Abstract
BACKGROUND:
Risk of recurrence among patients with oropharyngeal cancer (OPC) who survive 5 years is low. The goal of this study was to assess long-term survival of patients with OPC alive without recurrence 5 years after diagnosis.
METHODS:
This study included newly diagnosed patients with OPC, who had been treated with radiation and were alive without recurrence 5 years after diagnosis. Overall survival (OS) probabilities beyond 5 years were estimated using the Kaplan-Meier method. Factors associated with OS were determined using Bayesian piecewise exponential survival regression. Standardized mortality ratios for all-cause death were estimated controlling for study year, age, and sex in the US general population.
RESULTS:
Among 1699 patients, the additional 2-year, 5-year, and 10-year OS probabilities were 94%, 83%, and 63%, respectively, and were lower than those in the general population. Patients who were older, were current or former smokers, had other than tonsil or base of tongue tumors, or had T4 tumors had a higher risk of death. Patients who had base of tongue tumors and had received intensity-modulated radiation therapy (IMRT) or lower-radiation doses had a lower risk of death. Standardized mortality ratios were higher among current and heavy smokers and lower among recipients of IMRT and lower radiation doses.
CONCLUSIONS:
In this large cohort, long-term survival among patients with OPC was good but lower than predicted for the general population. Patients treated with IMRT and those with less tobacco exposure had better outcomes.
Keywords: conditional survival, head and neck neoplasms, oropharyngeal cancer, radiation therapy, standardized mortality ratios
INTRODUCTION
Patients with head and neck cancers (HNCs) commonly present with local, regionally advanced disease. Recurrence rates depend on primary tumor site and stage; regardless, the majority of recurrences manifest within 2 years after treatment, and the likelihood of recurrence after 5 years is <2%.1
Patients with human papillomavirus (HPV)–related oropharyngeal cancer (OPC), including p16-positive tumors, have higher survival rates than patients with HPV-unrelated disease2–4; however, the Radiation Therapy Oncology Group (RTOG) has demonstrated that p16-positive and p16-negative patients have similar time to recurrence, with >80% occurring within 2 years after therapy.5 Several groups have described late recurrences among patients with HPV-related disease, but these events are uncommon.6 Thus, the probability of recurrence beyond 5 years after completion of therapy is very low.
In a recent randomized trial of patients with HPV-related OPC, 5-year overall survival (OS) was approximately 80%.7 This has led investigators to focus on reducing treatment toxicity, and though a laudable goal in and of itself, long-term results from past RTOG studies have hinted that toxicity reduction may also improve long-term survival.8,9
Conditional survival, the probability of additional survival given that a patient has already survived a specified amount of time,10 is one metric for understanding the long-term prognosis of cancer survivors who have remained recurrence-free for many years after treatment. It can be reported in absolute terms with Kaplan-Meier techniques that define survival rates from a time point after diagnosis (eg, completion of treatment) in a patient who has survived to that new time point, and in relative terms, comparing survival with that of an age- and period-adjusted normal population.
Recognizing that the majority of patients with OPC survive at least 5 years (≈80%) and have a low probability of recurrence, we sought to identify factors that influenced long-term survival in a cohort of patients with OPC treated with radiation who survived recurrence-free for at least 5 years after diagnosis.
MATERIALS AND METHODS
The study was a retrospective database review approved by the institutional review board of The University of Texas MD Anderson Cancer Center. A database maintained in the Department of Radiation Oncology was searched using the following criteria: 1) incident squamous cell OPC with radiation treatment to the oropharynx, 2) diagnosis during 1980–2012, 3) survival after diagnosis at least 5 years, 4) gross disease at the primary site and/or neck at the time of treatment start (patients with unknown primary site were excluded), and 5) no cancer event during the 5 years after diagnosis. The majority of patients who presented to the institution during the study period were treated with radiation; patients who were not irradiated at the institution (<15%) or were treated with primary surgery (<2%) were not included in the analysis.
Statistical Analysis
Patient characteristics were summarized using the median and interquartile range (IQR) for continuous variables and counts and percentages for categorical variables. OS time was defined as the time from 5 years after diagnosis to death. Vital status (date of death, last follow-up, and time to progression) was determined through review of medical records that included information collected through periodic review of the Social Security Death Index, the State Bureau of Vital Statistics, and the State Cancer Registry. Because of the difficulty of distinguishing between second primary tumors and recurrence in head and neck mucosal sites, all occurrences of squamous malignancies in a head and neck site and all metastatic squamous cell cancer were considered recurrences.
The Kaplan-Meier method was used to estimate OS probabilities beyond 5 years. Bayesian piecewise exponential survival regression models11 were fit to assess the effects of patient covariates on OS time. The covariates included age, sex, smoking status at diagnosis, primary tumor site, T category, N category, radiation therapy dose, and receipt of intensity-modulated radiation therapy (IMRT), neck dissection, or chemotherapy. To evaluate possible interactions between T category and radiation dose, a second model also included 2 indicator variables (T1-T2 and radiation dose 20–66 Gy vs not; T2-T3 and radiation dose >67 vs not). Neither of the interaction terms were statistically significant and were not included in the final model. To avoid collinearity and an unstable fitted model, period of diagnosis (1980–1989, 1990–1999, 2000–2013) and pack-years of smoking were not included because 1) the period of diagnosis was highly correlated with IMRT (no patient received IMRT prior to 2000), and 2) pack-years was highly correlated with smoking status and was missing for 63 patients. Each model assumed a noninformative gamma prior for the hazard parameters and noninformative normal priors for the covariate coefficients. Separate models with 1 to 10 subintervals were fit, and the model with the smallest deviance information criterion,12 which had 7 subintervals, was selected (additional details provided in Supporting Information). A graphical user interface (GUI) to compute posterior estimates of OS based on individual patient covariates was constructed using the fitted survival model (https://biostatistics.mdanderson.org/shinyapps/BayesianSurvival/).
Standardized mortality ratios (SMRs) for all-cause death13–15 were estimated for specified subgroups, controlling for study year, age, and sex in the US general population.16,17 CIs and significance tests for SMRs were calculated using a normal approximation. The indirect standardization method, which uses the stratum-specific (study year, age, and sex) rate estimates in the reference population to compute the expected number of events in the study population, was used. The Ederer II method18 was used to calculate adjusted expected survival curves from US mortality data, and the Kaplan-Meier method19 was used to estimate 2-, 5-, and 10-year unadjusted OS probabilities for the study population. SAS 9.4 (SAS, Inc) was used for all statistical computations.
RESULTS
Of 3100 patients with OPC, 1699 met the inclusion criteria (Supporting Fig. 1). Patient characteristics are summarized in Table 1. The median age was 60 years (IQR, 55–68 years). HPV (by in situ hybridization) and/or p16 immunohistochemistry testing was only available for patients diagnosed after 1999. Of 477 patients, 429 (90%) had an HPV-related tumor. The median radiation dose was 70 Gy (IQR, 66–70.5 Gy).
TABLE 1.
Demographic and Clinical Characteristics of Patients With Oropharyngeal Cancer Who Survived at Least 5 Years After Diagnosis
| Characteristic | No. of Patients (%) |
|---|---|
| Age at diagnosis, median (IQR), y | 60 (55–68) |
| Radiation dose, median (IQR), Gy | 70 (66–70.5) |
| Sex | |
| Male | 1407 (82.8) |
| Female | 292 (17.2) |
| Period of diagnosis | |
| 1980–1989 | 133 (7.8) |
| 1990–1999 | 311 (18.3) |
| 2000–2013 | 1255 (73.9) |
| Smoking status | |
| Never | 744 (44.2) |
| Former | 571 (33.9) |
| Current | 370 (22.0) |
| Missing | 14 |
| Smoking, pack-years | |
| ≤10 | 956 (58.4) |
| >10 | 680 (41.6) |
| Missing | 63 |
| Site | |
| Tonsil | 773 (45.5) |
| Base of tongue | 821 (48.3) |
| Other | 105 (6.2) |
| HPV status | |
| Negative | 48 (10.1) |
| Positive | 429 (89.9) |
| Missing | 1222 |
| T category | |
| T1 | 523 (30.8) |
| T2 | 639 (37.6) |
| T3 | 353 (20.8) |
| T4 | 183 (10.8) |
| Missing | 1 |
| N category (AJCC 7th ed.) | |
| N0 | 218 (12.9) |
| N1 | 330 (19.4) |
| N2 | 1045 (61.5) |
| N3 | 105 (6.2) |
| Missing | 1 |
| Neck dissection | |
| No | 1306 (76.9) |
| Yes | 393 (23.1) |
| IMRT | |
| No | 621 (36.6) |
| Yes | 1078 (63.4) |
| Fractionation | |
| Conventional | 1164 (68.7) |
| Concomitant boost | 456 (26.9) |
| Other | 75 (4.4) |
| Missing | 4 |
| Radiation dose, Gy | |
| >70 | 440 (26.0) |
| >66–70 | 622 (36.7) |
| ≤66 | 633 (37.3) |
| Missing | 4 |
| Chemotherapy | |
| None | 726 (42.7) |
| Adjuvant only | 266 (15.7) |
| Concurrent only | 485 (28.5) |
| Both | 222 (13.1) |
| Recurrencea | |
| None | 1614 (95.0) |
| Local | 53 (3.1) |
| Regional | 9 (0.5) |
| Distant | 35 (2.1) |
| Second primary tumor | |
| No | 1408 (84.5) |
| Yes | 258 (15.5) |
| Missing | 33 |
Abbreviations: AJCC, American Joint Committee on Cancer; HPV, human papillomavirus; IMRT, intensity-modulated radiation therapy; IQR, interquartile range.
Groups not mutually exclusive.
A total of 308 patients (18%) had a cancer event after time zero (ie, after having survived 5 years from diagnosis; Table 1). Eighty-five patients developed an HNC recurrence: 45 solely in a head and neck site, 5 with isolated nodal recurrences, and 35 with distant metastases. Twelve of the 35 patients with distant metastases had a known history of a second primary (non-head and neck) squamous cancer, a potential source of the metastases in some cases. Patients who suffered a cancer event were more likely to have ever smoked than those who did not (67% vs 53%; P < .001).
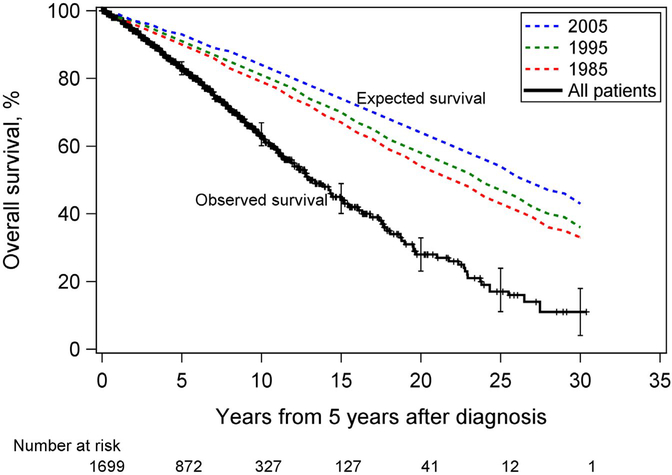
A total of 496 patients (29%) died during the study follow-up, including 197 (64%) with a cancer event. The median follow-up time from 5 years after diagnosis for all patients was 6.7 years (95% CI, 6.4–7.0; ie, 11.7 years from diagnosis). Figure 1 shows Kaplan-Meier estimates of OS for all patients along with age- and sex-adjusted expected survival probabilities based on US mortality data for 1985, 1995, and 2005. Patients had significantly worse survival over time compared with the general population. The unadjusted 2-, 5-, and 10-year survival probabilities were 94%, 83%, and 63%, respectively, for the full study population (Table 2).
Figure 1.
Overall survival of patients with oropharyngeal cancer who had already survived 5 years after diagnosis. Age- and sex-adjusted expected survival curves for the general US population for 1985, 1995, and 2005 are also shown. Vertical bars are 95% CIs. The time scale begins at 5 years after diagnosis.
TABLE 2.
Kaplan-Meier Estimates of 2-, 5-, and 10-Year Unadjusted Survival Probabilities of Patients With Oropharyngeal Cancer Who Survived at Least 5 Years After Diagnosis
| Survival Probability (95% CI) |
|||
|---|---|---|---|
| Subgroup | 2 y | 5 y | 10 y |
| Full study population | 0.94 (0.93–0.95) | 0.83 (0.81–0.85) | 0.63 (0.60–0.67) |
| Age at diagnosis, y | |||
| <55 | 0.97 (0.94–0.98) | 0.91 (0.87–0.93) | 0.80 (0.74–0.84) |
| ≥55 | 0.93 (0.92–0.95) | 0.81 (0.78–0.83) | 0.57 (0.53–0.61) |
| Period of diagnosis | |||
| 2000–2013 | 0.96 (0.95–0.97) | 0.87 (0.85–0.89) | 0.67 (0.62–0.71) |
| 1990–1999 | 0.92 (0.88–0.94) | 0.78 (0.73–0.82) | 0.58 (0.52–0.63) |
| 1980–1989 | 0.86 (0.79–0.91) | 0.70 (0.62–0.77) | 0.54 (0.45–0.62) |
| Sex | |||
| Male | 0.94 (0.93–0.96) | 0.84 (0.82–0.86) | 0.64 (0.60–0.68) |
| Female | 0.93 (0.90–0.96) | 0.80 (0.75–0.85) | 0.60 (0.53–0.67) |
| Smoking status | |||
| Never | 0.97 (0.95–0.98) | 0.92 (0.89–0.94) | 0.76 (0.71–0.80) |
| Former | 0.95 (0.93–0.97) | 0.81 (0.77–0.85) | 0.61 (0.55–0.67) |
| Current | 0.88 (0.85–0.91) | 0.71 (0.66–0.76) | 0.47 (0.41–0.54) |
| Smoking, pack-y | |||
| >10 | 0.91 (0.88–0.93) | 0.74 (0.70–0.77) | 0.50 (0.45–0.55) |
| ≤10 | 0.97 (0.95–0.98) | 0.91 (0.89–0.93) | 0.75 (0.71–0.79) |
| Site | |||
| Tonsil | 0.94 (0.92–0.95) | 0.84 (0.80–0.86) | 0.64 (0.59–0.68) |
| Base of tongue | 0.95 (0.94–0.97) | 0.86 (0.83–0.89) | 0.69 (0.64–0.73) |
| Other | 0.87 (0.78–0.92) | 0.63 (0.52–0.72) | 0.34 (0.24–0.45) |
| T category | |||
| T1 | 0.96 (0.94–0.98) | 0.89 (0.85–0.92) | 0.71 (0.65–0.77) |
| T2 | 0.94 (0.92–0.96) | 0.85 (0.81–0.88) | 0.69 (0.64–0.74) |
| T3 | 0.93 (0.90–0.96) | 0.79 (0.74–0.83) | 0.53 (0.46–0.60) |
| T4 | 0.91 (0.86–0.95) | 0.72 (0.63–0.79) | 0.43 (0.32–0.53) |
| N category (AJCC 7th ed.) | |||
| N0 | 0.88 (0.83–0.92) | 0.71 (0.64–0.77) | 0.51 (0.43–0.58) |
| N1 | 0.94 (0.90–0.96) | 0.84 (0.79–0.88) | 0.64 (0.57–0.71) |
| N2 | 0.96 (0.95–0.97) | 0.87 (0.84–0.89) | 0.67 (0.62–0.71) |
| N3 | 0.88 (0.79–0.93) | 0.75 (0.65–0.83) | 0.56 (0.44–0.67) |
| Neck dissection | |||
| No | 0.94 (0.92–0.95) | 0.82 (0.80–0.85) | 0.62 (0.58–0.65) |
| Yes | 0.95 (0.92–0.97) | 0.86 (0.82–0.89) | 0.69 (0.63–0.75) |
| IMRT | |||
| No | 0.91 (0.88–0.93) | 0.77 (0.73–0.80) | 0.58 (0.53–0.61) |
| Yes | 0.97 (0.95–0.98) | 0.89 (0.86–0.91) | 0.69 (0.62–0.75) |
| Radiation dose, Gy | |||
| >70 | 0.93 (0.90–0.95) | 0.80 (0.76–0.83) | 0.58 (0.53–0.63) |
| >66–70 | 0.92 (0.90–0.94) | 0.80 (0.75–0.83) | 0.60 (0.53–0.65) |
| ≤66 | 0.97 (0.95–0.98) | 0.90 (0.87–0.92) | 0.72 (0.65–0.77) |
| Chemotherapy | |||
| None | 0.93 (0.91–0.95) | 0.81 (0.78–0.84) | 0.63 (0.59–0.67) |
| Adjuvant only | 0.96 (0.92–0.98) | 0.89 (0.84–0.93) | 0.71 (0.62–0.79) |
| Concurrent only | 0.94 (0.91–0.96) | 0.83 (0.79–0.87) | 0.58 (0.50–0.65) |
| Both | 0.97 (0.93–0.98) | 0.88 (0.81–0.93) | 0.61 (0.39–0.77) |
Abbreviations: AJCC, American Joint Committee on Cancer; IMRT, intensity-modulated radiation therapy.
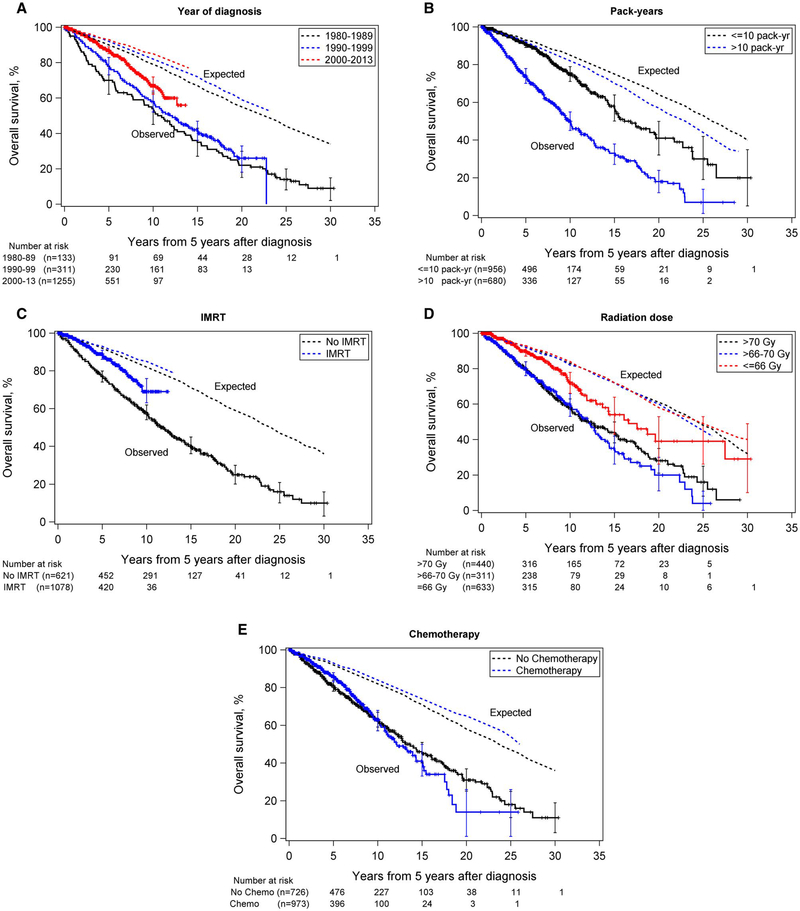
Figure 2 shows Kaplan-Meier estimates of OS by period of diagnosis, pack-years of smoking, IMRT, radiation dose, and chemotherapy along with corresponding expected survival probabilities from the general population. Although patients had worse survival than the general population regardless of the decade of diagnosis, survival improved over time; 67% of those diagnosed from 2000 through 2013 survived at least 10 years from time zero, compared with 58% from 1990–1999 and 54% from 1980–1989 (Fig. 2A and Table 2).
Figure 2.
Overall survival of patients with oropharyngeal cancer who had already survived 5 years after diagnosis by (A) year of diagnosis, (B) pack-years of smoking (including never smokers), (C) receipt of intensity-modulated radiation therapy (IMRT), (D) radiation dose, and (E) receipt of chemotherapy. Solid lines represent patients; dotted lines represent the corresponding study year-, age-, and sex-adjusted expected survival rates for the general US population. Vertical bars are 95% CIs. The time scale begins at 5 years after diagnosis.
Heavy smokers had significantly worse survival than light/never smokers (10-year rate: 50% for >10 vs 75% for ≤10 pack-years), and both groups had poorer survival than the general population (Fig. 2B and Table 2). Only patients diagnosed after 2000 received IMRT and had better survival than those who did not receive IMRT (Fig. 2C and Table 2). Lower radiation doses were associated with improved survival (10-year rate: 72% for ≤66 Gy vs 58% for >70 Gy, although some overlap between groups was observed; Fig. 2D and Table 2). Patients who received concurrent chemotherapy had the lowest 10-year rate, and patients who received adjuvant chemotherapy had the highest, although significant overlap was observed between groups (Fig. 2E and Table 2).
The fitted Bayesian piecewise exponential model is summarized in Table 3, including each covariate’s posterior mean hazard ratio, 95% Bayesian credible interval, and posterior probability of a beneficial effect (PBE) on OS for a larger value of the covariate. For example, PBE = 0.05 indicates that the covariate is associated with shorter survival, whereas PBE = 0.95 indicates that there is a 0.95 probability of longer survival time for larger values of the covariate. Older age, current/former smoking, other than tonsil or base of tongue tumor site, and T4 category were all independently associated with increased risk of death (lower PBE). Base of tongue tumor site, use of IMRT, and lower radiation dose (≤66 Gy vs >70 Gy) were associated with lower risk of death (larger PBE).
TABLE 3.
Factors Associated With Overall Survival of Patients With Oropharyngeal Cancer Who Survived at Least 5 Years After Diagnosis
| Subgroup | Posterior Mean HR (95% CRI) | Posterior Probability of Longer Overall Survival |
|---|---|---|
| Age (per 1-year increase) | 1.080 (1.069–1.092) | 0.0000 |
| Sex | ||
| Male | 1.000 | |
| Female | 0.932 (0.734–1.161) | 0.7422 |
| Smoking status | ||
| Never | 1.000 | |
| Former | 1.276 (1.002–1.597) | 0.0238 |
| Current | 2.158 (1.701–2.713) | 0.0000 |
| Site | ||
| Tonsil | 1.000 | |
| Base of tongue | 0.747 (0.606–0.908) | 0.9982 |
| Other | 1.337 (0.986–1.764) | 0.0303 |
| T category | ||
| T1 | 1.000 | |
| T2 | 1.024 (0.765–1.343) | 0.4630 |
| T3 | 1.224 (0.880–1.653) | 0.1195 |
| T4 | 1.482 (1.008–2.094) | 0.0225 |
| N category (AJCC 7th ed.) | ||
| N0 | 1.000 | |
| N1 | 1.056 (0.787–1.388) | 0.3812 |
| N2 | 1.009 (0.775–1.298) | 0.5026 |
| N3 | 1.259 (0.834–1.799) | 0.1411 |
| Neck dissection | ||
| No | 1.000 | |
| Yes | 0.990 (0.778–1.235) | 0.5539 |
| IMRT | ||
| No | 1.000 | |
| Yes | 0.776 (0.588–1.004) | 0.9735 |
| Radiation dose, Gy | ||
| >70 | 1.000 | |
| >66–70 | 1.067 (0.851–1.320) | 0.3004 |
| ≤66 | 0.718 (0.522–0.962) | 0.9863 |
| Chemotherapy | ||
| None | 1.000 | |
| Adjuvant only | 1.020 (0.728–1.375) | 0.4783 |
| Concurrent only | 1.019 (0.768–1.316) | 0.4679 |
| Both | 0.907 (0.528–1.427) | 0.6898 |
Abbreviations: AJCC, American Joint Committee on Cancer; CRI, Bayesian credible interval; HR, hazard ratio; IMRT, intensity-modulated radiation therapy.
For all subgroups except HPV-negative status, the SMRs were significantly larger than 1 compared with the general population, indicating a significantly higher mortality rate among patients (Table 4). Although the SMR for the HPV-negative group was >1, this was not statistically significant. Overall, mortality was 4 times higher among patients than the general population. Notably, the SMR for patients diagnosed in earlier periods was higher than for those more recently diagnosed (5.6 for 1980–1989 vs 2.9 for 2000–2013; Table 4). Current and high intensity of smoking were both associated with higher SMRs (7.4 for current smoker vs 2.8 for never smoker; 5.4 for >10 vs 2.7 for ≤10 pack-years). The SMR for patients receiving IMRT was 2.3, compared with 5.2 for those not receiving IMRT, and was lowest for patients who received a radiation dose in the lowest tertile (2.4 for ≤66 Gy vs 5.0 for >70 Gy). We believe that the nonsignificant result for HPV-negative patients is due to insufficient statistical power caused by the small sample size (N = 48).
TABLE 4.
Standardized Mortality Ratio Estimates for All-Cause Death of Patients With Oropharyngeal Cancer Who Survived at Least 5 Years After Diagnosis
| Subgroup | No. of Patients | Total Person-Years | No. of Observed Deaths | No. of Expected Deaths | SMR (95% CI) | P |
|---|---|---|---|---|---|---|
| Full study population | 1699 | 10,671.05 | 496 | 125.67 | 3.95 (3.60–4.29) | <.0001 |
| HPV status | ||||||
| Negative | 48 | 176.40 | 4 | 2.04 | 1.96 (0.04–3.88) | .3277 |
| Positive | 429 | 941.34 | 21 | 11.45 | 1.83 (1.05–2.62) | .0372 |
| Period of diagnosis | ||||||
| 1980–1989 | 133 | 1531.88 | 112 | 20.10 | 5.57 (4.54–6.60) | <.0001 |
| 1990–1999 | 311 | 3171.69 | 186 | 36.80 | 5.05 (4.33–5.78) | <.0001 |
| 2000–2013 | 1255 | 5967.48 | 198 | 68.77 | 2.88 (2.48–3.28) | <.0001 |
| Age at diagnosis, y | ||||||
| <55 | 401 | 3201.20 | 81 | 13.01 | 6.23 (4.87–7.58) | <.0001 |
| ≥55 | 1298 | 7469.85 | 415 | 112.66 | 3.68 (3.33–4.04) | <.0001 |
| Sex | ||||||
| Male | 1407 | 8625.97 | 377 | 102.67 | 3.67 (3.30–4.04) | <.0001 |
| Female | 292 | 2045.08 | 119 | 23.00 | 5.17 (4.24–6.10) | <.0001 |
| Smoking status | ||||||
| Never | 744 | 4692.57 | 136 | 49.37 | 2.75 (2.29–3.22) | <.0001 |
| Former | 571 | 3514.43 | 163 | 49.64 | 3.28 (2.78–3.79) | <.0001 |
| Current | 370 | 2411.98 | 194 | 26.10 | 7.43 (6.39–8.48) | <.0001 |
| Smoking, pack-y | ||||||
| >10 | 680 | 4220.84 | 292 | 54.09 | 5.40 (4.78–6.02) | <.0001 |
| ≤10 | 956 | 5913.59 | 171 | 62.47 | 2.74 (2.33–3.15) | <.0001 |
| Site | ||||||
| Tonsil | 773 | 4781.10 | 230 | 52.39 | 4.39 (3.82–4.96) | <.0001 |
| Base of tongue | 821 | 5184.03 | 194 | 62.65 | 3.10 (2.66–3.53) | <.0001 |
| Other | 105 | 705.92 | 72 | 10.63 | 6.77 (5.21–8.34) | <.0001 |
| T category | ||||||
| T1 | 523 | 3132.47 | 98 | 34.35 | 2.85 (2.29–3.42) | <.0001 |
| T2 | 639 | 4158.36 | 173 | 46.77 | 3.70 (3.15–4.25) | <.0001 |
| T3 | 353 | 2346.27 | 151 | 31.02 | 4.87 (4.09–5.64) | <.0001 |
| T4 | 183 | 1006.48 | 73 | 13.44 | 5.43 (4.18–6.68) | <.0001 |
| N category (AJCC 7th ed.) | ||||||
| N0 | 218 | 1621.86 | 124 | 25.49 | 4.86 (4.01–5.72) | <.0001 |
| N1 | 330 | 2388.96 | 101 | 27.45 | 3.68 (2.96–4.40) | <.0001 |
| N2 | 1045 | 5934.37 | 228 | 66.24 | 3.44 (3.00–3.89) | <.0001 |
| N3 | 105 | 698.39 | 42 | 6.41 | 6.56 (4.57–8.54) | <.0001 |
| Neck dissection | ||||||
| No | 1306 | 7966.37 | 387 | 97.50 | 3.97 (3.57–4.36) | <.0001 |
| Yes | 393 | 2704.67 | 109 | 28.17 | 3.87 (3.14–4.60) | <.0001 |
| IMRT | ||||||
| No | 621 | 6086.91 | 369 | 71.38 | 5.17 (4.64–5.70) | <.0001 |
| Yes | 1078 | 4584.13 | 127 | 54.30 | 2.34 (1.93–2.75) | <.0001 |
| Radiation dose, Gy | ||||||
| >70 | 440 | 3918.13 | 222 | 44.69 | 4.97 (4.31–5.62) | <.0001 |
| >66–70 | 622 | 3057.03 | 169 | 38.40 | 4.40 (3.74–5.07) | <.0001 |
| ≤66 | 633 | 3643.70 | 102 | 41.89 | 2.43 (1.96–2.91) | <.0001 |
| Chemotherapy | ||||||
| No | 726 | 5989.76 | 301 | 72.29 | 4.16 (3.69–4.63) | <.0001 |
| Yes | 973 | 4681.29 | 195 | 53.38 | 3.65 (3.14–4.17) | <.0001 |
Abbreviations: AJCC, American Joint Committee on Cancer; HPV, human papillomavirus; IMRT, intensity-modulated radiation therapy; SMR, standardized mortality ratio.
DISCUSSION
Among 1699 patients with newly diagnosed OPC treated with definitive radiation therapy (with or without chemotherapy) and alive without recurrence 5 years after diagnosis, the likelihood of living an additional 5 years was >80% and the likelihood of living an additional 10 years was 60%.
Although long-term survival rates for this population were good, they were lower than those of the general population. Based on this finding, we offer the following interpretations: 1) although patients with OPC who survive at least 5 years after diagnosis have very good long-term outcomes with low recurrence rate, their mortality rate nonetheless remains higher than that of the general population due to their index cancer; 2) unaccounted-for effects of treatment contribute to mortality; or 3) our population of patients was inherently at greater risk for death.
Regarding our first interpretation, although several authors have cautioned that for HPV-related OPC particularly, late recurrences can occur,6 the likelihood is very low.1,5 Only 5% of our patients had an HNC event in the time interval of interest. In many patients, it was difficult to distinguish between recurrent disease and a second primary tumor. In particular, hematogenous recurrences accounted for <2% of cancer events overall and approximately 40% of the HNC events, and we speculate that some hematogenous recurrences were related to second primary cancers. Finally, these few HNC events, even if they had all led to death (which they did not), would have accounted for <25% of the deaths observed in addition to the number of deaths expected.
Regarding our second interpretation, as more patients survive many years following treatment, more data are becoming available from which to gain insight into the long-term sequelae of high-dose radiation therapy, which is often combined with chemotherapy.20–22 Our group and others have described chronic radiation-associated dysphagia and late cranial neuropathies contributing to dysphagia.22–26 These late dysphagia events, particularly in older patients,27 can lead to aspiration, which either directly or indirectly can lead to a mortal event.28,29 Based on our results, we speculate that radiation sequelae contribute to mortality. Increased risk of death with higher T category is commonly attributed to higher risk of recurrence. However, the vast majority of our patients remained recurrence-free. Although it is possible that the T category was a surrogate for a larger volume of pharynx receiving high doses of radiation, we found no evidence of a joint effect of T category and radiation dose. Perhaps a more plausible explanation is that T4 tumors frequently present with functional compromise that does not completely resolve following treatment.
An important finding about the long-term sequelae of aggressive therapy is that IMRT, specifically designed to avoid structures within the head and neck that do not need radiation, resulted in 5-year and 10-year OS rates >10% higher than in patients treated with non-IMRT techniques. SEER-Medicare analyses from our group have demonstrated both improved cause-specific survival rates and lower gastrostomy rates in patients with HNC treated with IMRT.30,31 Although the improvement in cause-specific survival was likely due to better target coverage, both analyses suggested that toxicity reduction with the use of IMRT was also important.
We also found that chemotherapy had only a weak negative impact on long-term outcomes. In the RTOG 91–11 report, the seminal RTOG trial testing nonsurgical approaches for advanced laryngeal cancer, deaths not attributed to cancer or obvious toxic effects were more frequent in patients treated concurrently with chemotherapy and radiation.8 The authors believed that this could have been secondary to fatal treatment-related episodes not identified by the monitoring techniques used. We did not reproduce this finding; in our study, patients who received concurrent chemotherapy or radiation alone had similar survival rates.
Our third interpretation relates to an increased risk of death within the study population irrespective of the index disease and therapy. The observed differences in survival based on smoking history support this. Ten-year survival rates were 75% for patients with a smoking history of ≤10 pack-years compared with 50% for those with >10 pack-years, and the SMR in those who smoked >10 pack-years was nearly double the SMR in those who smoked less or not at all. The prevalence of smoking among adults in the United States in 2005 was 21%,32 whereas 22% of our patients were current and 34% were former smokers, suggesting that our population had a greater exposure to cigarettes that, in part, led to a higher SMR. Additionally, the lower prevalence of smoking among patients diagnosed during 2000–2013 (52%) compared with those diagnosed prior to 2000 (69% for 1980–1989 and 64% for 1990–1999) could have contributed to improved survival over time. However, despite clear correlation of poorer outcome with smoking, our never-smoker subgroup had an SMR = 2.8.
One limitation of our study was that cause of death was not captured, allowing only speculation on the causes of lower long-term survival rates in patients than the general population. As mentioned, late cranial neuropathies and dysphagia with associated aspiration are clear effects of therapy. Conversely, an increased risk of stroke among patients who undergo irradiation of the neck has been observed,33–35 yet carotid ischemic events are a common cause of morbidity and mortality in the general population, so attribution of our observation to radiation, particularly in the absence of pretreatment and posttreatment carotid imaging, is a challenge. Additional potentially life-threatening sequelae of irradiation of the neck, including baroreceptor dysfunction,36 are just starting to be recognized. Ultimately, the hypothesized negative impact on conditional survival of having OPC treated with definitive radiation is multifactorial.
Another limitation was the lack of known HPV status for the majority of patients. HPV prevalence in OPC tumors has increased dramatically over the past few decades in the United States, and HPV positivity is a strong independent predictor of better survival.2,3 However, this is mainly due to better disease control in the first few years from treatment. It is unlikely that tumor HPV status directly impacts conditional survival, though the underlying demographics of patients with HPV-related tumors (younger age, less comorbidity, less tobacco exposure) may have some impact on our findings.
Although our finding of improved survival among patients with OPC in the more recent time period could be ascribed to stage migration (ie, patients more recently diagnosed being assigned a higher stage due to advancements in technology allowing detection of more disease), this is unlikely. Under the assumption that a patient does not die of disease, stage can only theoretically be impactful if 1) it influenced the intensity of treatment, and survival is impacted by treatment intensity (ie, more intense correlates and late toxicity that impacts survival); or 2) the original stage had not only more extensive disease but that disease caused damage that influenced late survival (eg, more extensive disease caused tongue impairment that led to the patient aspirating). In conclusion, the overall 10-year conditional survival rate (15 years from diagnosis) was 63% among 1699 patients with OPC treated with radiation and cancer-free 5 years after diagnosis. Patients treated with IMRT and those with less tobacco exposure had better outcomes but still had poorer outcomes when compared with the general population.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
We acknowledge funding contributions from the Christopher and Susan Damico Chair in Viral Associated Malignancies (MD Anderson Cancer Center). This research was conducted within the Oropharynx Program at MD Anderson Cancer Center and funded in part through the Stiefel Oropharyngeal Research Fund. Our work is supported by the National Institutes of Health/National Cancer Institute under award number P30CA016672.
CONFLICT OF INTEREST DISCLOSURES
Kristina R. Dahlstrom reports other support from Roche Diagnostics outside the submitted work. Clifton D. Fuller reports grants from the National Science Foundation and from the National Institutes of Health (NIH) during the conduct of the study; grants from NIH; grants, personal fees, and nonfinancial support from Elekta AB; personal fees from the American Association of Physicists in Medicine, the National Institutes of Health, Tianjin Medical University Cancer Institute and Hospital, University of Illinois-Chicago, and the Netherlands Cancer Institute outside the submitted work. Katherine A. Hutcheson reports grants from Patient Center Outcomes Research Institute, NIH/NCI, and NIH/NIDCR; a MD Anderson Institutional Research Grant Program Survivorship Seed Monies Award; grant from ASH (sub. award through Stanford University); University of Texas MD Anderson Cancer Center Institutional Research Grant; grants from the University of Texas MD Anderson Cancer Center-Oropharynx Cancer Program, generously supported by Mr. and Mrs. Charles W. Stiefel; and personal fees from Medbridge, Inc, outside the submitted work. Steven J. Frank reports grants from Hitachi and Eli Lilly; personal fees from Varian and Boston Scientific; grants, personal fees, and other support from C4 Imaging; other support from NCCN outside the submitted work. Renata Ferrarotto reports personal fees from Regeneron-Sanofi, Ayala, Klus, Carevive, Prelude, and Medscape outside the submitted work. Erich M. Sturgis reports other support from Roche Diagnostics, Inc, outside the submitted work. All other authors have nothing to disclose.
Footnotes
We used the MD Anderson Cancer Center Biostatistics Resource Group. Stephanie Deming of the Department of Scientific Publications at MD Anderson provided editorial assistance.
Additional supporting information may be found in the online version of this article.
REFERENCES
- 1.Pignon JP, le Maitre A, Maillard E, Bourhis J. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother Oncol 2009;92: 4–14. [DOI] [PubMed] [Google Scholar]
- 2.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst 2008;100:261–269. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ang KK, Sturgis EM. Human papillomavirus as a marker of the natural history and response to therapy of head and neck squamous cell carcinoma. Semin Radiat Oncol 2012;22:128–142. [DOI] [PubMed] [Google Scholar]
- 5.Fakhry C, Zhang Q, Nguyen-Tan PF, et al. Human papillomavirus and overall survival after progression of oropharyngeal squamous cell carcinoma. J Clin Oncol 2014;32:3365–3373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.O’Sullivan B, Adelstein DL, Huang SH, et al. First site of failure analysis incompletely addresses issues of late and unexpected metastases in p16-positive oropharyngeal cancer. J Clin Oncol 2015;33:1707–1708. [DOI] [PubMed] [Google Scholar]
- 7.Gillison ML, Trotti AM, Harris J, et al. Radiotherapy plus cetuximab or cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet. 2019;393:40–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Forastiere AA, Zhang Q, Weber RS, et al. Long-term results of RTOG 91–11: a comparison of three nonsurgical treatment strategies to preserve the larynx in patients with locally advanced larynx cancer. J Clin Oncol 2013;31:845–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beitler JJ, Zhang Q, Fu KK, et al. Final results of local-regional control and late toxicity of RTOG 9003: a randomized trial of altered fractionation radiation for locally advanced head and neck cancer. Int J Radiat Oncol Biol Phys 2014;89:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hieke S, Kleber M, Konig C, Engelhardt M, Schumacher M. Conditional survival: a useful concept to provide information on how prognosis evolves over time. Clin Cancer Res 2015;21:1530–1536. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim JG, Chen M-H, Sinjha D. Bayesian Survival Analysis. Springer Series in Statistics, 2001. [Google Scholar]
- 12.Spiegelhalter DJ, Best NG, Carlin BP, Van Der Linde A. Bayesian measures of model complexity and fit. J R Stat Soc Series B Stat Methodol 2002;64:583–639. [Google Scholar]
- 13.Gail MH, Ware JH. Comparing observed life table data with a known survival curve in the presence of random censorship. Biometrics. 1979;35:385–391. [Google Scholar]
- 14.Woolson RF. Rank tests and a one-sample logrank test for comparing observed survival data to a standard population. Biometrics. 1981;37:687–696. [Google Scholar]
- 15.Finkelstein DM, Muzikansky A, Schoenfeld DA. Comparing survival of a sample to that of a standard population. J Natl Cancer Inst 2003;95:1434–1439. [DOI] [PubMed] [Google Scholar]
- 16.Human Mortality Database. University of California, Berkeley (USA), and Max Planck Institute for Demographic Research (Germany). Available at www.mortality.org. [Accessed June 17, 2019]. [Google Scholar]
- 17.National Center for Health Statistics. Vital Statistics of the United States, Volume II: Mortality, Part A. Washington, D.C.: Government Printing Office, 1980–2018. [Data obtained through the Human Mortality Database, www.mortality.org. [Accessed June 17, 2019]. [Google Scholar]
- 18.Cho H, Howlader N, Mariotto A. Estimating Relative Survival for Cancer Patients from the SEER Program Using Expected Rates Based on Ederer I Versus Ederer II Method. National Cancer Institute Surveillance Research Program; 2011. Technical report #2011–01. Available at https://surveillance.cancer.gov/reports/tech2011.01.pdf. [Accessed July 15, 2019]. [Google Scholar]
- 19.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 20.Bonner JA, Giralt J, Harari PM, et al. Association of human papillomavirus and p16 status with mucositis and dysphagia for head and neck cancer patients treated with radiotherapy with or without cetuximab: assessment from a phase 3 registration trial. Eur J Cancer. 2016;64:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ringash J, Fisher R, Peters L, et al. Effect of p16 status on the quality-of-life experience during chemoradiation for locally advanced oropharyngeal cancer: a substudy of randomized trial Trans-Tasman Radiation Oncology Group (TROG) 02.02 (HeadSTART). Int J Radiat Oncol Biol Phys 2017;97:678–686. [DOI] [PubMed] [Google Scholar]
- 22.Truong MT, Zhang Q, Rosenthal DI, et al. Quality of life and performance status from a substudy conducted within a prospective phase 3 randomized trial of concurrent accelerated radiation plus cisplatin with or without cetuximab for locally advanced head and neck carcinoma: NRG Oncology Radiation Therapy Oncology Group 0522. Int J Radiat Oncol Biol Phys 2017;97:687–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hutcheson KA, Lewin JS, Barringer DA, et al. Late dysphagia after radiotherapy-based treatment of head and neck cancer. Cancer. 2012;118:5793–5799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goepfert RP, Lewin JS, Barrow MP, et al. Long-term, prospective performance of the MD Anderson Dysphagia Inventory in “low-intermediate risk” oropharyngeal carcinoma after intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys 2017;97:700–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hutcheson KA, Yuk M, Hubbard R, et al. Delayed lower cranial neuropathy after oropharyngeal intensity-modulated radiotherapy: a cohort analysis and literature review. Head Neck. 2017;39:1516–1523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dong Y, Ridge JA, Ebersole B, et al. Incidence and outcomes of radiation-induced late cranial neuropathy in 10-year survivors of head and neck cancer. Oral Oncol 2019;95:59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.MD Anderson Head and Neck Cancer Symptom Working Group. Chronic radiation-associated dysphagia in oropharyngeal cancer survivors: towards age-adjusted dose constraints for deglutitive muscles. Clin Transl Radiat Oncol 2019;18:16–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aggarwal P, Zaveri JS, Goepfert RP, et al. Symptom burden associated with late lower cranial neuropathy in long-term oropharyngeal cancer survivors. JAMA Otolaryngol Head Neck Surg 2018;144:1066–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggarwal P, Zaveri JS, Goepfert RP, et al. Swallowing-related outcomes associated with late lower cranial neuropathy in long-term oropharyngeal cancer survivors: cross-sectional survey analysis. Head Neck. 2019;41:3880–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Beadle BM, Liao KP, Elting LS, et al. Improved survival using intensity-modulated radiation therapy in head and neck cancers: a SEER-Medicare analysis. Cancer. 2014;120:702–710. [DOI] [PubMed] [Google Scholar]
- 31.Beadle BM, Liao KP, Giordano SH, et al. Reduced feeding tube duration with intensity-modulated radiation therapy for head and neck cancer: a surveillance, epidemiology, and end results-Medicare analysis. Cancer. 2017;123:283–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jamal A, King BA, Neff LJ, Whitmill J, Babb SD, Graffunder CM. Current cigarette smoking among adults—United States, 2005–2015. MMWR Morb Mortal Wkly Rep 2016;65:1205–1211. [DOI] [PubMed] [Google Scholar]
- 33.Smith GL, Smith BD, Buchholz TA, et al. Cerebrovascular disease risk in older head and neck cancer patients after radiotherapy. J Clin Oncol 2008;26:5119–5125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Plummer C, Henderson RD, O’Sullivan JD, Read SJ. Ischemic stroke and transient ischemic attack after head and neck radiotherapy: a review. Stroke. 2011;42:2410–2418. [DOI] [PubMed] [Google Scholar]
- 35.Arthurs E, Hanna TP, Zaza K, Peng Y, Hall SF. Stroke after radiation therapy for head and neck cancer: what is the risk? Int J Radiat Oncol Biol Phys 2016;96:589–596. [DOI] [PubMed] [Google Scholar]
- 36.Shah-Becker S, Pennock M, Sinoway L, Goldenberg D, Goyal N. Baroreceptor reflex failure: review of the literature and the potential impact on patients with head and neck cancer. Head Neck. 2017;39:2135–2141. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.