Regenbogen SE, et al. NCCN Guidelines Insights: Genetic/Familial High‐Risk Assessment: Colorectal, Version 3.2017. J Natl Compr Canc Netw. 2017;15:1465‐1475. [DOI] [PubMed] [Google Scholar]
- 30. 23andMe . 23andMe receives FDA clearance for direct‐to‐consumer genetic test on a hereditary colorectal cancer syndrome. Accessed August 29, 2020. https://mediacenter.23andme.com/press‐releases/23andme‐receives‐fda‐clearance‐for‐direct‐to‐consumer‐genetic‐test‐on‐a‐hereditary‐colorectal‐cancer‐syndrome/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1


