Abstract
Introduction:
Despite rapid population ageing, there are currently limited data on the incidence of ageing-related cognitive impairment in sub-Saharan Africa. We aimed to determine the incidence of cognitive impairment and its distribution across key demographic, social, and health-related factors among older adults in rural South Africa.
Methods:
Data were from in-person interviews with 3,856 adults aged ≥40 who were free from cognitive impairment at baseline in the population-representative cohort, “Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa” (HAALSI), in Agincourt sub-district, Mpumalanga province, South Africa (2014−−19). Cognitive impairment was defined as scoring <1.5 standard deviations below the mean of the baseline distribution of orientation and episodic memory scores. Incidence rates (IRs) and rate ratios (IRRs) for cognitive impairment were estimated according to key demographic, social, and health-related factors, adjusted for age, sex/gender, and country of birth.
Results:
The incidence of cognitive impairment was 25.7/1,000 person-years (PY; 95% confidence interval [CI]: 23.0−−28.8), weighted for mortality (12%) and attrition (6%) over the 3.5-year mean follow-up (range: 1.5 to 4.8 years). Incidence increased with age, from 8.9/1,000 PY (95% CI: 5.2−−16.8) among those aged 40−−44 to 93.5/1,000 PY (95% CI: 75.9−−116.3) among those aged 80+, and age-specific risks were similar by sex/gender. Incidence was strongly associated with formal education and literacy, as well as marital status, household assets, employment, and alcohol consumption, but not with history of smoking, hypertension, stroke, angina, heart attack, diabetes, or, prevalent HIV.
Conclusions:
This study presents some of the first incidence rate estimates for ageing-related cognitive impairment in rural South Africa. Social disparities in incident cognitive impairment rates were apparent in patterns similar to those observed in many high-income countries.
Keywords: Africa, Cognitive impairment, Cohort studies, Community-based study, Epidemiology, Incidence
Introduction
By 2050, nearly 75% of all cases of Alzheimer’s disease and related dementias are projected to occur in low- and middle-income countries (LMICs), such as those in sub-Saharan Africa (1). Dementia is a syndrome marked by cognitive impairment that limits an individual’s everyday function. Cognitive impairment occurs when when a person has trouble remembering, learning new things, concentrating, or making decisions (2). While cognitive impairment in the absence of functional limitations has many aetiologies, in older adults it will often lead to dementia or Alzheimer’s disease, particularly when memory is involved (3). Preserving older adults’ cognitive function is important for the health, productivity, and well-being of ageing societies (4). Population-based data from LMICs are urgently needed to aid in the design and distribution of resources and policies to protect the cognitive health of ageing populations in these global regions (1,5,6).
Although prevalence estimates for cognitive impairment and dementia for some sub-Saharan African regions have been provided over the past decade (7–15), there remains a paucity of longitudinal, population-based data on the incidence of cognitive impairment or dementia. A 2014 systematic review identified four longitudinal studies, all in Nigeria, which observed dementia incidence ranging from 8.7 to 21.8 cases per 1000 person-years, and one study, which observed incidence of mild cognitive impairment of 16.4 cases per 1000 person-years (14). To the best of our knowledge, there is not more recent population-based evidence on the incidence of ageing-related cognitive impairment or dementia in sub-Saharan Africa.
Previous research in high-income countries suggests that the incidence of cognitive impairment with and without dementia is decreasing over time, which is thought to be in large part due to increasing educational attainment across successive birth cohorts (16,17). It is unclear whether this will also be the case in sub-Saharan Africa. Older adults born in the early-to-mid-twentieth century in sub-Saharan Africa have different cumulative lifetime exposures to cognitive risk factors than older adults in high-income countries, including access to formal education (18–21). Other factors relevant to ageing populations in some global settings, such as chronic HIV infection, are poorly understood as potential risk factors for cognitive ageing outcomes.
We thus aimed to determine the incidence of ageing-related cognitive impairment and its distribution across key sociodemographic, social, and health-related factors at the first follow-up of “Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa” (HAALSI).
Materials and Methods
Study design and population
HAALSI is a population-representative, longitudinal cohort of adults aged ≥40 in rural Agincourt sub-district, Mpumalanga province, South Africa (22). HAALSI is an International Partner Study to the US Health and Retirement Study (HRS). HAALSI is representative of its underlying sampling frame of the Agincourt Health and socio-Demographic Surveillance System (AHDSS), which covers a population of approximately 120 000 people living across 31 villages in an area of approximately 450km2 (22–24). Data collection involves in-person interviews in the Shangaan language (xiTsonga) with trained, local fieldworkers using computer-assisted personal interviewing.
Neuropsychological evaluation
A brief cognitive function assessment adapted from the US HRS was administered in the study interview at the baseline (wave 1) and follow-up (wave 2) (25,26). Orientation was assessed as the ability to state the current day, month, year, and South African president (one point each), and episodic memory was assessed as immediate and delayed recall of 10 words read out loud by the study interviewer (20 points total). At wave 2, three immediate recall trials were conducted; we used score on the first trial for comparability with the wave 1 assessment.
Cognitive impairment classification
Cognitive impairment was defined as scoring ≥1.5 standard deviations (SD) below the mean of the baseline cognitive function distribution on the cognitive assessment administered at wave 2, or, requiring a proxy interview at wave 2 with proxy-reported “fair” or “poor” memory and/or having the reason for the proxy interview recorded as due to cognitive impairment (27). Our outcome thus refers to any cognitive impairment, including probable dementia as well as cognitive impairment without dementia (CIND). Proxy interviews were conducted with a family member or friend when the participant was unable to complete the interview, with the main reasons being severe cognitive impairment, severe physical illness, or deafness. Of the 171 new proxy interviews at wave 2, 141 (82%) were classified as newly cognitively impaired, with 122/171 (71%) having proxy-reported “fair” or “poor” memory and 73/171 (43%) recorded as having the proxy interview due to cognitive impairment.
Sociodemographic, social, and health-related characteristics
We described incident cognitive impairment according to baseline demographic, social, and health-related factors relevant to this study population that are known or plausible risk factors for cognitive impairment and dementia: age, sex/gender, country of birth, years of formal education, literacy, employment status, marital status, asset-based household wealth quintile (28), smoking status, frequency of alcohol consumption, previous physician diagnosis of stroke, angina, or heart attack, hypertension, diabetes, and HIV status. In this population, country of birth is an important social determinant of health as a substantial number of people, also from the Shangaan ethnic group, came to the Agincourt region as refugees from the 1977−−1992 Mozambique civil war (29).
All variables were self-reported during the interview except for hypertension and HIV status. Hypertension was defined as a measured mean systolic blood pressure >140 mmHg or mean diastolic blood pressure >90 mmHg, or controlled blood pressure with self-reported use of hypertension medication. HIV status was determined through HIV enzyme-linked immunosorbent assays on dried blood spots (30). Diabetes was defined as any of a fasting glucose ≥7.0 mmol/L, a random plasma glucose ≥11.1 mmol/L, a self‐reported diagnosis of diabetes, or, self‐reported use of a prescribed diabetes medication.
Statistical analyses
At-risk person-time was calculated as years accrued between the baseline and follow-up interviews, with incident outcomes assumed to occur halfway between interviews. We estimated incidence rates (IRs) of cognitive impairment per 1000 person-years, overall and according to covariates. We estimated age-specific IRs by sex/gender. Poisson regression models with a log link function and robust error variance were used to estimate incidence rate ratios (IRRs) and 95% confidence intervals (CIs) for cognitive impairment (31). Models for age were not adjusted for other variables, models for sex/gender were age-adjusted, models for country of birth were age- and sex/gender-adjusted, and models for all other covariates were adjusted for age, sex/gender, and country of birth. The probabilities of mortality in the full baseline sample and drop-out among the surviving baseline sample were used to create inverse probability of censoring weights that jointly accounted for the probabilities of mortality and study drop-out over the follow-up period. All models incorporated these weights.
We examined the prevalence of limitations to activities of daily living (ADLs) and instrumental activities of daily living (IADLs) at the follow-up according to incident cognitive impairment status. We cannot attribute the cause of any ADL or IADL limitation to cognitive impairment, but we would expect to see substantially higher ADL and IADL limitations among older adults with an incident cognitive impairment, potentially indicative of dementia. We conducted a sensitivity analyses to assess the incidence of severe cognitive impairment using a threshold of scoring ≥2.0 SD below the baseline mean distribution, and examined ADL and IADL limitations according to this threshold. All analyses were conducted using StataSE 16.0 (College Station, TX). The HAALSI data are publicly available at https://www.haalsi.org.
Results
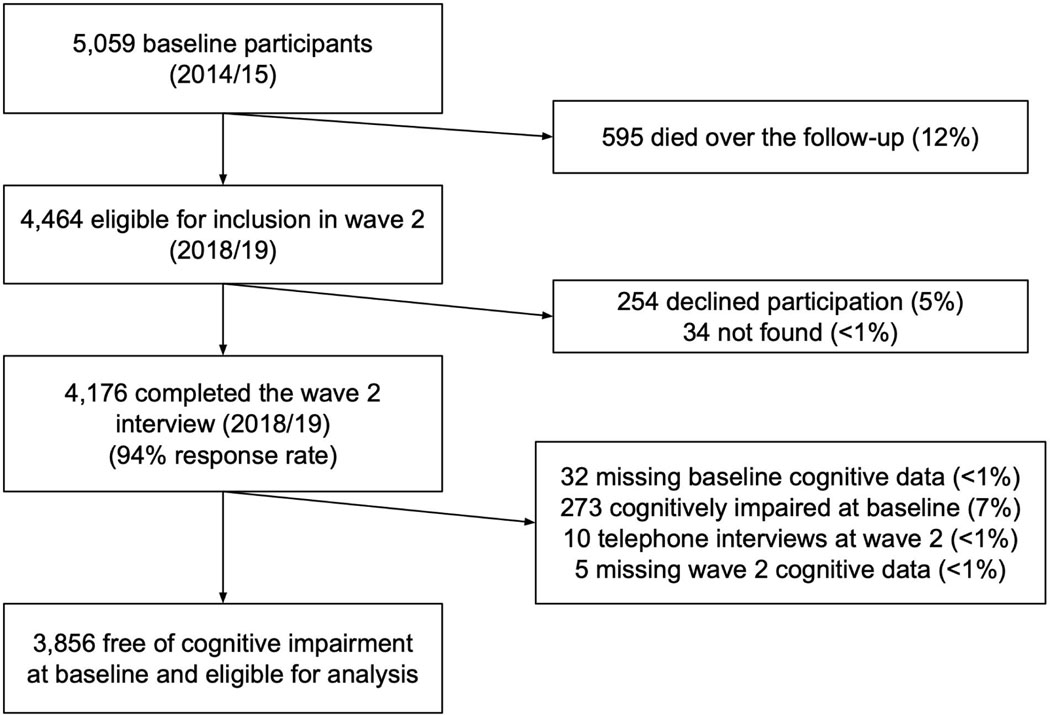
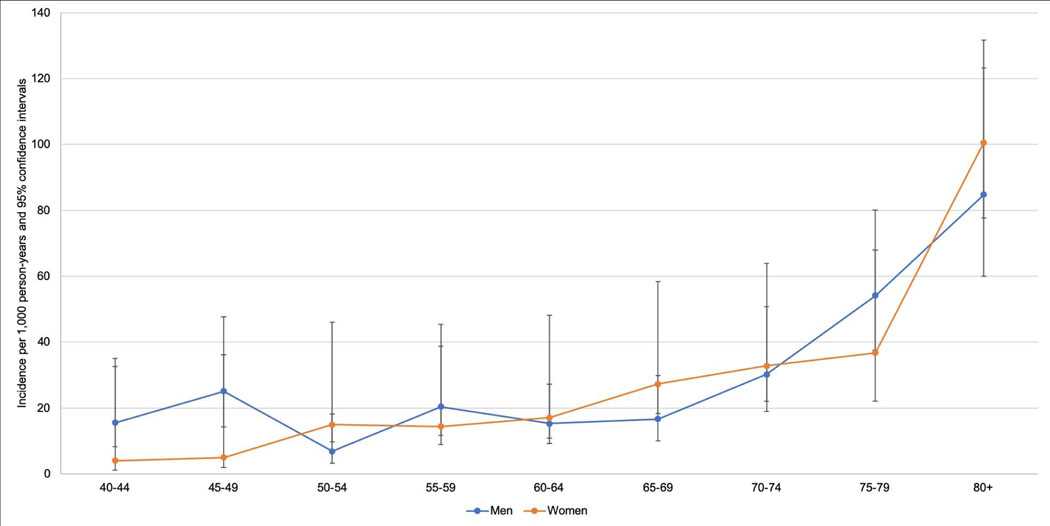
A total of 3,856 participants were included (Figure 1). Mean age at baseline was 60.1 years (SD: 12.2 years) and 55% were female (2,121/3,856; Table 1). Over a mean follow-up of 3.5 years of at-risk person-time (SD: 0.5; range: 1.5−−4.8), 8% of participants newly developed cognitive impairment (320/3,856). This translated to an incidence rate (IR) of 25.7/1,000 person-years (PY) of observation (95% CI: 23.0–28.8; Table 2). There was a significant linear trend in age-specific incidence, with IRR = 1.34 (95% CI: 1.28−−1.41) per 5-year age increase (Table 2). We observed minimal sex/gender differences in the age-specific risks of incident cognitive impairment (Figure 2; interaction P-value = 0.07).
Fig. 1.
Study flow diagram.
Table 1.
Baseline characteristics, “Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa” (HAALSI), Agincourt, South Africa, 2014–19
| Baseline Characteristic | Total eligible, at-risk participants |
|---|---|
| Overall, No. % | 3,856 (100%) |
| Age, y | |
| Mean (SD, range) | 60.1 (12.2, 40–111) |
| 40–44 | 377 (10%) |
| 45–49 | 372 (10%) |
| 50–54 | 590 (15%) |
| 55–59 | 526 (14%) |
| 60–64 | 555 (14%) |
| 65–69 | 485 (13%) |
| 70–74 | 375 (10%) |
| 75–79 | 273 (7%) |
| 80+ | 303 (8%) |
| Sex/gender, No. % | |
| Female | 2,121 (55%) |
| Male | 1,735 (45%) |
| Country of birth, No. % | |
| South Africa | 2,730 (71%) |
| Mozambique or other | 1,123 (29%) |
| Educational attainment, No. % | |
| Mean years (SD, range) | 3.9 (4.3, 0–14) |
| 0 years | 1,607 (42%) |
| 1–7 years | 1,386 (36%) |
| 8–11 years | 487 (13%) |
| 12+ years | 368 (10%) |
| Literacy, No. % | |
| Cannot read and/or write | 1,716 (45%) |
| Can read and write | 2,138 (55%) |
| Employment status, No. % | |
| Employed | 662 (17%) |
| Not working | 2,765 (72%) |
| Homemaker | 418 (11%) |
| Marital status, No. % | |
| Married or living as married | 2,066 (54%) |
| Not married | 1,788 (46%) |
| Household assets quintile, No. % | |
| 5 (richest) | 806 (21%) |
| 4 | 792 (21%) |
| 3 | 762 (20%) |
| 2 | 754 (20%) |
| 1 (poorest) | 742 (19%) |
| Smoking history, No. % | |
| Never smoker | 3,065 (80%) |
| Current or former smoker | 787 (20%) |
| Alcohol consumption ≥five days/week, No. % | |
| Yes | 218 (6%) |
| No | 3,635 (94%) |
| Hypertension, No. % | |
| Yes | 2,195 (58%) |
| No | 1,599 (42%) |
| History of stroke, angina, or heart attack, No. % | |
| Yes | 193 (5%) |
| No | 3,661 (95%) |
| History of diabetes, No. % | |
| Yes | 404 (11%) |
| No | 3,179 (89%) |
| HIV status (DBS), No. % | |
| Positive | 850 (24%) |
| Negative | 2,682 (76%) |
Note: The N for each baseline characteristic may not sum to 3,856, due to a small number of missing observations
Table 2.
Incidence of cognitive impairment overall and according to key baseline characteristics, “Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa” (HAALSI), Agincourt, South Africa, 2014–19
| Baseline characteristic | N cases, unweighted | Unweighted person-years | Weighted person-years | Incidence rate (IR), per 1000-person years (95% CI) | Incidence rate ratio (IRR) (95% CI) |
|---|---|---|---|---|---|
| Overall | 320 | 13,579.9 | 16,170.1 | 25.7 (23.0, 28.8) | - |
| Age | |||||
| 40–44 | 12 | 1,352.3 | 1,537.8 | 8.9 (5.2, 16.8) | 1.00 (ref) |
| 45–49 | 16 | 1,325.5 | 1,491.5 | 13.6 (8.4, 23.6) | 1.51 (0.72, 3.17) |
| 50–54 | 25 | 2,123.7 | 2,449.9 | 11.6 (7.9, 17.6) | 1.30 (0.66, 2.56) |
| 55–59 | 30 | 1,866.3 | 2,179.9 | 17.0 (11.8, 25.5) | 1.88 (0.97, 3.67) |
| 60–64 | 32 | 1,998.7 | 2,325.1 | 16.2 (11.6, 23.4) | 1.82 (0.95, 3.50) |
| 65–69 | 37 | 1,720.0 | 2,030.6 | 22.0 (16.0, 30.8) | 2.42 (1.28, 4.60) |
| 70–74 | 40 | 1,304.6 | 1,596.8 | 31.5 (23.3, 43.7) | 3.41 (1.82, 6.42) |
| 75–79 | 42 | 928.2 | 1,195.3 | 47.3 (35.2, 64.9) | 4.99 (2.66, 9.33) |
| 80+ | 86 | 960.6 | 1,363.2 | 93.5 (75.9, 116.3) | 9.21 (5.13, 16.5) |
| Per 5-year age increase | - | - | - | - | 1.34 (1.28, 1.41) |
| P for linear trend | - | - | - | - | <0.0001 |
| Sex/gender | |||||
| Male | 144 | 6,087.5 | 7,531.9 | 26.7 (22.6, 31.7) | 1.00 (ref) |
| Female | 176 | 7,492.4 | 8,638.1 | 24.9 (21.5, 29.0) | 0.98 (0.80, 1.20) |
| Country of birth | |||||
| South Africa | 179 | 9,687.8 | 11,618.3 | 20.6 (17.8, 24.0) | 1.00 (ref) |
| Mozambique or other | 141 | 3,880.8 | 4,539.0 | 38.8 (33.0, 46.1) | 1.62 (1.31, 2.00) |
| Educational attainment | |||||
| 0 years | 232 | 5,486.2 | 6,610.6 | 45.8 (40.3, 52.3) | 1.00 (ref) |
| 1–7 years | 61 | 5,010.8 | 5,926.4 | 13.5 (10.5, 17.6) | 0.44 (0.33, 0.58) |
| 8–11 years | 20 | 1,731.3 | 2,035.9 | 12.0 (7.8, 19.2) | 0.54 (0.33, 0.88) |
| 12+ years | 5 | 1,324.9 | 1,564.4 | 3.8 (1.6, 11.3) | 0.21 (0.09, 0.53) |
| Per year of education | – | – | 0.90 (0.86, 0.94) | ||
| Literacy | |||||
| Can read and write | 82 | 7,671.1 | 9,055.7 | 11.6 (9.4, 14.6) | 1.00 (ref) |
| Cannot read and/or write | 238 | 5,901.0 | 7,104.9 | 43.8 (38.5, 49.9) | 2.36 (1.80, 3.10) |
| Employment status | |||||
| Not working | 268 | 9,687.4 | 11,632.8 | 30.3 (26.8, 34.3) | 1.00 (ref) |
| Homemaker | 35 | 1,475.7 | 1,721.5 | 25.5 (18.4, 34.3) | 0.87 (0.62, 1.20) |
| Employed | 16 | 2,378.4 | 2,769.8 | 6.8 (4.2, 11.6) | 0.46 (0.28, 0.77) |
| Marital status | |||||
| Married | 122 | 7,362.5 | 8,677.7 | 17.7 (14.9, 21.3) | 1.00 (ref) |
| Not married | 197 | 6,211.9 | 7,484.6 | 34.8 (30.2, 40.2) | 1.84 (1.42, 2.39) |
| Household asset quintile | |||||
| 5 (richest) | 27 | 2,900.0 | 3,420.9 | 9.9 (6.9, 14.9) | 1.00 (ref) |
| 4 | 47 | 2,830.6 | 3,342.1 | 18.1 (13.6, 24.4) | 1.62 (1.02, 2.55) |
| 3 | 62 | 2,684.6 | 3,207.3 | 26.0 (20.3, 33.9) | 2.15 (1.38, 3.34) |
| 2 | 82 | 2,621.8 | 3,169.6 | 34.6 (27.8, 43.5) | 2.64 (1.73, 4.05) |
| 1 (poorest) | 102 | 2,542.9 | 3,030.0 | 42.5 (35.0, 51.9) | 3.27 (2.14, 5.01) |
| Per quintile decrease | - | - | - | 1.31 (1.20, 1.42) | |
| Smoking history | |||||
| Never smoker | 258 | 10,785.3 | 12,732.6 | 26.1 (23.1, 29.6) | 1.00 (ref) |
| Current or former smoker | 62 | 2,780.8 | 3,419.6 | 24.6 (19.2, 32.1) | 1.09 (0.81, 1.49) |
| Alcohol consumption ≥5 days/week | |||||
| No | 288 | 12,829.1 | 15,248.1 | 24.6 (21.9, 27.7) | 1.00 (ref) |
| Yes | 32 | 740.3 | 905.5 | 45.2 (32.2, 65.2) | 1.68 (1.19, 2.37) |
| Hypertension | |||||
| No | 109 | 5,689.3 | 6,728.6 | 21.2 (17.5, 25.8) | 1.00 (ref) |
| Yes | 206 | 7,676.5 | 9,174.8 | 29.2 (25.4, 33.6) | 1.03 (0.83, 1.29) |
| History of stroke, angina, or heart attack | |||||
| No | 298 | 12,900.3 | 15,344.8 | 25.1 (22.4, 28.2) | 1.00 (ref) |
| Yes | 22 | 672.9 | 817.0 | 38.5 (25.3, 61.3) | 1.45 (0.96, 2.19) |
| History of diabetes | |||||
| No | 259 | 11,215.2 | 13,319.9 | 25.0 (22.1, 28.4) | 1.00 (ref) |
| Yes | 39 | 1,415.7 | 1,698.7 | 30.3 (22.1, 42.5) | 1.05 (0.77, 1.44) |
| HIV status (DBS) | |||||
| Negative | 245 | 9,419.2 | 11,142.2 | 28.2 (24.9, 32.0) | 1.00 (ref) |
| Positive | 52 | 3,030.7 | 3,619.3 | 19.1 (14.5, 25.8) | 1.03 (0.76, 1.40) |
Note: Person-years for each baseline characteristic may not add to the total person-years, due to a small number of missing observations.
Note: IRs and IRRS are weighted to account for the probabilities of mortality and study attrition over the follow-up.
Note: Model for age is unadjusted; Model for sex/gender is age-adjusted; Model for country of birth is age- and sex/gender-adjusted; Models for all other variables are adjusted for age, sex/gender, and country of birth.
Fig. 2.
Age-specific incidence rates of cognitive impairment, by sex/gender (weighted).
Incident cognitive impairment was strongly graded according to formal education (IRR = 0.21, 95% CI: 0.09−−0.53 for 12+ years vs. 0 years) and literacy (IRR = 2.36; 95% CI: 1.80−−3.10 for non-literate vs. literate), as well as marital status, household assets, employment status, and frequency of alcohol consumption (Table 2). There was a weakly positive but imprecise association between incident cognitive impairment and history of stroke, angina, or heart attack (Table 2). ADL and IADL limitations strongly differed by cognitive impairment status (Table 3). Three-quarters of ADL limitations among those with an incident cognitive impairment were also incident over the follow-up (73%; 79/108), suggesting that they co-occurred with the cognitive impairment. The incidence of severe cognitive impairment was 17.4/1000 PY (95% CI: 15.2−−20.0), representing one-third of all cases (Supplementary Material).
Table 3.
Prevalence of ADL and IADL impairment according to incident cognitive impairment, “Health and Ageing in Africa: A Longitudinal Study of an INDEPTH Community in South Africa” (HAALSI), Agincourt, South Africa, 2014–19
| Prevalent ADL and IADL limitations | Incident cognitive impairment | No incident cognitive impairment | P-value |
|---|---|---|---|
| Any ADL limitation (N=3,824) | <0.0001 | ||
| Yes | 109 (35%) | 230 (7%) | |
| No | 202 (65%) | 3,283 (93%) | |
| Any IADL limitation (N=3,420) | <0.0001 | ||
| Yes | 172 (72%) | 1,020 (32%) | |
| No | 68 (28%) | 2,160 (68%) |
Discussion
Consistent with previous research in sub-Saharan Africa, as well as many other diverse global regions, we found that older age, low education, illiteracy, and other markers of social disadvantage were associated with higher incidence of cognitive impairment. As some of the first longitudinal evidence on the incidence of ageing-related cognitive impairment in sub-Saharan Africa, this study indicates that cognitive ageing is strongly related to the social determinants of health in this setting.
Comparison to existing literature
Direct comparisons of our findings to existing incidence studies is difficult due to differences in study population age structures, reporting of age intervals, and outcome classification criteria. In the 70+ age intervals, our incidence rate estimates are very similar to those estimated for 6-year dementia incidence but lower than those estimated for CIND incidence in the Ageing, Demographic, and Memory Study (ADAMS), a national probability sample of US adults aged ≥72 years (3). Hence, our incidence rates, which represent dementia as well as CIND, may be underestimates if the true incidence in this older South African population is similar to that in the older US population.
There are reasons to hypothesize that the age-specific incidence in this population could, in truth, be either higher or lower than in the US and other high-income countries. Low education and illiteracy in the non-white South African population as a result of the apartheid educational system could mean that the incidence of cognitive impairment or dementia may be higher than observed in high-income settings (32,33). Alternatively, as life expectancy remains in the mid-60s in South Africa (34), those who survive to an age where dementia risk begins to meaningfully increase may be a special segment of the population who have characteristics that promote survival and reduce dementia risk (35,36). Predicting the future dementia burden in sub-Saharan Africa may be difficult, as the determinants of mortality, a competing risk to dementia, are changing, along with the distributions of dementia risk factors both within birth cohorts over time and across subsequent birth cohorts (37,38).
There are few existing studies on the incidence of cognitive impairment or dementia in sub-Saharan Africa to compare against our results. We observed higher incidence rates of cognitive impairment than did the four studies in Nigeria identified in the 2014 systematic review (14). These discrepant results could be due to true population differences in incidence, or artificial differences induced by different outcome classification criteria or study methodologies. Our findings are consistent with cross-sectional studies associating low education and illiteracy with higher prevalence of cognitive impairment and dementia in South Africa and other sub-Saharan African countries, but inconsistent with those identifying sex/gender-based disparities (7–9,13,27,39–41). Our results are mostly consistent with a longitudinal study of cognitive decline over two years among adults aged 65 and over in a rural Tanzania (42).
We expected to see stronger positive relationships between cardiovascular risk factors and incident impairment (43). Survival rates from stroke or heart attack may be relatively low in our study population (44), and survivors may disproportionately have characteristics that protect against cardiovascular mortality and subsequent cognitive impairment or dementia, with the effect of biasing our estimates towards the null (35,36). Similarly, the HIV-positive individuals in this study may represent a select group of long-term cases who have been living with chronic HIV managed by anti-retroviral therapies. Future research that captures incident cardiovascular conditions and HIV infection is needed to elucidate the causal role of these factors in cognitive impairment and dementia risk among older adults in sub-Saharan Africa.
Limitations and strengths
There remains no consensus on the best methods to ascertain cognitive health status among older adults in sub-Saharan Africa (45). We employed a widely used cognitive screening measure designed for use in large population-based epidemiological studies, which has been validated against clinical diagnostic data and avoids floor or ceiling effects (25,26). However, cognitive impairment and dementia in this study population should be validated against diagnostic data, such as Clinical Dementia Ratings, in the future. Our outcome includes individuals with dementia and CIND, as well as those who may be cognitively impaired for reasons other than dementia or other neurodegenerative disorder. We may have missed cognitive impairment in domains other than orientation and memory, although amnestic cognitive impairment is common among those with CIND and is a hallmark of dementia. Baseline risk factors were self-reported and may be subject to measurement error. Small numbers of incident cognitive impairment in some risk factor categories may have limited our ability to observe incidence rate ratios of weak magnitude. We may have underestimated the incidence of cognitive impairment, as between-wave cognitive test practice, as well as within-wave practice from the three repeated immediate recall trials that were introduced in wave 2 could lead to an underestimate of cognitive impairment incidence between waves (46).
Strengths include this study’s large sample size, representativeness of a rapidly ageing, low-income population in rural South Africa, and its rich, in-person survey measures. We used weights to minimize potential bias that could be introduced into our estimates by between-wave mortality and drop-out, although rates of both were low. Results expand the global scope of existing evidence in the cognitive ageing literature, which disproportionately represents older adults from Western and high-income populations. The cognitive outcome measure used here is included in other HRS International Partner Studies in countries such as Mexico, India, China, Brazil, Indonesia, which will facilitate the international comparison of cognitive ageing outcomes.
Conclusion
Our results indicate that cognitive impairment affects a meaningful proportion of older adults as they age in rural South Africa, and that key social determinants of health including education and literacy are strongly related to incident cognitive impairment. Future studies should investigate the causal roles of these and other factors in dementia risk in sub-Saharan African populations (47). Our findings are broadly generalizable to other rural regions of southern Africa at similar levels of economic development. As some of the first longitudinal data on cognitive ageing in a rapidly ageing sub-Saharan African population, this study provides a snapshot into the future dementia burden and the population distribution of this burden that is to come in this global region.
Supplementary Material
Acknowledgements
The authors are grateful to all those involved in the successful field operations in HAALSI and the Agincourt HDSS, including field staff, data analysts, and, most importantly, the study participants themselves.
Funding Sources
HAALSI is supported by the NIA of the NIH (P01AG041710 to LFB). HAALSI is nested within the Agincourt Health and socio‐Demographic Surveillance System, with funding from The Wellcome Trust (058893/Z/99/A; 069683/Z/02/Z; 085477/Z/08/Z; 085477/B/08/Z), University of the Witwatersrand, and Medical Research Council, South Africa. RGW has received funding from the South African National Research Foundation (119234). The funders had no role in the preparation of data or the manuscript.
Footnotes
Statement of Ethics
All study participants gave informed consent to participate, and the study was conducted according to the principles embodied in the Declaration of Helsinki. Ethical approval was granted by the University of the Witwatersrand Human Research Ethics Committee (Medical), the Harvard T. H. Chan School of Public Health Office of Human Research Administration, and the Mpumalanga Provincial Research and Ethics Committee.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
References
- 1.Guerchet M, Mayston R, Lloyd-Sherlock P, Prince M, Aboderin I, Akinyemi R, et al. Dementia in sub-Saharan Africa: Challenges and opportunities. London; 2017. [Google Scholar]
- 2.U.S. Department of Health and Human Services and Centers for Disease Control and Prevention. Cognitive impairment: a call for action, now! [Internet]. Healthy Brain Initiative. 2011. Available from: https://www.cdc.gov/aging/pdf/cognitive_impairment/cogImp_poilicy_final.pdf
- 3.Plassman BL, Langa KM, McCammon RJ, Fisher GG, Potter GG, Burke JR, et al. Incidence of dementia and cognitive impairment, not dementia in the United States. Ann Neurol. 2011;70(3):418–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skirbekk V, Loichinger E, Weber D. Variation in cognitive functioning as a refined approach to comparing aging across countries. Proc Natl Acad Sci USA. 2012;109(3):770–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.de Jager CA, Joska JA, Hoffman M, Borochowitz KE, Combrinck MI. Dementia in rural South Africa: A pressing need for epidemiological studies. S Afr Med J. 2015;105(3):189–90. [DOI] [PubMed] [Google Scholar]
- 6.Walker R, Paddick SM. Dementia prevention in low-income and middle-income countries: a cautious step forward. Lancet Glob Heal. 2019;7(5):e538–9. [DOI] [PubMed] [Google Scholar]
- 7.Guerchet M, Houinato D, Paraiso MN, Von Ahsen N, Nubukpo P, Otto M, et al. Cognitive impairment and dementia in elderly people living in rural Benin, West Africa. Dement Geriatr Cogn Disord. 2009;27(1):34–41. [DOI] [PubMed] [Google Scholar]
- 8.De Jager CA, Msemburi W, Pepper K, Combrinck MI. Dementia Prevalence in a Rural Region of South Africa: A Cross-Sectional Community Study. J Alzheimers Dis. 2017;60(3):1087–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Olayinka OO, Mbuyi NN. Epidemiology of Dementia among the Elderly in Sub-Saharan Africa Epidemiology of Dementia among the Elderly in Sub-Saharan Africa. 2016;2014(August 2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Paddick SM, Kisoli A, Samuel M, Higginson J, Gray WK, Dotchin CL, et al. Mild cognitive impairment in rural Tanzania: prevalence, profile, and outcomes at 4-year follow-up. Am J Geriatr Psychiatry. 2015;23(9):950–9. [DOI] [PubMed] [Google Scholar]
- 11.Paddick SM, Longdon AR, Kisoli A, Dotchin C, Gray WK, Dewhurst F, et al. Dementia prevalence estimates in sub-Saharan Africa: comparison of two diagnostic criteria. Glob Health Action. 2013;6(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paraïso MN, Guerchet M, Saizonou J, Cowppli-Bony P, Mouanga AM, Nubukpo P, et al. Prevalence of dementia among elderly people living in Cotonou, an urban area of Benin (West Africa). Neuroepidemiol. 2011;36(4):245–51. [DOI] [PubMed] [Google Scholar]
- 13.Adeloye D, Auta A, Ezejimofor M, Oyedokun A, Harhay MO, Rudan I, et al. Prevalence of dementia in Nigeria: a systematic review of the evidence. J Glob Heal Rep. 2019;176(3):e2019014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lekoubou A, Echouffo-Techeugui J, Kengne A. Epidemiology of neurodegenerative diseases in sub-Saharan Africa: a systematic review. BMC Public Health. 2014;14:653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.George-Carey R, Adeloye D, Chan KY, Paul A, Kolčić I, Campbell H, et al. An estimate of the prevalence of dementia in Africa: a systematic analysis. J Glob Heal. 2012;2(2):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Langa KM, Larson EB, Crimmins EM, Faul JD, Levine DA, Kabeto MU, et al. A comparison of the prevalence of dementia in the United States in 2000 and 2012. JAMA Intern Med. 2017;177(1):51–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weden MM, Shih RA, Kabeto MU, Langa KM. Secular Trends in Dementia and Cognitive Impairment of U.S. Rural and Urban Older Adults. Am J Prev Med. 2017;54(2):164–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mukadam N, Sommerlad A, Huntley J, Livingston G. Population attributable fractions for risk factors for dementia in low-income and middle-income countries: an analysis using cross-sectional survey data. Lancet Glob Heal. 2019;7(5):e596–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Patterson C World Alzheimer Report 2018: The state of the art of dementia research: New frontiers. London; 2018. [Google Scholar]
- 20.Tollman SM, Norris SA, Berkman LF. Commentary: The value of life course epidemiology in low-and middle-income countries: an ageing perspective. Int J Epidemiol. 2016;45(4):997–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ben-Shlomo Y, Cooper R, Kuh D. The last two decades of life course epidemiology, and its relevance for research on ageing. Int J Epidemiol. 2016;45(4):973–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gómez-Olivé FX, Montana L, Wagner RG, Kabudula CW, Rohr JK, Kahn K, et al. Cohort Profile: Health and Ageing in Africa: a Longitudinal Study of an INDEPTH Community in South Africa (HAALSI). Int J Epidemiol. 2018;1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahn K, Collinson MA, Xavier Gómez-olivé F, Mokoena O, Twine R, Mee P, et al. Profile: Agincourt health and socio-demographic surveillance system. Int J Epidemiol. 2012;41(4):988–1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.United Nations. Apartheid in South Africa: Summary of the Report of the Special Committee on the Policies of Apartheid of the Government of South Africa. New York: United Nations; 1963. 47 p. [Google Scholar]
- 25.Ofstedal MB, Fisher G, Herzog A, HRS Health Working Group. Documentation of Cognitive Functioning Measures in the Health and Retirement Study. Ann Arbor; 2005. [Google Scholar]
- 26.Crimmins EM, Kim JK, Langa KM, Weir DR. Assessment of cognition using surveys and neuropsychological assessment: the Health and Retirement Study and the Aging, Demographics, and Memory Study. J Gerontol B Psychol Sci Soc Sci. 2011;66 Suppl 1:162–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kobayashi LC, Mateen FJ, Montana L, Wagner RG, Kahn K, Tollman SM, et al. Cognitive function and impairment in older, rural South African adults: Evidence from “Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in Rural South Africa.” Neuroepidemiol. 2019;52(1–2):32–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Riumallo-Herl C, Canning D, Kabudula C. Health inequalities in the South African elderly: the importance of the measure of social-economic status. J Econ Ageing. 2019;14:100191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sartorius K, Sartorius B, Tollman S, Schatz E, Kirsten J, Collinson M. Rural Poverty Dynamics and Refugee Communities in South Africa: A Spatial-Temporal Model. Popul Space Place. 2013;19(1):103–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.World Health Organization. Guidelines for using HIV testing technologies in surveillance: selection, evaluation, and implementation: 2009 update. Geneva; 2009. [PubMed] [Google Scholar]
- 31.Zou G A modified poisson regression approach to prospective studies with binary data. Am J Epidemiol. 2004;159(7):702–6. [DOI] [PubMed] [Google Scholar]
- 32.Badat S, Sayed Y. Post-1994 South African Education. Ann Am Acad Pol Soc Sci. 2014;652(1):127–48. [Google Scholar]
- 33.Christie P, Collins C. Bantu education: Apartheid ideology and labour reproduction. In: Kallaway P, editor. Apartheid and Education. Braamfontein: Raven Press; 1984. p. 160–83. [Google Scholar]
- 34.Statistics South Africa. Mid-year population estimates 2017 [Internet]. Mid-year population estimates. Pretoria; 2018. Available from: www.statssa.gov.za [Google Scholar]
- 35.Stovitz SD, Banack HR, Kaufman JS. “Depletion of the susceptibles” taught through a story, a table and basic arithmetic. BMJ Evidence-Based Med. 2018;23(5):199. [DOI] [PubMed] [Google Scholar]
- 36.Banack HR, Kaufman JS, Wactawski-Wende J, Troen BR, Stovitz SD. Investigating and Remediating Selection Bias in Geriatrics Research: The Selection Bias Toolkit. J Am Ger Soc. 2019;67(9):1970–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kabudula CW, Houle B, Collinson MA, Kahn K, Gómez-Olivé FX, Clark SJ, et al. Progression of the epidemiological transition in a rural South African setting: findings from population surveillance in Agincourt, 1993–2013. BMC Public Health. 2017;17(1):424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kabudula CW, Tollman S, Mee P, Ngobeni S, Silaule B, Xavier Gómez-Olivé F, et al. Two decades of mortality change in rural northeast South Africa. Glob Health Action. 2014;7(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Paddick SM, Longdon A, Gray WK, Dotchin C, Kisoli A, Chaote P, et al. The association between educational level and dementia in rural Tanzania. Dement Neuropsychol. 2014;8(2):117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Peltzer K, Phaswana-Mafuya N. Cognitive functioning and associated factors in older adults in South Africa. S Afr J Psychiatr. 2012;18(4):157–63. [Google Scholar]
- 41.Tianyi FL, Agbor VN, Njamnshi AK, Atashili J. Factors associated with the prevalence of cognitive impairment in a rural elderly cameroonian population: a community-based study in sub-Saharan Africa. Dement Geriatr Cogn Disord. 2019;47(1–2):104–13. [DOI] [PubMed] [Google Scholar]
- 42.Heward J, Stone L, Paddick SM, Mkenda S, Gray WK, Dotchin CL, et al. A longitudinal study of cognitive decline in rural Tanzania: rates and potentially modifiable risk factors. Int Psychogeriatr. 2018;30(9):1333–43. [DOI] [PubMed] [Google Scholar]
- 43.Akinyemi RO, Owolabi MO, Ihara M, Damasceno A, Ogunniyi A, Dotchin C, et al. Stroke, cerebrovascular diseases and vascular cognitive impairment in Africa. Brain Res Bull. 2019;145(May 2018):97–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mayosi BM, Benatar SR. Health and Health Care in South Africa — 20 Years after Mandela. N Engl J Med. 2014;371(14):1344–53. [DOI] [PubMed] [Google Scholar]
- 45.Paddick SM, Gray WK, McGuire J, Richardson J, Dotchin C, Walker RW. Cognitive screening tools for identification of dementia in illiterate and low-educated older adults, a systematic review and meta-analysis. Int Psychogeriatr. 2017;29(6):897–929. [DOI] [PubMed] [Google Scholar]
- 46.Weuve J, Proust-Lima C, Power MC, Gross AL, Hofer SM, Thiébaut R, et al. Guidelines for reporting methodological challenges and evaluating potential bias in dementia research. Alzheimers Dement. 2015;11(9):1098–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Livingston G, Huntley J, Sommerlad A, Ames D, Ballard C, Banerjee S, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.