Abstract
Purpose:
Intraoperative drain placement during an open transversus abdominis release (TAR) is common practice. However, evidence detailing the optimal timing of drain removal is lacking. Surgical dogma teaches that drains should remain in place until output is minimal. This practice increases the risk of drain associated complications (infection, pain and skin irritation) and prolongs the burden of surgical drain maintenance. The objective of this study is to review infectious outcomes following TAR with early or late drain removal.
Methods:
Patients who underwent an open bilateral TAR from 1/2018–1/2020 were eligible for the study. Prior to 2019, one of two intraoperative drains were left in place at discharge. In 2019, clinical practice shifted to remove both drains at hospital discharge irrespective of output. The rate of infectious morbidity was compared between the two cohorts.
Results:
A total of 184 patients were included: 89 late and 95 early drain removal. No differences in wound complications existed between the two cohorts: Surgical site occurrence (SSO): 21.3% vs. 18.9% (p=0.68); surgical site infection (SSI): 14.6% vs. 10.5% (p=0.40); abscess: 8.9% vs. 4.2% (p=0.20); seroma: 6.7% vs. 10.5% (p=0.36); cellulitis: 14.6% vs. 8.4% (p=0.19%); or SSO requiring procedural intervention (SSOPI): 5.6% vs. 5.2% (p=0.92). Rates of antibiotic prescription and 30-day readmission were also similar (p=0.69 and p=0.89).
Conclusions:
Early removal of abdominal wall surgical drains at discharge irrespective of drain output does not increase the prevalence of infectious morbidity following TAR. It is likely safe to remove all drains at discharge regardless of drain output.
Keywords: Incisional hernia repair, transversus abdominis release, component separation, surgical drain, wound infection
Introduction
Incisional hernias are extremely common and occur following 11%−50% of all laparotomies (1). The newest technique gaining significant popularity for complex incisional hernia repairs is the posterior component separation - transversus abdominis release (TAR). Developed in 2012 by Novitsky and colleagues, TAR is based on the Rives-Stoppa-Wantz retrorectus repair that utilizes a potential space between the posterior rectus sheath and rectus muscle that extends laterally beyond the linea semilunaris by dividing the transversus abdominis allowing for wide mesh overlap (1, 2). TAR has excellent long-term recurrence rates at less than 10%, far superior to other techniques previously described (1). Unfortunately, wound complications are quite common following all types of ventral hernia repairs, reported in as high as 20–41% of patients, and mesh infections can complicate nearly 10% of cases (1, 3, 4). Although wound infections are typically less severe following a TAR and often can be treated with antibiotics alone, wound complications remain a concern (5).
The extensive abdominal wall dissection required during a TAR places patients at risk for developing postoperative seromas and possible wound complications (6, 7). In an attempt to reduce fluid accumulation that can affect the ingrowth of mesh to native abdominal wall muscle/tissue, closed suction drains are often placed intraoperatively between the mesh implant and the rectus muscle (8). Surgical drain placement following all types of open incisional hernia repairs is routine practice, but a drain’s effectiveness in preventing wound infections remains controversial (8). Proponents argue that drains reduce postoperative accumulation of blood and fluid in the potential space created by the abdominal wall dissection (8). Opponents argue that drains are ineffective at draining the wound and can increase postoperative pain, generate a foreign body reaction, and act as a nidus for infection allowing bacteria to colonize mesh (7). However, the clinical dilemma is not only limited to whether or not to place a drain, but also how long the drain should remain in place. As drains can cause patients added pain and local skin irritation, and drain maintenance can be an additional burden on patients and their families, prompt drain removal may be beneficial if it does not also lead to increased wound complications (9).
Peer-reviewed evidence informing the optimal timeline for surgical drain removal postoperatively remains lacking and has not previously been studied following a TAR. Surgical dogma teaches that drains should remain in place until their output is minimal (less than 30cc/day for 2 consecutive days) and, thus, in many practices, drains are removed at postoperative clinic visits up to one month following the initial surgery. The practice of leaving drains in place for this extended period places a significant burden on patients who must maintain the drain after discharge and endure potential drain site irritation, drain dislodgment, and pain secondary to drain presence (10). Only one retrospective cohort study has attempted to address optimal timing of drain removal following an incisional hernia repair. The authors found that patients who had drains in place for greater than two weeks postoperatively had an increased incidence of wound complications (6). As multiple surgical techniques, mesh types, and mesh placement locations were utilized in this study, the relationship between drain duration and TAR, specifically, is unclear. In an effort to better understand the relationship between infectious outcomes and the timing of drain removal following a TAR, we compared infectious outcomes of patients with early drain removal at the time of discharge to patients whose final drain was removed at the patient’s first postoperative clinic visit. We have the opportunity to improve patients’ overall postoperative satisfaction and quality of life as well as simplify their recovery at home if we can demonstrate that drain removal at time of discharge does not affect infectious morbidity.
Methods
Study Population
This project was approved by our Institutional Review Board and all patients underwent verbal and written informed consent prior to surgery. Patients were included if they underwent an open bilateral TAR by one of two fellowship trained abdominal wall specialists at a quaternary care academic medical center from January 2018 to January 2020. Combined, multi-specialty cases were included in the study (i.e. combined colon and rectal procedures and TAR, urologic/gynecologic procedures and TAR, or parastomal hernia repair and TAR). Only patients who had non-absorbable, synthetic mesh placed in the sublay fashion were studied. All mesh weights (light-weight, mid-weight, and heavy-weight) were included, but patients were excluded if biologic or biosynthetic mesh was used. Additionally, all patients undergoing minimally invasive TAR (robotic or laparoscopic) were excluded, as were patients with cirrhosis or active/ongoing infections, including an enterocutaneous fistula, at the time of surgery. Perioperative surgical care was standardized according to our institution’s abdominal wall repair recovery pathway and was identical for all patients in the study.
Operative technique was similar between both senior surgeons and was modelled on the technique previously described by Novitsky et al (2). Notably, all patients had a large piece of macroporous, monofilament, non-coated synthetic mesh laid flat upon the posterior rectus sheath in a diamond formation and two 19-French (19-F) closed suction drains placed anterior to the mesh in the extraperitoneal space (Figure 1). In both cohorts, if a large subcutaneous cavity existed after hernia sac resection, a small 15-French (15-F) drain, in addition to the standard two 19-F drains, was placed into the subcutaneous space to minimize fluid accumulation. The clinical management of these 15-F drains was not altered for the purposes of the study and the decision on when to remove these drains was based on the clinical judgement of the attending surgeon. If an additional drain was placed by another surgical team during a combined procedure (colorectal, gynecological, etc.), post-operative drain management was deferred to the other surgical team; only the two 19-F drains placed by the two abdominal wall surgeons were influenced by the study parameters and were considered when calculating drain output totals.
Figure 1:
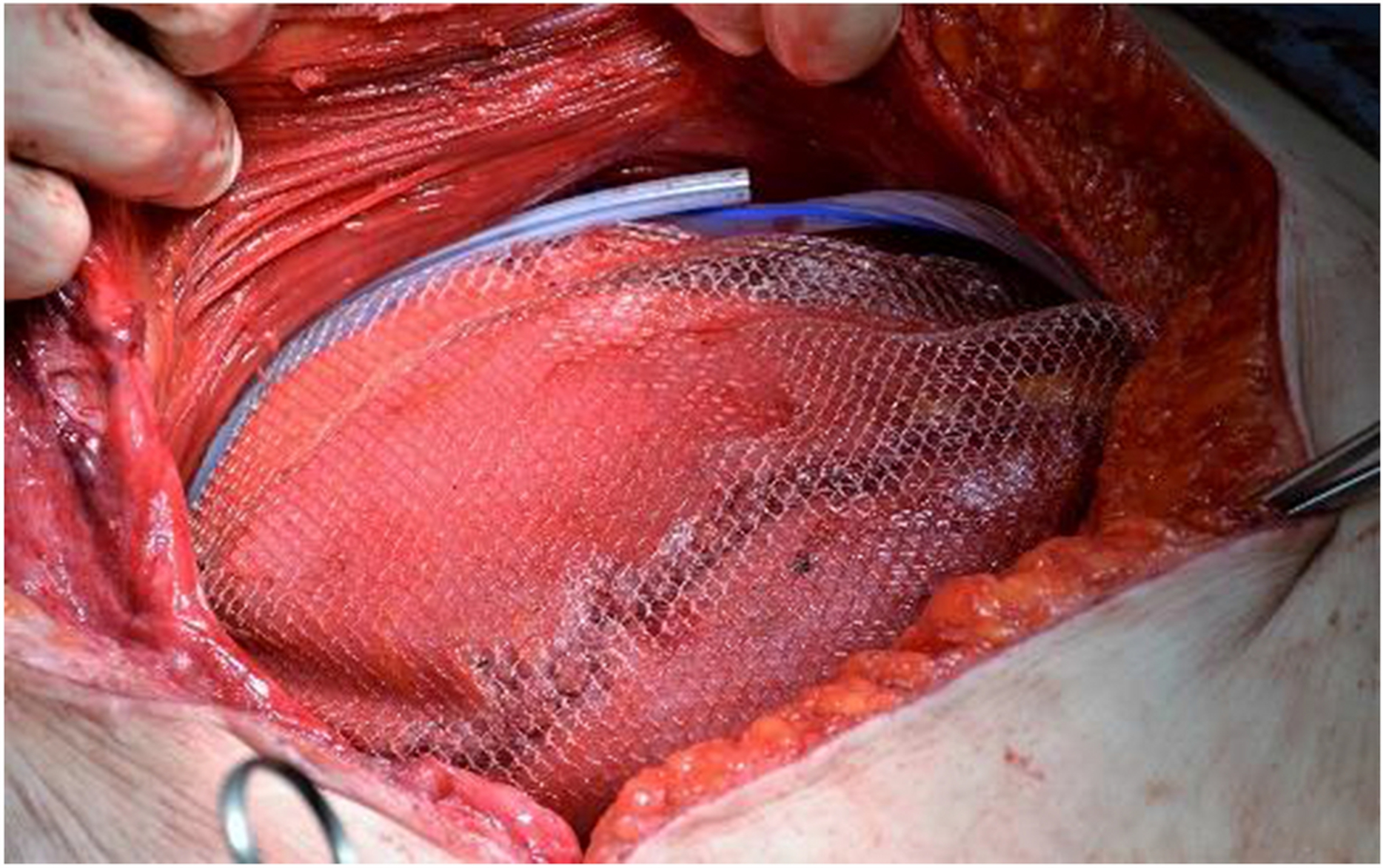
Two closed suction drains are placed anterior to the mesh superiorly and inferiorly after closure of the posterior sheath and mesh placement.
To study whether the timing of drain removal affected patient’s postoperative wound outcomes, eligible patients were retrospectively divided into two cohorts based on the calendar year their surgery was completed: Either 2018 (surgery dates from January 1, 2018-December 31, 2018) or 2019 (January 1, 2019-December 31, 2019). From 2018 to 2019 our team’s clinical practice regarding the timing of drain removal changed. During 2018, our clinical practice was to leave at least one (of two total) intraoperatively placed 19-F surgical drain in place at the time of discharge (the drain with the greatest daily output). At the first postoperative visit, approximately two weeks after surgery, the remaining drain was removed. However, starting January 1, 2019, surgeon clinical practice was altered so that both intraoperatively placed drains were removed on the day of discharge, irrespective of their output. Therefore, patients who underwent a TAR during calendar year 2018 were included in the cohort “late drain removal” and patients who underwent surgery during calendar year 2019 were included in the “early drain removal” cohort.
Data Collection
To examine the impact of drain removal timing on infectious outcomes, we compared the two cohorts: early versus late. Electronic health records were retrospectively analyzed for baseline demographic and patient comorbidity data (age, body mass index (BMI), diabetes mellitus (DM), obstructive sleep apnea (OSA), hypertension (HTN), chronic obstructive pulmonary disease (COPD), preoperative immunosuppression, history of solid organ transplant (liver or kidney), and tobacco use (current user; former user, meaning smoking cessation for greater than 1 month preoperatively; and never user). Perioperative clinical data was also collected: preoperative recurrent ventral hernias, defect size (cm2), operating room (OR) time, length of hospital stay (LOS), percentage of combined procedures (TAR + additional procedure), number of in-hospital complications (Clavien-Dindo grade 1–5), percentage of Clavien-Dindo complications grade 2 or higher, and total drain output (ml) on day of discharge or at time of all drain removal (11). Additionally, total 19-F drain output was recorded for each hospital day for the first 7 postoperative days (POD).
The infectious complications reviewed included surgical site occurrence (SSO) at 30 days, surgical site infection (SSI), abdominal wall or deep abscess (abscess), seroma/hematoma, wound cellulitis, surgical site occurrence requiring procedural intervention (SSOPI), antibiotic prescription, all-cause 30-day readmission rate, and wound-related 30-day readmission rate. SSOs included any SSI as well as wound cellulitis, non-healing incisional wound, fascial disruption, skin or soft tissue ischemia/necrosis, wound serous or purulent discharge, stitch abscess, seroma, hematoma, infected or exposed mesh, or the development of an enterocutaneous fistula (7). SSI rate was calculated from the number of SSOs minus the number of sterile seromas/hematomas. SSOPI is defined as any SSO that required opening of the wound, wound debridement, percutaneous drainage, suture excision, or partial or complete mesh removal (7). Seroma/hematoma is defined as a bulge or fluctuation without signs of infection or the presence of a non-infected abdominal wall fluid collection found on imaging. Antibiotic prescription and all-cause 30-day readmission were considered positive even if the reason for antibiotic prescription or admission was unrelated to the patient’s hernia operation.
Statistical Analysis
Data was extracted to and analyzed by Microsoft Excel 2018 (Microsoft, Redmond, WA, USA). Multivariate logistic regression was performed using SPSS versions (IBM Corporation Released 2015. IBM SPSS Statistics for Mac, Version 23.0. Armonk, NY: IBM Corporation). Continuous variables were expressed as mean ± standard deviation and compared via student’s t test or as medians and compared via the Wilcox Rank Sum test using interquartile range (IQR). Categorical variables were reported using percentages and compared via chi-squared tests. Patient demographics and infectious outcomes of both groups (early and late drain removal) were compared using chi-squared analysis. Multivariate logistic regression was used to examine the association between hernia risk factors (BMI, defect size, active smoking, and DM) and calendar year of surgery (proxy for drain removal timing) with SSO rate. Results are reported in odds ratios (OR) and 95% confidence intervals (CI). A p-value of <0.05 was considered statistically significant.
Results
Between January 2018 to December 2020, 184 patients met study criteria and underwent an open bilateral TAR: 95 patients underwent early drain removal (calendar year 2019), and 89 patients had a late drain removal (calendar year 2018). The average POD of final drain removal for the early cohort was POD 5.91 ± 5.16 and, for the late cohort, was POD 16.62 ± 5.82 (p<0.01). The average BMI of our entire population was 32.25 ± 5.20 kg/m2 and the average age was 59.15 ± 11.02 years (Table 1). The background demographics/comorbidities between the late and early cohorts were similar in all categories except for percentage of former smokers (Early: 31.6%; Late: 46.1%, p=0.04) (Table 1). The perioperative clinical characteristics were also similar between the two groups except for OR time and LOS: patients in the early cohort had longer median LOS (Early: 4.0 (IQR: 3–6); Late: 4.0 (IQR: 3–5), p=0.01) and OR time (minutes) (Early: 363.10 ± 70.87; Late: 319.03 ± 95.81, p=<0.01). However, there was no difference in the percentage of patients staying in the hospital between 3–5 days (expected LOS based on postoperative TAR pathway) (Early: 67.3%; Late: 76.4%, p=0.17). The groups were well matched in terms of defect size, percentage of preoperative recurrent hernias, and the percentage of cases requiring a subcutaneous 15-F drain (Tables 2). Additionally, total daily drain output was similar between the two cohorts on all PODs (POD 2: p=0.08; POD 3: p=0.45; POD 4: p=0.50; POD 5: 0.78; POD 6: p=0.98; POD 7: p=0.25), except for POD 1 in which the late cohort had slightly greater total drain output (253.98 vs. 224.6, p=0.03) (Figure 2).
Table 1:
Patient Baseline Demographics
| All Patients | Early | Late | p-value | |
|---|---|---|---|---|
| Number of Patients | 184 | 95 | 89 | |
| POD of Final Drain Removal (x ± SD) | 11.09 (7.67) | 5.91 (5.16) | 16.62 (5.82) | <0.01 |
| BMI (x ± SD) | 32.25 (5.20) | 31.82 (5.00) | 32.70 (5.36) | 0.23 |
| Age (x ± SD) | 59.15 (11.02) | 59.48 (10.41) | 58.80 (11.68) | 0.66 |
| DM (%) | 51 (27.7) | 25 (27.4) | 26 (29.1) | 0.66 |
| OSA (%) | 50 (27.2) | 26 (27.3) | 24 (30.0) | 0.95 |
| HTN (%) | 113 (61.4) | 63 (66.3) | 50 (56.2) | 0.16 |
| COPD (%) | 30 (10.3) | 14 (14.7) | 16 (18.0) | 0.55 |
| Immunosuppression (%) | 33 (17.9) | 16 (16.8) | 17 (19.1) | 0.69 |
| Prior Transplant | ||||
| Liver (%) | 15 (8.2) | 9 (9.5) | 6 (6.7) | 0.50 |
| Kidney (%) | 4 (2.2) | 2 (2.1) | 2 (2.2) | 0.95 |
| Tobacco Use | ||||
| Current (%) | 17 (9.2) | 11 (11.6) | 6 (6.7) | 0.26 |
| Former (%) | 71 (38.6) | 30 (31.6) | 41 (46.1) | 0.04 |
| Never (%) | 67 (34.4) | 54 (56.8) | 42 (47.2) | 0.19 |
POD of Final Drain Removal- postoperative day when final 19-F drain was removed; BMI-body mass index (kg/m2); x-mean; SD-standard deviation; Age (years); DM- Diabetes Mellitus; OSA-Obstructive Sleep Apnea, HTN- Hypertension; COPD-Chronic Obstructive Pulmonary Disease; Former Tobacco Use- tobacco cessation ≥1 month. p<0.05 is considered statistically significant.
Table 2:
Perioperative Patient Characteristics
| All Patients | Early | Late | p-value | |
|---|---|---|---|---|
| Number of Patients | 184 | 95 | 89 | |
| Preoperative Recurrent Hernia (%) | 108 (58.7) | 54 (56.8) | 54 (60.7) | 0.60 |
| Defect size (x ± SD) | 336.11 (210.15) | 337.99 (212.80) | 334.11 (208.46) | 0.60 |
| 15-F Subcutaneous Drain Presence | 50 (27.2) | 24 (25.2) | 26 (29.2) | 0.55 |
| LOS (median (IQR)) | 4.0 (3–6) | 4.0 (3–6) | 4.0 (3–5) | 0.01 |
| LOS (x+SD) | 5.2 (4.1) | 5.9 (5.1) | 4.8 (2.4) | 0.01 |
| LOS 3–5 days (%) | 132 (71.7) | 64 (67.4) | 68 (76.4) | 0.17 |
| OR Time (x ± SD) | 341.76 (86.49) | 363.10 (70.87) | 319.03 (95.81) | <0.01 |
| In-hospital Complications (%) | 67 (36.4) | 35 (36.8) | 32 (36.0) | 0.90 |
| Clavien-Dindo ≥ 2 (%) | 44 (24.9) | 26 (27.4) | 18 (20.2) | 0.26 |
| Discharge/Removal Drain Output (x ± SD) | 84.64 (62.10) | 79.28 (59.32) | 90.43 (64.76) | 0.25 |
| Combined Procedures (%) | 49 (26.6) | 23 (24.2) | 26 (29.2) | 0.82 |
x-mean; SD-standard deviation; IQR- interquartile range; Defect Size (cm2); LOS – length of stay (days); OR time-operating room time (minutes); drain output (ml). p<0.05 is considered statistically signific
Figure 2:
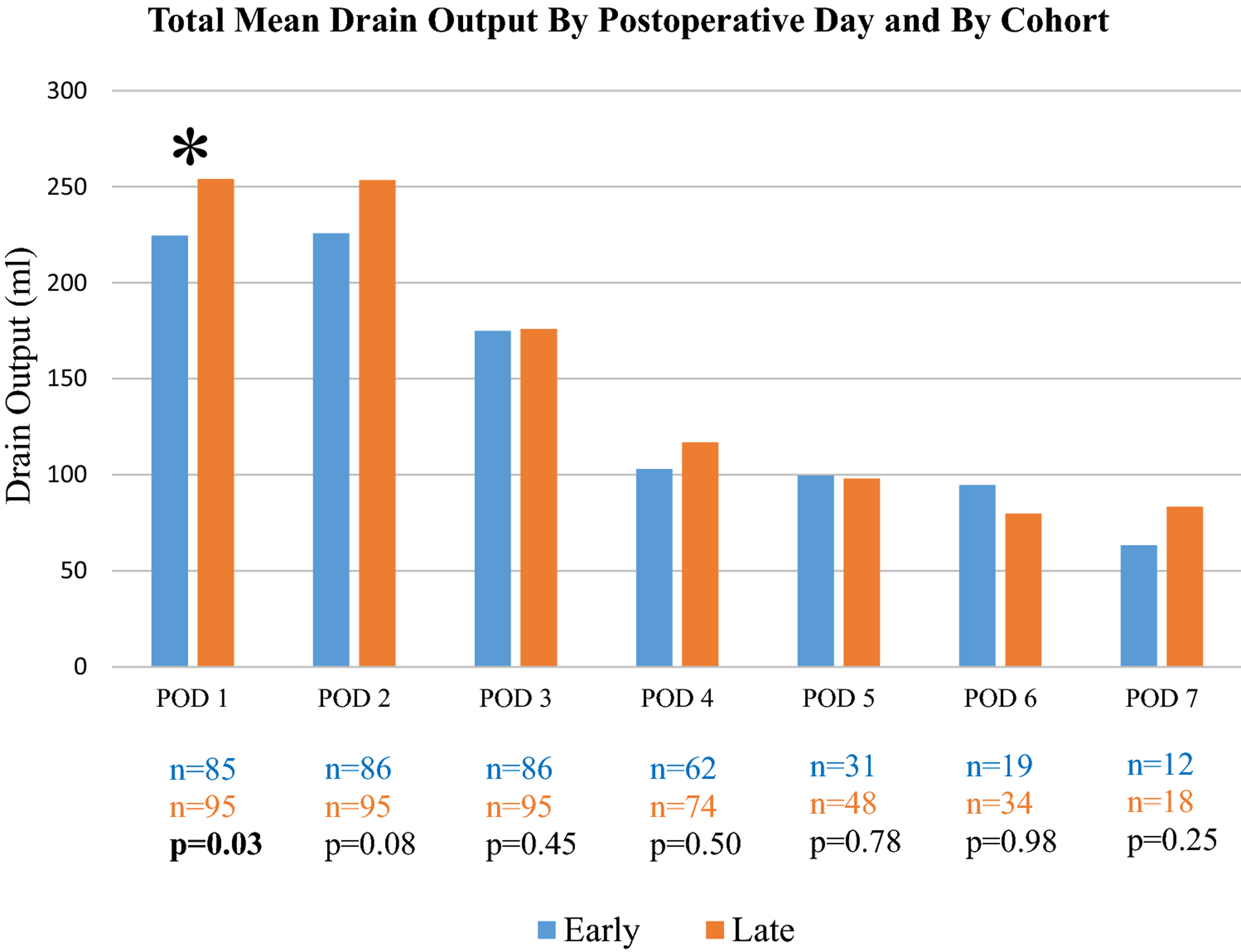
Comparison of the mean total 19-F drain output (anterior to the mesh) by postoperative day (milliliters) and by drain removal timing.
POD= Postoperative Day; n= number of patients; * = statistically significant; p<0.05 is considered statistically significant.
There were no statistical differences in any infectious outcome between patients who had early drain removal vs. late drain removal: SSO: 18.9% vs. 21.3% (p=0.68); SSI: 10.5% vs. 14.6% (p=0.40), SSOPI: 5.2% vs. 5.6% (p=0.92); abscess/deep infection: 4.2% vs. 9.0% vs. (p=0.19); seroma/hematoma: 10.5% vs. 6.7% (p=0.36); cellulitis: 8.4% vs. 14.6% (p=0.19); antibiotic prescription: 17.9% vs. 20.2% (p=0.69); and 30-day readmission: 8.4% vs. 7.9% (p=0.89). In our total population of 184, 37 patients (20.1%) had an SSO, 23 patients (12.5%) had an SSI, and 10 patients (5.4%) required a procedural intervention for their SSO. Overall, the most common SSO was superficial cellulitis (11.4%). In total, 19.0% of patients received an antibiotic prescription within the first 30 days of discharge and 8.1% required readmission. Complete infectious outcomes are listed in Table 3.
Table 3:
Wound complications between Early Drain Removal vs. Late Drain Removal cohort
| All Patients | Early | Late | p-value | |
|---|---|---|---|---|
| Number of Patients | 184 | 95 | 89 | |
| SSO (%) | 37 (20.1) | 18 (18.9) | 19 (21.3) | 0.68 |
| SSI (%) | 23 (12.5) | 10 (10.5) | 13 (14.6) | 0.40 |
| SSOPI (%) | 10 (5.4) | 5 (5.2) | 5 (5.6) | 0.92 |
| Abscess/Deep Infection (%) | 12 (6.5) | 4 (4.2) | 8 (9.0) | 0.19 |
| Seroma/Hematoma (%) | 16 (8.7) | 10 (10.5) | 6 (6.7) | 0.36 |
| Cellulitis (%) | 21 (11.4) | 8 (8.4) | 13 (14.6) | 0.19 |
| Antibiotic Prescription (%) | 35 (19.0) | 17 (17.9) | 18 (20.2) | 0.69 |
| 30-Day All Cause Readmission (%) | 15 (8.1) | 8 (8.4) | 7 (7.9) | 0.89 |
| 30-Day Readmission for Wound Related Complications (%) | 9 (4.9) | 4 (4.2) | 5 (5.6) | 0.66 |
SSO- surgical site occurrence, SSI- surgical site infection, SSOPI- surgical site occurrence requiring procedural intervention. p<0.05 is considered statistically significant.
On multivariate logistic regression, when controlling for active smoking, DM, defect size, and BMI, there was no association between SSO rate and calendar year of surgery (p=0.78, OR=1.12, 95% CI 0.52–2.38). While active smoking had a trend towards an increased risk of SSO (p=0.06, OR: 2.94, 95% CI 0.93–9.31), this trend did not reach statistical significance. Likewise, DM, being overweight (BMI 25–30), obesity (BMI>35), and defect size were not independently associated with increased SSO risk: DM (p=0.78, OR=1.12, 95% CI 0.52–2.38), overweight (p=0.29, OR= 3.48, 95% CI= 0.35–34.5), obesity (p=0.25, OR=3.59, 95% CI 0.40–32.0), and defect size (p=0.36, OR=1.52, 95% CI=0.62–3.68). Lastly, to ensure that patients with extended hospital stays did not skew the main outcomes of interest, subgroup analysis of only patients with a length of stay 6 days or fewer was completed. Of the 184 total eligible patients, 152 (82.6%) had hospital stays less than 7 days. In the subgroup analysis, the average POD of final drain removal in the early cohort was POD 4.10 ± 1.07 and, for the late cohort, was POD 16.23 ± 5.56 (p<0.01). There were no statistical difference in any infectious outcomes between the two cohorts (Table 4).
Table 4:
Wound complications of patients with lengths of hospital stays less than 7 days
| All Patients | Early | Late | p-value | |
|---|---|---|---|---|
| Number of Patients | 152 | 77 | 75 | |
| POD of Final Drain Removal (x ± SD) | 10.21 (7.29) | 4.10 (1.07) | 16.23 (5.57) | <0.01 |
| SSO (%) | 27 (17.8) | 12 (15.6) | 15 (20.0) | 0.48 |
| SSI (%) | 16 (10.5) | 5 (6.5%) | 11 (14.7) | 0.10 |
| SSOPI (%) | 4 (2.6) | 1 (1.3) | 3 (4.0) | 0.30 |
| Abscess/Deep Infection (%) | 5 (3.3) | 1 (1.3) | 4 (5.3) | 0.16 |
| Seroma/Hematoma (%) | 12 (7.9) | 7 (9.1) | 5 (6.7) | 0.58 |
| Cellulitis (%) | 18 (11.8) | 7 (9.1) | 11 (14.7) | 0.29 |
| Antibiotic Prescription (%) | 25 (16.4) | 11 (14.3) | 14 (18.7) | 0.47 |
| 30-Day All Cause Readmission (%) | 8 (5.3) | 4 (5.2) | 4 (5.3) | 0.97 |
| 30-Day Readmission for Wound Related Complications (%) | 3 (2.0) | 1 (1.3) | 2 (2.7) | 0.55 |
POD of Final Drain Removal- postoperative day when final 19-F drain was removed, SSO- surgical site occurrence, SSI- surgical site infection, SSOPI- surgical site occurrence requiring procedural intervention. p<0.05 is considered statistically significant.
Discussion
Although drain placement has become common practice following a TAR, peer-reviewed evidence is lacking detailing the optimal timing of drain removal postoperatively. Surgical dogma teaches that drains must remain in place until their output is minimal: often below 30–40 mL for two consecutive days. This leaves patients to care for their drains for extended periods during recovery as well as often adding postoperative pain or triggering local skin irritation around drain sites. In the present study, we found that there were no differences in any infectious complications, including SSO, SSI, and seromas/hematomas when drains were removed early at the time of discharge compared to at the time of the patient’s first follow-up appointment (two weeks postoperatively). To date, this is the largest study evaluating the timing of drain removal following an incisional hernia repair and the first study to evaluate drain duration specifically for patients undergoing a TAR. Our results suggest that it is likely safe to remove patients’ abdominal wall drains at discharge irrespective of drain output, thus eliminating patients’ discomfort and irritation associated with drain care at home.
The present study’s findings suggest that early drain removal, at the time of hospital discharge, does not increase infection risk. Drains can act as a foreign body increasing infection risk and can easily become colonized with bacteria (10, 12, 13). Additionally, many patients’ main complaint at follow-up visits is pain and/or redness/local skin irritation around cutaneous drain sites and the added burden of drain care responsibilities at home. Early drain removal could increase patient’s overall satisfaction without also increasing infectious complications. The only other group to address this question was Plymale et al. The authors found that wound complications increased linearly if drains were left in place greater than 14 days following an abdominal wall reconstruction. Although their study ultimately analyzed only 64 cases and included several different mesh types, mesh placement locations, and repair techniques, it also supports that early drain removal is beneficial and certainly not detrimental (6). Based on our results and the limited data available, it is now our team’s standard of practice to remove both 19-F retromuscular drains prior to discharge regardless of their daily output (unless specific contraindications exist such as active infection or history of cirrhosis). Ultimately, large randomized control trials are needed to determine whether early drain removal is associated with reduced long-term mesh infections and wound complications before official recommendations can be offered. If surgeons are to contemplate not leaving a drain following an abdominal wall reconstruction, mesh placement and surgical technique must be considered. While no drain placement may be possible after a TAR, the creation of large lipocutaneous flaps or mesh placement in the subcutaneous space would certainly preclude this strategy. Additionally, the study of minimally invasive TARs may provide insight into the question of drain utility. Many centers, including our own, are not routinely using drains following a robotic TAR. As robotic TARs increase in popularity and are offered to more patients who would have otherwise undergone an open TAR, it will be interesting to observe whether clinical practice regarding drain placement in open TARs evolve.
TAR is quickly being adopted by surgeons worldwide for the repair of large incisional hernias and has shown excellent long-term recurrence rates that are far superior to other techniques previously described (less than 10%) (1). The technique affords the surgeon a large potential space between the posterior rectus sheath and rectus muscle for wide prosthetic mesh overlap, as well as the ability to exclude the mesh from the viscera. While TAR is associated with fewer complications and wound infections than other component separation techniques, seroma and wound complications are still common, and mesh infections remain a dreaded complication (1, 3, 5, 14). The total SSO, SSI, and seroma/hematoma rate in the present study was 20.1%, 12.5% and 8.7%, respectively. Of the 37 SSOs, 10 (27%) required procedural intervention. These rates are slightly better than what has been reported by other teams for all open abdominal wall reconstructions and is likely a result of several key advantages of the TAR technique (6, 7, 10, 15–17). In contrast to an external oblique release, TAR does not require the creation of large lipocutaneous skin flaps which are often associated with tremendous wound morbidity (18). Furthermore, with native tissue apposition on both sides of the mesh, the risk for mesh infection is reduced in comparison with other reinforcement techniques (underlay or intraperitoneal), and even if the mesh were to be contaminated, mesh salvage with antibiotics alone is possible (14, 19).
There are several limitations to the present study. This study was a retrospective review of a single center’s experience. Comparison data was retrospective and was based on calendar year rather than a true head-to-head randomized or matched comparison of drain removal timing. Although it is possible that infectious results could have been biased from temporal variations, such as changes in surgeon’s experience or other institutional practices unaccounted for, this would be unlikely given surgeon staff consistency, unchanged surgical technique, and stable postoperative patient pathways throughout the study time-period. Furthermore, BMI, DM, immunosuppression, and current tobacco use (the largest determinants of wound morbidity following a ventral hernia repair), were all similar between the two groups as was hernia defect size. Another possible limitation is the presence of the 15-F subcutaneous drain. Although it is possible that the presence of this drain could have reduced seroma formation, both groups had a similar percentage of patients with 15-F drain, making it unlikely to affect our main infectious outcomes. A third limitation of our study was that the two cohorts had statistically different LOS. However, the percentage of patients staying 3–5 days postoperatively (expected LOS based on our institution’s TAR pathway) was not statistically significant. This likely reflects the fact that the LOS difference observed was truly a reflection of a few outlier patients and not a direct result of drain removal timing; ultimately, this difference was unlikely to affect the infectious outcomes of interest. Lastly, drain removal in the early cohort was not standardized by output or by postoperative day but based solely on a patient’s discharge day. In the early cohort, 81% (77/95) of patients stayed 6 days or fewer and thus, the vast majority, had their drain removed by the 7th postoperative day. This endpoint for drain removal was chosen to act as a realistic patient care endpoint: by removing the drain at discharge, patients would be free from drain care responsibilities at home, while also providing the longest possible benefit of fluid accumulation. Furthermore, subgroup analysis of patients who had been in the hospital for less than 7 days showed no difference in infectious outcomes suggesting our results were unlikely skewed by patients with prolonged hospital stays.
An equally important question is whether drain placement at all following a TAR is beneficial. While it has become common practice to place two closed suction drains anterior to the mesh in attempt to eliminate fluid accumulation and, potentially, reduce infectious morbidity, whether this reduces SSOs remains controversial. Again, there is insufficient peer-reviewed evidence to fully answer this question: In a Cochrane review of randomized clinical trials, published in 2013, the authors found only one randomized trial of 24 patients eligible for their review. Unfortunately, this one study was only a comparison of two different drain types, rather than a comparison of whether drain placement or no drain placement is advantageous (20). The limited data that does exist is mixed. Krpata et al. utilized data from the American Hernia Society Quality Collaborative (AHSQC) and found that patients undergoing incisional hernia repair with a drain were less likely to develop non-infectious SSOs and there was no resultant increase in SSIs. Alternatively, Ramshaw et al. found less wound and pulmonary complications, shorter hospital stays, and less opioid use when no drains were used following an abdominal wall reconstruction. Lastly, a review of infectious outcomes following onlay mesh repair randomized 42 patients to either subcutaneous drain placement or progressive tension sutures and found no differences in seroma formation or infections. However, the authors reported higher than average early seroma rates for both groups (greater than 70%) (15). While it has been our anecdotal experience that drain placement can reduce seroma formation in the early post-operative period and may potentially reduce long-term wound complications, the limited data available makes official recommendations challenging and further investigation is warranted.
Our work is the first study to evaluate whether early drain removal following a TAR affects patients’ postoperative infectious morbidity. Our data suggests that early abdominal wall surgical drain removal irrespective of drain output does not increase the prevalence of infectious morbidity following TAR. Therefore, it is likely safe to remove patient’s retromuscular abdominal wall drains at discharge irrespective of drain output, thus eliminating patient burdens associated with drain care. Future prospective randomized trials should focus on studying early drain removal following abdominal wall reconstruction to verify our results and also examine if abdominal wall drains after TAR are necessary.
Acknowledgements:
Support: Research for this study was supported by the Washington University School of Medicine Surgical Oncology Basic Science and Translational Research Training Program grant T32CA009621 from the National Cancer Institute (EEO).
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Conflicts of interest: Dr. Blatnik has an honorary speaking and teaching appointment with Bard International (BD) and Intuitive Surgical. The other authors have no conflicts to report.
Consent to Participate:
• The Institutional Review Board at the Washington University in St. Louis approved this study. Due to the low patient risk of the study and its retrospective nature, an exception for formal written informed consent was provided by the IRB and a waiver for HIPPAA Authorization was granted per section 164.512(i) of the Privacy Rule. All patients underwent verbal and written informed consent prior to surgery. IRB ID # 202002069.
References
- 1.Pauli EM, Rosen MJ. Open ventral hernia repair with component separation. Surg Clin North Am. 2013;93(5):1111–33. [DOI] [PubMed] [Google Scholar]
- 2.Novitsky YW, Elliott HL, Orenstein SB, Rosen MJ. Transversus abdominis muscle release: a novel approach to posterior component separation during complex abdominal wall reconstruction. Am J Surg. 2012;204(5):709–16. [DOI] [PubMed] [Google Scholar]
- 3.Kao AM, Arnold MR, Augenstein VA, Heniford BT. Prevention and Treatment Strategies for Mesh Infection in Abdominal Wall Reconstruction. Plast Reconstr Surg. 2018;142(3 Suppl):149S–55S. [DOI] [PubMed] [Google Scholar]
- 4.Maloney SR, Schlosser KA, Prasad T, Kasten KR, Gersin KS, Colavita PD, et al. Twelve years of component separation technique in abdominal wall reconstruction. Surgery. 2019;166(4):435–44. [DOI] [PubMed] [Google Scholar]
- 5.Jones CM, Winder JS, Potochny JD, Pauli EM. Posterior Component Separation with Transversus Abdominis Release: Technique, Utility, and Outcomes in Complex Abdominal Wall Reconstruction. Plast Reconstr Surg. 2016;137(2):636–46. [DOI] [PubMed] [Google Scholar]
- 6.Plymale MA, Harris JW, Davenport DL, Smith N, Levy S, Scott Roth J. Abdominal Wall Reconstruction: The Uncertainty of the Impact of Drain Duration upon Outcomes. Am Surg. 2016;82(3):207–11. [PubMed] [Google Scholar]
- 7.Krpata DM, Prabhu AS, Carbonell AM, Haskins IN, Phillips S, Poulose BK, et al. Drain Placement Does Not Increase Infectious Complications After Retromuscular Ventral Hernia Repair with Synthetic Mesh: an AHSQC Analysis. J Gastrointest Surg. 2017;21(12):2083–9. [DOI] [PubMed] [Google Scholar]
- 8.Gurusamy KS, Samraj K. Wound drains after incisional hernia repair. Cochrane Db Syst Rev. 2007(1). [DOI] [PubMed] [Google Scholar]
- 9.Addison P, Nauka PC, Fatakhova K, Amodu L, Kohn N, Rodriguez Rilo HL. Impact of Drain Placement and Duration on Outcomes After Pancreaticoduodenectomy: A National Surgical Quality Improvement Program Analysis. J Surg Res. 2019;243:100–7. [DOI] [PubMed] [Google Scholar]
- 10.Ramshaw B, Dean J, Forman B, Heidel E, Gamenthaler A, Fabian M. Can Abdominal Wall Reconstruction Be Safely Performed Without Drains? Am Surg. 2016;82(8):707–12. [PubMed] [Google Scholar]
- 11.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reiffel AJ, Pharmer LA, Weinstein AL, Spector JA. A Prospective Analysis of the Association Between Indwelling Surgical Drains and Surgical Site Infection in Plastic Surgery. Ann Plas Surg. 2013;71(5):561–5. [DOI] [PubMed] [Google Scholar]
- 13.Gurusamy KS, Allen VB. Wound drains after incisional hernia repair. Cochrane Db Syst Rev. 2013(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair LJ, Cox TC, Huntington CR, Groene SA, Prasad T, Lincourt AE, et al. The effect of component separation technique on quality of life (QOL) and surgical outcomes in complex open ventral hernia repair (OVHR). Surg Endosc. 2017;31(9):3539–46. [DOI] [PubMed] [Google Scholar]
- 15.Westphalen AP, Araujo AC, Zacharias P, Rodrigues ES, Fracaro GB, Lopes Filho Gde J. Repair of large incisional hernias. To drain or not to drain. Randomized clinical trial. Acta Cir Bras. 2015;30(12):844–51. [DOI] [PubMed] [Google Scholar]
- 16.Misra MC, Bansal VK, Kulkarni MP, Pawar DK. Comparison of laparoscopic and open repair of incisional and primary ventral hernia: results of a prospective randomized study. Surg Endosc. 2006;20(12):1839–45. [DOI] [PubMed] [Google Scholar]
- 17.Memon AA, Khan A, Zafar H, Murtaza G, Zaidi M. Repair of large and giant incisional hernia with onlay mesh: Perspective of a tertiary care hospital of a developing country. Int J Surg. 2013;11(1):41–5. [DOI] [PubMed] [Google Scholar]
- 18.Krpata DM, Blatnik JA, Novitsky YW, Rosen MJ. Posterior and open anterior components separations: a comparative analysis. Am J Surg. 2012;203(3):318–22. [DOI] [PubMed] [Google Scholar]
- 19.Blatnik JA, Krpata DM, Novitsky YW. Transversus Abdominis Release as an Alternative Component Separation Technique for Ventral Hernia Repair. Jama Surg. 2016;151(4):383–4. [DOI] [PubMed] [Google Scholar]
- 20.Gurusamy KS, Allen VB. Wound drains after incisional hernia repair. Cochrane Database Syst Rev. 2013(12):CD005570. [DOI] [PMC free article] [PubMed] [Google Scholar]


