Abstract
Bariatric surgery is associated with changing food preferences, but it is not known whether these changes differ by type of operation or are associated with weight loss. The current study presents validation results for a new 27-item scale, Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED). This scale measured enjoyment, craving, and intolerance changes for nine food and beverage categories common to dietary habits in the Southern California region of the U.S. one year following bariatric surgery in the Bariatric Experience Long Term (BELONG) study. Validation of BSATED was done using exploratory factor analyses, construct validity with other conceptually related survey instruments, and criterion validity using hypothesized differences for operation type and percent total weight loss (%TWL) at 12 −18 months after surgery. Participants (n = 999) were 86% female, 41% non-Hispanic White, with a mean age of 43.1 ± 11.3 years and a body mass index (BMI) of 43.4 ± 6.8 kg/m2 at the time of surgery. Participants reported less enjoyment and craving for high-fat meats (62%), grains (54%), candy and other desserts (e.g. candy bars, chocolate, ice cream) (52%), and sweet baked goods (48%) 12 months after surgery. These changes were more common among participants undergoing Roux-en-Y gastric bypass (RYGB) compared to those receiving sleeve gastrectomy (SG). Participants who reported decreased enjoyment and craving for foods and beverages that post-bariatric patients are counseled to reduce or avoid had greater %TWL at 12–18 months following surgery (p < .001 and p = .003 respectively). The foods and beverages in BSATED that post-bariatric patients are counseled to reduce or avoid could be used to understand how changes in enjoyment, craving and tolerability of these foods/beverages contribute to weight loss following surgery.
Keywords: hedonic eating, validation, self-report
Introduction
Bariatric surgery is the most effective treatment for patients with severe obesity (Colquitt, Pickett, Loveman, & Frampton, 2014; Maciejewski et al., 2016); however the amount and durability of surgical weight loss varies considerably between individuals (Ahmed et al., 2018; Arterburn et al., 2018; Maciejewski et al., 2016). For example, after the gold standard Roux-en-Y gastric bypass (RYGB) operation, percent total weight loss (%TWL) ranges from 15–40% (Courcoulas et al., 2013; Wolfe, Kvach, & Eckel, 2016), and up to 10% of patients having the most common restrictive operation, sleeve gastrectomy (SG), lose only 5% TWL after five years (Arterburn et al., 2018).
Reasons for the variable weight response to bariatric surgery are poorly understood, but eating behaviors are believed to play a large role (Ghaferi, Woodruff, & Arnould, 2016; Jensen et al., 2014; Mitchell et al., 2016). Bariatric patients generally report lower dietary energy intakes early after surgery (Giusti et al., 2016; Kanerva, Larsson, Peltonen, Lindroos, & Carlsson, 2017), with energy intake increasing gradually after the immediate post-operative period. The composition of the diet also changes after surgery, with less energy coming from simple sugars and more from lean proteins (Coupaye et al., 2014; Ernst, Thurnheer, Wilms, & Schultes, 2009; Sarwer et al., 2008). These changes may be due to dietary recommendations made by bariatric care providers (Dagan et al., 2017) and to the restrictive effects of all bariatric operations.
Changes in sensory and behavioral responsiveness to food may also influence post-operative dietary intake and weight loss (Ahmed, Penney, Darzi, & Purkayastha, 2018; Behary & Miras, 2015; Graham, Murty, & Bowrey, 2014; Nance, Acevedo, & Pepino, 2020). Studies of post-bariatric taste preference changes include animal models (Ahmed et al., 2018) and human fMRI studies (Behary & Miras, 2015; Holsen et al., 2018) of the brain’s response to various food stimuli. In addition, several different survey scales have been used to explore changes in dietary and taste preferences following bariatric surgery (Crowley et al., 2012; Hubert et al., 2019; Sudan, Sudan, Lyden, & Thompson, 2017). Despite the broad range in study methodology, a common finding was an initial heightened sensitivity to, decreased enjoyment and craving for, and increased intolerance for sweets and fatty foods which varied across bariatric operations (Boerlage, van de Laar, Westerlaken, Gerdes, & Brandjes, 2017; Goldenshluger et al., 2017; Kvehaugen & Farup, 2018; Ruiz-Tovar et al., 2018).
The mechanisms driving post-surgery sensory responsiveness to food are not completely understood. It is thought that changes in gastrointestinal anatomy following surgery may provoke unpleasant symptoms and side effects after the ingestion of certain foods and beverages (deAGodoy et al., 2018; Goldenshluger et al., 2017). These foods and beverages then become aversive and the hedonic response is reduced. The durability of changes in taste function and how people consume (Nance et al., 2020) and their association with surgical weight loss (Hubert et al., 2019; Kittrell et al., 2018; Papasavas, Rawal, Ng, Tishler, & Duffy, 2015) remain uncertain. Operations that bypass the small intestine (e.g. RYGB) may have more profound influence on taste function than operations that simply restrict the size of the stomach (e.g. SG). This hypothesis has not been studied extensively (Behary & Miras, 2015). Finally, most studies in this area have consisted of relatively small (<100) homogenous samples of patients (≥ 90% White), and older purely restrictive operations (laparoscopic banding) which are no longer being used in practice. It is unknown whether results would generalize to a larger population, typical of contemporary clinical practice, which is increasingly more racially and ethnically diverse and predominately SG operations.
To better understand changes in food and beverage taste and consumption after bariatric surgery, we developed and administered a 27-item instrument, Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED), designed to measure changes in enjoyment, craving and intolerance for nine food/beverage categories one year after bariatric surgery. This scale was part of a larger longitudinal survey study: The Bariatric Experience Long Term (BELONG)(Moore et al., 2020). We developed the BSATED instrument with the goal of maximizing its clinical relevance and face validity by working with BELONG study investigators who were also bariatric clinicians (KHL and SBM) and/or who had researched similar constructs in non-bariatric populations (AD). We also worked with a post-bariatric patient who was also a research scientist (CLC).
The instrument was designed to measure three constructs related to the experience of consuming foods/beverages after bariatric surgery. The first construct was change in a patient’s “enjoyment” of foods/beverages which was intended to be directly related to the hedonic experience (Berridge, Ho, Richard, & DiFeliceantonio, 2010; Gearhardt, Rizk, & Treat, 2014) of consuming these types of foods/beverages. Second, we wanted to measure a related construct of “craving” (Batra et al., 2013; Berridge et al., 2010; Gearhardt et al., 2014) which describes how much a patient desires or wants to consume a food/beverage. Finally, we measured “tolerability” to different food/beverage categories, characterized as feeling physically ill after consuming a food/beverage.
The current study presents the validation results for the BSATED instrument using exploratory factor analyses, construct validity with other conceptually related survey instruments, and criterion validity using hypothesized differences for operation type and percent total weight loss (%TWL) at 12 −18 months after surgery. Based upon clinical experience and the literature in this area (reviewed above), we tested the following a priori hypotheses as part of the validation: 1) Participants would report increases in enjoyment and craving for types of foods/beverages recommended for post-bariatric patients (lean proteins and fruits and vegetables); while reporting decreases in enjoyment and craving for types of foods/beverages that post-bariatric patients are counseled to reduce or avoid (candy and other desserts [e.g. candy bars, chocolate, ice cream] and high-fat meats); 2) that these changes would be more pronounced in participants who had RYGB when compared to SG; and 3) that participants who reported less craving and enjoyment, and less tolerability for foods/beverages that post-bariatric patients are counseled to reduce or avoid would have higher %TWL 12–18 months after surgery compared to participants who did not experience these changes or reported the opposite pattern.
2. Methods
2.1. Setting and Study Population
Participants were patients in a large integrated healthcare system in the Southern California region of the U.S. preparing to have bariatric surgery recruited as part of the larger parent BELONG study. Inclusion criteria for the BELONG study were: 1) being enrolled in a 12-week (one hour per week) surgical preparation course; 2) planning a first bariatric operation within six months of the baseline survey; 3) being an adult ≥ 18 years old at the time of the baseline survey; and 4) meeting general eligibility criteria for weight loss surgery based on national recommendations (NIDDK., 2016). These criteria were having a body mass index (BMI) ≥ 40 kg/m2 or 35–39 kg/m2 with at least one major obesity-related comorbid condition such as diabetes or sleep apnea. Surgeons could still elect not to perform bariatric surgery if: 1) they determined that the patient had an excessively high risk for surgery, and/or 2) the patient had not lost at least 5% TWL during a required 12-week preparation course. In rare cases, patients could have surgery if their BMI was 32 kg/m2 with serious obesity-related comorbid conditions.
In addition to these pre-surgical criteria, eligible participants for the BELONG study could not have had surgery before the baseline survey and could not have had surgery more than 12 months from the baseline survey. The healthcare system institutional review board for human subjects approved all study procedures and waived the requirement for signed informed consent (protocol #10865). Participants provided verbal consent at the time of recruitment for the baseline survey.
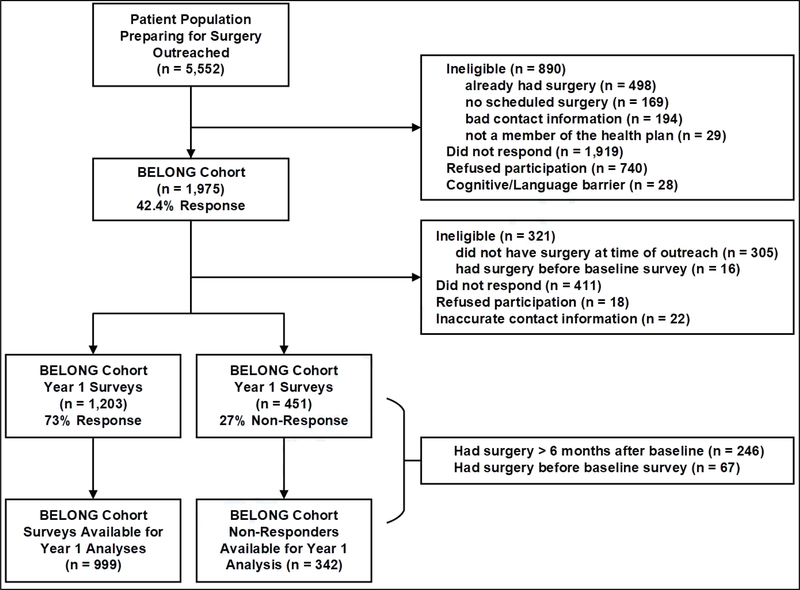
Figure 1 provides the details of the eligibility, recruitment, enrollment, and year 1 survey respondents in the BELONG study. The BSATED instrument was only administered in the year 1 survey. A total of 5,552 participants were approached during their bariatric preparation classes between March 1, 2015 and August 30, 2016 with a one-time in-class announcement and distribution of study flyers. This was followed by four outreach attempts over a period of eight weeks consisting of emails, postal mailed letters and phone calls. Participants were identified as enrolled in the pre-surgical classes using the electronic medical record (EMR) as well as information obtained from course instructors. Of the 5,552 outreached, 890 were not eligible; over half of whom had already had surgery (56.0%; n = 498). Of the 4,662 eligible outreached patients, 1,975 participants were consented and completed a baseline survey (42.4% response rate).
Figure 1.
Sample selection process for the Bariatric Experiences Long Term (BELONG) study and the sample used for the validation of the Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED) instrument.
Of the 1,975 participants in the BELONG baseline cohort, 321 were not eligible for the year 1 survey, did not respond (n = 411), refused (n = 18), or did not have current contact information (n = 22) (n = 451 non-responders). The remaining BELONG cohort participants (n = 1,203) completed a year 1 survey (73% response rate). From these 1,203 survey respondents, 204 were further eliminated because they had either had surgery more than six months after their baseline survey (n = 153) or had undergone surgery before their baseline survey (n = 51). From the n = 451 non-responders, 109 patients were further eliminated for the same reasons. The final sample with BSATED responses was n = 999 and n = 342 non-responders.
2.2. Survey Procedures
Year 1 surveys were administered using a Computer-Aided Telephone Interview (CATI) system or a self-directed website and took approximately 75 minutes to complete. Participants could stop and return to the web survey or stop and reschedule a separate phone call to complete the phone survey. If participants started the web survey but did not complete it, they were called to complete the survey by phone. Most of the participants in the analytic sample (63%; n = 630) completed the survey using the website. Participants were provided with a $25 gift certificate for their time.
2.3. Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED) Instrument Structure and Scoring
We asked participants about post-operative change in each of three constructs (enjoyment, craving, tolerability), for nine different food/beverage categories. Categories were chosen to align with post-bariatric dietary recommendations (Dagan et al., 2017), and were based on the typical American diet, which would be familiar to the participants having bariatric operations in the Southern California region of the U.S. These categories were (1) milk and dairy (milk, cheese, yogurt), (2) lean proteins (grilled chicken, baked, lean beef, fish, tofu), (3) higher-fat meats (burgers, hot dogs, fried chicken, bacon, sausages), (4) grains/cooked grains (breads/rolls, tortillas, pasta, rice, pizza crust), (5) sweet baked goods (cookies, cakes, brownies), (6) fresh fruits and vegetables (apples, bananas, salads, tomatoes, broccoli), (7) candy and other desserts (candy bars, chocolate, ice cream), (8) salty snacks (tortilla chips, potato chips, popcorn), and (9) non-alcoholic sweetened drinks (juice, soda, sweetened coffee/tea, sports drinks, penafiel, manzanita, refrescos).
The root question for the enjoyment and craving constructs was “For the following questions we would like you to tell us about your enjoyment and craving of certain kinds of foods now as compared to before surgery” and response options were “more”, “less”, “no change”, “never eat/ate”, and “not sure”. To measure the construct of tolerability, respondents were asked whether they “Sometimes now feel physically ill when [they] eat/drink even a small amount of” and response options were “yes”, “no”, “never eat/ate”, and “not sure”.
2.4. Measures Used for Scale Validation
All measures used for scale validation are shown in the Appendix.
2.4.1. Construct Validity
2.4.1.1. Self-Reported weight management strategies.
We used a version of the Weight Control Strategies Scale (WCSS) modified for bariatric patients to assess patient behaviors predictive of weight loss and maintenance (Mitchell et al., 2016; Pinto, Fava, Raynor, LaRose, & Wing, 2013). The overall modified WCSS assessed 27 behaviors/strategies for which participants endorsed a frequency over the past 30 days, ranging from “never” to “always” or “don’t know/remember”.
2.4.1.2. Emotional eating and loss of control of eating.
We used the 18 item three-factor eating questionnaire (Angle et al., 2009) to test if emotional eating and loss of control of eating were associated with enjoyment and craving. We expected that enjoyment and craving could be related to behaviors involved in using foods/beverages as a coping strategy for stress and related to the loss of control of consuming foods/beverages that were particularly enjoyable or that a participant would crave.
2.4.1.3. Self-reported dietary recall and rating of diet quality.
A brief assessment of dietary intake was done using the short form of the Rapid Eating and Activity Assessment for Participants (REAP-S) scale (Segal-Isaacson, Wylie-Rosett, & Gans, 2004). This scale asked about how frequently participants engaged in certain dietary behaviors in a typical week using response options of “usually/often”, “sometimes”, “rarely/never” and “does not apply to me”. The behaviors were skipping breakfast, eating out, eating whole grains/high fiber foods, eating fruits, eating vegetables, eating/drinking dairy, eating lean meats, eating processed meats, eating chips, eating fried foods, adding butter/margarine to food, eating sweet baked goods, and drinking sweetened beverages.
In order to determine if participants were also following nutritional guidelines after bariatric surgery, we added eating meal replacements, drinking water, eating frozen foods, and drinking diet beverages. Self-rated dietary quality was assessed using a single question from the National Health and Nutrition Examination Survey (NHANES) that asks “In general how healthy is your overall diet?” and response categories are “excellent”, “very good”, “good”, “fair”, and “poor”(Woglom, Gray, Hill, & Wang, 2020).
2.4.2. Criterion Validity
2.4.2.1. Percent total weight loss (%TWL).
The main measure used to establish criterion validity of the BSATED instrument was percent total weight loss (%TWL) calculated using the following formula: [(weight at follow-up – weight at surgery)/weight at surgery]*100. Body weight and height were obtained from the EMR for one year before surgery and all years following surgery. Body weight was measured by clinical staff and height was primarily self-reported. Negative values indicated weight loss and positive values indicated weight gain. This is a standardized outcome measure for bariatric studies (Brethauer et al., 2015). For the current study, %TWL was calculated for the period of 12 – 18 months following surgery, because the data were from clinical follow-up visits, which are typically spread across several months even if they are intended to represent a particular follow-up time (e.g. 12 months).
2.4.2.2. Control variables for %TWL models.
We used standard control variables collected in the baseline period of the BELONG study that were known to be related to %TWL and may have confounded the relationship between craving, enjoyment and tolerability and %TWL. These included %TWL in the 12 months before surgery from the EMR, and self-reported age, sex, and race/ethnicity using standardized instruments we have used in our previous work with bariatric patients (Coleman & Brookey, 2014; Coleman et al., 2017). We also included socioeconomic status (SES) calculated using the Hollingshead Index of Social Status (Hollingshead, 1975), which used categories of self-reported education and classification of the status of a person’s type of self-reported employment (including a category for nonworking) to create a weighted composite measure ([education level*3] + [work status*5]) of social status that ranged from 6 – 87. We have used this instrument in our previous work with bariatric patients (Coleman & Brookey, 2014; Coleman et al., 2017).
Finally, we accounted for comorbidity burden at the time of surgery using the Charlson Comorbidity Index (Charlson, Pompei, Ales, & MacKenzie, 1987). The health conditions used for this score were abstracted from the EMR using diagnosis codes. A score of ≥ 6 indicated that a patient had a very high probability of mortality in the following 12 months. We also used the type of surgical operation (RYGB vs. SG) from the EMR as a control variable in these analyses, however this was also used to determine construct validity by testing a hypothesis that enjoyment, craving, and tolerability were different between operations (see section 2.5).
2.5. Data Analysis
Descriptive statistics (mean ± standard deviation for continuous and frequencies for categorical variables) were used to describe the study sample. To determine if the participants who completed the BSATED instrument (n = 999) were different than those who did not (n = 342), t-tests for continuous variables and the Chi square statistic for categorical variables were used and presented in Table 1.
Table 1.
Comparison of baseline characteristics between participants who completed the Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED) instrument (n = 999) and those who did not (n = 342). Data are presented as frequencies (n [%]) or mean ± standard deviations.
| Characteristic | Non-Respondents (n = 342) | Respondents (n = 999) | p |
|---|---|---|---|
| Age (years) | 42.1 ± 10.4 | 43.8 ± 11.6 | .015 |
| Baseline Body Mass Index (BMI) kg/m2 | 43.6 ± 6.6 | 42.9 ± 6.4 | .07 |
| % Total Weight Loss (%TWL) 12 months Before Baseline | 6.4 ± 4.7 | 6.6 ± 4.6 | .35 |
| Gender (Female) | 290 (85) | 860 (86) | .53 |
| Race/Ethnicity | .10 | ||
| Non-Hispanic Black | 636 (18) | 153 (15) | |
| Hispanic | 141 (41) | 368 (37) | |
| Non-Hispanic White | 119 (35) | 433 (43) | |
| Other/Mixed/Unknown | 19 (6) | 45 (5) | |
| Baseline Socioeconomic Status | 38 ± 13 | 38 ± 12 | .40 |
| Baseline Comorbidity Burden | .10 | ||
| 0 | 135 (40) | 359 (36) | |
| 1 | 126 (37) | 347 (35) | |
| ≥ 2 | 81 (24) | 293 (29) | |
| Bariatric Operation | .14 | ||
| Roux-en-Y Gastric Bypass (RYGB) | 95 (28) | 305 (31) | |
| Sleeve Gastrectomy (SG) | 245 (72) | 693 (69) | |
| Other | 2 (<1) | 1 (<1) | |
| % Total Weight Loss (%TWL) 12 – 18 months after Baseline | 24.2 ± 9.6 | 26.3 ± 8.7 | < .001 |
For BSATED scoring, each craving or enjoyment response of “more” was scored as “+1”, responses of “less” as “−1”, and “no change” as “0”. For the tolerability categories, each response of “yes” was scored as “+1”, “no” as “−1”, and “no change” as “0”. Responses of “never ate/eat” and “not sure” were included in descriptive statistics (see Table 2), however exploratory factor analysis loadings, inter-item correlations, and Cronbach’s alpha were calculated only using data from the participants who had responses other than “never ate/do not know” on all items (n = 534; see Table 3 for these results). Pearson bivariate correlations were generated to test construct validity between the BSATED instrument and behaviors measured by the modified WCSS and emotional and loss of control of eating.
Table 2.
Self-reported changes in enjoyment, craving, and tolerability for 9 food/beverage categories 12 – 18 months after bariatric surgery for participants in the Bariatric Experiences Long Term (BELONG) study (n = 999) as measured with the Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED) instrument. Respondents were prompted as follows for the enjoyment and craving questions: “For the following questions we would like you to tell us about your enjoyment and craving of certain kinds of foods now as compared to before surgery. If you have never eaten/drank the items in the food/beverage category before or after surgery please choose ‘never eaten/drank’. If you never enjoyed or craved a food/beverage before surgery and you still do not enjoy/crave it then choose ‘no change’.” For the tolerability questions the prompt was as follows: “Sometimes now feel physically ill when [they] eat/drink even a small amount of”.
| Enjoyment | Craving | Tolerability | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| More | No change | Less | Never ate/Not sure | More | No change | Less | Never ate/Not sure | Yes | No | Never ate/Not sure | |||
| Food category | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | n (%) | ||
| Milk and Dairy (milk, cheese, yogurt) | 217 (22) | 425 (43) | 327 (33) | 30 (3) | 166 (17) | 448 (45) | 344 (34) | 41 (5) | 260 (26) | 673 (67) | 66 (7) | ||
| Lean Proteins (grilled chicken, baked, lean beef, fish, tofu) | 517 (52) | 318 (32) | 150 (15) | 14 (2) | 341 (34) | 434 (43) | 202 (20) | 22 (3) | 172 (17) | 781 (78) | 46 (5) | ||
| Higher-Fat Meats (burgers, hot dogs, fried chicken, bacon, sausages) | 69 (7) | 173 (17) | 673 (67) | 84 (8) | 74 (7) | 237 (24) | 621 (62) | 67 (6) | 489 (49) | 393 (39) | 117 (12) | ||
| Grains (breads/rolls, tortillas, pasta, rice, pizza crust) | 91 (10) | 234 (23) | 615 (62) | 59 (6) | 132 (13) | 281 (28) | 535 (54) | 51 (5) | 399 (40) | 510 (51) | 88 (9) | ||
| Sweet Bakery Products (cookies, cakes, brownies) | 131 (13) | 267 (27) | 532 (53) | 69 (7) | 198 (20) | 266 (27) | 479 (48) | 56 (5) | 436 (44) | 467 (47) | 96 (9) | ||
| Fruits/Vegetables (apples, bananas, salads, tomatoes, broccoli) | 547 (55) | 322 (32) | 116 (12) | 14 (2) | 436 (44) | 401 (40) | 146 (15) | 16 (2) | 102 (10) | 858 (86) | 39 (4) | ||
| Candy and Other desserts (candy bars, chocolate, ice cream) | 124 (12) | 245 (25) | 548 (55) | 82 (8) | 167 (17) | 247 (25) | 522 (52) | 63 (6) | 408 (41) | 488 (49) | 103 (10) | ||
| Salty Snacks (tortilla chips, potato chips, popcorn) | 194 (19) | 313 (31) | 430 (43) | 62 (7) | 236 (24) | 329 (33) | 390 (39) | 44 (4) | 202 (20) | 706 (71) | 91 (9) | ||
| Non-Alcoholic Sweet Drinks (juice, soda, sweetened coffee/tea, sports drinks, penafiel, manzanita, refrescos) | 97 (10) | 219 (22) | 496 (50) | 187 (19) | 106 (11) | 221 (22) | 517 (52) | 155 (16) | 286 (29) | 476 (48) | 237 (24) | ||
Table 3.
Results from exploratory factor analysis of the Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED) instrument (n = 534) from the Bariatric Experience Long Term (BELONG) study. Participants were measured one year after bariatric surgery.
| Scale | Cronbach’s Alpha | Inter-Item Correlations |
|---|---|---|
| All Items (n = 27) | .77 | −.03 to .42 |
| Factor 1* Food/Beverage Categories (n = 9) | .60 | .20 to .37 |
| Factor 2* Food/Beverage Categories (n = 18) | .79 | .25 to .52 |
| Enjoyment Scale Items (n = 9) | .54 | −.19 to .50 |
| Enjoyment Scale: Factor 1 Foods/Beverages Subscale (n = 3) | .21 | .01 to .20 |
| Enjoyment Scale: Factor 2 Foods/Beverages Subscale (n = 6) | .75 | .42 to .60 |
| Craving Scale Items (n = 9) | .61 | .01 to .48 |
| Craving Scale: Factor 1 Foods/Beverages Subscale (n = 3) | .28 | .10 to .22 |
| Craving Scale: Factor 2 Foods/Beverages Subscale (n = 6) | .71 | .32 to .53 |
| Intolerance Scale Items (n = 9) | .80 | .32 to .62 |
| Intolerance Scale: Factor 1 Foods/Beverages Subscale (n = 3) | .47 | .23 to .46 |
| Intolerance Scale: Factor 2 Foods/Beverages Subscale (n = 6) | .80 | .51 to .66 |
Factor 1 Items: Milk and Dairy (milk, cheese, yogurt); Lean Proteins (grilled chicken, baked, lean beef, fish, fish, tofu); and Fresh Fruits and Vegetables (apples, bananas, salads, tomatoes, broccoli). Factor 2 Items: Higher-Fat Meats (burgers, hot dogs, fried chicken, bacon, sausages); Grains/Cooked Grains (breads/rolls, tortillas, pasta, rice, pizza crust); Sweet Baked Goods (cookies, cakes, brownies); Candy and Other Desserts (candy bars, chocolate, ice cream); Salty Snacks (tortilla chips, potato chips, popcorn); Non-Alcoholic Sweetened Drinks (juice, soda, sweetened coffee/tea, sports drinks, penafiel, manzanita, refrescos).
Independent sample t-tests and the Chi square statistic were used to test the hypothesis that decreases in enjoyment and cravings for foods/beverages that bariatric patients are counseled to reduce or avoid (e.g. candy and other desserts [candy bars, chocolate, ice cream], higher-fat meats) would be more pronounced in participants who had RYGB compared to those who had SG. The hypothesis that reductions in enjoyment and cravings for foods/beverages that bariatric patients are counseled to reduce or avoid would be associated with higher %TWL 12–18 months after surgery was tested using separate linear regressions. Each summary construct (enjoyment, craving, and tolerability) was treated as a predictor along with sex, age, race/ethnicity, surgical operation, BMI at the time of surgery, %TWL in the 12 months before surgery, and comorbidity burden. Standardized estimates are presented in regression results.
3. Results
3.1. Descriptive Findings
Table 1 provides summary statistics for participants who completed a BSATED instrument (n = 999) and those who did not (n = 342). Participants who completed the BSATED instrument were primarily women (86%), either Non-Hispanic Black or Hispanic (52%), and a majority underwent SG (69%). At the time of surgery, these participants were 43.8 ± 11.6 years old, had a BMI of 42.9 ± 6.4 kg/m2, and were primarily upper-middle class SES (38 ± 12 out of a scale of 8 – 67). They had an average %TWL of 6.6 ± 4.6% in the year before surgery and an average %TWL of 26.3 ± 8.7% at 12 – 18 months after surgery. Compared to participants who did not complete the BSATED instrument (n = 342), those who did (n = 999) were older (p = 0.015) and had more %TWL 12– 18 months after surgery (p < .001).
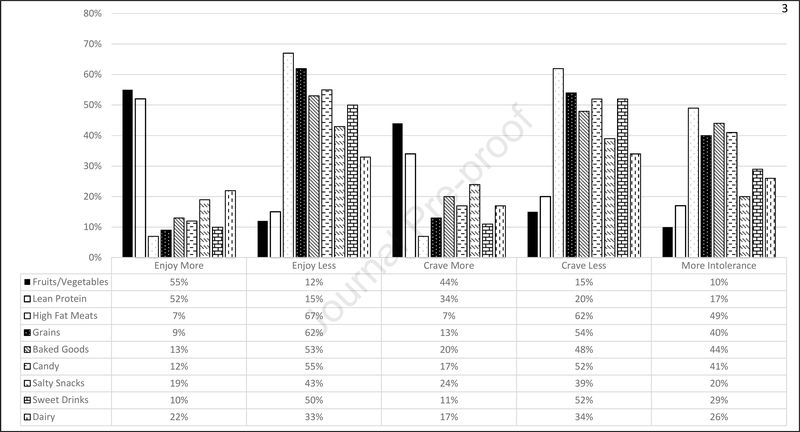
Responses on all BSATED items are shown in Table 2 and Figure 2. There were clear patterns of decreased enjoyment for higher-fat meats (67% reported enjoying less), grains/cooked grains (62%), sweet baked goods (53%), candy and other desserts (e.g. candy bars, chocolate, ice cream) (55%), salty snacks (43%), and non-alcoholic sweetened drinks (50%) one year after surgery. Just over half of participants (52%) reported more enjoyment of lean proteins as well as fresh fruits and vegetables (55%). Responses about enjoyment of milk and dairy were the most variable with 22%, 33%, and 43% reporting more, less, or no change in enjoyment respectively.
Figure 2.
Descriptive frequencies for all responses used for the exploratory factor analysis of the Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED) instrument (n = 534) from the Bariatric Experience Long Term (BELONG) study.
Many patients also reported decreased craving for higher-fat meats (62%), grains/cooked grains (54%), sweet baked goods (48%), candy and other desserts (e.g. candy bars, chocolate, ice cream) (52%), salty snacks (39%), and non-alcoholic sweetened drinks (52%) one year after surgery. Many participants (43%) also reported no change in craving for lean proteins. Cravings for fresh fruits and vegetables increased for 44% of participants, while 40% reported no change. As with enjoyment, reports of cravings for milk and dairy were variable with 17%, 45%, and 34% reporting more, same, or less cravings. Participants only reported less tolerability (higher rates of intolerance) 12 months after surgery for higher-fat meats (49%). For all other food/beverage categories, participants mostly reported no changes for tolerability.
3.2. Factor Analysis
Summary results of the exploratory factor analysis are shown in Table 3. Exploratory factor analysis of participants who reported consuming all nine food/beverage categories (n = 534) indicated a two-factor solution for each of the scale constructs (enjoyment, craving, tolerability). The first factor (Factor 1) included milk and dairy, fresh fruits and vegetables, and lean proteins, which appeared to align closely with recommended foods/beverages for post-bariatric patients. The second factor (Factor 2) included the foods/beverages that post-bariatric patients are counseled to reduce or avoid. Using this two factor solution, Cronbach’s alpha and inter-item correlations were examined (see Table 3 and Table A1). For the three BSATED constructs (enjoyment, craving, tolerability), Factor 1 had poor internal consistency (inter-item correlations ranged from .01 to .46) and reliability (Cronbach’s alpha ranged from .21 to .47). For the three BSATED constructs, Factor 2 had good internal consistency (inter-item correlations ranged from .32 to .66) and reliability (Cronbach’s alpha ranged from .71 to .80). In general, the Cronbach’s alpha did not change when items were removed from the factors (see Table A1 in the appendix). Based on these results, construct and criterion validity were only determined using the items from Factor 2 for each of the constructs (enjoyment, craving, tolerability).
3.3. Construct Validity
Using the criterion of r = .20 as a minimum meaningful correlation (Wuensch, 2019), there were no meaningful relationships between BSATED food/beverage categories or between the enjoyment, craving, and tolerability constructs in Factor 2 and weight control strategies or emotional eating. More craving of sweet baked goods (r = −.21; p< .001) and candy and other desserts (e.g. candy bars, chocolate, ice cream) (r = −.20; p<.001) were related to lower self-rated diet quality. Increased craving of grains/cooked grains (r = .21; p<.001), sweet baked goods (r = .21; p<.001), candy and other desserts (r = .23; p<.001), and salty snacks (r = .23; p<.001) were all related to self-reported loss of control of eating. If a participant self-reported eating more chips they craved salty snacks more (r = .23; p<.001). If they reported eating more sweet baked goods, then they craved candy and other desserts (r = .22; p<.001) and sweet baked goods (r = .21; p<.001) more, and enjoyed sweet baked goods more (r = .21; p<.001).
3.4. Criterion Validity – Surgical Operation
When examining the hypothesized differences in reported changes for enjoyment, craving, and tolerability of foods/beverages that post-bariatric patients are counseled to reduce or avoid between RYGB and SG operations (see Table 4), participants who had RYGB reported enjoying (p = .03) and craving (p = .01) higher-fat meats less and having less tolerability (higher intolerance) for these foods (p = .001) one year after surgery when compared to SG patients. This was also true for candy and other desserts (e.g. candy bars, chocolate, ice cream) (p = .005, p = .04, p = .001 for enjoyment, craving, and tolerability respectively). For salty snacks, the pattern was reversed such that patients who had RYGB reported enjoying (p = .003) and craving (p = .001) salty snacks more one year after surgery compared to patients who had SG. They still reported less tolerability (higher intolerance) of salty snacks than participants who had SG (p = .001). Finally, there were no differences between participants who had RYGB or SG for reported enjoyment and craving of grains/cooked grains, sweet baked goods, and non-alcoholic sweetened drinks. However, participants who had RYGB did report less tolerability (more intolerance) for sweet baked goods (p = .001) and non-alcoholic sweetened drinks (p = .008) than participants who had SG.
Table 4.
Comparison of Roux-en-Y gastric bypass (RYGB) and sleeve gastrectomy (SG) with respect to changes in enjoyment, craving, and tolerability (n (%) yes) for Factor 2 food/beverage categories one year after bariatric surgery for participants in the Bariatric Experiences Long Term (BELONG) study (n = 999). Significance levels are presented for the comparison of RYGB and SG within enjoyment, craving, and tolerability for each type of food/beverage category.
| Enjoyment | Craving | Tolerability | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Surgery type | Surgery type | Surgery type | |||||||||||
| RYGB | SG | RYGB | SG | RYGB | SG | ||||||||
| More | Less | More | Less | More | Less | More | Less | Yes | No | ||||
| Food category | n (%) | n (%) | n (%) | n (%) | p | n (%) | n (%) | n (%) | n (%) | p | n (%) | n (%) | p |
| Higher-fat meats (burgers, hot dogs, fried chicken, bacon, sausages) | 22 (7) | 205 (67) | 47 (7) | 468 (68) | 0.03 | 23 (8) | 188 (62) | 51 (8) | 433 (63) | 0.01 | 169 (56) | 320 (46) | 0.001 |
| Grains/Cooked grains (breads/rolls, tortillas, pasta, rice, pizza crust) | 30 (10) | 185 (61) | 60 (9) | 430 (62) | 0.17 | 37 (12) | 158 (52) | 95 (14) | 376 (15) | 0.17 | 137 (45) | 261 (38) | 0.08 |
| Sweet baked goods (cookies, cakes, brownies) | 35 (12) | 170 (56) | 96 (14) | 362 (52) | 0.72 | 51 (17) | 149 (49) | 147 (21) | 338 (48) | 0.34 | 165 (55) | 271 (39) | 0.001 |
| Candy and other desserts (candy bars, chocolate, ice cream) | 32 (11) | 183 (60) | 91 (13) | 365 (53) | 0.005 | 44 (14) | 175 (57) | 123 (18) | 346 (50) | 0.04 | 161 (53) | 247 (36) | 0.001 |
| Salty snacks (tortilla chips, potato chips, popcorn) | 69 (23) | 120 (39) | 125 18) | 310 (45) | 0.003 | 82 (27) | 119 (39) | 154 (22) | 271 (39) | 0.001 | 76 (25) | 126 (18) | 0.001 |
| Non-alcoholic Sweetened drinks (juice, soda, sweetened coffee/tea, sports drinks, penafiel, manzanita, refrescos) | 39 (10) | 152 (50) | 67 (10) | 344 (50) | 0.58 | 34 (11) | 151 (50) | 72 (10) | 366 (53) | 0.22 | 102 (34) | 184 (27) | 0.008 |
3.5. Criterion Validity – Percent Total Weight Loss (%TWL)
Across all foods/beverages that post-bariatric patients are counseled to reduce or avoid (Factor 2) enjoyment at one year after bariatric surgery was related to 13% higher TWL 12 – 18 months after surgery (F(8,686) = 8.21; β = .13; p = .001). There were also effects for specific foods/beverages in Factor 2 and %TWL. Participants who reported enjoying higher-fat meats less had 10% higher TWL than those with no change or who enjoyed them more (F(8,888) = 10.05; β= .10; p = .003). Participants who reported enjoying grains/cooked grains less had 15% higher TWL than those with no change or who enjoyed them more (F(8,914) = 11.24; β = .15; p < .001). Participants who reported enjoying sweet baked goods less had 7% higher TWL than those with no change or who enjoyed them more (F(8,903) = 9.68; β = .07; p = .02). Participants who reported enjoying candy and other desserts (e.g. candy bars, chocolate, ice cream) less had 6% higher TWL than those with no change (F(8,891) = 9.56; β = .06; p = .047). There was no relationship between changes in enjoyment of salty snacks or non-alcoholic sweetened beverages and %TWL 12 – 18 months after surgery.
Similar to enjoyment, decreased craving across all foods/beverages that post-bariatric patients are counseled to reduce or avoid (Factor 2) was related to 11% higher TWL 12 – 18 months after surgery (F(8,734) = 9.28; β = .11; p = .002). Participants who reported less craving for higher-fat meats had 7% higher TWL than those with no change or who craved them more (F(8,905) = 10.16; β = .07; p = .03). Participants who reported craving grains/cooked grains less had 10% higher TWL than those with no change or who craved them more (F(8,922) = 10.29; β = .10; p = .002). Participants who reported craving sweet baked goods less had 10% higher TWL than those with no change or who craved them more (F(8,916) = 10.40; β = .10; p = .003). Participants who reported craving salty snacks less had 10% higher TWL than those with no change or who craved them more (F(8,929) = 11.69; β = .10; p = .002). There was no relationship between changes in craving of candy and other desserts (e.g. candy bars, chocolate, ice cream) or non-alcoholic sweetened beverages one year after surgery and %TWL 12 – 18 months after surgery.
In general, less tolerability (more intolerance) for Factor 2 foods/beverages was related to 10% higher TWL 12 – 18 months after surgery (F(1,626) = 7.28; β = .10; p = .02). Participants who reported more intolerance of higher-fat meats had 9% higher TWL than those without intolerance (F(8,856) = 9.73; β = .09; p = .008). Participants who reported more intolerance of grains/cooked grains had 11% higher TWL than those without intolerance (F(8,884) = 9.25; β = .11; p = .001). Participants who reported more intolerance of candy and other desserts (e.g. candy bars, chocolate, ice cream) had 10% higher TWL than those without intolerance (F(8,871) = 9.73; β = .10; p = .003). There was no relationship between lower tolerability of sweet baked goods, salty snacks, and non-alcoholic sweetened beverages one year after surgery and %TWL 12 – 18 months after surgery.
4. Discussion
In this development and validation study for the Bariatric Surgical Alterations in Tolerability, Enjoyment and Cravings in the Diet (BSATED) instrument, over half of bariatric patients in the BELONG study reported reduced enjoyment and cravings for food/beverage categories that post-bariatric patients are counseled to reduce or avoid such as higher-fat meats, sweet baked goods, grains/cooked grains, and candy and other desserts (e.g. candy bars, chocolate, ice cream) one year after surgery. BSATED food/beverage categories that post-bariatric patients are counseled to reduce or avoid were associated in the expected direction with %TWL at 12–18 months (e.g. enjoy and crave less leads to more %TWL). Compared to patients undergoing SG, those who had RYGB were more likely to report decreased enjoyment and craving for foods/beverages post-bariatric patients are counseled to reduce or avoid and less tolerability of these same foods/beverages. Our findings align with the neuroscience findings in this area that there is a tendency toward decreased activation of the brain’s reward centers to highly energy-dense foods after bariatric surgery, as well as a decreased hedonic response or preference for sweets and fatty foods (Ahmed et al., 2018). In addition, other work has also shown that bariatric patients reported decreased preference and/or craving for sweets and higher-fat meats in the first year after surgery and such changes varied by bariatric operation (Ahmed et al., 2018; Behary & Miras, 2015; Hansen et al., 2016; Nance et al., 2020; Primeaux, de Silva, Tzeng, Chiang, & Hsia, 2016).
The differences between operations could be explained by a reduced hedonic drive to eat highly palatable foods in patients who had RYGB (Hansen et al., 2016). In addition, biologic mechanisms for altered hedonic response to food may include surgically-induced changes in bile acid production and delivery to the distal ileum, with resulting direct and indirect (via hormones like GLP-1 and Peptide YY) impacts on satiety (Penney, Kinross, Newton, & Purkayastha, 2015; Wang et al., 2019). These bile acid-mediated changes in satiety appear more pronounced and enduring after RYGB compared to SG (Batterham & Cummings, 2016; Miras & le Roux, 2013). Our findings address an area of identified need by prior reviews (Behary & Miras, 2015) with the largest, most diverse cohort of post-bariatric changes in food/beverage preference and tolerability following the SG operation. Differences between SG and RYGB in food preference (e.g. greater reduction in enjoyment of sweet baked goods for patients having RYGB) (Ahmed et al., 2018; Nielsen et al., 2019) are important to study because they may shed further light on the known differences in weight loss between these operations (Arterburn et al., 2018).
As was the case with a recent publication (Sudan et al., 2017), we were not able to reliably measure changes in enjoyment, craving, and tolerability for foods/beverages that are more often recommended as regular parts of the post-bariatric diet (Factor 1: milk and dairy, lean proteins, fresh fruits and vegetables). One possible reason for the poor performance of Factor 1 was the small number of items it included (n = 3). Also, the majority of participants may not have associated the constructs of enjoyment and craving with food categories like fresh fruits and vegetables and lean proteins (as compared to more highly-palatable foods like sweet baked goods) (Gearhardt et al., 2014).
Similarly, questions about enjoyment and craving for milk and dairy foods may have produced inconsistency in terms of grouping with the other Factor 1 items due to a high prevalence of lactose intolerance among adults, as well as heterogeneity of the types of foods/beverages in this broad category. For example, butter or cream could be perceived very differently from plain yogurt or cottage cheese. Our use of a single item asking about both fruits and vegetables could have also been problematic because participants may have viewed these two food types very differently both in terms of level of enjoyment (fruits as sweet, vegetables as primarily bitter), and perceived healthfulness (vegetables > fruits). Future research would need to address these distinctions more carefully to understand changes in enjoyment, craving, and tolerability for foods/beverages more often recommended as regular parts of the post-bariatric diet.
We observed low correlations between individual BSATED food/beverage items and broad constructs such as self-rated dietary quality and emotional eating. This did not support our hypotheses nor did these findings agree with previous research that found an association between liking of sweets and a greater degree of disinhibited/unrestrained eating (Lahteenmaki & Tuorila, 1995). The observed lack of strong correlation in our data could be because BSATED examined post-surgical changes in enjoyment (not the absolute level of liking or enjoyment), or because individual food categories (e.g. higher-fat meats) represented only a small portion of the total dietary intake for an individual, thus not fully explaining broader constructs such as overall perceived dietary quality or emotional eating. Our results could also suggest that, despite enjoying and craving certain foods such as sweet baked goods or salty snacks, most bariatric patients are still able to adhere to dietary patterns that align with clinical guidelines.
There are several important limitations of our study that should be considered. First, the BSATED instrument was developed for use in a post-operative bariatric sample, rather than for administration before and after surgery, so it is not a true measure of change over time. A second limitation was that BSATED was developed using terminology and food categories familiar to people in the Southern California region of the U.S. which might have different meaning or relevance to respondents in other areas of the U.S. or throughout the world. A third limitation is the retrospective nature of the instrument (asking participants to compare a current state to a past state) which could result in recall bias. Participants may be more likely to have noticed changes in foods/beverages that they viewed as either particularly problematic or helpful with respect to weight regulation.
Finally, an important limitation is that our findings may not reflect the broader population of people having bariatric surgery. Compared to the BSATED respondents (n = 999), BELONG study participants who did not complete the instrument (n = 342) were younger and had less %TWL 12 – 18 months after surgery (see Table 1). There was also a further sample loss when conducting the factor analysis because we could only analyze responses from participants who endorsed ever eating all nine categories of foods/beverages (i.e. we did not use data from people with even a single response of “never ate/eat” out of 27 items). This requirement led to a loss of almost half of the 999 respondents. Future studies should be done with other bariatric populations with the BSATED instrument to improve generalizability.
5. Conclusions
In this large and diverse cohort of post-bariatric surgery patients, a majority reported changes in enjoyment, cravings and tolerability of specific food categories, which were consistent with prior literature. This study supported the emerging idea that RYGB may be associated with more pronounced changes in taste and liking of certain foods compared to SG, and that these changes may be associated with the greater weight loss seen with RYGB compared to SG at 12–18 months after surgery (Arterburn et al., 2018). The BSATED instrument may provide an efficient way to measure multiple related constructs for post-bariatric patients with respect to their experience of consuming, particularly for foods/beverages that they are counseled to reduce or avoid after surgery. Future studies, including later follow-up in our own cohort, could examine the durability of these changes after surgery, and the ongoing association between food/beverage enjoyment, craving, tolerability and weight loss and maintenance following bariatric surgery.
Supplementary Material
Acknowledgements
We thank the patients who participated in this study without whom this research would not be possible.
Statement of Funding: This work was funded by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) #R01DK108522. The funder was not involved in any part of the study after granting the award.
Footnotes
Declarations of Interest: None
Other Declarations: This manuscript is not under review elsewhere. The corresponding author can provide the original data for review, however, a data use agreement must be put in place and IRB approval must be obtained for us to share this data with staff at Appetite even if it is fully de-identified.
Ethical Approval: The healthcare system institutional review board for human subjects approved all study procedures and waived the requirement for signed informed consent (protocol #10865). Participants provided verbal consent at the time of recruitment.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Kristina H. Lewis, Division of Public Health Sciences, Department of Epidemiology & Prevention, Wake Forest University Health Sciences, Winston-Salem, NC.
Ming Ji, College of Nursing, University of South Florida, Tampa, FL.
Yun Bai, College of Nursing, University of South Florida, Tampa, FL.
David E. Arterburn, Health Research Institute, Kaiser Permanente Washington, Seattle, WA.
Bhumi B. Bhakta, Department of Research and Evaluation, Kaiser Permanente Southern California, Pasadena, CA.
Melissa Cornejo, Kaiser Permanente Southern California, Department of Research and Evaluation, Pasadena, CA.
Cecelia L. Crawford, Regional Nursing Research Program, Kaiser Permanente Southern California, Pasadena, CA.
Adam Drewnowski, Center for Public Health Nutrition, University of Washington, Seattle, WA.
Marlaine Figueroa Gray, Center for Health Research, Kaiser Permanente Washington, Seattle, WA.
Darren D. Moore, Marriage and Family Therapy Program, Touro University Worldwide, Los Alamitos, CA.
Sameer B. Murali, Center for Healthy Living, San Bernardino Medical Center, Kaiser Permanente Southern California, Fontana, CA.
Silvia R. Paz, Kaiser Permanente Southern California, Department of Research and Evaluation, Pasadena, CA.
Brianna Taylor, Kaiser Permanente Southern California, Department of Research and Evaluation, Pasadena, CA.
Tae K. Yoon, Kaiser Permanente Southern California, Department of Research and Evaluation, Pasadena, CA.
Deborah Rohm Young, Kaiser Permanente Southern California, Department of Research and Evaluation, Pasadena, CA.
Karen J. Coleman, Kaiser Permanente Southern California, Department of Research and Evaluation, Pasadena, CA.
References
- Ahmed B, King WC, Gourash W, Belle SH, Hinerman A, Pomp A, … Courcoulas, A. P. (2018). Long-term weight change and health outcomes for sleeve gastrectomy (SG) and matched Roux-en-Y gastric bypass (RYGB) participants in the Longitudinal Assessment of Bariatric Surgery (LABS) study. Surgery, 164(4), 774–783. doi: 10.1016/j.surg.2018.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed K, Penney N, Darzi A, & Purkayastha S (2018). Taste Changes after Bariatric Surgery: a Systematic Review. Obes Surg, 28(10), 3321–3332. doi: 10.1007/s11695-018-3420-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angle S, Engblom J, Eriksson T, Kautiainen S, Saha MT, Lindfors P, … Rimpela A (2009). Three factor eating questionnaire-R18 as a measure of cognitive restraint, uncontrolled eating and emotional eating in a sample of young Finnish females. Int J Behav Nutr Phys Act, 6, 41. doi: 10.1186/1479-5868-6-41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arterburn D, Wellman R, Emiliano A, Smith SR, Odegaard AO, Murali S, … Collaborative, P. C. B. S. (2018). Comparative Effectiveness and Safety of Bariatric Procedures for Weight Loss: A PCORnet Cohort Study. Ann Intern Med, 169(11), 741–750. doi: 10.7326/M17-2786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batra P, Das SK, Salinardi T, Robinson L, Saltzman E, Scott T, … Roberts SB (2013). Relationship of cravings with weight loss and hunger. Results from a 6 month worksite weight loss intervention. Appetite, 69, 1–7. doi: 10.1016/j.appet.2013.05.002 [DOI] [PubMed] [Google Scholar]
- Batterham RL, & Cummings DE (2016). Mechanisms of Diabetes Improvement Following Bariatric/Metabolic Surgery. Diabetes Care, 39(6), 893–901. doi: 10.2337/dc16-0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behary P, & Miras AD (2015). Food preferences and underlying mechanisms after bariatric surgery. Proc Nutr Soc, 74(4), 419–425. doi: 10.1017/S0029665115002074 [DOI] [PubMed] [Google Scholar]
- Berridge KC, Ho CY, Richard JM, & DiFeliceantonio AG (2010). The tempted brain eats: pleasure and desire circuits in obesity and eating disorders. Brain Res, 1350, 43–64. doi: 10.1016/j.brainres.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerlage TC, van de Laar AW, Westerlaken S, Gerdes VE, & Brandjes DP (2017). Gastrointestinal symptoms and food intolerance 2 years after laparoscopic Roux-en-Y gastric bypass for morbid obesity. Br J Surg, 104(4), 393–400. doi: 10.1002/bjs.10419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brethauer SA, Kim J, el Chaar M, Papasavas P, Eisenberg D, Rogers A, … Committee, A. C. I. (2015). Standardized outcomes reporting in metabolic and bariatric surgery. Surg Obes Relat Dis, 11(3), 489–506. doi: 10.1016/j.soard.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Charlson ME, Pompei P, Ales KL, & MacKenzie CR (1987). A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis, 40(5), 373–383. doi: 10.1016/0021-9681(87)90171-8 [DOI] [PubMed] [Google Scholar]
- Coleman KJ, & Brookey J (2014). Gender and racial/ethnic background predict weight loss after Roux-en-Y gastric bypass independent of health and lifestyle behaviors. Obes Surg, 24(10), 1729–1736. doi: 10.1007/s11695-014-1268-0 [DOI] [PubMed] [Google Scholar]
- Coleman KJ, Caparosa SL, Nichols JF, Fujioka K, Koebnick C, McCloskey KN, … Levy SS (2017). Understanding the Capacity for Exercise in Post-Bariatric Patients. Obes Surg, 27(1), 51–58. doi: 10.1007/s11695-016-2240-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman KJ, Huang YC, Hendee F, Watson HL, Casillas RA, & Brookey J (2014). Three-year weight outcomes from a bariatric surgery registry in a large integrated healthcare system. Surg Obes Relat Dis, 10(3), 396–403. doi: 10.1016/j.soard.2014.02.044 [DOI] [PubMed] [Google Scholar]
- Colquitt JL, Pickett K, Loveman E, & Frampton GK (2014). Surgery for weight loss in adults. Cochrane Database Syst Rev(8), CD003641. doi: 10.1002/14651858.CD003641.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupaye M, Riviere P, Breuil MC, Castel B, Bogard C, Dupre T, … Ledoux S (2014). Comparison of nutritional status during the first year after sleeve gastrectomy and Roux-en-Y gastric bypass. Obes Surg, 24(2), 276–283. doi: 10.1007/s11695-013-1089-6 [DOI] [PubMed] [Google Scholar]
- Courcoulas AP, Christian NJ, Belle SH, Berk PD, Flum DR, Garcia L, … Longitudinal Assessment of Bariatric Surgery, C. (2013). Weight change and health outcomes at 3 years after bariatric surgery among individuals with severe obesity. JAMA, 310(22), 2416–2425. doi: 10.1001/jama.2013.280928 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowley NM, LePage ML, Goldman RL, O’Neil PM, Borckardt JJ, & Byrne TK (2012). The Food Craving Questionnaire-Trait in a bariatric surgery seeking population and ability to predict post-surgery weight loss at six months. Eat Behav, 13(4), 366–370. doi: 10.1016/j.eatbeh.2012.07.003 [DOI] [PubMed] [Google Scholar]
- Dagan S, Goldenshluger A, Globus I, Schweiger C, Kessler Y, G. S, … Sinai T (2017). Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv Nutr, 8(2), 382–394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deAGodoy CM, Aprigio LCS, de Godoy EP, Furtado MC, Coelho D, de Souza LBR, & de Oliveira AMG (2018). Food Tolerance and Eating Behavior After Roux-en-Y Gastric Bypass Surgery. Obes Surg, 28(6), 1540–1545. doi: 10.1007/s11695-017-2850-z [DOI] [PubMed] [Google Scholar]
- Ernst B, Thurnheer M, Wilms B, & Schultes B (2009). Differential changes in dietary habits after gastric bypass versus gastric banding operations. Obes Surg, 19(3), 274–280. doi: 10.1007/s11695-008-9769-3 [DOI] [PubMed] [Google Scholar]
- Gearhardt AN, Rizk MT, & Treat TA (2014). The association of food characteristics and individual differences with ratings of craving and liking. Appetite, 79, 166–173. doi: 10.1016/j.appet.2014.04.013 [DOI] [PubMed] [Google Scholar]
- Ghaferi AA, Woodruff M, & Arnould J (2016). The Behavior and Biology Behind Bariatric Surgery Outcomes. JAMA Surg, 151(8), 758. doi: 10.1001/jamasurg.2016.0479 [DOI] [PubMed] [Google Scholar]
- Giusti V, Theytaz F, Di Vetta V, Clarisse M, Suter M, & Tappy L (2016). Energy and macronutrient intake after gastric bypass for morbid obesity: a 3-y observational study focused on protein consumption. Am J Clin Nutr, 103(1), 18–24. doi: 10.3945/ajcn.115.111732 [DOI] [PubMed] [Google Scholar]
- Goldenshluger M, Goldenshluger A, Keinan-Boker L, Cohen MJ, Ben-Porat T, Gerasi H, … Elazary R (2017). Postoperative Outcomes, Weight Loss Predictors, and Late Gastrointestinal Symptoms Following Laparoscopic Sleeve Gastrectomy. J Gastrointest Surg, 21(12), 2009–2015. doi: 10.1007/s11605-017-3585-9 [DOI] [PubMed] [Google Scholar]
- Graham L, Murty G, & Bowrey DJ (2014). Taste, smell and appetite change after Roux-en-Y gastric bypass surgery. Obes Surg, 24(9), 1463–1468. doi: 10.1007/s11695-014-1221-2. [DOI] [PubMed] [Google Scholar]
- Hansen TT, Jakobsen TA, Nielsen MS, Sjodin A, Le Roux CW, & Schmidt JB (2016). Hedonic Changes in Food Choices Following Roux-en-Y Gastric Bypass. Obes Surg, 26(8), 1946–1955. doi: 10.1007/s11695-016-2217-x [DOI] [PubMed] [Google Scholar]
- Hollingshead A (1975). Four Factor Index of Social Status Retrieved from https://pdfs.semanticscholar.org/a63d/541e697f6f743749437cbcaf0e8372f53db4.pdf.
- Holsen LM, Davidson P, Cerit H, Hye T, Moondra P, Haimovici F, … Stoeckel LE. (2018). Neural predictors of 12-month weight loss outcomes following bariatric surgery. Int J Obes (Lond), 42(4), 785–793. doi: 10.1038/ijo.2017.190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hubert PA, Papasavas P, Stone A, Swede H, Huedo-Medina TB, Tishler D, & Duffy VB (2019). Associations between Weight Loss, Food Likes, Dietary Behaviors, and Chemosensory Function in Bariatric Surgery: A Case-Control Analysis in Women. Nutrients, 11(4). doi: 10.3390/nu11040804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen MD, Ryan DH, Apovian CM, Ard JD, Comuzzie AG, Donato KA, … Obesity S (2014). 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation, 129(25 Suppl 2), S102–138. doi: 10.1161/01.cir.0000437739.71477.ee [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanerva N, Larsson I, Peltonen M, Lindroos AK, & Carlsson LM (2017). Changes in total energy intake and macronutrient composition after bariatric surgery predict long-term weight outcome: findings from the Swedish Obese Subjects (SOS) study. Am J Clin Nutr, 106(1), 136–145. doi: 10.3945/ajcn.116.149112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kittrell H, Graber W, Mariani E, Czaja K, Hajnal A, & Di Lorenzo PM (2018). Taste and odor preferences following Roux-en-Y surgery in humans. PLoS One, 13(7), e0199508. doi: 10.1371/journal.pone.0199508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvehaugen AS, & Farup PG (2018). Changes in gastrointestinal symptoms and food tolerance 6 months following weight loss surgery: associations with dietary changes, weight loss and the surgical procedure. BMC Obes, 5, 29. doi: 10.1186/s40608-018-0206-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lahteenmaki L, & Tuorila H (1995). Three-factor eating questionnaire and the use and liking of sweet and fat among dieters. Physiol Behav, 57(1), 81–88. doi: 10.1016/0031-9384(94)00210-v [DOI] [PubMed] [Google Scholar]
- Maciejewski ML, Arterburn DE, Van Scoyoc L, Smith VA, Yancy WS Jr., Weidenbacher HJ, … Olsen MK. (2016). Bariatric Surgery and Long-term Durability of Weight Loss. JAMA Surg, 151(11), 1046–1055. doi: 10.1001/jamasurg.2016.2317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miras AD, & le Roux CW (2013). Mechanisms underlying weight loss after bariatric surgery. Nat Rev Gastroenterol Hepatol, 10(10), 575–584. doi: 10.1038/nrgastro.2013.119 [DOI] [PubMed] [Google Scholar]
- Mitchell JE, Christian NJ, Flum DR, Pomp A, Pories WJ, Wolfe BM, … Belle SH. (2016). Postoperative Behavioral Variables and Weight Change 3 Years After Bariatric Surgery. JAMA Surg, 151(8), 752–757. doi: 10.1001/jamasurg.2016.0395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore DD, Arterburn DE, Bai Y, Cornejo M, Crawford CL, Drewnowski A, … Coleman KJ (2020). The Bariatric Experience Long Term (BELONG): Factors Related to Having Bariatric Surgery in a Large Integrated Healthcare System. Obes Surg. doi: 10.1007/s11695-020-05045-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nance K, Acevedo MB, & Pepino MY (2020). Changes in taste function and ingestive behavior following bariatric surgery. Appetite, 146, 104423. doi: 10.1016/j.appet.2019.104423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIDDK. (2016). Potential Candidates for Bariatric Surgery. Retrieved from https://www.niddk.nih.gov/health-information/weight-management/bariatric-surgery/potential-candidates
- Nielsen MS, Andersen I, Lange B, Ritz C, le Roux CW, Schmidt JB, … Bredie WLP. (2019). Bariatric Surgery Leads to Short-Term Effects on Sweet Taste Sensitivity and Hedonic Evaluation of Fatty Food Stimuli. Obesity (Silver Spring), 27(11), 1796–1804. doi: 10.1002/oby.22589 [DOI] [PubMed] [Google Scholar]
- Papasavas PS, Stone H, A., Rawal S, Ng J, Tishler D, & Duffy V (2015). Successful weight loss post bariatric surgery associates with greater affinity for healthy dietary and activity behaviors-preliminary support from a case-controlled study. Surg Obes Relat Dis, 11(S). doi: 10.1016/j.soard.2015.08.270 [DOI] [Google Scholar]
- Penney NC, Kinross J, Newton RC, & Purkayastha S (2015). The role of bile acids in reducing the metabolic complications of obesity after bariatric surgery: a systematic review. Int J Obes (Lond), 39(11), 1565–1574. doi: 10.1038/ijo.2015.115 [DOI] [PubMed] [Google Scholar]
- Pinto AM, Fava JL, Raynor HA, LaRose JG, & Wing RR (2013). Development and validation of the weight control strategies scale. Obesity (Silver Spring), 21(12), 2429–2436. doi: 10.1002/oby.20368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Primeaux SD, de Silva T, Tzeng TH, Chiang MC, & Hsia DS (2016). Recent advances in the modification of taste and food preferences following bariatric surgery. Rev Endocr Metab Disord, 17(2), 195–207. doi: 10.1007/s11154-016-9365-0 [DOI] [PubMed] [Google Scholar]
- Ruiz-Tovar J, Bozhychko M, Del-Campo JM, Boix E, Zubiaga L, Munoz JL, & Llavero C (2018). Changes in Frequency Intake of Foods in Patients Undergoing Sleeve Gastrectomy and Following a Strict Dietary Control. Obes Surg, 28(6), 1659–1664. doi: 10.1007/s11695-017-3072-0 [DOI] [PubMed] [Google Scholar]
- Sarwer DB, Wadden TA, Moore RH, Baker AW, Gibbons LM, Raper SE, & Williams NN (2008). Preoperative eating behavior, postoperative dietary adherence, and weight loss after gastric bypass surgery. Surg Obes Relat Dis, 4(5), 640–646. doi: 10.1016/j.soard.2008.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal-Isaacson CJ, Wylie-Rosett J, & Gans KM (2004). Validation of a short dietary assessment questionnaire: the Rapid Eating and Activity Assessment for Participants short version (REAP-S). Diabetes Educ, 30(5), 774, 776, 778 passim. doi: 10.1177/014572170403000512 [DOI] [PubMed] [Google Scholar]
- Sudan R, Sudan R, Lyden E, & Thompson JS (2017). Food cravings and food consumption after Roux-en-Y gastric bypass versus cholecystectomy. Surg Obes Relat Dis, 13(2), 220–226. doi: 10.1016/j.soard.2016.09.001 [DOI] [PubMed] [Google Scholar]
- Wang W, Cheng Z, Wang Y, Dai Y, Zhang X, & Hu S (2019). Role of Bile Acids in Bariatric Surgery. Front Physiol, 10, 374. doi: 10.3389/fphys.2019.00374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woglom C, Gray V, Hill M, & Wang L (2020). Significant Relationships Exist between Perceived and Objective Diet Quality in Young Adults. J Acad Nutr Diet, 120(1), 103–110. doi: 10.1016/j.jand.2019.06.002 [DOI] [PubMed] [Google Scholar]
- Wolfe BM, Kvach E, & Eckel RH (2016). Treatment of Obesity: Weight Loss and Bariatric Surgery. Circ Res, 118(11), 1844–1855. doi: 10.1161/CIRCRESAHA.116.307591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuensch K (2019). Cohen’s Conventions for Small, Medium, and Large Effects.. Retrieved from http://core.ecu.edu/psyc/wuenschk/docs30/EffectSizeConventions.pdf.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.