Abstract
Clustering is a prominent feature of receptors at the plasma membrane (PM). It plays an important role in signaling. Liquid-liquid phase separation (LLPS) of proteins is emerging as a novel mechanism underlying the observed clustering. Receptors/transmembrane signaling proteins can be core components essential for LLPS (such as LAT or nephrin) or clients enriched at the phase separated condensates (for example at the postsynaptic density or tight junctions). Condensate formation has been shown to regulate signaling in multiple ways, including by increasing protein binding avidity and by modulating the local biochemical environment. Moving forward, it is important to study protein LLPS at the PM of living cells, its interplay with other factors underlying receptor clustering, and its signaling and functional consequences.
Graphical Abstract
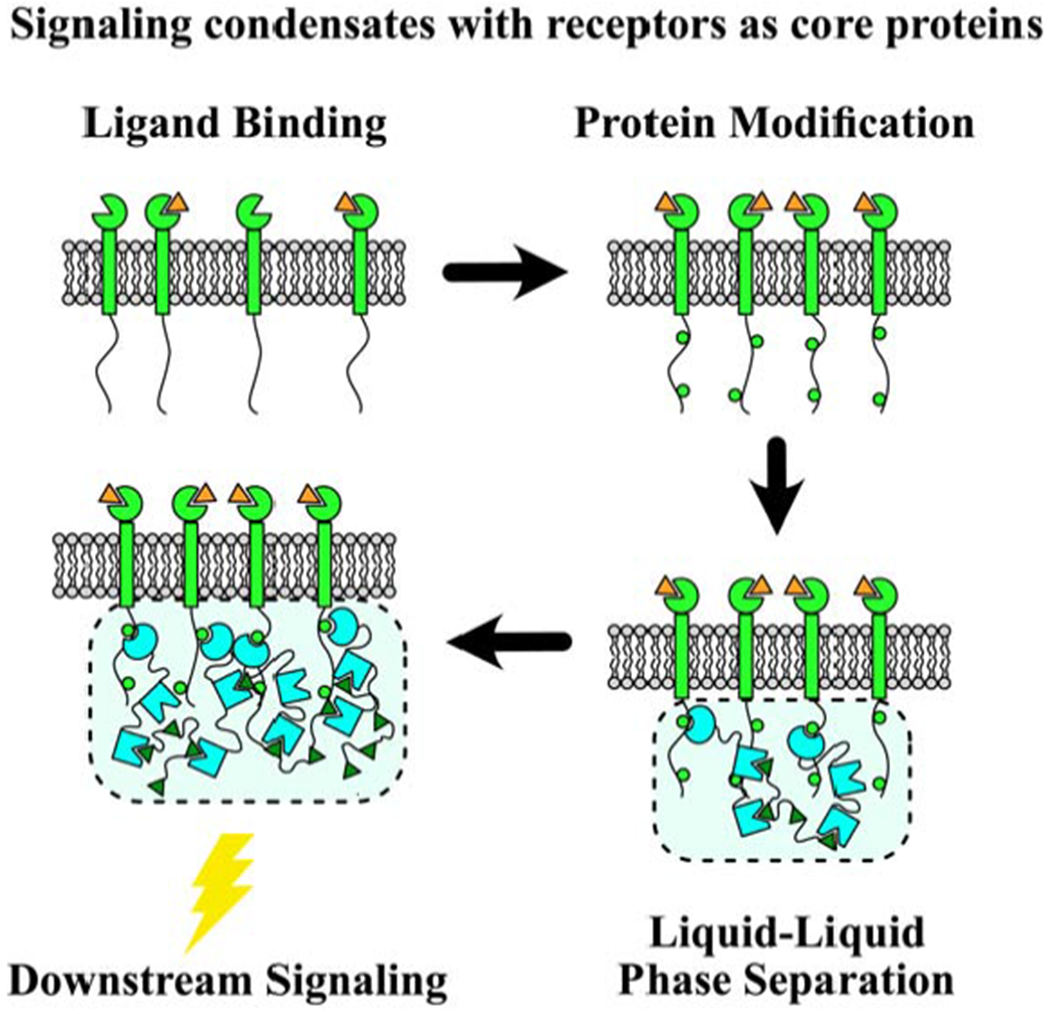
Introduction
Clustering is a prominent feature of receptors at the plasma membrane (PM) [1,2]. Multiple mechanisms may lead to receptor clustering, such as dimerization, oligomerization and complex formation, co-compartmentalization within nanodomains, and clustering mediated by the cortical cytoskeleton [3,4]. Recently, mounting evidence indicates that another mechanism is also at play, namely liquid-liquid phase separation (LLPS) of proteins at the PM, where weak multivalent interactions between proteins drive the formation of biomolecular condensates [5]. For many systems shown to undergo LLPS in vitro, similar mechanisms have been shown to promote cluster formation in cells [6–8]. This indicates that the molecular elements responsible for LLPS play an important role in protein clustering in cells. In this review, we discuss the different classes of condensates at the PM, and what has been discovered thus far of their signaling and functional consequences. We also highlight major open questions in the field, especially in terms of understanding the nature, regulation and functional consequences of LLPS in cells.
Condensates of transmembrane proteins and signaling partners
In one class of PM-associated condensates, LLPS is initiated by phosphorylation (i.e. activation) of transmembrane proteins (Fig. 1A). Two such systems are signaling complexes of the T cell adaptor protein LAT [6,9–11] and the kidney slit diaphragm protein nephrin [8,12–14]. LLPS is driven by multivalent phospho-tyrosine-SH2 domain interactions on the transmembrane proteins and their cytosolic binding partners (Grb2 and Gads for LAT; Nck for nephrin), followed by multivalent SH3 domain-Proline Rich Motif (PRM) interactions with downstream binding partners (Sos1 and SLP-76 for Grb2 and Gads; N-WASP for Nck). LAT condensates comprise a second layer of LLPS, involving phospho-SLP-76, Nck and WASP. In a somewhat analogous manner, the ABC transporter Rv1747 in M. tuberculosis undergoes LLPS, mainly driven by multivalent phospho-threonine-Forkhead Associated (FHA) domain interactions, although in this case both are on the cytosolic tail of Rv1747 [15].
Figure 1. Two classes of phase separated condensates at the PM.
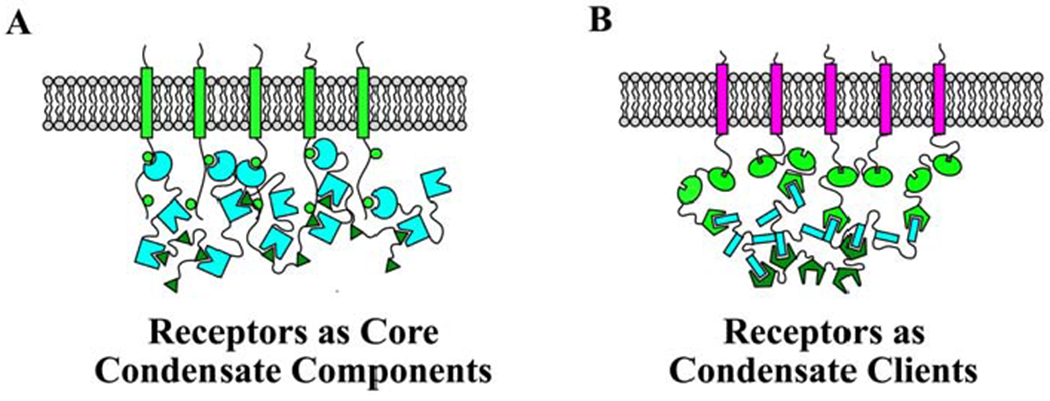
Green to Cyan colors indicate core proteins that are required for LLPS. Magenta colors indicate client proteins that localize to the condensate but are not required for LLPS. A) Schematic of a condensate in which receptors/transmembrane signaling proteins are a core component required for LLPS. Examples of such condensates are those initiated by LAT or nephrin phosphorylation. B) Schematic of a condensate formed by multivalent interactions between scaffolding proteins, which then organize receptors. In this condensate, receptors are clients that localize to the condensate at the PM, but are not required for condensate formation.
For these systems, stimulation induces phosphorylation of specific residues to generate multiple binding sites to promote LLPS of signaling proteins. This couples upstream signaling to LLPS, which in turn regulates downstream signaling. The dependence on phosphorylation implies that LLPS will be greatly reduced or abolished in the absence of stimulation. Some molecules, e.g. LAT, exhibit low levels of basal phosphorylation [16]; however it is unclear if the phospho-tyrosines that are basally phosphorylated drive LLPS. Therefore, this class of condensates most likely does not underlie the clustering observed for many receptors, potentially with their downstream effectors, prior to stimulation [1,17]. However, such pre-clustering increases the local concentration of the proteins involved in LLPS, and it might enable LLPS upon stimulation, even if the average concentration at the PM is below what is required. For example, both LAT and nephrin (through its binding partner podocin) have an affinity for cholesterol-enriched membrane nanodomains [18–20]. Thus it will be important to investigate how protein and lipid phase separation may cooperatively promote the formation of membrane-associated condensates, where both lipids and proteins promote signaling [21,22].
Many transmembrane receptors, such as receptor tyrosine kinases (RTKs), are phosphorylated in their intracellular domains upon stimulation. This enables binding of multidomain scaffolding proteins, which may promote LLPS. Thus, LLPS might be a widespread feature of receptor activation and signaling [23]. Indeed, for the RTK EphB2, there is evidence that, upon stimulation, it forms small oligomers that then condensate into larger clusters [24]. In this case, cluster condensation serves to terminate signaling. Also, a recent study of FGFR2 suggests that it phase separates into signaling-competent condensates together with Shp2 and PLCγ1, by a process analogous to LAT and nephrin LLPS [25,26]. Because many RTKs consist of analogous domains and motifs, it will be important to determine the role of LLPS in the regulation of RTK signaling and cellular responses to numerous extracellular stimuli.
Condensates of scaffolding proteins that help organize receptors
In another class of PM-associated condensates, the phase separating proteins are scaffolding proteins (in the signaling sense) that organize receptors at the PM, and are thus critical for downstream signaling (Fig. 1B). One such system are proteins involved in the formation of the postsynaptic density (PSD), in particular through MAGUK family proteins (SAP-97, PSD-93, SAP-102 and PSD-95), which then help localize receptors such as AMPAR and NMDAR [27,28]. The receptors in this case are clients in the condensates [29]. With this, the condensates control cellular responses to neurotransmitter binding to receptors, such as actin polymerization [27,28,30] (Fig. 2). Another system are the Zona Occludens (ZO) 1 and 2 proteins, also of the MAGUK family, which drive the formation of tight junction complexes [7,31]. In both systems, LLPS requires a combination of PDZ, SH3 and GuK domains. ZO protein LLPS is reduced by intrinsically disordered region (IDR) phosphorylation [7]. It would be interesting to know whether phosphorylation of MAGUK family proteins in the PSD also inhibits LLPS.
Figure 2. Multiple LLPS modules play a role in signaling in dendritic spines.
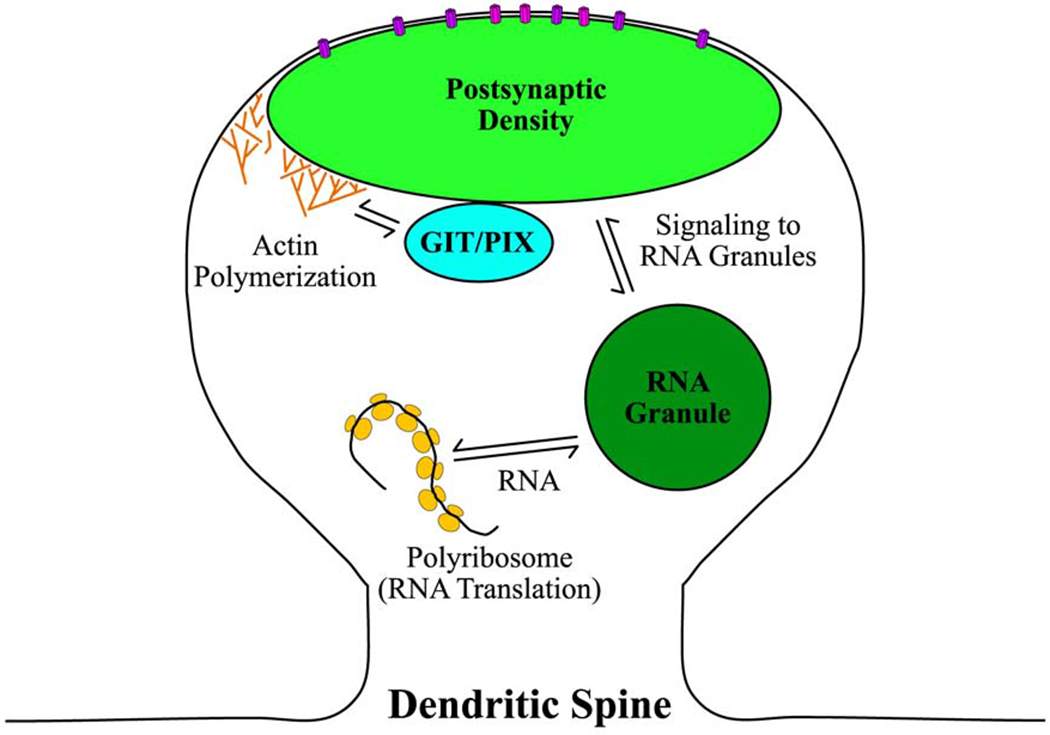
The PSD is a protein-rich condensate that resides at the plasma membrane in dendritic spines. Phase separation of core PSD proteins controls NMDAR (magenta) and AMPAR (violet) localization on the dendritic spine membrane. Binding of neurotransmitters to these receptors initiates signaling pathways to control local actin polymerization and RNA translation. Phase separated GIT/PIX condensates localize in dendritic spines and coordinate with the PSD to control actin polymerization by regulating Rho GTPases [38]. Both GIT/PIX and PSD condensates contain actin regulatory proteins, suggesting that they can simultaneously contribute to controlling local actin polymerization. Signaling in dendritic spines also triggers RNA granules, another condensate that localizes to dendritic spines, to process translationally repressed RNA [58]. The processed RNA can then be translated by polyribosomes in dendritic spines.
Of note, the concentration needed for spontaneous LLPS of ZO proteins is higher than the concentration of ZO proteins in cells, suggesting that an active process triggers phase separation [7]. To better understand the role of LLPS in tight junction formation, it will be important to determine what processes trigger ZO protein LLPS, and to also elucidate the interplay between ZO protein LLPS and other protein clustering mechanisms at the PM, such as lipid phase separation, as several tight junction proteins have an affinity for cholesterol enriched nanodomains [32].
LLPS also plays an important organizational role in presynaptic boutons, where synapsin LLPS organizes synaptic vesicles [33], and RIM/RIM-BP LLPS organizes Ca2+-voltage gated channels at an active zone condensate [34]. Both Synapsin and RIM/RIM-BP phase separate through IDR-SH3 domain interactions. Recently, the phase separation of C. elegans active zone core proteins SYD-2 and ELKS-1 was shown to be essential for proper active zone mixing and composition [35]. Specific IDRs within both SYD-2 and ELKS-1 drove phase separation of these proteins. Because multiple components of presynaptic boutons undergo LLPS and use LLPS to regulate active zone composition, more comprehensive investigations into the functional importance of LLPS in regulating neurotransmitter release will be essential for understanding the mechanisms that regulate neuronal signaling.
Furthermore, LLPS appears to play a role in cell polarity and asymmetry, for example through Par3/Par6 and Numb/Pon condensates at the cell cortex of Drosophila neuroblasts [36,37]. aPKC is a client in Par3/Par6 condensates, yet when it phosphorylates Par3, this leads to condensate dispersal [36]. Numb/Pon LLPS controls Notch degradation to antagonize Notch signaling and promote neuroblast differentiation [37]. The specific mechanisms by which LLPS of these condensates contributes to cell polarity and receptor signaling requires further investigation.
Modular condensates that assist with signaling at the PM
While not at the PM, the small GTPases GIT and PIX, which mediate signaling just downstream of proteins affiliated with focal adhesions, cell junctions and the PSD (Fig. 2), have been shown to phase separate [38]. GIT phase separates on its own, but β-Pix enhances LLPS by binding tightly to GIT and by forming a trimer itself. β-Pix alone does not phase separate. In cells, disruption of GIT/PIX LLPS results in reduced cell migration [38], suggesting that LLPS of these proteins disrupts cellular signal transduction.
Condensates enhance avidity at the molecular level to promote signaling
One prominent consequence of LLPS is that the concentration and proximity of proteins within condensates increases the dwell time (or residence time) of proteins involved in signaling events (Fig. 3). This consequence is consistent with previous predictions from modeling and experimental observations in the context of receptor clustering [3,39]. One consequence of increased dwell time is that it increases the likelihood of activation and signaling. For example, increased dwell time of WASP-family proteins at PM-associated condensates increases their assembly with the Arp2/3 complex. This increases the Arp2/3 complex’s actin nucleation activity at nephrin-based condensates (Fig. 3), which is a rate-limiting step in actin assembly [8,40]. This mechanism is likely responsible for enhanced actin polymerization at LAT condensates as well [11]. Importantly, the activity of the Arp2/3 complex is regulated by condensate stoichiometry [8], thus providing a route for the regulation of signaling leading to actin polymerization. These findings explain previous observations made through quantitative live-cell microscopy and computational modeling [41], highlighting the novel biophysical understanding afforded by studying protein clustering in the context of LLPS. As another example, increased dwell time of SOS1 at LAT condensates increases the probability of autoinhibition release to catalyze nucleotide exchange in Ras [9] (Fig. 3). This mechanism enables kinetic proofreading, where long-dwelling SOS1 is activated to a greater degree than short-dwelling SOS1.
Figure 3. Composition and molecular-level functions of PM-associated condensates.
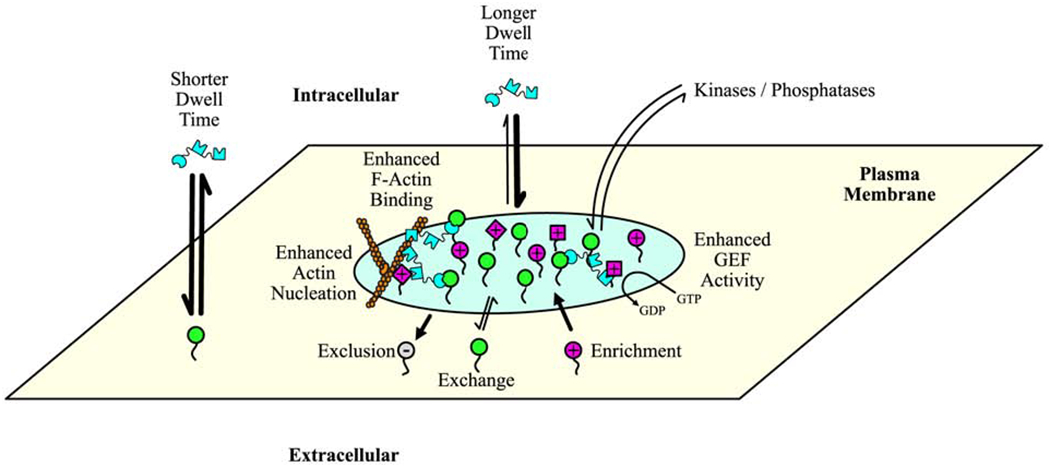
In this illustration, the condensate (light green) is composed of membrane-associated (green) and soluble (cyan) core proteins, as well as several client proteins (magenta, different shapes represent different proteins) [8,9]. Core proteins can freely exchange with the surrounding membrane or solution. Because of enhanced rebinding, exchange of soluble components is slower in the condensate, which results in increased dwell times when compared to exchange outside of the condensate (rate represented by arrow width). Increased dwell time enhances various functions, such as actin polymerization (illustrated on left side of condensate) and exchange of GTP for GDP on GTPases (illustrated on right side of condensate) [8,9]. Enhanced rebinding also increases the collective binding affinity of condensate components to cytoskeletal structures, such as actin filaments (illustrated on top-left side of condensate) [6]. Condensates can also exclude molecules (gray, (−) in circle on membrane protein) or enrich molecules (magenta, (+) in circle on membrane protein). In some instances, exclusion and enrichment are based on electrostatic repulsion and attraction, respectively [11], while in other instances the mechanism is yet to defined [27]. Other potential drivers of exclusion or enrichment include size and hydrophobicity.
Another consequence of increased avidity is that it increases the collective binding affinity of condensate components to cytoskeletal structures, most notably actin filaments (Fig. 3). For example, while Nck and WASP family proteins bind actin only weakly as individual molecules, their collective binding affinity within condensates increases significantly, allowing LAT or nephrin cluster movement driven by actin filament movement [6,13]. This increased collective binding affinity might explain also PSD association with actin [27] and enable the translocation of ZO clusters to tight junctions via actin [7,31]. Further investigations into the molecular-level consequences of LLPS are necessary to determine the mechanisms that enable actin association with these condensates.
Other signaling-related functions of condensates at the PM
Condensates also regulate signaling through their composition. For example, LAT condensates actively segregate proteins in a charge-dependent manner (Fig. 3); positively charged proteins, e.g. the zeta-chain TCR tail and kinase ZAP-70, which promotes LAT phosphorylation and LLPS, are enriched, while negatively charged proteins, e.g. the phosphatase CD45, which dephosphorylates LAT to prevent LLPS, are excluded [11]. Likewise, reconstituted PSDs can be enriched with CaMKII, a kinase that promotes signaling within the PSD, while excluding gephyrin, a protein that inhibits excitatory synapse formation [42,43]. These data strongly suggest that LLPS of signaling proteins can promote specific signaling pathways by creating unique biochemical environments for these pathways.
Condensates can also change composition, through which they might regulate signaling. For example, LAT condensates lose Nck (and consequently WASP) as they traverse the immunological synapse (IS) [6]. This weakens their binding to the actin cytoskeleton, which aids the condensates in continuing their movement toward the IS center despite the changing nature of the actin cortex [6]. Loss of Nck and WASP is also expected to reduce actin polymerization at LAT condensates [8,40]. Because actin polymerization at LAT condensates promotes PLCγ function [44], it will be interesting to determine whether this signaling function of LAT condensates diminishes with the loss of Nck and WASP.
Condensates may also imbue cell signaling with spatial memory. Using elegant optogenetic manipulations of the RTK FGFR1, Dine et al. [45] showed that, if cells are exposed to a localized stimulus that triggers LLPS, PM-associated condensates will form at the stimulus location, thus depleting monomers from other regions. Because of Ostwald Ripening, larger condensates will grow bigger while smaller condensates lose molecules. In the context of a cell, this leads to asymmetry that can persist even after the asymmetric stimulus is gone. In the case of FGFR1, the authors showed that this leads to asymmetric cytoskeletal responses [45].
Conclusions/outlook
Recent studies have revealed that LLPS of membrane-associated signaling complexes can spatially and biochemically control signaling pathways. Some are triggered by PM protein activation, some contribute to receptor organization as needed for signaling, while others act downstream of PM-associated components. In vitro reconstitution of membrane-associated condensates provides key insights into condensate formation and function. However, in the cellular environment, protein LLPS is more complex [46], occurring in the presence of many other factors that regulate PM-associated protein dynamics and organization. This includes lipid phase separation, as discussed above, as well as the cortical cytoskeleton. The cortical cytoskeleton has a wide range of effects on the PM, from confining molecular movement, to promoting protein clustering, to influencing lipid phase separation [3,47–49]. Therefore, it will be important to determine the interdependence between PM-associated protein LLPS, lipid phase separation, and the cortical cytoskeleton. For this, and for gaining a deeper understanding of the functional and signaling consequences of condensate formation and regulation, quantitative light microscopy experiments combined with mathematical modeling (Box 1) and controlled system manipulation [45,50,51] will be key. Investigating cellular signaling through the lens of phase separation will likely result in many exciting discoveries and provide novel biophysical insight into the mechanisms that control cellular signaling and function.
Box 1: Characterizing PM protein LLPS and signaling in cells.
Condensates formed by LLPS are highly dynamic structures. Quantitative light microscopy is ideally suited to probe their properties, regulation, and signaling consequences in cells. Singlemolecule imaging and fluorescence correlation spectroscopy are powerful approaches to probe the behavior and consequences of condensates at the molecular level, whether it be formation properties, movement properties, dwell times or activation times [9–11,52]. While most of the existing quantitative studies of condensates have been in vitro, similar studies should be feasible in cells. However, condensate/cluster behavior in cells is generally more complex than condensate behavior in vitro. For example, LAT condensates can be relatively stationary on a supported lipid bilayer, but they are highly mobile in cells [6]. Therefore, advances in computer vision and computational data analysis will be critical for addressing these questions in cells. Comparing single-molecule behaviors in the context of condensates/clusters in cells to those in vitro will shed light on additional factors that may regulate condensate/cluster formation in cells. In addition, because LLPS is well understood in a polymer chemistry context [53], it is possible in many situations to predict – through analytical means or through mathematical modeling and simulations – the expected behavior of condensates and their constituent molecules [8–10,54,55]. Thus, comparing the expected behaviors to those in cells will reveal the extent to which the behavior of clusters in cells is truly reflective of LLPS [56]. Such comparisons are not limited to the single-molecule level. For example, fluorescence recovery after photobleaching (FRAP) experiments have shown that ZO dynamics at tight junctions are slower than expected for a liquid phase, indicating that additional interactions constrain ZO proteins [7]. As for investigating the signaling consequences of LLPS, activity biosensors that allow the monitoring of molecular activity in living cells with high spatial and temporal resolution will be critical moving forward [57].
Protein phase separation is a mechanism for plasma membrane receptor clustering
Membrane proteins can be core phase separating components or enriched clients
Increased binding avidity at condensates promotes signaling molecule activation
Phase separation regulates actin nucleation, enzyme activity and RNA processing
Signaling complexes and pathways may consist of multiple phase separating modules
Acknowledgments
Funding: This work was supported by the National Institutes of Health [grant R35 GM119619] and the UT Southwestern Endowed Scholars program to KJ, and by the Hospital for Sick Children Research Institute to JAD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest
None.
References
- 1.Garcia-Parajo MF, Cambi A, Torreno-Pina JA, Thompson N, Jacobson K: Nanoclustering as a dominant feature of plasma membrane organization. Journal of Cell Science 2014, 127:4995–5005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Casaletto JB, McClatchey AI: Spatial regulation of receptor tyrosine kinases in development and cancer. Nature Reviews Cancer 2012, 12:387–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jaqaman K, Grinstein S: Regulation from within: the cytoskeleton in transmembrane signaling. Trends Cell Biol 2012, 22:515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sezgin E, Levental I, Mayor S, Eggeling C: The mystery of membrane organization: composition, regulation and roles of lipid rafts. Nature Reviews Molecular Cell Biology 2017, 18:361–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li PL, Banjade S, Cheng HC, Kim S, Chen B, Guo L, Llaguno M, Hollingsworth JV, King DS, Banani SF, et al. : Phase transitions in the assembly of multivalent signalling proteins. Nature 2012, 483:336–U129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Ditlev JA, Vega AR, Köster DV, Su X, Tani T, Lakoduk AM, Vale RD, Mayor S, Jaqaman K, Rosen MK: A composition-dependent molecular clutch between T cell signaling condensates and actin. Elife 2019, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses in vitro reconstitution and cellular experiments to show that the composition of LAT condensates regulates their interaction with moving actin networks, which is a novel functional consequence of phase separation. In cells, changes in LAT condensate composition enable two different actin networks to move clusters across the immunological synapse to regulate the response of activated T cells to antigens.
- *7.Beutel O, Maraspini R, Pombo-García K, Martin-Lemaitre C, Honigmann A: Phase Separation of Zonula Occludens Proteins Drives Formation of Tight Junctions. Cell 2019, 179:923–936.e911. [DOI] [PubMed] [Google Scholar]; The authors use in vitro reconstitution and cell biology to show that phase separation of ZO proteins drives tight junction formation. Tight junction condensates can be enriched in adhesion receptors, cytoskeletal adaptors, and transcription factors.
- **8.Case LB, Zhang X, Ditlev JA, Rosen MK: Stoichiometry controls activity of phase-separated clusters of actin signaling proteins. Science 2019, 363:1093–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that the increased dwell time of N-WASP and Arp2/3 in nephrin condensates promotes local actin nucleation by Arp2/3. N-WASP dwell time is controlled by the stoichiometry of nephrin, Nck, and N-WASP within condensates, thus linking condensate composition to signaling outcome.
- **9.Huang WYC, Alvarez S, Kondo Y, Lee YK, Chung JK, Lam HYM, Biswas KH, Kuriyan J, Groves JT: A molecular assembly phase transition and kinetic proofreading modulate Ras activation by SOS. Science 2019, 363:1098–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors demonstrate that the dwell time of Sos1 in LAT condensates controls Sos1 GEF activity. Sos1 autoinhibition release requires ~50s; thus long dwell times within LAT condensates, resulting from multivalent interactions with Grb2, increase the probability that Sos1 will be activated at the membrane. This provides a novel mechanism for kinetic proofreading in transmembrane signal transduction.
- 10.Huang WYC, Chiang HK, Groves JT: Dynamic Scaling Analysis of Molecular Motion within the LAT:Grb2:SOS Protein Network on Membranes. Biophys J 2017, 113:1807–1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su X, Ditlev JA, Hui E, Xing W, Banjade S, Okrut J, King DS, Taunton J, Rosen MK, Vale RD: Phase separation of signaling molecules promotes T cell receptor signal transduction. Science 2016, 352:595–599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Banjade S, Rosen MK: Phase transitions of multivalent proteins can promote clustering of membrane receptors. Elife 2014, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim S, Kalappurakkal JM, Mayor S, Rosen MK: Phosphorylation of nephrin induces phase separated domains that move through actomyosin contraction. Mol Biol Cell 2019, 30:2996–3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin CE, New LA, Phippen NJ, Keyvani Chahi A, Mitro AE, Takano T, Pawson T, Blasutig IM, Jones N: Multivalent nephrin-Nck interactions define a threshold for clustering and tyrosine-dependent nephrin endocytosis. J Cell Sci 2020, 133. [DOI] [PubMed] [Google Scholar]
- *15.Heinkel F, Abraham L, Ko M, Chao J, Bach H, Hui LT, Li H, Zhu M, Ling YM, Rogalski JC, et al. : Phase separation and clustering of an ABC transporter in Mycobacterium tuberculosis. Proc Natl Acad Sci U S A 2019, 116:16326–16331. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that the bacterial ABC transporter cytoplasmic region can phase separate, providing evidence that phase separation can regulate protein clustering and activity on bacterial membranes.
- 16.Lin J, Weiss A: Identification of the minimal tyrosine residues required for linker for activation of T cell function. J Biol Chem 2001, 276:29588–29595. [DOI] [PubMed] [Google Scholar]
- 17.Githaka JM, Vega AR, Baird MA, Davidson MW, Jaqaman K, Touret N: Ligand-induced growth and compaction of CD36 nanoclusters enriched in Fyn induces Fyn signaling. Journal of Cell Science 2016, 129:4175–4189. [DOI] [PubMed] [Google Scholar]
- 18.Diaz-Rohrer BB, Levental KR, Simons K, Levental I: Membrane raft association is a determinant of plasma membrane localization. Proc Natl Acad Sci U S A 2014, 111:8500–8505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hancock JF: Ras proteins: different signals from different locations. Nat Rev Mol Cell Biol 2003, 4:373–384. [DOI] [PubMed] [Google Scholar]
- 20.Martin CE, Jones N: Nephrin Signaling in the Podocyte: An Updated View of Signal Regulation at the Slit Diaphragm and Beyond. Front Endocrinol (Lausanne) 2018, 9:302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chung JK, Huang WYC, Carbone CB, Nocka LM, Parikh AN, Vale RD, Groves JT: Coupled Membrane Lipid Miscibility and Phosphotyrosine-Driven Protein Condensation Phase Transitions. Biophys J 2020, 119:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee IH, Imanaka MY, Modahl EH, Torres-Ocampo AP: Lipid Raft Phase Modulation by Membrane-Anchored Proteins with Inherent Phase Separation Properties. ACS Omega 2019, 4:6551–6559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Case LB, Ditlev JA, Rosen MK: Regulation of Transmembrane Signaling by Phase Separation. Annu Rev Biophys 2019, 48:465–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ojosnegros S, Cutrale F, Rodríguez D, Otterstrom JJ, Chiu CL, Hortigüela V, Tarantino C, Seriola A, Mieruszynski S, Martínez E, et al. : Eph-ephrin signaling modulated by polymerization and condensation of receptors. Proc Natl Acad Sci U S A 2017, 114:13188–13193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lin C-C, Suen KM, Jeffrey P-A, Wieteska L, Stainthorp A, Seiler C, Koss H, Molina-París C, Miska E, Ahmed Z, et al. : Receptor tyrosine kinases regulate signal transduction through a liquid–liquid phase separated state. bioRxiv 2019:783720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zeng L, Palaia I, Šarić A, Su X: PLCγ1 promotes phase separation of the T cell signaling clusters. bioRxiv 2020:2020.2006.2030.179630. [Google Scholar]
- **27.Zeng M, Chen X, Guan D, Xu J, Wu H, Tong P, Zhang M: Reconstituted Postsynaptic Density as a Molecular Platform for Understanding Synapse Formation and Plasticity. Cell 2018, 174:1172–1187.e1116. [DOI] [PubMed] [Google Scholar]; This study demonstrates that phase separation of the postsynaptic density core proteins PSD-95, Homer1, Shank3, and DLGAP (or GKAP) drives the formation of postsynaptic density-like condensates. These PSD-like condensates can associate with actin filaments and sort molecules such as CaMKII and Gephyrin.
- 28.Zeng M, Díaz-Alonso J, Ye F, Chen X, Xu J, Ji Z, Nicoll RA, Zhang M: Phase Separation-Mediated TARP/MAGUK Complex Condensation and AMPA Receptor Synaptic Transmission. Neuron 2019, 104:529–543.e526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banani SF, Lee HO, Hyman AA, Rosen MK: Biomolecular condensates: organizers of cellular biochemistry. Nat Rev Mol Cell Biol 2017, 18:285–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen X, Wu X, Wu H, Zhang M: Phase separation at the synapse. Nat Neurosci 2020, 23:301–310. [DOI] [PubMed] [Google Scholar]
- *31.Schwayer C, Shamipour S, Pranjic-Ferscha K, Schauer A, Balda M, Tada M, Matter K, Heisenberg CP: Mechanosensation of Tight Junctions Depends on ZO-1 Phase Separation and Flow. Cell 2019, 179:937–952.e918. [DOI] [PubMed] [Google Scholar]; This study shows that, in cells, ZO-1 condensates associate with and are moved by the actin cytoskeleton to tight junctions where they confer mechanosensitivity to the tight junction. Mechanosensivity is required for the spreading of epithelial tissue in zebrafish.
- 32.Zihni C, Mills C, Matter K, Balda MS: Tight junctions: from simple barriers to multifunctional molecular gates. Nat Rev Mol Cell Biol 2016, 17:564–580. [DOI] [PubMed] [Google Scholar]
- *33.Milovanovic D, Wu Y, Bian X, De Camilli P: A liquid phase of synapsin and lipid vesicles. Science 2018, 361:604–607. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors show that the phase separation of synapsin organizes synaptic vesicles that contain neurotransmitters in presynaptic boutons. Phosphorylation of synapsin causes dissambly of synapsin condensates, which may promote exocytosis of neurotransmitters to trigger neuronal signal transduction in the neighboring synapse.
- 34.Wu XD, Cai QX, Shen ZY, Chen XD, Zeng ML, Du SW, Zhang MJ: RIM and RIM-BP Form Presynaptic Active-Zone-like Condensates via Phase Separation. Molecular Cell 2019, 73:971-+. [DOI] [PubMed] [Google Scholar]
- 35.McDonald NA, Fetter RD, Shen K: Assembly of synaptic active zones requires phase separation of scaffold molecules. Nature 2020. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Yang Y, Gu A, Xu J, Mao Y, Lu H, Hu W, Lei QY, Li Z, Zhang M, et al. : Par complex cluster formation mediated by phase separation. Nat Commun 2020, 11:2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shan Z, Tu Y, Yang Y, Liu Z, Zeng M, Xu H, Long J, Zhang M, Cai Y, Wen W: Basal condensation of Numb and Pon complex via phase transition during Drosophila neuroblast asymmetric division. Nat Commun 2018, 9:737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhu J, Zhou Q, Xia Y, Lin L, Li J, Peng M, Zhang R, Zhang M: GIT/PIX Condensates Are Modular and Ideal for Distinct Compartmentalized Cell Signaling. Mol Cell 2020, 79:782–796.e786. [DOI] [PubMed] [Google Scholar]
- 39.Oh D, Ogiue-Ikeda M, Jadwin JA, Machida K, Mayer BJ, Yu J: Fast rebinding increases dwell time of Src homology 2 (SH2)-containing proteins near the plasma membrane. Proceedings of the National Academy of Sciences of the United States of America 2012, 109:14024–14029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith BA, Padrick SB, Doolittle LK, Daugherty-Clarke K, Correa IR, Xu MQ, Goode BL, Rosen MK, Gelles J: Three-color single molecule imaging shows WASP detachment from Arp2/3 complex triggers actin filament branch formation. Elife 2013, 2:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ditlev JA, Michalski PJ, Huber G, Rivera GM, Mohler WA, Loew LM, Mayer BJ: Stoichiometry of Nck-dependent actin polymerization in living cells. Journal of Cell Biology 2012, 197:643–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kennedy MB: Signal-processing machines at the postsynaptic density. Science 2000, 290:750–754. [DOI] [PubMed] [Google Scholar]
- 43.Tyagarajan SK, Fritschy JM: Gephyrin: a master regulator of neuronal function? Nature Reviews Neuroscience 2014, 15:141–156. [DOI] [PubMed] [Google Scholar]
- 44.Kumari S, Depoil D, Martinelli R, Judokusumo E, Carmona G, Gertler FB, Kam LC, Carman CV, Burkhardt JK, Irvine DJ, et al. : Actin foci facilitate activation of the phospholipase C-gamma in primary T lymphocytes via the WASP pathway. Elife 2015, 4:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dine E, Gil AA, Uribe G, Brangwynne CP, Toettcher JE: Protein Phase Separation Provides Long-Term Memory of Transient Spatial Stimuli. Cell Syst 2018, 6:655–663.e655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Riback JA, Zhu L, Ferrolino MC, Tolbert M, Mitrea DM, Sanders DW, Wei MT, Kriwacki RW, Brangwynne CP: Composition-dependent thermodynamics of intracellular phase separation. Nature 2020, 581:209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]; The authors use optogenetic tools to evaluate cellular phase separation. They show that in complex condensates (i.e. composed of more than two components) found in living cells, there is no fixed critical, or saturation, concentration above which phase separation will occur. Rather, as the composition of components is varied, the critical concentration for phase separation changes. These results have broad implications for understanding cellular control of phase separation-dependent signaling processes.
- 47.Honigmann A, Sadeghi S, Keller J, Hell SW, Eggeling C, Vink R: A lipid bound actin meshwork organizes liquid phase separation in model membranes. Elife 2014, 3:e01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fujiwara TK, Iwasawa K, Kalay Z, Tsunoyama TA, Watanabe Y, Umemura YM, Murakoshi H, Suzuki KG, Nemoto YL, Morone N, et al. : Confined diffusion of transmembrane proteins and lipids induced by the same actin meshwork lining the plasma membrane. Mol Biol Cell 2016, 27:1101–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Koster DV, Husain K, Iljazi E, Bhat A, Bieling P, Mullins RD, Rao M, Mayor S: Actomyosin dynamics drive local membrane component organization in an in vitro active composite layer. Proceedings of the National Academy of Sciences of the United States of America 2016, 113:E1645–E1654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *50.Bracha D, Walls MT, Wei MT, Zhu L, Kurian M, Avalos JL, Toettcher JE, Brangwynne CP: Mapping Local and Global Liquid Phase Behavior in Living Cells Using Photo-Oligomerizable Seeds. Cell 2019, 176:407. [DOI] [PubMed] [Google Scholar]; The authors develop an optogenetically controlled ‘Corelet’ experimental system to map intracellular phase diagrams. Coupling of this technology with signaling condensates at the membrane will provide researchers with the ability to measure signaling outputs at precise locations within phase diagrams for their proteins of interest.
- 51.Lin Y, Currie SL, Rosen MK: Intrinsically disordered sequences enable modulation of protein phase separation through distributed tyrosine motifs. J Biol Chem 2017, 292:19110–19120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Peng S, Li W, Yao Y, Xing W, Li P, Chen C: Phase separation at the nanoscale quantified by dcFCCS. Proc Natl Acad Sci U S A 2020, 117:27124–27131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hyman AA, Brangwynne CP: Beyond Stereospecificity: Liquids and Mesoscale Organization of Cytoplasm. Developmental Cell 2011, 21:14–16. [DOI] [PubMed] [Google Scholar]
- 54.Freeman Rosenzweig ES, Xu B, Kuhn Cuellar L, Martinez-Sanchez A, Schaffer M, Strauss M, Cartwright HN, Ronceray P, Plitzko JM, Förster F, et al. : The Eukaryotic CO(2)-Concentrating Organelle Is Liquid-like and Exhibits Dynamic Reorganization. Cell 2017, 171:148–162.e119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Harmon TS, Holehouse AS, Rosen MK, Pappu RV: Intrinsically disordered linkers determine the interplay between phase separation and gelation in multivalent proteins. Elife 2017, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.McSwiggen DT, Mir M, Darzacq X, Tjian R: Evaluating phase separation in live cells: diagnosis, caveats, and functional consequences. Genes Dev 2019, 33:1619–1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Greenwald EC, Mehta S, Zhang J: Genetically Encoded Fluorescent Biosensors Illuminate the Spatiotemporal Regulation of Signaling Networks. Chem Rev 2018, 118:11707–11794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *58.Kim TH, Tsang B, Vernon RM, Sonenberg N, Kay LE, Forman-Kay JD: Phospho-dependent phase separation of FMRP and CAPRIN1 recapitulates regulation of translation and deadenylation. Science 2019, 365:825–829. [DOI] [PubMed] [Google Scholar]; The authors show that different patterns of phosphorylation of CAPRIN1 tyrosine residues and FMRP serine/threonine residues controls condensate association with RNA and can promote the formation of multi-layered condensates that tune RNA deadenylation and translation. These results demonstrate how signaling pathways can work together to regulate RNA processing.


